Abstract
Aims
Clinical trials for acute heart failure syndromes (AHFS) have traditionally enrolled patients well after emergency department (ED) presentation. We hypothesized a large proportion of patients would undergo changes in clinical profiles during the first 24 hours of hospitalization and these changes would be associated with adverse events.
Methods
We evaluated a prospective cohort of patients with clinical data available at ED presentation and 12–24 hours after ED treatment for AHFS. Patients were categorized into distinct clinical profiles at these time points based on: 1) systolic blood pressure- a. hypertensive [>160 mmHg]; b. normotensive [100–159 mmHg]; or c. hypotensive [<100 mmHg]; 2) moderate to severe renal dysfunction [GFR ≤ 60 ml/min/1.73 m2]; and 3) presence of troponin positivity. A composite outcome of 30-day cardiovascular events was determined by phone follow-up.
Results
In the 370 patients still hospitalized with data available at the 12–24 hour time point, 196 (53.0%) had changed their clinical profiles, with 117 (59.7%) improving and 79 (40.3%) worsening. The composite 30-day event rate was 16.9%. Patients whose clinical profile started and stayed abnormal had a significantly greater proportion of events than those who started and stayed normal (26.1% vs. 11.3%; p=0.03).
Conclusion
Patients with abnormal clinical profiles at presentation that remain abnormal throughout the first 12–24 hours of hospitalization are at increased risk of 30-day adverse events. Future clinical trials may need to consider targeting these patients, as they may be the most likely to benefit from experimental therapy.
Introduction
Clinical trials in patients with acute heart failure syndromes (AHFS) have been universally disappointing. Only one trial (VMAC) has resulted in the Food and Drug Administration (FDA) clearing a new therapy (nesiritide) for use in AHFS [1]. However, questions about nesiritide's safety and overly conservative dosing of nitroglycerin in the trial's standard care arm raised doubt about its role in AHFS [1–3]. While ASCEND-HF demonstrated nesiritide's safety, it did not demonstrate efficacy based on the primary endpoint [4]. This is consistent with other neutral therapeutic trials and, despite strong evidence of disease heterogeneity and concerns over confounding due to standard care, AHFS trials have largely not changed their approach. Patients continue to be enrolled long after their emergency department (ED) stay, when the majority have experienced significant improvement in dyspnea and a subset have already experienced adverse events which may be related to early therapy [5–8]. The barrier preventing early enrollment has traditionally been the inability to enroll patients early in their ED course at the time of or prior to initial therapy. However, with the advent of strong alliances between emergency medicine and cardiology this barrier has been largely overcome [9,10].
Recent clinical trial results suggest early enrollment and targeted therapy may result in improved outcomes in ED patients with AHFS. In a Phase II study of Relaxin, 234 patients with moderate renal dysfunction were randomized to standard care plus a 48-hour infusion of placebo or Relaxin [11]. Patient were enrolled a median of 6.6 hours from presentation, which is much earlier than other randomized AHFS trials. This early enrollment may have contributed to the observed dyspnea improvement and blood pressure control seen in treated patients at 6, 12, and 24 hours after therapy, as well as the reduced number of cardiovascular events at day 60 [11]. Similar results were seen in ASCEND-HF, where patients enrolled less than 15.5 hours from presentation had significantly less dyspnea at 6 hours after drug initiation than those who were enrolled after 15.5 hours (p=0.008) [4]. Finally, a prospective trial has recently suggested an approach using high-dose nitroglycerin in ED patients with AHFS and hypertension is safe and efficacious [12]. This raises the possibility that when studying patients who receive experimental therapy early in their clinical course, at a time when standard therapy is normally administered, different results will be seen than when enrolling patients much later in their clinical course. Such differing treatment effects may arise from: 1) an interaction between initial standard and subsequent experimental therapy; or 2) a change in clinical characteristics resulting from early non-experimental treatment, with a subsequent change in likelihood of responding to therapy in the expected manner. Importantly, enrolling patients earlier and targeting those who are likely to not respond to standard therapy, and benefit most from experimental therapy, is crucial to improving our success rate in current clinical trials.
Patients who are enrolled in clinical trials 12–24 hours after initial therapy are exposed to experimental therapy which targets their profile at the time of recruitment, but this is not necessarily the same as their profile at ED presentation. For example, a patient who was hypertensive with normal renal function at ED presentation may be relatively normotensive with renal dysfunction 12–24 hours later. While this subject may have been excluded due to renal dysfunction at the time of enrollment, they would have been an ideal candidate for inclusion at ED presentation, because they would represent a subject who would have subsequently failed to respond safely to initial therapy.
Three prognostic variables readily available at ED presentation, which have been associated with patients who fail to respond safely to initial therapy, include systolic blood pressure, renal function and troponin release [13–17]. The impact of ED therapy on these variables, and, more importantly, how these variable changes may influence subsequent treatment response, has not been well studied in a prospective manner that begins with ED enrollment. We hypothesize that patients who present to the ED with AHFS have initial clinical profiles which change substantially over the first 12–24 hours after initial therapy. We further hypothesize that changes in these clinical profiles will be associated with the risk of adverse events.
Methods
This was a secondary analysis of data from a large, prospective observational cohort study conducted at the time of ED presentation in patients with AHFS [10]. It was approved by institutional review boards at each site and each patient provided written, informed consent.
Setting and Selection of Subjects
In the cohort study, ED patients with signs and symptoms of AHFS presenting to 4 EDs, 2 in Nashville, Tennessee and 2 in Cincinnati, Ohio, were approached for enrollment and consent. Patients were approached for enrollment at the time of ED evaluations. All EDs were staffed by physicians from The University of Cincinnati or Vanderbilt University. This cohort represents a socioeconomically and demographically diverse group of subjects. Patients at Vanderbilt University Medical Center represent a highly educated, tertiary care cohort where 55% are female and a predominantly bi-racial population (approximately 60% white and 30% black). The Veterans Administration Tennessee Valley Healthcare System serves care to veterans predominantly in Middle Tennessee. Their patients seen in the ED are almost entirely male with an average age of 70. Cincinnati's University Hospital is an urban, tertiary care, teaching hospital, with a substantial indigent patient population. The ED population is bi-racial with both black and white patients; there is a mix of self-pay, insured, and Medicare and Medicaid patients. Cincinnati's Jewish Hospital is a community hospital that serves a largely white, higher socioeconomic status, insured population.
Study assistants and research coordinators, trained in patient identification, recruitment, and data collection, prospectively gather data during the participant's ED and hospital course. The study assistants approach the patient shortly after ED evaluation and then follow the patient during their inpatient stay. In addition to clinical presentation and post-treatment variables, medications administered during the first 96 hours are also tracked using the nurses' medication administration record. Patients were treated and dispositioned at the discretion of the emergency medicine and inpatient physician.
To be eligible for this analysis patients had to: 1) have AHFS recorded as either a primary or secondary diagnosis by the treating ED physician and 2) have received diuretic or vasodilator treatment for AHFS within three hours of ED presentation. Treating physicians make their AHFS diagnosis based on data routinely available in the ED, which typically includes history and physical examination, an electrocardiogram (ECG), chest radiography, and results from serum chemistries, renal function and b-type natriuretic peptide testing.
Data Collection and Processing
The study team records clinical variables upon ED presentation, and at 12–24, and 72–96 hours after ED treatment. Vital signs and urine output are routinely recorded every 2–4 hours in the ED and on the hospital floor, and often hourly in the ICU. Laboratory parameters are ordered on a daily basis in the majority of patients. However, patients also have blood drawn for the study if laboratory tests are not run as part of routine care. The cardiac troponin assay utilized for the study was the Abbott i-STAT, which has a 99th percentile reference limit of 0.08 μg/L [18].
Criterion Standard
Patient characteristics were categorized at baseline and at 12–24 hours based on: 1) systolic blood pressure- a. hypertensive [>160 mmHg]; b. normotensive [100–159 mmHg]; or c. hypotensive [<100 mmHg]; 2) moderate to severe renal dysfunction (MRD) [ GFR ≤ 60 ml/min/1.73 m2]; and 3) troponin positivity (TNP)[a maximal concentration of cardiac troponin exceeding the 99th percentile of values for a reference control group] [19,20].
Patients' clinical profiles were categorized as being initially normal or abnormal. Normal was defined as the absence of TNP, MRD, hypertension, or hypotension. Presence of one or more of those characteristics at baseline resulted in categorization as abnormal. Patients were then re-categorized based on the presence or absence of these characteristics at the 12–24 hour point, with patients falling into four primary categories: 1) started abnormal and stayed abnormal; 2) started abnormal and became normal; 3) started normal and stayed normal; 4) started normal and became abnormal.
A composite 30-day event was used as the primary outcome measure for this analysis. An event comprised: 1) an unscheduled ED revisit or rehospitalization for AHFS; 2) acute coronary syndrome (ACS); 3) percutaneous coronary intervention or coronary artery bypass grafting; 4) death; 5) defibrillation; 6) intubation; or 7) cardiopulmonary resuscitation. These events were determined by study investigators who performed telephone follow-up at 5- and 30-days after their ED presentation and confirmed events in the medical record; follow up was conducted in person for hospitalized patients.
Statistical Analysis
The sample was described using median and range for continuous variables and frequencies and percents for categorical variables. Chi Square tests were used to compare categorical variables between groups. Where 95% confidence intervals (95CI) for proportions are given, these were computed using the score method with continuity correction. This analysis was largely descriptive in nature and was not powered to look at a comprehensive multivariable analysis. Analyses were conducted using SPSS v18.0 (SPSS Inc., Chicago, II).
Results
Of the 455 patients available for analysis, the median age was 65 years, 56% were Caucasian, and 60% were male (Table 1). Of these patients, 359 (78.9%) had a primary diagnosis of AHFS and 96 (21.1%) had a secondary diagnosis of AHFS. A primary diagnosis of NSTEMI occurred in 8 (1.8%), STEMI in 0 (0%), and 1 (0.2%) with a ventricular arrhythmia. There were no deaths in the ED and 11 (2.4%) discharged directly from the ED. Hypertension and pre-existing heart failure were common, and beta-blockers and diuretics were used frequently. Median systolic blood pressure at presentation was 147 mmHg. The majority of patients had an initial blood pressure in the normotensive (270, 59.3%) or hypertensive (174, 38.2%) range. The median BNP was 805 pg/ml, and congestion on chest radiograph and on clinical exam was common.
Table 1.
Baseline characteristics in those patients included in the analysis. Data are presented with median and range or count and proportion as appropriate.
| Median (range) | Count (%) | |
|---|---|---|
| Age | 65 (25–99) | |
| Male | 273 (60.0) | |
| Caucasian | 253 (55.6%) | |
| PMH | ||
| Heart Failure | 327 (71.9%) | |
| Renal Dysfunction | 100 (22.1%) | |
| Hypertension | 380 (83.5%) | |
| Myocardial Infarction | 157 (34.5%) | |
| Medications | ||
| Beta-blockers | 293 (64.8%) | |
| ACE Inhibitors | 185 (40.8%) | |
| Diuretics | 293 (65.1%) | |
| Vitals | ||
| Pulse Rate (beats/min) | 88 (45–173) | |
| Systolic Blood Pressure (mmHg) | 147 (89–262) | |
| Respiratory Rate (breaths/min) | 20 (10–90) | |
| Exam Findings | ||
| Jugular Venous Distension | 89 (19.6%) | |
| S3 Gallop | 46 (10.1%) | |
| Rales or Crackles | 297 (65.3%) | |
| Extremity Edema | 302 (66.4%) | |
| Lab findings | ||
| BNP (pg/ml) | 805 (5–6486) | |
| Creatinine (mg/dl) | 1.3 (0.5–10.4) | |
| BUN (mg/dl) | 22.0 (2.0–105.0) | |
| Sodium (mEq/L) | 139 (100–147) | |
| Ejection Fraction (%) | 43 (5–75) | |
| Chest Radiograph Findings | ||
| Cardiomegaly | 270 (60.0%) | |
| Pleural Effusion | 149 (33.1%) | |
| Interstitial Edema | 67 (14.9%) | |
| Pulmonary Edema | 126 (28.0%) | |
| Initial ED Therapy | ||
| Furosemide | 291 (64.0%) | |
| Nitroglycerin SL/topical | 205 (45.0%) | |
| Nitroglycerin IV | 7 (1.5%) | |
| Dobutamine | 0 (0%) |
Initial Clinical Profiles
All 455 patients were categorized into one of twelve distinct, baseline clinical profiles (Table 2). Most were either normal (101, 22.2%) or had isolated MRD (126, 27.7%). Of the 208 patients with MRD, 126 (60.6%) were normotensive and 75 (36.1%) were hypertensive. The frequency of TNP patients who presented with hypertension was similar to that in normotensive patients (17.8% compared with 16.0%).
Table 2.
Initial patient profiles categorized by blood pressure and further stratified by the presence or absence of TNP or moderate-to-severe renal dysfunction.
| Initial Patient Profile | Blood Pressure Category |
||
|---|---|---|---|
| < 100 mm Hg n=11 | 100–159 mm Hg n=270 | > 160 mm Hg n=174 | |
| n (%) | n (%) | n (%) | |
| MRD and TNP | 1 (9) | 26 (10) | 17 (10) |
| MRD only | 7 (64) | 126 (47) | 75 (43) |
| TNP only | 0 (0) | 17 (6) | 14 (8) |
| Neither MRD nor TNP | 3 (27) | 101 (37) | 68 (39) |
TNP= Troponin positivity
MRD- moderate-to-severe renal dysfunction
Changes in Clinical Profiles over the first 12–24 hours of Therapy
Of the 370 patients who were admitted to the hospital and who had data available at the 12–24 hour time point, 196 (53.0%, 95CI 47.8–58.1%) had changed their clinical profiles, with 117 (59.7%, 95CI 52.7–66.3%) improving and 79 (40.3%, 95CI 33.7–47.3%) worsening (Table 3). Of those with worsening clinical profiles, 24 (30.4%, 95CI 21.3–41.2%) developed hypotension, 25 (31.7%, 95CI 22.5–42.6%) developed new TNP, and 26 (32.9% 95CI 23.6–43.9%) developed subsequent MRD. In the 174 patients whose clinical profile remained unchanged at 12–24 hours, 110 (63.2%, 95CI 55.8–70.0%) started abnormal and stayed abnormal, while 64 (36.8%, 95CI 30.0–44.2%) started normal and stayed normal.
Table 3.
Changing AHFS Phenotypes over the first 24 hours of Therapy
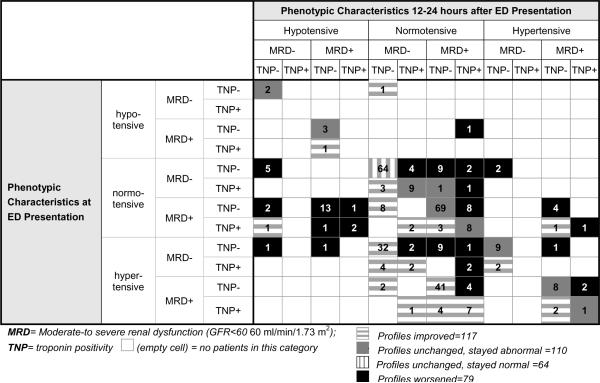 |
Changes in Clinical Profiles and their Association with 30-day events
The 30-day event rate in those with data available at 12–24 hours was 16.9%. All but 4 of the patients were discharged from their index stay by the 30-day follow-up. Of the 41 patients admitted to the intensive care unit 4 (9.8%) experienced a 30-day event. Of these, 45 (10.0%) had an unscheduled ED visit for AHFS, 52 (11.0%) were hospitalized for AHFS, 2 (0.4%) had sudden death with defibrillation or CPR, 2 (0.4%) had ACS, 4 (1.0%) had PCI, 4 (1.0%) were intubated, 3 (1.0%) received coronary artery bypass grafting, and 15 (3.3%) had all-cause death. Patients who had worsening of their clinical profile had a slightly greater proportion of adverse events than those with improved clinical profiles (20.5% vs. 17.6%; p= 0.7) Importantly, patients who started with an abnormal clinical profile and stayed abnormal had a significantly greater proportion of events than those who started normal and stayed normal (26.1% vs. 11.3%; p=0.03).
Discussion
Based on this secondary analysis, over 50% of patients treated in the ED for AHFS undergo changes in their clinical profiles within the first 24 hours of therapy. Approximately 40% of these changes resulted in the development of MRD or TNP, identifying a cohort of patients who failed to safely respond to initial therapy. Conversely, nearly 60% had clinical improvement, suggesting initial therapy was beneficial in this subset. Adverse event rates were high overall, indicating that such clinical changes may be harmful for some patients, while outcomes in others may be more influenced by comorbidities or precipitants [21].
Our findings suggest that those patients whose profiles started abnormal and stayed normal are significantly more likely to have an adverse event compared to those who started and stayed normal. However, while those patients who had worsening of their clinical profile had a great number of events than those who improved (20.5% vs 17.6%) this was not statistically significant. This represents a total of 37 patients, and perhaps in a larger cohort this absolute difference of 2.9% would be clinically significant.
We chose renal function, blood pressure and troponin release a priori not only because of their prognostic significance, but also because they are readily available at ED presentation. Previous studies have already demonstrated the magnitude of changes in dyspnea over time, and indicate that the earlier treatment is initiated, the greater and more rapid the dyspnea improvement [4,11,22]. However, the findings in our current study also suggest that early treatment is also associated with changes in clinical characteristics in a significant proportion of patients. Further, these changes, or lack thereof, appear to be associated with a patient's clinical course. Together, these results imply we need to regard not only a patient's current clinical profile at the time of enrollment, but also their clinical profile when they presented to the ED.
These findings may also have implications for clinical trial design. Our data suggest that 40% of patients may not respond safely to initial therapy, and targeting this group with experimental therapy to safely improve symptoms should be considered. If patients can be identified early, they may be eligible for enrollment in clinical trials that had previously excluded them when they were enrolled 12–24 hours after initial therapy. Moreover, this would remove the spectrum bias towards excluding those patients who do not safely respond to standard therapy. For example, our data suggests those patients with persistent abnormal clinical profiles after initial therapy, who have an increased adverse event rate, may be good candidates for therapeutic trials. Importantly, these findings also suggest that exclusion from a trial because of an anticipated poor clinical course may be premature. In fact, these patients may be the cohort we want to enroll; as they are unlikely to respond to standard therapy, but may respond preferentially to experimental therapy. Further, while some patients do develop worsening renal function during their hospital stay, others improve, and changes may only be transient and may not impact short-term clinical outcomes [15]. Further, patients who would currently be considered ineligible because of renal dysfunction or an elevated troponin could be enrolled to receive therapy aimed at minimizing on-going renal or cardiac injury, and the increased adverse clinical events that occur if improvement is not apparent. Such individualized therapy is a critical next step as we tailor treatment to those patients who respond poorly to traditional management strategies or show signs of organ injury and dysfunction.
However, in order to safely enroll patients early in clinical trials we need to better understand the interaction between their phenotypic presentation and underlying pathophysiology, as well as how this interaction impacts their response to therapy and clinical trajectory. This identification process starts with categorizing patients based on several characteristics, such as initial disease severity, comorbidities, precipitants, and perhaps novel biomarkers [21]. Once categorized, patients can then be followed to determine whether their allocated treatment results in end-organ dysfunction or an adverse event. To achieve this, further examination of the categorization process is needed with a specific focus on those variables that can rapidly and accurately help differentiate high- and low-risk patients.
Individualized treatment in AHFS randomized trials has been traditionally problematic, but targeting this population with a single therapy has not been successful to date. Furthermore, a study design which focuses on a single therapy does not readily generalize to the typical ED patient with AHFS and multiple comorbidities. As our findings suggest, heterogeneity defines this patient population; an issue that is compounded by the fact that in many, the initial clinical profile will change soon after presentation. Studies that match specific patient profiles with individualized treatment approaches are needed, and contemporary clinical trial approaches may facilitate this. For example, Bayesian adaptive trial design provides us with a unique opportunity to study multiple patient profiles with a variety of treatment approaches, while simultaneously reducing the sample size [23]. Under this design, as the trial progresses, patient profiles and treatments may be removed (or added), both for futility and efficacy, until all modes of therapy have been adequately tested across the patient profiles. Although such a trial does require significant upfront planning and collaboration by the investigative team, including emergency physicians, heart failure physicians and biostatisticians, the potential upside is tremendous.
Limitations
While our results suggest that a large proportion of patient's profiles changed during the first 12–24 hours of an acute presentation, there are some noteworthy limitations worth discussing. First, significant adverse events after the 30-day time period were not recorded. However, it is often difficult to determine the association between the index visit and events that occur 60 to 90 days later. Secondly, as an observational study, treatment was not controlled and has also not been considered in analysis; understanding the association between treatment and outcomes was not the goal of this analysis. Subsequent studies that aim to examine these profiles in detail should consider the relationship between initial treatment and subsequent endpoints. Further, we do not have the exact date of the subjects' events, so it is hard to determine whether the subjects experienced events while hospitalized or as an outpatient. We do know that approximately 75% of patients were discharged within 5 days and 6% of patients had events at 5-days. Because this study was observational there is a possibility that differences in outpatient management, patient behaviors, and the protective effect of hospitalization could have confounded our findings.
While we utilized strict definitions of moderate-to-severe renal dysfunction and TNP, troponin rise in AHFS can occur for many reasons and an elevated troponin alone may not be indicative of TNP. In particular, troponin release may be an on-going process in patients with more severe heart failure and thus independent of cardiac injury due to microvascular or macrovascular to coronary artery disease [24]. Nonetheless, it is clear that, regardless of mechanism, patients with AHFS and an elevated troponin have a far worse prognosis [25]. Finally, the limited sample size prohibits definitive conclusions about whether the individual components of clinical profile (i.e., TNP, renal dysfunction, or hemodynamics) contributed to 30-day events with an equivalent or differential effect. This was a descriptive paper about three readily-available ED variables and not a comprehensive analysis about all variables available during the first 24 hours of hospital admission. Finally, our subjects were enrolled early in their clinical course, within 3 hours of initial ED evaluation and treatment. While there may be a discrepancy in ED and hospital discharge diagnosis in 10–15% of patients, our data represent were collected prospectively during a time period that is often overlooked and underrepresented in registries and clinical trials.
Conclusion
Heterogeneity defines ED patients with AHFS, an issue that is compounded by the fact that in over 50% of these patients their initial clinical profile will change within the first 12 to 24 hours of acute presentation. Many of these changes are related to development of MRD or TNP. Patients with abnormal clinical profiles at presentation that remain abnormal throughout the first 12–24 hours of hospitalization are at significantly higher risk of 30-day adverse events than those whose profiles remain normal during this time period. Future clinical trials may need to consider targeting these patients, as they may be the most likely to benefit from experimental therapy.
Acknowledgments
The authors would like to acknowledge Lyndi Goette, MBA, for her help in preparation of the manuscript.
Sean Collins: Consultant: Abbott Point of Care, Bayer, The Medicines Company, Trevena, Novartis;
Grant Support: NIH/NHLBI, Abbott Point of Care, BG Medicine
Alan Storrow: Current Grant Support: Abbott Diagnostics, NIH / NHLBI (R01HL088459-02). NIH / NHLBI (K23HL085387-01A2), NIH / NHLBI (K12HL1090-01), Centers for Disease Control, Corthera, Roche Diagnostics, Abbott Diagnostics, Thermo Fisher
Current Consultant: Roche Diagnostics
Greg Fermann: Grant support: Dyax, Corthera, The Medicines Company
Advisory Board: Quest Diagnostics/Hemocue, Daiichi Sankyo
Frank Peacock: Research Grants (>$10,000) Abbott, Alere, BAS, Brahms, EKR, Nanosphere, The Medicine's Compnay;
Consultant (<$10,000) Abbott, Alere, Beckman Coulter, Electrocore, The Medicine's Company; Speaker's Bureau (<$10,000) Abbott, Alere; Ownership Interest (<$10,000) Comprehensive Research Associates LLC, Vital Sensors, Emergencies in Medicine LLC.
Peter Pang: Dr. Pang has been a consultant for Bayer, J&J, Medtronic, Novartis, Otsuka, SigmaTau, and Trevena and a member of the DSMB for J&J and the Medicines Company. He has received research support from Abbott.
Phil Levy: Consultant: The Medicines Company (Data Safety Monitoring Board), Corthera, Inc., Bayer Schering Pharma AG, EKR Therapeutics, Trevena, Inc
Research support/grants: The Cleveland Clinic Foundation, Nile Therapeutics, Corthera, Inc., Bayer Schering Pharma AG
Speakers Bureau: The Society of Chest Pain Centers
Chris Lindsell: Grant support: Abbott Point of Care
Mihai Gheorghiade: Consultant (modest): Abbott Labs, Astellas, Astra Zeneca, Bayer Schering Pharma AG, CorThera, Inc., Cytokinetics, Inc., DebioPharm S.A., Errekappa Terapeutici (Milan, Italy), Glaxo Smith Kline, Johnson & Johnson, Medtronic, Merck, Novartis Pharma AG, Otsuka Pharmaceuticals, Pericor Therapeutics, Protein Design Laboratories, Sanofi Aventis, Sigma Tau, Solvay Pharmaceuticals.
Dr. Gheorghiade receives $10,000 or more as a consultant from the following companies:Bayer Schering Pharma AG, DebioPharm S.A., Medtronic, Novartis Pharma AG, Otsuka Pharmaceuticals, Sigma Tau, Solvay Pharmaceuticals, DebioPharm S.A., Pericor Therapeutics.
This work was supported in part by National Heart, Lung and Blood Institute grant K23HL085387 and an institutional Clinical and Translational Science Award NIH/NCRR Grant Number 1UL1RR026314-01.
Footnotes
Author Disclosures Drs. Doug Sawyer and Neal Weintraub have no conflicts of interest or financial ties to disclose.
References
- 1.VMAC Investigators Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: A randomized controlled trial. Jama. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 2.Collins SP, Hinckley WR, Storrow AB. Critical review and recommendations for nesiritide use in the emergency department. The Journal of emergency medicine. 2005;29:317–329. doi: 10.1016/j.jemermed.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: A pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr., Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Wilson WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 5.Mebazaa A, Pang PS, Tavares M, Collins SP, Storrow AB, Laribi S, Andre S, Mark Courtney D, Hasa J, Spinar J, Masip J, Frank Peacock W, Sliwa K, Gayat E, Filippatos G, Cleland JG, Gheorghiade M. The impact of early standard therapy on dyspnoea in patients with acute heart failure: The urgent-dyspnoea study. Eur Heart J. 2010;31:832–841. doi: 10.1093/eurheartj/ehp458. [DOI] [PubMed] [Google Scholar]
- 6.Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O'Connor CM, Califf RM, Adams KF., Jr. Relation between dose of loop diuretics and outcomes in a heart failure population: Results of the escape trial. Eur J Heart Fail. 2007;9:1064–1069. doi: 10.1016/j.ejheart.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottlieb SS, Abraham W, Butler J, Forman DE, Loh E, Massie BM, O'Connor CM, Rich MW, Stevenson LW, Young J, Krumholz HM. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. 2002;8:136–141. doi: 10.1054/jcaf.2002.125289. [DOI] [PubMed] [Google Scholar]
- 8.Weintraub NL, Collins SP, Pang PS, Levy PD, Anderson AS, Arslanian-Engoren C, Gibler WB, McCord JK, Parshall MB, Francis GS, Gheorghiade M. Acute heart failure syndromes: Emergency department presentation, treatment, and disposition: Current approaches and future aims: A scientific statement from the american heart association. Circulation. 2010;122:1975–1996. doi: 10.1161/CIR.0b013e3181f9a223. [DOI] [PubMed] [Google Scholar]
- 9.Collins SP, Levy PD, Lindsell CJ, Pang PS, Storrow AB, Miller CD, Naftilan AJ, Thohan V, Abraham WT, Hiestand B, Filippatos G, Diercks DB, Hollander J, Nowak R, Peacock WF, Gheorghiade M. The rationale for an acute heart failure syndromes clinical trials network. J Card Fail. 2009;15:467–474. doi: 10.1016/j.cardfail.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Storrow AB, Collins S, Lindsell CJ, Disalvo T, Han J, Weintraub NL. Improving heart failure risk stratification in the ed: Stratify 1r01hl088459-01; treatment endpoints in acute decompensated heart failure 1k23hl085387-01a2. 2007. [Google Scholar]
- 11.Teerlink JR, Metra M, Felker GM, Ponikowski P, Voors AA, Weatherley BD, Marmor A, Katz A, Grzybowski J, Unemori E, Teichman SL, Cotter G. Relaxin for the treatment of patients with acute heart failure (pre-relax-ahf): A multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase iib study. Lancet. 2009;373:1429–1439. doi: 10.1016/S0140-6736(09)60622-X. [DOI] [PubMed] [Google Scholar]
- 12.Levy P, Compton S, Welch R, Delgado G, Jennett A, Penugonda N, Dunne R, Zalenski R. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: A feasibility and outcome analysis. Annals of emergency medicine. 2007;50:144–152. doi: 10.1016/j.annemergmed.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Peacock WFt, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, Wu AH. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117–2126. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 14.Voors AA, Davison BA, Felker GM, Ponikowski P, Unemori E, Cotter G, Teerlink JR, Greenberg BH, Filippatos G, Teichman SL, Metra M. Early drop in systolic blood pressure and worsening renal function in acute heart failure: Renal results of pre-relax-ahf. Eur J Heart Fail. 13:961–967. doi: 10.1093/eurjhf/hfr060. [DOI] [PubMed] [Google Scholar]
- 15.Aronson D, Burger AJ. The relationship between transient and persistent worsening renal function and mortality in patients with acute decompensated heart failure. J Card Fail. 2010;16:541–547. doi: 10.1016/j.cardfail.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–2226. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 17.Fonarow GC, Adams KF, Jr., Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for inhospital mortality in acutely decompensated heart failure: Classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 18.Apple FS. A new season for cardiac troponin assays: It's time to keep a scorecard. Clinical chemistry. 2009;55:1303–1306. doi: 10.1373/clinchem.2009.128363. [DOI] [PubMed] [Google Scholar]
- 19.Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, Wu AH, Christenson RH. National academy of clinical biochemistry laboratory medicine practice guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115:e356–375. doi: 10.1161/CIRCULATIONAHA.107.182882. [DOI] [PubMed] [Google Scholar]
- 20.Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation. 2007;115:949–952. doi: 10.1161/CIRCULATIONAHA.106.683110. [DOI] [PubMed] [Google Scholar]
- 21.Gheorghiade M, Braunwald E. A proposed model for initial assessment and management of acute heart failure syndromes. JAMA. 2011;305:1702–1703. doi: 10.1001/jama.2011.515. [DOI] [PubMed] [Google Scholar]
- 22.Collins SP, Pang PS, Lindsell CJ, Kyriacou DN, Storrow AB, Hollander JE, Kirk JD, Miller CD, Nowak R, Peacock WF, Tavares M, Mebazaa A, Gheorghiade M. International variations in the clinical, diagnostic, and treatment characteristics of emergency department patients with acute heart failure syndromes. Eur J Heart Fail. 12:1253–1260. doi: 10.1093/eurjhf/hfq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry DA. Bayesian clinical trials. Nature reviews. Drug discovery. 2006;5:27–36. doi: 10.1038/nrd1927. [DOI] [PubMed] [Google Scholar]
- 24.Potluri S, Ventura HO, Mulumudi M, Mehra MR. Cardiac troponin levels in heart failure. Cardiol Rev. 2004;12:21–25. doi: 10.1097/01.crd.0000089981.53961.cf. [DOI] [PubMed] [Google Scholar]
- 25.Peacock WFt, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, Wu AH, Investigators A, Peacock WFt, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, Wu AHB. Cardiac troponin and outcome in acute heart failure. New England Journal of Medicine. 2008;358:2117–2126. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]


