Abstract
Glioblastoma multiforme (GBM) is the most malignant brain tumor. Microglia/macrophages are found within human GBM where they likely promote tumor progression. We report that CCL5, CCR1, and CCR5 are expressed in glioblastoma. Individual deletion of CCR1 or CCR5 had little to no effect on survival of tumor bearing mice, or numbers of glioblastoma-infiltrated microglia/macrophages or lymphocytes. CCL5 promoted in vitro migration of wild type, CCR1- or CCR5-deficient microglia/macrophages that was blocked by the dual CCR1/CCR5 antagonist, Met-CCL5. These data suggest that CCL5 functions within the glioblastoma microenvironment through CCR1 and CCR5 in a redundant manner.
Keywords: glioblastoma, GL261, microglia, Met-CCL5, RANTES
1. Introduction
Among more than 120 types of brain tumor, glioblastoma multiforme (GBM) is the most aggressive and lethal form due to its rapid growth rate, high invasiveness to surrounding normal tissues, and ability to escape the host immune system (Kelly et al. 1984, Prados, 2000, Furnari et al., 1995, Kleihues et al., 1995, Holland, 2001). Although several therapeutic approaches have been applied, including surgery, radiotherapy, and chemotherapy, none of these treatments are very effective at halting tumor progression (Marks, 1989, Mangiola et al., 2010, Hosli et al., 1998, Nicholas et al., 2011). Of interest, a large proportion of microglia/macrophages cells are found within this type of malignant human glioma as well as mouse models of the disease (Graeber et al., 2002, Liu et al., 2009, Charles et al., 2011). Generally, these myeloid-derived, macrophage-like microglial cells function as the main form of immune defense in the central nervous system (Gehrmann et al., 1995, Streit, 2001, Alliot et al., 1999, Chan et al., 2007, Rivest, 2009). When activated by pathological changes in the brain, they transform from a resting state to a pro-inflammatory phenotype that is capable of phagocytosis, cytotoxicity, and antigen presentation through the high expression of major histocompatibility complex (MHC) class II molecules (Davalos et al., 2005, Fetler and Amigorena, 2005, Gehrmann et al., 1995, Nimmerjahn et al., 2005, Stoll et al., 2006). Opposite to their normal function in the immune system, glioma infiltrated microglia/macrophages show an anti-inflammatory phenotype, in which the expression of MHC class II molecules is down-regulated, especially in brain tumors with high malignancy (Badie et al., 2002, Bettinger et al., 2002, Flügel et al., 1999, Morantz et al., 1979, Tran et al., 1998, Badie and Schartner, 2001, Watters et al., 2005, Roggendorf et al., 1996), possibly by glioma-secreted factors, including IL-4, IL-6, IL-10, transforming growth factor β (TGFβ), and prostaglandin E2, (Charles et al., 2011). A positive correlation between the number of infiltrating microglia/macrophages and the proliferation rate of the tumor is also evident (Graeber et al., 2002, Morimura et al., 1990). Thus, these immune cells are believed to contribute to the local immunosuppressive milieu of glioma, as well as promote tumor progression (Hussain et al., 2006, Yang et al., 2010, Markovic et al., 2005). Despite the potential importance of microglia/macrophages in glioma tumorigenesis, the mechanism by which microglia/macrophages infiltrate into the tumor is still unknown. Increasing evidence supports the hypothesis that glioma regulates this mechanism through different factors, including hepatocyte growth factor/scatter factor (HGF/SF), colony-stimulating factor 1 (CSF-1), granulocyte-colony stimulatory factor (G-CSF) (Badie et al., 1999, Alterman and Standley, 1994, Nitta et al., 1992), or possibly MMP-2 and MMP-9, which are found abundantly within glioma (Nakada et al., 2003). Current studies strongly suggest this localization may require the presence of chemokines, a family of chemoattractant cytokines, in the tumor microenvironment (Sciumè et al., 2010, Kielian et al., 2002, Platten et al., 2003, Leung et al., 1997).
Due to their chemoattractant property, chemokines and chemokine receptors are well-known for guiding the migration of cells (Allen et al., 2007). During tumorigenesis, chemokine networks play important roles in many processes required for tumor development, such as tumor growth, proliferation, invasion, and angiogenesis (Balkwill, 2004, Kakinuma and Hwang, 2006, Koizumi et al., 2007, Mantovani et al., 2010, Bajetto et al., 2002). In the case of glioblastoma, treatment with the CXCR4 antagonist, AMD 3100, inhibits growth of murine intracranial glioblastoma and reduces the proliferation of tumor cells (Rubin et al., 2003). We have determined that the CXCR3 antagonist, NBI-74330, decreases tumor growth and prolongs survival in glioma-bearing mice (Liu et al., 2011). With regard to the interaction between microglia/macrophages and glioma, increasing evidence supports the participation of chemokines in glioma-microglia/macrophages crosstalk (Boehme et al., 2000, Semple et al., 2010). For example, CCL2 is a well described candidate for this function as an anti-CCL2 antibody prevented the migration of microglia/macrophages in response to CCL2 (Kielian et al., 2002, Platten et al., 2003). In contrast, recent evidence indicated that CCL7 (monocyte chemo-attractant protein-3), but not CCL2, is indeed the chemokine responsible for this function since CCL7 was predominantly expressed in glioma cell lines and the expression level of CCL7 is correlated to the percentage of GIM (glioma infiltrated microglia/macrophages) in glioma tissues (Okada et al., 2009).
Another member of the CC family, CCL5 (alternatively named RANTES) also promotes macrophage and lymphocyte infiltration in various types of human cancers (Robinson et al., 2003, Soria and Ben-Baruch, 2008). In a transplantable model of breast carcinoma, CCL5 was determined to be expressed by tumor cells, while its receptor, CCR5, was localized to infiltrating macrophages and lymphocytes. Furthermore, the dual CCR1/CCR5 antagonist, Met-CCL5, was able to reduce tumor growth and inhibit the migration of macrophages and lymphocytes into 410.4 tumors, suggesting a potential role of CCL5 in tumor-promoting macrophage/lymphocyte infiltration (Robinson et al., 2003). A query of the NCI REMBRANDT (National Cancer Institution REpository for Molecular BRAin Neoplasia DaTa) database using CCL5 as the search term, revealed an association between CCL5 expression and survival outcomes in “All Glioma” patients. A significantly shorter mean survival time was evident in tumors having high CCL5 as compared to those characterized by intermediate levels of CCL5; insufficient numbers of patients with low CCL5 limited analysis of this patient population. The observations suggest a significant role of CCL5 within glioma, as well as a potential mechanism of immune and tumor cells interaction, through this ligand.
Although the expression of CCL5 and its receptors, CCR1 and CCR5, is documented in different cancer models, no study on the role of the CCL5/CCR1/CCR5 axis in glioblastoma has been reported. Herein, we establish that CCL5 is highly expressed in murine and human GBM cell lines, and found abundantly in tumors of glioblastoma bearing mice where CCR1 and CCR5 are also expressed. Individual deletion of either CCR1 or CCR5 had little to no impact on survival rates of glioblastoma-bearing or the numbers of tumor-infiltrated immune CD11b+, CD4+, and CD8+ cells. An in vitro analysis showed that CCR1 and CCR5 were expressed by primary microglia suggesting functional redundancy in this system. Indeed, the dual CCR1/CCR5 antagonist, Met-CCL5, was able to inhibit CCL5-dependent migration of wild type, CCR1- and CCR5-deficient microglia. Collectively, the results indicate that the infiltration of microglia/macrophages into glioblastoma, as well as the survival of tumor bearing mice, does not solely depend on either CCR1 or CCR5 but suggests a potential mechanism of redundancy, where CCL5 directs the infiltration of microglia/macrophages into glioblastoma through both CCR1 and CCR5.
2. Materials and Methods
2.1. Animals
Wild type (WT) C57BL/6 mice were obtained from either JAX Laboratory (Bar Harbor, ME) or Taconic Inc. (Hudson, NY). CCR1- and CCR5-deficient mice, backcrossed to the C57BL/6 background for greater than 10 generations, were purchased from Taconic Inc. and JAX Laboratory, respectively. Experimental endpoints from CCR1-deficient mice were compared to WT mice purchased from Taconic Inc. while endpoints from CCR5-deficient mice were compared to WT mice obtained from the JAX Laboratory. All procedures involving animal housing and surgical protocols were followed according to the guidelines of the University of Florida Institutional Animal Care and Use Committee (IACUC).
2.2. Cell culture
The human glioblastoma cell lines T98G and U87 were maintained in Eagle’s minimum essential medium (EMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% penicillin–streptomycin, 1% sodium pyruvate and 2mM L-glutamine. The U118 GBM cell line was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated FBS, 1% penicillin-streptomycin and 2mM L-glutamine. Primary patient derived GBM cell lines L0, L1, and L2, generously provided by Dr. Brent A. Reynolds (Department of Neurosurgery, University of Florida), were cultured in DMEM/F12 medium supplemented with 2% B27, 20ng/ml of epidermal growth factor (EGF) and 1% penicillin-streptomycin. The GL261 murine glioblastoma cell line was maintained in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 1% penicillin-streptomycin, and 4mM L-glutamine. To generate gliomaspheres, GL261 glioblastoma cells were cultured in DMEM/F12 medium supplemented with 2% B27, 20ng/ml of epidermal growth fctor (EGF) and basic fibroblast growth factor (bFGF), 5 µg/ml of heparin and 1% penicillin-streptomycin, using non Poly-D-Lysine treated T75 flasks. All cells were grown in a humidified incubator at 37°C with 5% CO2. DMEM, EMEM, RPMI-1640, DMEM/F12 medium, B27, EGF, bFGF, L-glutamine and antibiotics were obtained from Gibco-BRL (Invitrogen, Carlsbad, CA). Sodium pyruvate and heparin were purchased from Sigma-Aldrich (St Louis, MO). FBS was from HyClone (Thermo Scientific, Waltham, MA)
2.3. Primary microglia isolation
Primary microglia were harvested from postnatal one day old mouse pups using a previously published protocol (Saura et al., 2003). Briefly, brain tissue was removed, mechanically and enzymatically dissociated, and kept in medium A containing 0.585% glucose (Sigma-Aldrich), 15mM HEPES, 100 υ/ml penicillin, and 100 µg/ml streptomycin in HBSS (Gibco). The finely minced brain tissue was incubated in 0.25% trypsin medium (Gibco) for 30–45 minutes at 37°C. The medium was aspirated and replaced with trypsin inhibitor medium (Invitrogen). After incubation for 4 min at room temperature, the tissue was triturated with a fire-polished glass pipette and then centrifuged for 15 min at 100×g. The supernatant was aspirated and the cell suspension was plated in T75 flasks with DMEM/F12 medium supplemented with 10% FBS, 1% sodium pyruvate, and 1% penicillin and streptomycin. Culture medium was changed every 3–4 days. After 15 days, cultures were treated with 0.0625% trypsin-EDTA (diluted in DMEM/F12) for 1 hour at 37°C to lift astrocytes and neurons from the flasks, leaving an essentially pure culture of primary microglia. The cultures were checked for purity and found to be greater than 97% microglia as measured by cell-type specific expression of CD11b. Purified primary microglia were collected using 2.5% trypsin with EDTA for RT-PCR and migration analyses.
2.4. Reverse transcription-polymerase chain reaction (RT-PCR)
To isolate total RNA from normal brain and tumor tissues, approximately 1cm3 fragments of normal and tumor tissues were removed from the brains of GL261-implanted mice and immersed immediately in TRIzol reagent (Invitrogen). The normal tissue was collected from the non-implanted hemisphere to avoid contamination of tumor tissue. All samples were homogenized with a homogenizer and processed to RNA isolation as indicated by the manufacturer’s instructions. Total RNA was also isolated from microglia and glioma cells with the TRIzol reagent. Genomic DNA contamination was removed by RQ1 RNase-free DNase treatment (Promega, Madison, WI). Total RNA was then quantified and stored at −80°C. RNA (1µg) was retrotranscribed with iScript complementary DNA (cDNA) synthesis kit (BioRad, Hercules, CA). Synthesized cDNA was subjected to PCR analysis. PCR was performed by heating for 96°C for 2 min, followed by amplification for 35 cycles: 96°C for 30 sec, 56°C for 1 min, and 72°C for 1 min. The following primers were used: murine CCL5: 5’ ggtaccatgaagatctctgca 3’ (forward) and 5’ agcaagccatgacagggaagc 3’ (reverse); human CCL5: 5’ cgtgcccacatcaaggag 3’ (forward) and 5’ ggacaagagcaagcagaaac 3’ (reverse); murine CCR1: 5’ gtggtgggcaatgtcctagt (forward) and 5’ tcagattgtagggggtccag 3’ (reverse); murine CCR5: 5’ tcagttccgacctatatctatg 3’ (forward) and 5’ gtggaaaatgaggactgcatgt 3’ (reverse); murine CX3CR1: 5’ atgccatgtgcaagctca 3’ (forward) and 5’ cttcatgtcacaactggg 3’ (reversed); murine glyceraldehyde 3-phosphate dehydrogenase: 5’ aaatggtgaaggtcggtgtg 3’ (forward) and 5’ tctccatggtggtgaagaca 3’ (reverse); human glycealdehyde 3-phosphate dehydrogenase: 5’ cgagatccctccaaaatcaa 3’ (forward) and 5’ tgctgtagccaaattcgttg 3’ (reverse). The RT-PCR analyses on tumor and normal brain tissues were repeated two times using two different animals. With primary cultured microglia, the experiments were independently performed at least three times with different microglia preparations.
2.5. Cytokine protein array
The presence of cytokines in GL261 cell conditioned medium was determined by mouse cytokine antibody array (R&D System, Minneapolis, MN). Membranes were treated with blocking buffer and incubated for 30 min at room temperature. The membrane was then exposed to an aliquot (1 ml) of GL261 conditioned medium and incubated for 1 h at room temperature. After this incubation period, the membranes were washed five times with a washing buffer and incubated for 1 h at room temperature with biotin-conjugated antibodies against murine cytokines (dilution: 1:250 dilution). Thereafter, the membranes were washed five times, incubated for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated streptavidin (dilution: 1:1000) and washed five times again. Finally, the reaction was developed in a mixture of SuperSignal West Pico luminol/enhancer and stable peroxide solutions (Thermo Scientific, Rockford, IL) and exposed to X-ray film. The densities of signals on films were then analyzed with ImageJ software (NIH). This experiment was performed in duplicate.
2.6. Glioma cell implantations
GL261 glioma cells (1×105) in a total volume not exceeding 3µl were injected 3mm deep into the right cerebral hemisphere (1mm posterior and 2mm lateral from Bregma) of wild type, CCR1-, and CCR5-deficient mice. For Kaplan-Meier survival rate analysis, percentages of surviving mice in the various groups of animals were recorded daily after GL261 glioma implantation. The endpoint was defined by a lack of physical activity and a body weight reduction of greater than 15%. The data were subjected to log-rank test in order to determine if significant differences existed in survival between the experimental groups. The numbers of animals used in each group are indicated in the graphs. For other experiments, glioma bearing mice (3 weeks after GL261 cell injection) were euthanized using sodium pentobarbital (32mg/kg) and subsequently perfused with 0.9% saline followed by buffered 4% paraformaldehyde (PFA). Brains were surgically removed and post-fixed with 4% PFA. After fixation, tissues were incubated in 30% sucrose solution at 4°C overnight followed by liquid nitrogen freezing. Frozen brains were then sectioned with a cryostat and subjected to either in situ hybridization or immunohistochemistry.
2.7. In situ hybridization and immunohistochemistry
In situ hybridization was performed as described previously (Harrison et al., 2003). Multiple sections from tumor bearing animals were analyzed and included the following number of mice: wild type (JAX Laboratory): 11; wild type (Taconic Inc): 9; CCR1−/−: 7; CCR5−/−: 2. For immunohistochemistry, brain sections were permeabilized with 0.5% of Triton X-100 in phosphate-buffered saline (PBS) for 15 min at room temperature followed by blocking with 10% goat serum in PBS for 30 min. The sections were then incubated in primary antibodies at room temperature. The following antibodies were used: rat anti-CD4 (dilution 1:50, BD Pharmingen), rat anti-CD8 (dilution 1:50, Serotec), rat anti-CD11b (dilution 1:50, Serotec). After 2 hours, sections were washed three times with PBS and incubated subsequently in goat anti-rat Alexa 594 (dilution 1:1000, BD Pharmingen). The sections were then washed three times with PBS and finally counterstained with DAPI. For quantification of CD4+, CD8+, and CD11b+ cells, the number of cells in three high-powered field, which was the visible area under 20× magnification, in three sections from multiple animals were calculated to determine the mean and standard error of mean. The data were subjected to statistical analysis (one tail T-test). The numbers of animals used in each group are indicated in the graphs.
2.8. In vitro migration
In vitro migration assays were performed using 24-well transwell units with 8-µm polycarbonated filters (BD Falcon, Franklin Lakes, NJ). After trypsinization with 2.5% trypsin, microglia (2×104 cells) were placed in the top compartment in 500µl of serum-free DMEM/F12 with or without 50nM of Met-CCL5. The bottom wells contained 500µl of serum-free DMEM/F12 with or without 20nM of CCL5. The plates were incubated for 24 hours at 37°C. Non-migrating cells were removed by wiping the upper side of the insert with a cotton swab. Migrating cells were fixed with 4% paraformaldehyde for 20 min at room temperature, stained with hematoxylin, and quantified by counting the number of cells on 4 random areas on the lower side of the membrane. Each experiment was performed in triplicate and repeated three times using different microglial preparations.
2.9. Statistical analysis
All statistical analyses were calculated using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA). All data are presented as mean and standard error of mean. P-values were calculated using Student’s t-test with two-tailed distribution. Survival data were subjected to log-rank test to determine statistically significant differences between groups. A p-value <0.05 was considered significant and is indicated by symbols depicted in the figures and figure legends.
3. Results
3.1. CCL5 is expressed by murine GL261 and human GBM cell lines
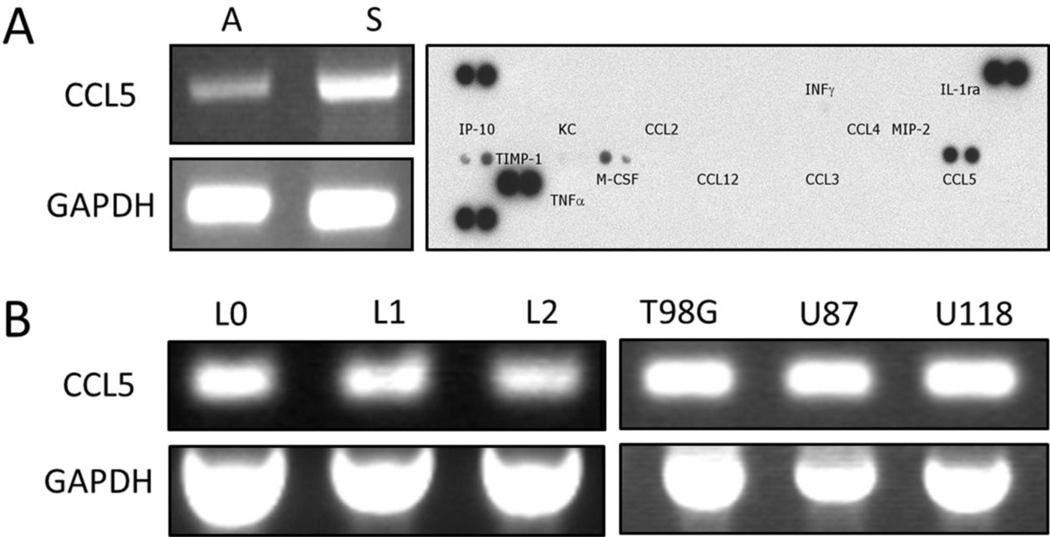
Because chemokine CCL5 has been found in various types of tumor, including prostate, cervical, and breast cancers (Vaday et al., 2006, Niwa et al., 2001), we were interested in determining if CCL5 was also present in glioblastoma. The expression of CCL5 in vitro was assessed by RT-PCR analysis in the murine GL261 glioblastoma cell line. The results showed that CCL5 mRNA was present in cells cultured as adherent monolayers and under culture conditions that favored formation of gliomaspheres; increased levels of CCL5 mRNA were noted in the gliomaspheres. In addition, CCL5 peptide was detected in the GL261 conditioned medium, using a cytokine protein array (Fig. 1A), indicating that GL261 cells are capable of both producing and releasing CCL5 protein. The expression of this chemokine was also identified in several different human glioblastoma cells. CCL5 mRNA, detected by RT-PCR analysis, was found in primary patient-derived lines L0, L1, and L2 that were cultured under serum-free conditions in the presence of EGF and bFGF, as well as the commonly used glioblastoma lines T98G, U87, and U118 cultured in the presence of serum (Fig. 1B). None of the lines expressed CCR1 and CCR5 (data not shown).
Figure 1.
Expression of CCL5 by murine GL261 and human GBM cell lines. A) Left: RT-PCR analysis detecting CCL5 mRNA in murine GL261 cells, cultured as either adherent monolayers (A) or gliomaspheres (S). GAPDH served as a control. Right: CCL5 protein detected in GL261 conditioned medium, using cytokine protein array. B) RT-PCR analysis of CCL5 mRNA in different human GBM cell lines, L0, L1, and L2 as well as commonly used standard glioblastoma cell lines, T98G, U87, and U118. GAPDH served as a control.
3.2. CCL5, CCR1, and CCR5 are present in murine GL261 glioblastomas
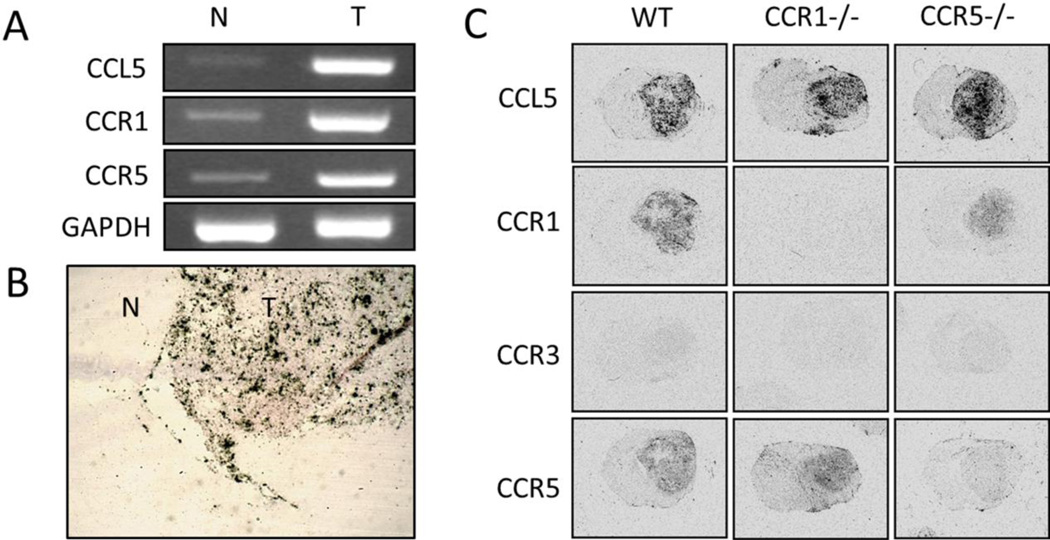
The in vivo expression of CCL5 in the tissues taken from GL261 glioblastoma-bearing wild type mice was evaluated by RT-PCR and in situ hybridization analyses. In both cases, mRNA levels of CCL5 were significantly elevated in the tumor tissue, when compared to the normal brain area (Fig. 2A, B). Together with the in vitro data above, these results suggested that tumor cells are the primary source for CCL5 production within glioblastoma. In addition, the two major receptors of this chemokine, CCR1 and CCR5, were strongly detected in the tumor tissues (Fig. 2A, C). We also evaluated the expression of CCR3, another low affinity CCL5 receptor. The lack of signal of this receptor from in situ hybridization analysis indicated that CCR3 was not expressed in intracranial GL261 glioblastomas (Fig. 2C). Immunohistochemical localization of CCR1 and CCR5 within the tumors was evaluated but the lack of availability of suitable antibodies precluded definitive identification of these receptors.
Figure 2.
Expression of CCL5 and its receptors CCR1 and CCR5 in murine GL261 glioblastomas. A) RT-PCR analysis in normal (N) and tumor (T) tissues. In situ hybridization (ISH) analysis depicting the expression of B) CCL5 in a section containing normal (N) and tumor (T) tissue from a glioma-bearing wild type (WT) C57BL/6 mouse, and C) CCL5, CCR1, CCR3, and CCR5 in WT, CCR1 and CCR5-deficient mice.
The expression of CCL5 and its receptors, CCR1 and CCR5, was also analyzed in the tissues collected from tumor bearing CCR1−/− and CCR5−/− mice. In situ hybridization analysis showed that CCL5 was expressed at high levels within tumors of these animals, suggesting that CCR1 and CCR5 deficiency did not affect the expression of this chemokine within the tumor. In contrast, while both CCR1 and CCR5 were present in the tumors from wild type mice, the signals for CCR1 and CCR5 were greatly diminished in glioblastomas from CCR1−/− and CCR5−/− mice, respectively (Fig. 2C). This observation indicates that intratumoral CCR1- and CCR5-expressing cells are derived from the recipient mice, and not from the culture-derived GL261 glioblastoma cells that were implanted into the animals.
3.3. Neither CCR1 nor CCR5 deficiency contributed to the infiltration of immune cells into glioblastoma, or impacted the survival of tumor bearing mice
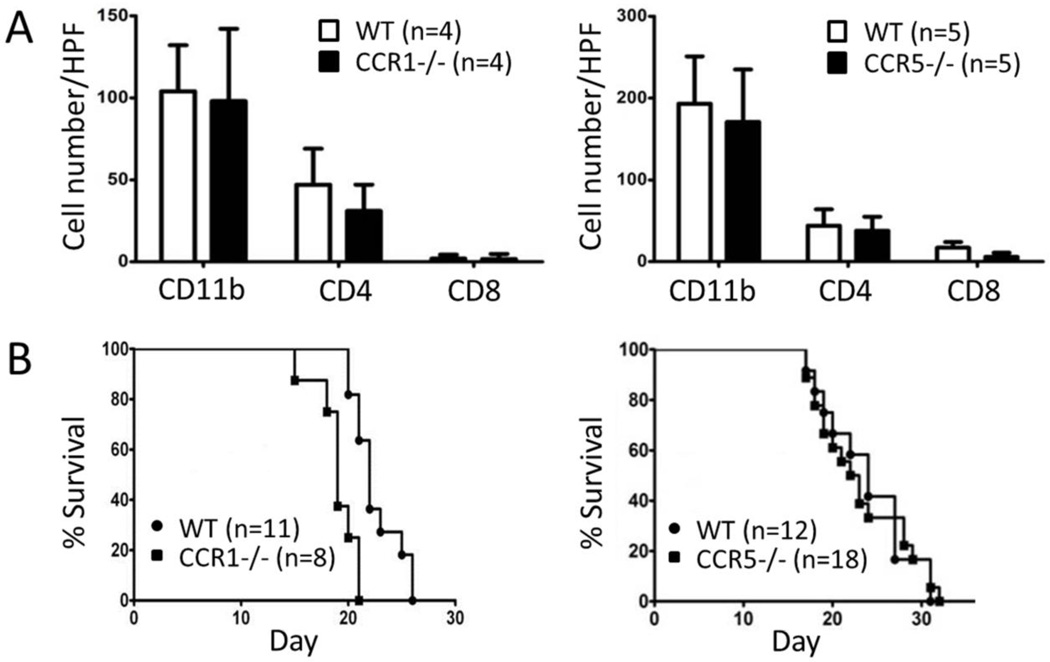
To evaluate the hypothesis that CCL5 directs the localization of microglia/macrophages into glioblastoma in a CCR1 or CCR5 dependent manner, we characterized immune cell infiltration and animal survival in tumor bearing CCR1- and CCR5-deficient mice. Immunohistochemical analysis was performed to investigate if CCR1 and CCR5 regulated the infiltration of immune cells, including CD11b+ microglia/macrophages, CD4+ and CD8+ T cells, into glioblastoma. Quantitative analysis did not show any statistically significant differences in the number of these cells located within the glioblastomas of tumor bearing CCR1−/− and CCR5−/− mice, as compared to the wild type animals (Fig. 3A); the supplementary figure depicts representative images of the immunohistochemistry. The effect of CCR1 and CCR5 deficiency on the survival of glioblastoma bearing mice among wild type, CCR1−/−, and CCR5−/− groups was also assessed using Kaplan-Meier survival analysis. No significant difference in the survival rate between tumor bearing wild type and CCR5−/− groups were evident (p value = 0.77). The life span of glioblastoma bearing CCR1−/− mice, however, was slightly shorter than the life span of the wild type (p value = 0.0007). The median survival time of tumor bearing CCR1−/− mice after GL261 cell implantation was 19 days, while that of glioblastoma bearing wild type mice was 22 days. Together, these results suggest that CCR1 and CCR5 deficiency had no impact on the infiltration of CD11+, CD4+, and CD8+ immune cells into glioblastoma, and at best, only a modest effect on the survival rate of tumor bearing mice.
Figure 3.
Effect of CCR1 and CCR5 deficiency on infiltration of immune cells into glioblastoma and survival of tumor bearing mice. A) Numbers of intra-glioblastoma CD11b+, CD4+, and CD8+ in CCR1 (left, black) and CCR5 (right, black)-deficient mice, compared to WT (white) mice. B) Kaplan-Meier survival plots of glioblastoma bearing CCR1 (left, square) and CCR5 (right, square)-deficient mice, compared to WT (circles) mice. The numbers of animals that were used in each group are indicated.
3.4. CCL5 interacts with its microglia-expressed receptors, CCR1 and CCR5, in a redundant manner
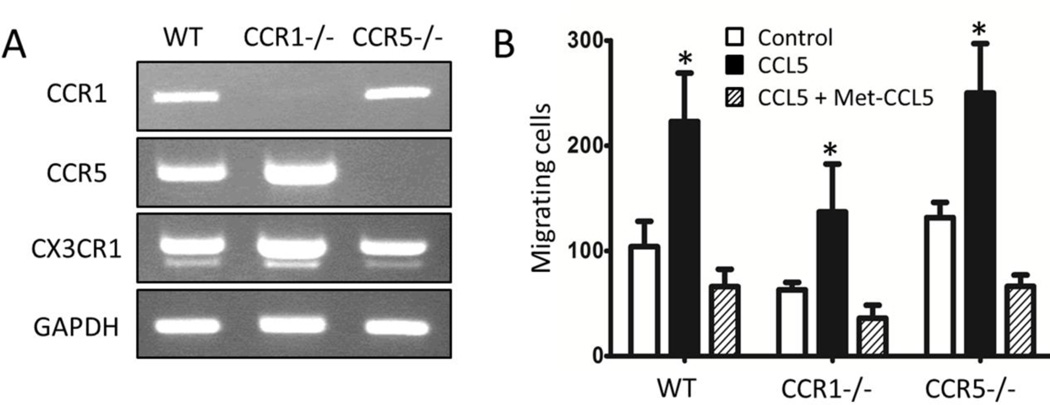
As neither CCR1 nor CCR5 contribute individually to the action of CCL5 on the localization of microglia/macrophages into glioblastoma, as well as the survival of tumor bearing mice, we next examined the possibility that CCL5 may interact with CCR1 and CCR5 in a redundant manner. The in vitro expression of CCR1 and CCR5 was determined on primary cultures of microglia derived from wild type, CCR1−/−, and CCR5−/− mice. Both CCR1 and CCR5 mRNAs were detected in microglia using RT-PCR analysis, suggesting that these CCL5 receptors are co-expressed by these cells in vitro (Fig. 4A). The absence of CCR1 and CCR5 mRNA signals in CCR1−/− and CCR5−/− mice, respectively, confirmed that these gene products were deleted in the microglia derived from the CCR1- and CCR5-deficient animals. CX3CR1, a microglia-expressed chemokine receptor, was detected in microglia cultures from all of the animals. We also pursued the expression of CCR1 and CCR5 protein by microglia in vitro, but similar to our attempts to detect these receptors in vivo, the lack of quality antibodies reactive towards murine CCR1 and CCR5 posed significant challenges to identifying these receptors. Thus, a functional analysis of CCL5-dependent migration of microglia was assessed using the modified Boyden chamber assay (Fig. 4B). Migration of wild type, as well as CCR1−/− and CCR5−/− microglia was stimulated by CCL5. Met-CCL5, a modified form of CCL5 that has antagonist activity at both CCR1 and CCR5, completely blocked the migration by CCL5 in all groups.
Figure 4.
In vitro expression of CCR1 and CCR5 by primary microglia cultures and the effect of Met-CCL5 on CCL5-stimulated migration of microglia. A) RT-PCR analysis of CCR1 and CCR5 expressed by WT, CCR1- (CCR1−/−), and CCR5- (CCR5−/−) deficient microglia. CX3CR1 and GAPDH served as controls. B) Migration of WT, CCR1-, and CCR5-deficient microglia treated with CCL5 (black), or with CCL5 and Met-CCL5 (stripe), compared to control (white). *p < 0.05, compared to either control or CCL5/Met-CCL5.
4. Discussion
The presence of a high number of infiltrated microglia/macrophages within glioma, confirmed by various studies both in human glioma and rodent models of the disease (Graeber et al., 2002, Liu et al., 2009, Charles et al., 2011), has prompted further investigations into the role of these immune competent cells within the tumor microenvironment. Accumulating evidence suggests that microglia/macrophages facilitate glioma growth by contributing to the immunosuppressive environment and directly assisting in tumor growth and invasion (Markovic et al., 2005, Markovic et al., 2009, Sliwa et al., 2007, Morimura et al., 1990, Zhai et al., 2011). Understanding the mechanisms by which microglia/macrophages cells are localized to glioma will provide novel therapeutic targets for intervention. Here we report on the expression of CCL5 and its receptors, CCR1 and CCR5, in glioblastoma. Our data demonstrate that CCL5 is highly expressed by human and murine GL261 GBM cells in vitro, as well within intracranial glioblastomas in vivo. Moreover, CCL5 is upregulated in GL261 cells cultured under condition that favors growth of the cells as spheres; cells cultured under this condition exhibit a more malignant phenotype (Lee et al., 2006, Pellegatta et al., 2006). Its receptors CCR1 and CCR5, on the other hand, are expressed by cultured microglia, but not by glioblastoma cells, and are also highly expressed within intracranial tumors. The loss of CCR1 and CCR5 in situ hybridization signals from tumor sections from CCR1- and CCR5-deficient mice also indicates that the glioblastoma cells do not express either of these receptors. These data support the hypothesis that glioblastoma-expressed CCL5 may be a key regulator in the crosstalk between glioblastoma and microglia/macrophages, through an interaction with microglia/macrophages-expressed CCR1 or CCR5, and that blocking either CCR1 or CCR5 could prevent the glioblastoma localization of microglia/macrophages.
Glioblastoma growth and numbers of intra-tumoral microglia/macrophages cells, together with CD4+ and CD8+ T cells, were evaluated in mice individually deficient in either CCR1 or CCR5. Our data showed that microglia/macrophages infiltration was not attenuated in either CCR1- or CCR5-deficient tumor-bearing animals. CD4+ and CD8+ cells were also similar in tumors from receptor deficient and wild type mice. Moreover, there was only a modest, in the case of CCR1-deficiency, or no difference, with CCR5-deficiency, in survival rates between glioblastoma-bearing wild type mice and the chemokine receptor-deficient tumor-bearing animals. Our explanation for these observations is that CCL5 has a high affinity for both CCR1 and CCR5, and blocking either one of them individually is not sufficient to inhibit the actions of this chemokine. Due to the promiscuous interactions of chemokines and chemokine receptors, this redundant mechanism needs to be considered. Indeed, this issue has been addressed in several studies (Iida et al., 2008, de Lemos et al., 2005, Remick et al., 2001, Repeke et al., 2010). In a recent study that characterized the role of these receptors in a periodontal disease model, Repeke and colleagues found that CCR1- and CCR5-deficient mice present a lower leukocyte infiltration and alveolar bone loss than wild type mice, yet inhibiting both of these receptors by Met-CCL5 (a dual CCR1/CCR5 antagonist) severely attenuated the inflammatory bone resorption (Repeke et al., 2010). A redundancy mechanism in glioblastoma is consistent with our in vitro investigation which indicates that CCR1 and CCR5 are co-expressed by primary microglia in vitro, and that the dual antagonist Met-CCL5 could completely block the migratory effect of CCL5 on these cells regardless of whether the cells expressed CCR1 or CCR5. Nonetheless, our data does not exclude the possibility that CCL5 may act through another unidentified receptor(s).
While the evidence for a cooperative role of CCR1 and CCR5 is suggested from the in vitro study, a redundancy of these chemokine receptors in this murine glioblastoma model has not yet been evaluated in vivo. A combined CCR1- and CCR5-deficient mouse would offer a model system to test the redundancy hypothesis. However, the CCR1 and CCR5 genes are located adjacent to each other on murine chromosome 9, providing a major limitation in generating the double knockout mice by simply breeding CCR1- and CCR5-deficient lines. Alternative pharmacologically-based approaches are worth considering but these also have limitations. Although Met-CCL5 has been used as a dual antagonist for both CCR1 and CCR5 in in vitro studies (Proudfoot et al., 1996, Robinson et al., 2003), effectively targeting these receptors located within the brain would require the peptide to penetrate the blood brain barrier, one of the most important obstacles that restricted the delivery of molecularly targeted therapy to glioma (Agarwal et al., 2011). A combined pharmacological and genetic approach is an alternative strategy worthy of consideration. In particular, CCR1-deficient mice could be treated with a CCR5 antagonist, e.g. TAK-779, while CCR5-deficient mice would be treated with the CCR1 antagonist, BX-741. Both of these drugs have been tested for their effective delivery to the CNS, when administered systemically (Liang et al., 2000, Longden et al., 2008, Maeda et al., 2006, Ni et al., 2009). However, as BX-741 and TAK-779 were specifically designed for human CCR1 and CCR5 respectively, the low binding affinity for mouse CCR1 and CCR5 is a primary concern. These obstacles together provide significant challenges for addressing the redundant hypothesis through in vivo experimentation.
The interaction between microglia/macrophages and glioblastoma is complex and likely to involve bi-directional signaling. After recruitment into the tumor milieu by glioblastoma cells, microglia/macrophages lose control of their immune surveillance property and, in turn, help facilitate tumor progression. Since the recruitment of microglia/macrophages likely depends on their interaction with numerous glioblastoma-secreted factors or by other components in the tumor microenvironment, it is not surprising that this process is regulated in a redundant manner. Our findings support the notion that the function of CCL5 in glioblastoma is not dependent on individual interactions with either CCR1 or CCR5. To the contrary, the CCL5/CCR1/CCR5 network likely involves a redundant mechanism, adding more complexity to glioblastoma-microglia/macrophages crosstalk and signaling a need to block both receptors to prevent the actions of this chemokine in this highly malignant form of brain cancer.
Supplementary Material
Acknowledgments
Acknowledgement of funding source: the studies were funded by a grant from the National Institutes of Health to J.K.H. (AI058256)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal S, Sane R, Oberoi R, Ohfest J, Elmquist W. Delivery of molecularly targetd therapy to malignant glioma, a disease of the whole brain. Expert Reviews in Molecular Medicine. 2011;13:1–27. doi: 10.1017/S1462399411001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J, Crown E, Handel M. Chemokine: receptor structure, interactions, and antagonism. Annual Review of Immunology. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Developmental Brain Research. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- Alterman R, Standley E. Colony stimulating factor-1 expression in human glioma. Molecular and Chemical Neuropathology. 1994;21:177–188. doi: 10.1007/BF02815350. [DOI] [PubMed] [Google Scholar]
- Badie B, Bartley B, Schartner J. Differential expression of MHC class II and B7 costimulatory molecules by microglia in rodent gliomas. Journal of Neuroimmunology. 2002;133:39–45. doi: 10.1016/s0165-5728(02)00350-8. [DOI] [PubMed] [Google Scholar]
- Badie B, Schartner J. Role of microglia in glioma biology. Microscopy Research and Technique. 2001;54:106–113. doi: 10.1002/jemt.1125. [DOI] [PubMed] [Google Scholar]
- Badie B, Schertner J, Klaver J, Vorpahl J. In vitro modulation of microglia motility by glioma cells is mediated by hepatocyte growth/scatter factor. Neurosurgery. 1999;44:1077–1082. doi: 10.1097/00006123-199905000-00075. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Schettini G. Characterization of chemokines and their receptors in the central nervous system: physiopathological implications. Journal of Neurochemistry. 2002;82:1311–1329. doi: 10.1046/j.1471-4159.2002.01091.x. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Cancer and the chemokine network. Nature Reviews. Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- Bettinger I, Thanos S, Paulus W. Microglia promote glioma migration. Acta Neuropathologica. 2002;103:351–355. doi: 10.1007/s00401-001-0472-x. [DOI] [PubMed] [Google Scholar]
- Boehme S, Lio F, Maciejewski-Lenoir D, Bacon K, Conlon P. The chemokine fractalkine inhibits Fas-mediated cell death of brain microglia. Journal of Immunology. 2000;165:397–403. doi: 10.4049/jimmunol.165.1.397. [DOI] [PubMed] [Google Scholar]
- Chan W, Kohsaka S, Rezaie P. The origin and cell lineage of microglia: new concepts. Brain Research Reviews. 2007;53:344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Charles N, Holland E, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;1180:1169–1180. doi: 10.1002/glia.21136. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim J, Zuo Y, Jung S, Littman D, Dustin M, Gan W. ATP mediates rapid microglial response to local brain injury in vivo. Nature Neuroscience. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- De Lemos C, Christensen J, Nansen A, Moos T, Lu B, Gerard Christensen, J, Thomsen A. Opposing effects of CXCR3 and CCR5 deficiency on CD8+ T cell-mediated inflammation in the central nervous system of virus-infected mice. Journal of Immunology. 2005;175:1767–1775. doi: 10.4049/jimmunol.175.3.1767. [DOI] [PubMed] [Google Scholar]
- Fetler L, Amigorena S. Brain Under Surveillance : The Microglia Patrol. Science. 2005;309:392–393. doi: 10.1126/science.1114852. [DOI] [PubMed] [Google Scholar]
- Flügel A, Labeur M, Grasbon-Frodl E, Kreutzberg G, Graeber M. Microglia only weakly present glioma antigen to cytotoxic T cells. International journal of Developmental Neuroscience. 1999;17:547–556. doi: 10.1016/s0736-5748(99)00020-9. [DOI] [PubMed] [Google Scholar]
- Furnari F, Huang H, Cavenee W. Genertics and malignant progression of human brain tumours. Cancer Surveys. 1995;25:233–275. [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg G. Microglia: Intrinsic immuneffector cell of the brain. Brain Research Reviews. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Graeber M, Scheithauer B, Kreutzberg G. Microglia in brain tumors. Glia. 2002;40:252–259. doi: 10.1002/glia.10147. [DOI] [PubMed] [Google Scholar]
- Harrison J, Luo D, Streit W. In situ hybridization analysis of chemokines and chemokine receptors in the central nervous system. Methods. 2003;29:312–318. doi: 10.1016/s1046-2023(02)00354-7. [DOI] [PubMed] [Google Scholar]
- Holland E. Gliomagenesis: genetic alterations and mouse models. Nature Reviews. Genetics. 2001;2:120–129. doi: 10.1038/35052535. [DOI] [PubMed] [Google Scholar]
- Hosli P, Sappino A, De Tribolet N, Dietrich P. Malignant glioma: Should chemotherapy be overthrown by experimental treatments. Annals of Oncology. 1998;9:589–600. doi: 10.1023/a:1008267312782. [DOI] [PubMed] [Google Scholar]
- Hussain S, Yang D, Suki D, Aldape K, Grimm E, Heimberger A. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro-Oncology. 2006;8:261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida N, Nakamoto Y, Baba T, Kakinoki K, Li Y, Wu Y, Matsushima K, Kaneko S, Mukaida N. Tumor cell apoptosis induces tumor-specific immunity in a CC chemokine receptor 1- and 5-dependent manner in mice. Journal of Leukocyte Biology. 2008;84:1001–1010. doi: 10.1189/jlb.1107791. [DOI] [PubMed] [Google Scholar]
- Kakinuma T, Hwang S. Chemokines, chemokine receptors, and cancer metastasis. Cancer. 2006;79:639–651. doi: 10.1189/jlb.1105633. [DOI] [PubMed] [Google Scholar]
- Kelly K, Kirkwood J, Kapp D. Glioblastoma multiforme: pathology, natural history and treatment. Cancer Treatment Reviews. 1984;11:1–26. doi: 10.1016/0305-7372(84)90014-8. [DOI] [PubMed] [Google Scholar]
- Kielian T, Van Rooijen N, Hickey W. MCP-1 expression in CNS-1 astrocytoma cells: implications for macrophage infiltration into tumors in vivo. Journal of Neuro-Oncology. 2002;56:1–12. doi: 10.1023/a:1014495613455. [DOI] [PubMed] [Google Scholar]
- Kleihues P, Soylemezoglu F, Schauble B, Burger P. Histopathology, classification, and grading of gliomas. Glia. 1995;15:211–221. doi: 10.1002/glia.440150303. [DOI] [PubMed] [Google Scholar]
- Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Science. 2007;98:1652–1658. doi: 10.1111/j.1349-7006.2007.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin N, Pastorino S, Purow B, Christopher N, Zhang W, Park J, Fine H. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Leung S, Wong M, Chung L, Chan A, S Y. Monocyte chemoattractant protein-1 expression and macrophage infiltration into gliomas. Acta Neurophathology. 1997:93. doi: 10.1007/s004010050647. [DOI] [PubMed] [Google Scholar]
- Liang M, Mallari C, Rosser M, Ng HP, May K, Monahan S, Bauman JG, Islam I, Ghannam A, Buckman B, Shaw K, Wei GP, Xu W, Zhao Z, Ho E, Shen J, Oanh H, Subramanyam B, Vergona R, Taub D, Dunning L, Harvey S, Snider RM, Hesselgesser J, Morrissey MM, Perez HD. Identification and characterization of a potent, selective, and orally active antagonist of the CC chemokine receptor-1. The Journal of Biological Chemistry. 2000;275:19000–19008. doi: 10.1074/jbc.M001222200. [DOI] [PubMed] [Google Scholar]
- Liu C, Luo D, Reynolds BA, Meher G, Katritzky AR, Lu B, Gerard CJ, Bhadha CP, Harrison JK. Chemokine receptor CXCR3 promotes growth of glioma. Carcinogenesis. 2011;32:129–137. doi: 10.1093/carcin/bgq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Luo D, Streit W, Harrison J. CX3CL1 and CX3CR1 in the GL261 murine model of glioma: CX3CR1 deficiency does not impact tumor growth or infiltration of microglia and lymphocytes. Journal of Neuroimmunology. 2009;198:98–105. doi: 10.1016/j.jneuroim.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longden J, Cooke E, Hill S. Effect of CCR5 receptor antagonists on endocytosis of the human CCR5 receptor in CHO-K1 cells. British Journal of Pharmacology. 2008;153:1513–1527. doi: 10.1038/sj.bjp.0707691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Das D, Ogata-Aoki H, Nakata H, Miyakawa T, Tojo Y, Norman R, Takaoka Y, Ding J, Arnold G, Arnold E, Mitsuya H. Structural and molecular interactions of CCR5 inhibitors with CCR5. The Journal of Biological Chemistry. 2006;281:12688–12698. doi: 10.1074/jbc.M512688200. [DOI] [PubMed] [Google Scholar]
- Mangiola A, Anike C, Pompucci A, Capone G, Rigante L, De Bonis P. Glioblastoma therapy: Going beyond Hercules Columns. Expert Reviews in Neurotherapy. 2010;10:507–514. doi: 10.1586/ern.09.158. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. The chemokine system in cancer biology and therapy. Cytokine & Growth Factor Reviews. 2010;21:27–39. doi: 10.1016/j.cytogfr.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Markovic D, Glass R, Synowitz M, Rooijen N, Kettenmann H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. Journal of Neuropathology and Experimental Neurology. 2005;64:754–762. doi: 10.1097/01.jnen.0000178445.33972.a9. [DOI] [PubMed] [Google Scholar]
- Markovic D, Vinnakota K, Chirasani S, Synowitz M, Raguet H, Stock K, Sliwa M, Lehmann S, Kälin R, Van Rooijen N, Holmbeck K, Heppner FL, Kiwit J, Matyash V, Lehnardt S, Kaminska B, Glass R, Kettenmann H. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. PNAS. 2009;106:12530–12535. doi: 10.1073/pnas.0804273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J. Radiation treatment of brain tumors: Concepts and strategies. Critical Reviews Neuroniology. 1989;5:93–112. [PubMed] [Google Scholar]
- Morantz R, Wood G, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. Journal of Neurosurgery. 1979;50:305–311. doi: 10.3171/jns.1979.50.3.0305. [DOI] [PubMed] [Google Scholar]
- Morimura T, Neuchrist C, Kitz K, Budka H. Monocyte subpopulations in human gliomas: expression of Fc and complement receptors and correlation with tumor proliferation. Acta Neuropathologica. 1990;80:287–294. doi: 10.1007/BF00294647. [DOI] [PubMed] [Google Scholar]
- Nakada M, Okada Y, Yamashita J. The role of matrix metalloproteinases in glioma invasion. Frontier in Bioscience. 2003;8:261–269. doi: 10.2741/1016. [DOI] [PubMed] [Google Scholar]
- Ni J, Zhu Y, Zhong X, Ding Y, Hou L, Tong X, Tang W, Ono S, Yang Y, Zuo J. The chemokine receptor antagonist, TAK-779, decreased experimental autoimmune encephalomyelitis by reducing inflammatory cell migration into the central nervous system, without affecting T cell function. British Journal of Pharmacology. 2009;158:2046–2056. doi: 10.1111/j.1476-5381.2009.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas M, Lukas R, Chmura S, Yamini B, Lesniak M, Pytel P. Molecular heterogeneity in glioblastoma: Therapeutic opportunities and challenges. Seminars in Oncology. 2011;38:243–253. doi: 10.1053/j.seminoncol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nitta T, Sata K, Allegretta M, Brocke S, Lim M, Mitchell D, Steinman L. Expression of granulocyte colony stimulating factor and grannulocyte-macrophage colony stimulating factor genes in human astrocytoma cell lines and in glioma specimens. Brain Research. 1992:571. doi: 10.1016/0006-8993(92)90505-4. [DOI] [PubMed] [Google Scholar]
- Niwa Y, Akamatsu H, Niwa H, Sumi H, Ozaki Y, Abe A. Correlation of tissue and plasma RANTES levels with disease course in patients with breast or cervical cancer. Clinical Cancer Research. 2001;7:285–289. [PubMed] [Google Scholar]
- Okada M, Saio M, Kito Y, Ohe N, Yano H, Yoshimura S, Iwama T, Takami T. Tumor-associated macrophage/microglia infiltration in human gliomas is correlated with MCP-3, but not MCP-1. International Journal of Oncology. 2009;34:1621–1627. doi: 10.3892/ijo_00000292. [DOI] [PubMed] [Google Scholar]
- Pellegatta S, Poliani P, Corno D, Menghi F, Ghielmetti F, Suarez-Merino B, Caldera V, Nava S, Ravanini M, Facchetti F, Bruzzone M, Finocchiaro G. Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Research. 2006;66:10247–10252. doi: 10.1158/0008-5472.CAN-06-2048. [DOI] [PubMed] [Google Scholar]
- Platten M, Kretz A, Naumann U, Aulwurm S, Egashira K, Isenmann S, Weller M. Monocyte chemoattractant protein-1 increases microglial infiltration and aggressiveness of gliomas. Annals of Neurology. 2003;54:388–392. doi: 10.1002/ana.10679. [DOI] [PubMed] [Google Scholar]
- Prados M. Biology and treatment of malignant glioma. Seminars in Oncology. 2000:27. [PubMed] [Google Scholar]
- Proudfoot A, Power C, Hoogewerf A, Montjovent M, Borlat F, Offord R, Wells T. Extension of recombinant human RANTES by the retention of the initiating methionine produces a potent antagonist. The Journal of Biological Chemistry. 1996;271:2599–2603. doi: 10.1074/jbc.271.5.2599. [DOI] [PubMed] [Google Scholar]
- Remick D, Green L, Newcomb D, Garg S, Bolgos G, Call D. CXC chemokine redundancy ensures local neutrophil recruitment during acute inflammation. The American Journal of Pathology. 2001;159:1149–1157. doi: 10.1016/S0002-9440(10)61791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repeke C, Ferreira S, Claudino M, Silveira E, De Assis G, Avila-Campos M, Silva J, Garlet G. Evidences of the cooperative role of the chemokines CCL3, CCL4 and CCL5 and its receptors CCR1+ and CCR5+ in RANKL+ cell migration throughout experimental periodontitis in mice. Bone. 2010;46:1122–1130. doi: 10.1016/j.bone.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nature Reviews. Immunology. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Robinson S, Scott K, Wilson J, Thompson R, Proudfoot A, Balkwill F. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Research. 2003;63:8360–8365. [PubMed] [Google Scholar]
- Roggendorf W, Strupp S, Paulus W. Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathologica. 1996;92:288–293. doi: 10.1007/s004010050520. [DOI] [PubMed] [Google Scholar]
- Rubin J, Kung A, Klein R, Chan J, Sun Y, Schmidt K, Kieran M, Luster A, Segal R. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. PNAS. 2003;100:13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura J, Tusell J, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia. 2003;44:183–189. doi: 10.1002/glia.10274. [DOI] [PubMed] [Google Scholar]
- Sciumè G, Santoni A, Bernardini G. Chemokines and glioma: invasion and more. Journal of Neuroimmunology. 2010;224:8–12. doi: 10.1016/j.jneuroim.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Semple B, Kossmann T, Morganti-Kossmann M. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. Journal of Cerebral Blood Flow and Metabolism. 2010;30:459–473. doi: 10.1038/jcbfm.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwa M, Markovic D, Gabrusiewicz K, Synowitz M, Glass R, Zawadzka M, Wesolowska A, Kettenmann H, Kaminska B. The invasion promoting effect of microglia on glioblastoma cells is inhibited by cyclosporin A. Brain. 2007;130:476–489. doi: 10.1093/brain/awl263. [DOI] [PubMed] [Google Scholar]
- Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Letters. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Stoll M, Capper D, Dietz K, Warth A, Schleich A, Schlaszus H, Meyermann R, Mittelbronn M. Differential microglial regulation in the human spinal cord under normal and pathological conditions. Neuropathology and Applied Neurobiology. 2006;32:650–661. doi: 10.1111/j.1365-2990.2006.00774.x. [DOI] [PubMed] [Google Scholar]
- Streit W. Microglia and macrophages in the developing CNS. Neurotoxicology. 2001;22:619–624. doi: 10.1016/s0161-813x(01)00033-x. [DOI] [PubMed] [Google Scholar]
- Tran C, Wolz P, Egensperger R, Kösel S, Imai Y, Bise K, Kohsaka S, Mehraein P, Graeber M. Differential expression of MHC class II molecules by microglia and neoplastic astroglia: relevance for the escape of astrocytoma cells from immune surveillance. Neuropathology and Applied Neurobiology. 1998;24:293–301. doi: 10.1046/j.1365-2990.1998.00120.x. [DOI] [PubMed] [Google Scholar]
- Vaday G, Peehl D, Kadam P, Lawrence D. Expression of CCL5 (RANTES) and CCR5 in prostate cancer. The Prostate. 2006;66:124–134. doi: 10.1002/pros.20306. [DOI] [PubMed] [Google Scholar]
- Watters J, Schartner J, Badie B. Microglia function in brain tumors. Journal of Neuroscience Research. 2005;81:447–455. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- Yang I, Han S, Kaur G, Crane C, Parsa A. The role of microglia in central nervous system immunity and glioma immunology. Journal of Clinical Neuroscience. 2010;17:6–10. doi: 10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H, Heppner F, Tsirka S. Microglia/macrophages promote glioma progression. Glia. 2011;59:472–485. doi: 10.1002/glia.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.