Abstract
The mouse has proven to be an advantageous animal model system in basic science research focused on aiding in development and evaluation of potential treatments; however, the small size of mouse tendons makes consistent and reproducible injury models and subsequent biomechanical evaluation challenging for studying tendon healing. In this study, we investigated the feasibility and reproducibility of multiple mouse tendon injury models. Our hypothesis was that incisional (using a blade) and excisional (using a biopsy punch) injuries would result in consistent differences in tendon material properties. At 16 weeks of age, 17 C57BL/6 mice underwent surgery to create defects in the flexor digitorum longus, Achilles, or patellar tendon. Each animal received 1–2 full-thickness, central-width incisional or excisional injuries per limb; at least one tendon per limb remained uninjured. The injuries were distributed such that each tendon type had comparable numbers of uninjured, incisionally-injured, and excisionally-injured specimens. Three weeks after injury, all animals were euthanized and tendons were harvested for mechanical testing. As hypothesized, differences were detected for all three different tendon types at three weeks post-injury. While all models created injuries that produced predictable outcomes, the patellar tendon model was the most consistent in terms of number and size of significant differences in injured tendons compared to native properties, as well as in the overall variance in the data. This finding provides support for its use in fundamental tendon healing studies; however, future work may use any of these models, based on their appropriateness for the specific question under study.
Keywords: tendon, biomechanics, injury, animal model, mouse
INTRODUCTION
Musculoskeletal disorders were reported by more than 100 million adults in the United States in 2005 alone (Jacobs et al., 2008). More specifically, tendon and other soft tissue injuries are known to cause considerable pain and disability. A better understanding of the healing response of these injuries will improve the ability to treat those affected as well as to better prevent injuries. Investigations into tendon mechanical properties and healing mechanics can provide valuable information as the field ventures further into the realm of tissue engineered constructs.
Animal models are often used to help understand human physiology and disease and to aid in development and evaluation of treatment modalities. The mouse has become a popular model system due to its similar physiology with humans, the ability to alter the mouse genome to mimic human disease, and the availability of a large number of biologic assays (Bedell et al., 1997). Despite these advantages, the small size of mouse tendons makes consistent and reproducible injury models and subsequent biomechanical evaluation challenging. Therefore, the objective of this study was to investigate the feasibility and reproducibility of multiple mouse tendon injury models by evaluating the hind flexor digitorum longus (FDL), Achilles, and a previously utilized patellar tendon injury model (Lin et al., 2006). With the goal of studying fundamental tendon healing processes at the basic science level, we aimed to develop and compare models that did not require suture repair as that might add to, or alter, the normal biological processes at the injury site (e.g., inflammation) and this study was aimed at developing and evaluating model systems for natural healing of these injuries. This technique was chosen rather than a complete laceration so that we would be able to assess the biomechanical efficacy of these injury models in a basic science context so that these fundamental studies might be used in a targeted fashion in future studies to address directly clinically relevant scenarios such as a complete laceration or other acute critical defect. Our hypothesis was that partial width laceration (incisional) and punch (excisional) injuries would result in consistent biomechanical differences in tendon material properties, providing multiple tendon injury models for use in our field.
METHODS
At 16 weeks of age, 17 C57BL/6 mice underwent surgery to create full-thickness, partial width central defects in the FDL, Achilles, or patellar tendon with IACUC approval. This age was chosen to represent a skeletally mature mouse. Each animal received 1–2 incisional or excisional injuries per limb, which were evenly distributed across groups. At least one tendon per limb remained uninjured. The different injury types were distributed such that each tendon type had approximately equal numbers of uninjured, incisionally injured, and excisionally injured specimens. Incisional injuries were created with a customized flat-edged scalpel blade designed to lacerate the central two-thirds of the tendon width (Figure 1). Excisional injuries were created using commercially available biopsy punches (Shoney Scientific, Waukesha, WI) to remove a small piece of tissue in the central two-thirds of the tendon width. Injuries were scaled according to the approximate width of the different sized tendons in order to standardize the percent of remaining tissue across tendon types, and hence provide a more consistent opportunity for the tendon to heal with regard to amount of intact tendon substructures.
Figure 1.
Incisionally injured (a) FDL, (b) Achilles, and (c) patellar tendons at the time of surgery and (d) illustrated schematically. The central two-thirds of each tendon was lacerated (or biopsied for excisional injuries) through the full thickness, t, of the tendon.
For FDL injuries, the tendon was surgically exposed through an incision on the plantar aspect of the hind foot and isolated by placing a plastic-coated backing underneath the tendon. A 0.5 mm laceration (incisional) or punch (excisional) was made, leaving a central defect. Usage of the plastic backing provided a stable surface for the laceration or punch and prevented the blade/punch from cutting through to underlying tissues. Achilles tendons were exposed and similarly isolated and injured using either a 0.5 mm blade or biopsy punch. For patellar tendon injuries, the tendon was isolated by making two incisions in the retinaculum parallel to each side of the tendon. The plastic backing was again used to support a 0.75 mm blade or punch. Since tendon struts were left intact on both sides of all defects, no suture repair was required to prevent in vivo rupture. Skin incisions were closed and mice were allowed normal cage activity. Three weeks after injury, all animals were euthanized and tendons were harvested for mechanical testing.
Following dissection, tendon cross-sectional areas were measured using a custom laser-based device which utilized two translational stages, two orthogonal linear variable differential transformers (LVDTs), and a charge-coupled device laser (Favata, 2006). Using the LVDT position data in the transverse direction in addition to the laser thickness data, average cross-sectional area was calculated by using the axial LVDT position data to interpolate between each of the five transverse passes across the tendon width using custom software. This allowed for actual cross-sectional area measurement without making approximations of specimen geometry. This area value was recorded and compared between groups. All tendons were then stamped into a dumbbell (i.e., dogbone) shape, removing the lateral and medial portions of the tendon that were not subjected to injury. In addition to helping isolate healed tissue from the injury, this also increased the likelihood of midsubstance failure during subsequent testing and avoidance of potentially confounding grip effects. Stain lines were applied to the tissue to allow optical measurement of local tissue strain within the dumbbell-stamped gauge length. Cross-sectional areas were again measured used to calculate material properties.
FDL tendons (n=13 for uninjured; n=11 for incisional; n=9 for excisional) were dissected and the defect, located near the distal end of the tendon, was positioned between two sandpaper-cyanoacrylate constructs and gripped in custom fixtures. Achilles tendons (n=12; n=11; n=11) were dissected free from muscle, while preserving the calcaneal insertion. The tendon end and calcaneus were each secured and clamped as with the FDL. Patellar tendons (n=14; n=9; n=8) were dissected to an intact patella-tendon-tibia complex. The tibia was potted in polymethylmethacrylate (PMMA) and placed in custom fixtures. The patella end was placed into a conical shaped custom grip as we have used previously (Lin et al., 2004). All tendons were submerged in a 37°C PBS bath and tensile tested in a mechanical test frame (Instron Corp., Norwood, MA). After an initial preload to remove slack, ten cycles of preconditioning were performed to provide a consistent strain history. A 300-second hold was followed by a stress-relaxation experiment in which the tendon was loaded to 5% strain at a rate of 5%/s and then relaxed over 600 s. After a relaxation period of 600 s, specimens were returned to initial gauge length and finally tested to failure at 0.1%/s. Tissue strain was measured optically.
A check of normality and variance in the data revealed that just over one quarter of the reported parameters were not normally distributed and did not possess equal variance. Given this violation of parametric analysis assumptions and to maintain consistency of analysis, all data were evaluated for differences in each tendon between injury groups using a non-parametric Kruskal-Wallis test with a Conover-Inman post-hoc test for multiple comparisons to remain conservative. Statistical significance was set at p30.05. In addition, coefficients of variation of the data were calculated as an overall assessment of the consistency and variation in each model.
RESULTS
Failure data for Achilles and uninjured patellar tendons were deemed unreliable due to several calcaneal fractures and tibial insertion failures, respectively (Table 1). For this reason, the reported results focus on area, percent relaxation, and modulus, which were reliable for all tendons in all groups and are the properties deemed most important for our assessment, since failure properties do not reflect the normal, functional response of the tissue.
Table 1.
Percentages of viable failure data (i.e., tendon failure within the gauge length of the stamped region) across tendons. Achilles and patellar tendons frequently failed at the bony insertions of the calcaneus and tibia, respectively.
| FDL | Achilles | Patellar | |
|---|---|---|---|
| Uninjured | 62% | 8% | 7% |
| Incisional | 64% | 0% | 44% |
| Excisional | 89% | 18% | 75% |
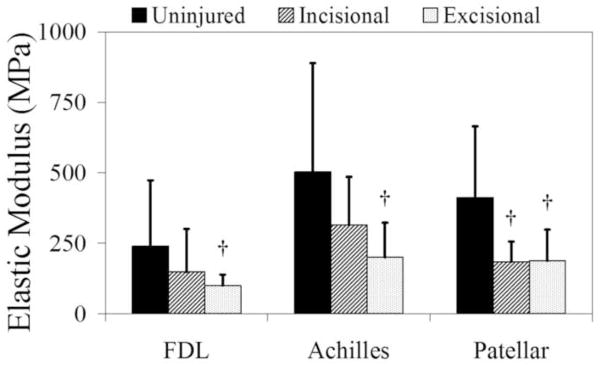
For the FDL at the time point studied, the excisional injury resulted in a decrease (p=0.02) in elastic modulus (Figure 2) and no difference in area or percent relaxation compared to uninjured (Table 2). The incisional injury showed no significant differences in any property. When comparing incisional and excisional injured tendons, FDL tendons showed no differences.
Figure 2.
Differences in moduli were seen in all three tendons between uninjured and both incisional and excisional injuries.† denotes a significant difference from uninjured.
Table 2.
Geometrical and biomechanical results for mouse FDL, Achilles, and patellar tendons (means ± standard deviations). Statistically significant differences in modulus were detected in all tendons subjected to excisional injuries. Only the patellar tendon resulted in significant differences following incisional injury.
| Area (mm2) | Relaxation (%) | Modulus (MPa) | ||
|---|---|---|---|---|
| FDL | Uninjured | 0.18 ± 0.03 | 34.4 ± 12.4 | 232.8 ± 201.4 |
| Incisional | 0.22 ± 0.05 | 23.4 ± 15.9 | 149.3 ± 145.0 | |
| Excisional | 0.18 ± 0.06 | 24.3 ± 17.9 | 100.6 ± 38.6† | |
| Achilles | Uninjured | 0.22 ± 0.02 | 37.5 ± 13.8 | 502.5 ± 386.8 |
| Incisional | 0.33 ± 0.08† | 48.9 ± 8.9† | 315.3 ± 169.8 | |
| Excisional | 0.42 ± 0.04†* | 49.2 ± 15.4 | 200.8 ± 122.0† | |
| Patellar | Uninjured | 0.27 ± 0.05 | 44.5 ± 16.6 | 412.6 ± 252.0 |
| Incisional | 0.52 ± 0.08† | 51.5 ± 9.6 | 191.5 ± 70.5† | |
| Excisional | 0.56 ± 0.06† | 64.3 ± 9.0† | 187.8 ± 110.5† | |
denotes statistical significance compared to uninjured.
denotes statistical significance of excisional compared to incisional.
For the Achilles tendons at the time point studied, incisional (p<0.0001) and excisional (p<0.0001) injuries resulted in a significant increase in area compared to control (Table 2). Incisional injuries showed an increase in percent relaxation compared to control (p=0.03). Elastic modulus in the excisionally-injured group were significantly reduced compared to control (p=0.003). Excisionally-injured tendons were also significantly larger (p=0.0002) than incisional. Moduli for injured Achilles tendons were not different from each other.
For the patellar tendon at the time point studied, areas showed significant increases (p<0.0001) for both injured groups compared to control, while injured areas were not different from each other (Table 2). Percent relaxation was significantly increased in the excisional group compared to control (p=0.0006). Patellar tendon moduli showed significant decreases from uninjured values for both incisional (p=0.02) and excisional (p=0.01) groups.
When comparing the models across tendons, the coefficients of variation for area and modulus revealed the patellar tendon model to have the lowest overall variance for each of the uninjured, incisional, and excisional groups (Table 2).
DISCUSSION
Material properties were assessed in mouse FDL, Achilles, and patellar tendons subjected to an incisional or excisional injury, or to no injury. As hypothesized, differences were detected for all three different injury models at three weeks post-injury. In most cases, reproducible incisional and excisional tendon injury model results were not different from each other, suggesting that both incisional and excisional models can be useful for tendon healing investigations, depending on the objectives and hypotheses of a particular study.
While all models created injuries that produced predictable outcomes, the patellar tendon model was the most consistent in terms of number and size of significant differences in injured tendons compared to native properties (Table 2; Fig. 2), as well as in the overall variance in the data (Table 3). This would have the advantage of having a better likelihood of detecting differences in various treatment modalities or other system perturbations. It is possible that the larger width of the patellar tendon compared to the FDL and Achilles tendons lends itself more to a reproducible injury simply due to the larger size, although blade and punch sizes were increased proportionally to account for this. In addition, the patellar and Achilles injuries require minimal soft tissue disruption to create the injury as the tendons are more superficial. The FDL, by contrast, requires retraction of soft tissues in order to visualize and isolate the tendon. Finally, the naturally shorter length of the patellar tendon allowed for visualization of the entire tendon with minimal surgical exposure as compared to the FDL and Achilles. This visualization of both the tibia and patella may have allowed for more consistent injury placement with respect to the long axis of the tendon and thus, may have contributed to more consistent findings.
Table 3.
Summary of combined coefficients of variation for area and modulus across tendons. Patellar tendon data had the lowest combined variation in all groups.
| FDL | Achilles | Patellar | |
|---|---|---|---|
| Uninjured | 57% | 44% | 40% |
| Incisional | 65% | 38% | 27% |
| Excisional | 37% | 36% | 35% |
One limitation of this work was that, in order to minimize the numbers of animals needed to address our study hypotheses, more than one injury was assigned to many of the limbs. Of particular concern could be the combination of FDL and Achilles injuries in the same limb, given their close proximity to each other and contrasting functions. To address this concern, we have performed an analysis comparing data from the reported properties in animals having injuries in both the FDL and Achilles tendons with those having either an Achilles or FDL injury (without consideration of whether a patellar tendon injury was present). This analysis showed no differences (p>0.4) for any reported property, indicating that there were minimal adverse effects, if any, of the implementation of multiple injuries per limb.
Another limitation of our methods is the differences in gripping techniques used between the different tendon types. The FDL and Achilles tendons were both gripped at both ends, whereas the patellar tendon was gripped at the patellar end using a special conical fixture as is our standard protocol for this tissue in our lab. The relatively short nature of the patellar tendon made gripping at both tendon ends an unviable technique due to the inability to isolate grip effects at the tendon ends from the gauge length in the midsubstance. The inherent, ligament-like nature of the patellar tendon (i.e., with bone on both ends) allowed for other techniques to be used so that testing of the tendon is feasible. While this technique could have advantages over standard gripping of tendon, FDL and Achilles tendons were marked at the grip interface and no notable slipping occurred during the tensile tests in our studies. Nonetheless, the noted differences in overall results between the patellar tendon and the other tendons should be mindful of this variation in methods.
A final limitation of this work is the lack of additional assays to assess potential differences in parameters such as cellularity, biochemistry, gene expression, or collagen fiber alignment. The evaluation of results of these studies would yield stronger confidence in recommendations made with respect to injury model performance. In addition to non-biomechanical assays, the current results are limited by the difficulties in obtaining reliable failure data, which could have also contributed useful information to this comparison.
In summary, we have successfully created and biomechanically evaluated incisional and excisional injuries in three different mouse tendons. The high consistency and significance of findings in the patellar tendon model provide support for its use in fundamental tendon healing biomechanical studies. However, future work may use any of these models, based on their appropriateness for the specific question under study.
Acknowledgments
This study was supported by NIH-NIAMS and the Penn Center for Musculoskeletal Disorders. These funding sources had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors, their immediate families, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bedell MA, Jenkins NA, Copeland NG. Mouse models of human disease. Part I: Techniques and resources for genetic analysis in mice. Genes & Development. 1997;11:1–10. doi: 10.1101/gad.11.1.1. [DOI] [PubMed] [Google Scholar]
- Favata M. PhD Thesis. University of Pennsylvania; 2006. Scarless healing in the fetus: implications and strategies for postnatal tendon repair. [Google Scholar]
- Jacobs JJ, Andersson GBJ, Bell J-E, et al. The Burden of Musculoskeletal Diseases in the United States. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008. Burden of Musculoskeletal Disease Overview; pp. 1–20. [Google Scholar]
- Lin TW, Cardenas L, Glaser DL, Soslowsky LJ. Tendon healing in interleukin-4 and interleukin-6 knockout mice. Journal of Biomechanics. 2006;39:61–69. doi: 10.1016/j.jbiomech.2004.11.009. [DOI] [PubMed] [Google Scholar]