Abstract
The innate immune system is a crucial component of inflammatory reactions, while the central nervous system (CNS) is the most vulnerable site of the body to inflammatory tissue injury. Neuroinflammatory brain pathologies are disorders in which the CNS is threatened by its own immune system. Chemokine receptor CXCR2 and its ligands have been implicated in several neuroinflammatory brain pathologies, as well as in neutrophil recruitment and in the developmental positioning of neural cells.
This review focuses on the basics of CXCR2, its regulating role in bone marrow neutrophil recruitment, oligodendrocyte progenitor cell positioning and neural repair mechanisms, as well as its diverse roles in neuroinflammatory brain pathologies.
Keywords: CXCR2, chemokines, neutrophil, brain pathology, multiple sclerosis
1. Introduction
Chemokines and chemokine receptors are potential therapeutic targets for a variety of diseases. The suggested positive effect of chemokine signaling blockade range from drugs preventing cancer metastasis or ameliorating multiple sclerosis (MS) symptoms, to treatments inhibiting HIV or medications acting on roughly any other disease in which the chemokines and/or chemokine receptors have been implicated (Bielecki et al., 2008; Charo and Ransohoff, 2006; Garin and Proudfoot, 2011; Singh et al., 2010; Vasilescu et al., 2007). Research in academia and pharmaceutical industry has vastly expanded since the roles of chemokines and chemokine receptors in immunity unraveled. Moreover, the disclosure of their functions in homeostasis and under inflammatory conditions, make these molecules salient targets for the regulation of immunity (Charo and Ransohoff, 2006).
Currently, 19 different chemokine receptors have been discovered, separated into four different subfamilies: C, CC, CXC and CX3C.. In humans, the CXC subfamily currently contains seven chemokine receptors and 15 ligands (Murphy et al., 2011). The CXC chemokine subfamily can be divided in a group of chemokines containing a glutamic acid-leucine-arginine (ELR) motif, and a group in which the ELR motif is absent (Hebert et al., 1993; Savarin-Vuaillat and Ransohoff, 2007). Seven of the 15 CXC ligands (CXCL1-3 and CXCL5-8) contain an ELR motif, all having high binding affinity for chemokine receptor CXCR2. Important roles in the innate immune system for CXCR2 were first described by Huber et al. shortly after CXCR2 was discovered as a neutrophil receptor (Huber et al., 1991; Murphy and Tiffany, 1991). Strong evidence for a role of CXCR2 in a neuroimmunological disease came a few years ago, when CXCR2 was shown to govern developmental positioning in the oligodendrocyte lineage (Robinson et al., 1998; Tsai et al., 2002). Extensive research in the past two decades has began to delineate the role CXCR2 plays in immunity, the central nervous system (CNS), and disease related contexts, all associated with the importance and the complexity of the functions of CXCR2.
Therefore, we will focus mainly on CXCR2, by addressing the importance of CXCR2 in neuroinflammatory pathologies, reviewing the basics of the receptor and its ligands, discussing expression of CXCR2 on neutrophils and the receptor’s role in the recruitment of immune cells to the CNS, and going over the differential roles CXCR2 plays in the development and pathologies of the CNS.
2. Biological functioning of CXCL1-CXCR2
2.1. Chemokine receptor structure
CXCR2 was first described as IL-8 receptor IL-8RB by Murphy et al. (Murphy and Tiffany, 1991). Simultaneously another receptor was described by Holmes et al. as IL-8RA (Holmes et al., 1991), now known as CXCR1. CXCR1 and CXCR2 show 77% amino acid identity and are both mapped on human chromosome 2q34-q35 (Ahuja et al., 1992). CXCR2, and in some cases CXCR1, is highly conserved among vertebrates (Catusse et al., 2003; Hipkin et al., 2004; Huising et al., 2003a; Lee et al., 1992; Pighetti and Rambeaud, 2006; Prado et al., 1994). Genome comparison has shown about 70% sequence identity for human with rat (Rattus norvegicus) and murine (Mus musculus) CXCR1 and CXCR2 (Bozic et al., 1995; Dunstan et al., 1996; Fan et al., 2007). Although both receptors are fairly similar in structure and are well conserved, studies with CXCR2 germ line knock-outs have shown an extensive and non-redundant function for CXCR2 (Cacalano et al., 1994; Liu et al., 2010a; Liu et al., 2010b; Tsai et al., 2002), leaving the role of CXCR1, and interaction with its ligands, CXCL6 in rodents, and CXCL6 and CXCL8 in humans, unclear.
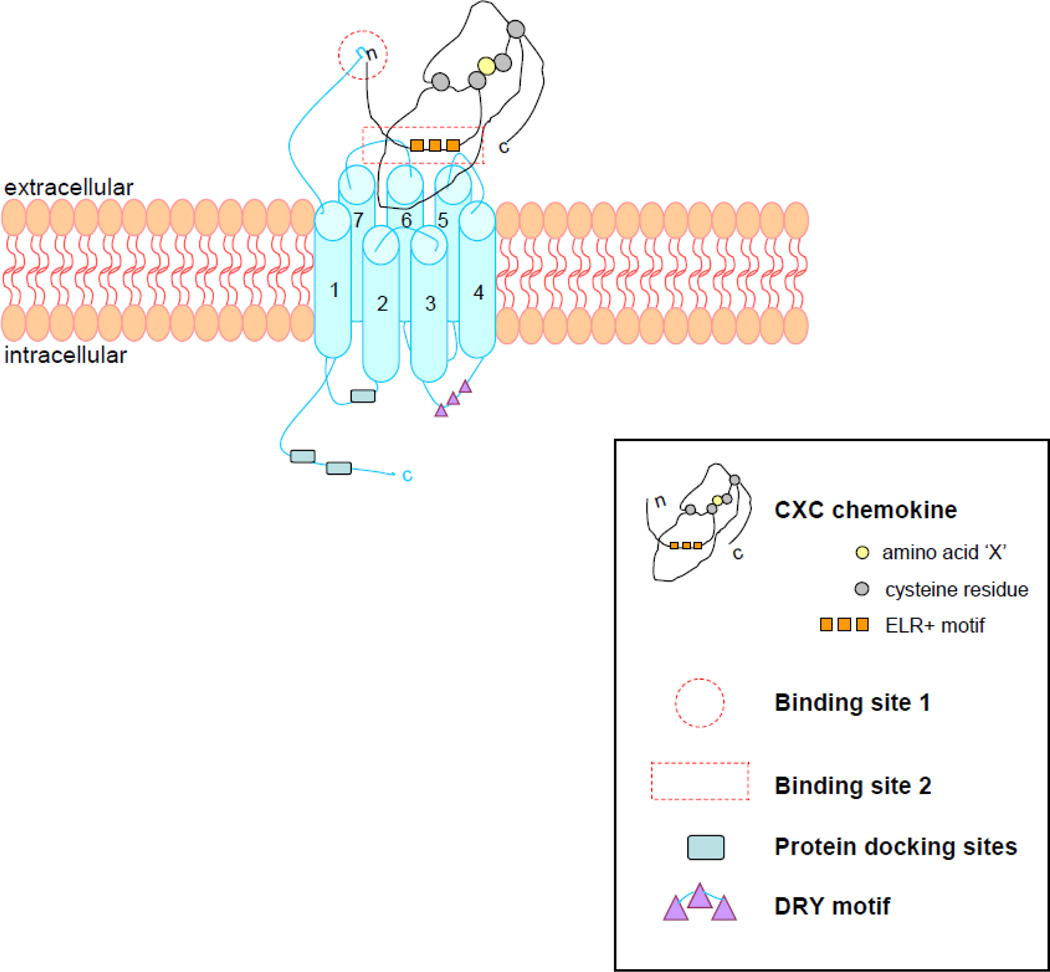
The DNA sequence of CXCR2 contains a single long open reading frame, encoding for protein domains which indicate membership in the G protein coupled receptors (GPCRs) of the rhodopsin superfamily (Holmes et al., 1991). CXCR2 is considered part of the rhodopsin Class A GPCR subfamily, which contains all chemokine receptors. The proposed structure of CXCR2 is mainly based on bovine rhodopsin (Palczewski et al., 2000), since studies investigating the structures of chemokine receptors have proven to be refractory so far (Lodowski and Palczewski, 2009). The receptor is comprised of seven transmembrane segments, three extracellular and three intracellular loops, an extracellular N-terminal domain, and a cytosolic C-terminal segment (Figure 1). The amino acid aspartate in the second extracellular loop and a LLKIL motif in the C-terminus are both required for rapid receptor internalization, while the second intracellular loop contains a DRY (Asp – Arg – Tyr) motif as the G protein docking site, giving the chemokine receptor the ability to signal upon ligand binding (Allen et al., 2007; Nasser et al., 2007).
Figure 1. CXCR2 structure and ligand binding concept.
The CXCR2 chemokine receptor is compromised of seven transmembrane segments, three extracellular and three intracellular loops, an extracellular N-terminal domain, and an intracellular C-terminal domain. It contains a DRY motif on the second intracellular loop, functioning as G-protein docking site, and other protein docking sites as the LLKIL motif on the C-terminus.
The CXCR2 ligand N-terminus binds with the N-terminal domain of the receptor (site 1), after which the ELR+ motif of the chemokine binds with the second and third extracellular loop of the receptor (site 2), to control the binding and to activate receptors signaling pathways.
2.2. Ligand biology
The characteristic ELR motif of the CXCR2 ligands, chemokines CXCL1-3 and CXCL5-8, assures binding with CXCR2 and is strongly associated with neutrophil attraction (Charo and Ransohoff, 2006). ELR+ CXC chemokines are located closely together in the human genome at chromosome 4q12-21, consistent with overlapping functions (Ransohoff, 2009).
An association between the CXC chemokines was already found in the late 1980’s, when Richmond et al., Sager et al. and Yoshimura et al. extracted and characterized the first ELR+ CXC chemokines (Anisowicz et al., 1987; Richmond et al., 1983; Richmond et al., 1985; Yoshimura et al., 1987). Rapidly, several other analogous chemokines were described, originally reported as MGSAαβγ/GROαβγ, ENA-78, GCP-2 and NAP-1 and 2 in human, and as KC/MIP-2 and CINC in mice and rats respectively (Table 1). Until now a murine CXCL8 homolog has not been reported. However all other ELR+ CXC chemokines have been detected in mice and ELR+ CXC chemokine analogs have also been described in several other vertebrates. Some CXC chemokine and receptor analogs have been reported in the earliest vertebrates, suggesting a function advantageous for vertebrate biology (Huising et al., 2003b).
Table 1. The CXCR2 ligands and their secondary names.
Murine (Mus musculus) CXC chemokines and rat (Rattus norvegicus) CXC chemokine.
| Ligands | Secondary names | Characterization |
|---|---|---|
| CXCL1 | Gro-1, Gro-α, MGSA-α, NAP-3, KC | Anisowciz et al., 1987 Richmond et al., 1988 |
| CXCL2 | Gro-2, Gro-β, MGSA-β, MIP-2α, MIP-2 | Haskill et al., 1990 |
| CXCL3 | Gro-3, Gro-γ, MGSA-γ, MIP-2β | Haskill et al., 1990 |
| CXCL5 | ENA-78 | Walz et al., 1991 |
| CXCL6 | GCP-2 | Proost et al., 1993 |
| CXCL7 | NAP-2, CTAP-III | Moser et al., 1991 |
| CXCL8 | IL-8, CINC | Baggiolini et al., 1989 |
The importance of CXC chemokines for leukocyte recruitment in the innate immune system can be concluded from the extensive research showing that all ELR+ CXC chemokines have equal CXCR2 binding affinities and neutrophil chemoattractant properties (Addison et al., 2000; Ahuja et al., 1996). This leads to the suggestion that CXC chemokines are highly redundant, and the suggestion that a rapid chemokine expansion was necessary to cope with a continuous treat of viral infections (Huising et al., 2003b). Chemokines are also important for the induction of effector functions, raising intracellular Ca2+ levels, releasing granule contents and forming respiratory burst (Baggiolini et al., 1995). The early extraction of the MGSA/GRO chemokines (CXCL1-3) from tumor-derived human cells by Richmond et al., showing its effect on melanoma cell growth and neural crest cells (Balentien et al., 1991; Bordoni et al., 1989), prefigured the importance of CXCL1-CXCR2 biology in cancer and CNS development (Horton et al., 2007; Tsai et al., 2002), while highly elevated CXCL1 levels during CXCR2 deficiency show the scavenging role of chemokine receptors, being one of their effector functions (Cardona et al., 2008).
2.3. Expression
The expression of CXCR2 on neutrophils and oligodendrocytes has been widely described for its roles in immunity, cancer growth and CNS development, but its expression has also been reported on human basophils, T-lymphocytes and endothelial cells (Krieger et al., 1992; Lippert et al., 2004; Raman et al., 2007; Ransohoff et al., 2007). Although this expression, as well as its murine expression on tissues and cells other than neutrophils and oligodendrocyte lineage cells, has been reported, characterization by immunocytochemistry has been shown to be of questionable specificity (Lindner et al., 2008; Liu et al., 2010a). Here we will mainly consider the functions CXCR2 exerts through its expression on neutrophils and in the oligodendrocyte lineage.
2.4. Activation, signaling and desensitization
Chemokine receptors are activated through signaling, based on the concept of a two-site interaction between the chemokine and its receptor (Rajagopalan and Rajarathnam, 2006). Although this interaction concept is based on ligand-receptor interaction studies of chemokine receptors CCR2 and CXCR4 (Crump et al., 1997; Monteclaro and Charo, 1996), more structures and binding modes of chemokine receptors and chemokines are currently solved (Wu et al., 2010; Salanga and Handel, 2011). For example, a recent study described similarities between the CXCR2 and CXCR4 binding modes (de Kruijf et al, 2011).
The first step in the ligand-receptor binding interaction is binding of the chemokine’s N-terminal residues preceding the first cysteine, with the N-terminal domain of the receptor (site 1), followed by the binding of the ligand’s ELR+ motif with the receptors 2nd and 3rd exoloops (site 2) (Figure 1). It appears that site 1 binding is essential for receptor selectivity and affinity (Rajagopalan and Rajarathnam, 2004), whereas the binding of the ELR+ motif of the chemokine with the 2nd binding site of the receptor stabilizes the binding and activates the signaling pathway (Kraemer et al., 2011).
Upon ligand-receptor binding, and subsequently receptor activation, the heterotrimeric G-protein, Gαβγ, separates into the subunits Gα and Gβγ, through the exchange of GDP for GTP on the Gα subunit. The Gβγ subunit then activates phospholipase C, which cleaves phosphatidylinositol (4,5)-biphosphate (PIP2) into secondary messengers inositol triphosphate (IP3) and diaglycerol. They in turn trigger signaling events such as the rise of intracellular calcium from intracellular stores, leading to responses like chemotaxis and degranulation (Murdoch and Finn, 2000). Next, depending on the cell type and chemokine receptor binding, activation of the classic mitogen-activated protein kinase (MAPK) signal transduction pathway takes place, leading to downstream transcription activation (Knall et al., 1996).
Similar to the binding of a chemokine with its receptor during activation, the induction of internalization of CXCR2, depends on the interactions between the N-terminal of the chemokine and the N-domain of the chemokine receptor (Prado et al., 2007). The efficiency of receptor internalization, as well as the choice for the intracellular trafficking pathway of CXCR2, depends on motifs on the C-terminus of the receptor (Baugher and Richmond, 2008). In vitro studies in HL60 and HEK293 cell lines show that binding of adaptor proteins and phosphorylation of serine residues in the receptor’s C-terminus facilitate intracellular movement of CXCR2 (Raman et al., 2009). Internalization of CXCR2 through clathrin-coated pits, and association with different cellular trafficking regulators, as Rab GTPases like Rab5, results in either the recycling or lysosomal sorting pathway of CXCR2 (Neel et al., 2005). In case of the recycling pathway, CXCR2 returns back to the cell surface after dephosphorylation, while, in case of the lysosomal sorting pathway, the receptor will be degraded by proteolytic enzymes (Fan et al., 2003).
3. Immunobiology of CXCR2
3.1. Bone marrow to lesion
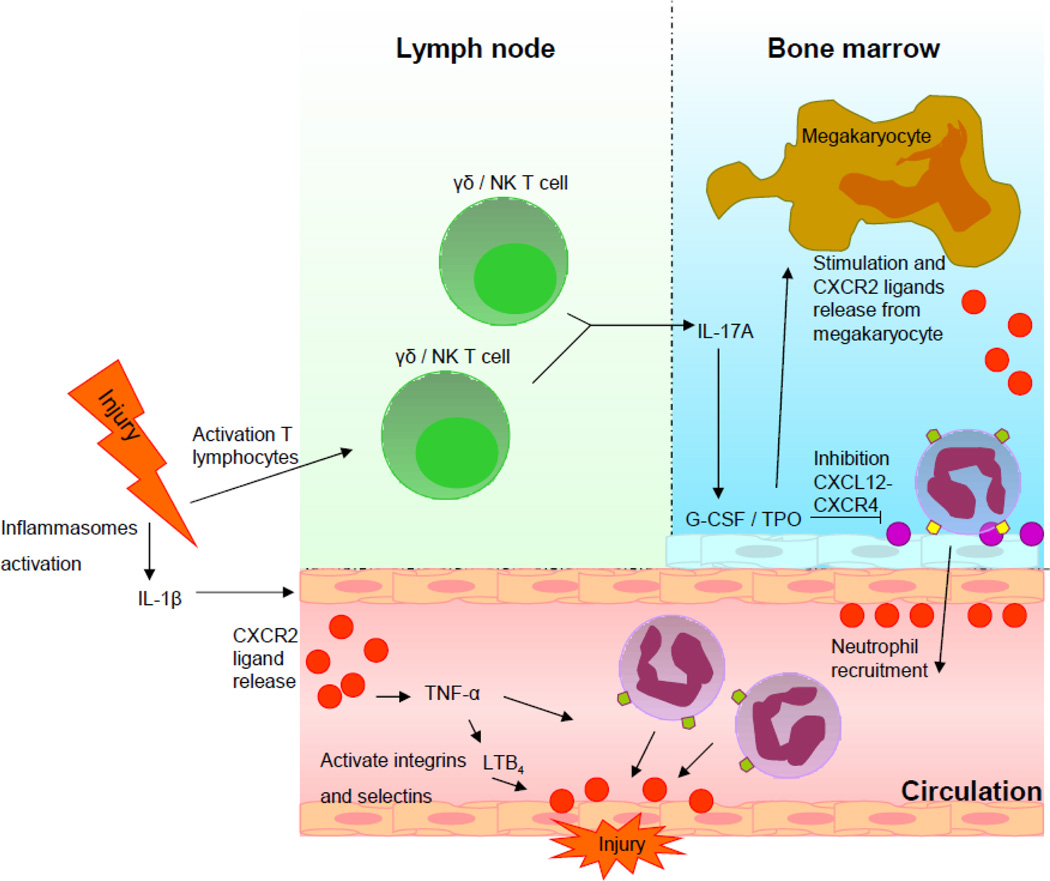
The regulation of neutrophil recruitment from the bone marrow to the site of inflammation is incompletely known, but research is pointing toward major roles for CXCR2 and CXCR4 (Borregaard, 2010). Upon acute injury, two different reactions take place simultaneously (Figure 2).
Figure 2. Hypothetical model of CXCR2’s regulating role in two separate recruiting events.
- Injury activates γδ and natural killer T cells, which produces IL-17A. This induces transcription of G-CSF in the bone marrow, which activates TPO. Stimulation by TPO inhibits CXCL12-CXCR4 binding and activates the release of CXCR2 ligands from megakaryocytes. The release of the CXCR2 ligands and weakening of the CXCR2 binding, leads to mobilization of neutrophils from the bone marrow.
- Activation of inflammasomes leads to IL-1β release and stimulation of local CXCR2 ligand production, inducing a subsequential production of TNF-α and LTB4, therewith activating integrins and selectins on the endothelial cells close the site of the lesion. CXCR2 ligands are presented at the site of the lesion, creating a chemotactic gradient for the recruitment of neutrophils through the interaction of CXCR2 and CXCR2 ligands.
First, pro-inflammatory cytokine interleukin-1β (IL-1β) is released within damaged tissue as a reaction to injury. IL-1β is processed from the inactive proIL-1β into the bioactive IL-1β by either inflammasome caspase-1 in monocytes and macrophages, or proteinase 3 in neutrophils (van de Veerdonk et al., 2011). Since macrophage are situated in a specialized environment with constant exposure to microbial stimuli, the macrophage inflammasome needs to be activated by two signals, Toll like receptor (TLR) stimulation and a secondary adenosine triphosphate “danger” signal. The monocyte inflammasome is activated through TLR stimulation only, because monocytes normally remain in a pathogen free environment (Netea et al., 2008). The release of IL-1β at the site of injury leads to activation of intracellular adhesion molecule-1, while the concurrent release of CXCR2 ligands induces a sequential assembly of tumor necrosis factor-α (TNF-α) and leukotriene B4 (LTB4), all leading to activation of adherence factors on endothelial cells at the site of the lesion, facilitating the migration of neutrophils to the site of injury (Coelho et al., 2008; Hu et al., 2010a; Hu et al., 2010b; McDonald et al., 2010; Ramos et al., 2006). Simultaneously, CXCR2 ligands are produced by macrophages and endothelial cells in the periphery or astrocytes in the CNS, in order to form a chemotactic gradient to guide neutrophils through the bloodstream to the injured site (McDonald et al., 2010; Oh et al., 1999; Vieira et al. 2009).
Secondly, G-CSF is produced quickly by the release of IL-17A from activated γδ T cells and natural killer T cells immediately after injury (Kerns et al., 2009; Ley et al., 2006). The release of G-CSF weakens the retention signal of CXCR4 with CXCL12 in the bone marrow, by decreasing CXCR4 and CXCL12 expression, concurrently inducing thrombopoietin (TPO) production by bone marrow stroma, and triggering the release of CXCR2 ligands by megakaryocytes, preparing the release of neutrophils from the bone marrow (Eash et al., 2010; Greenbaum and Link, 2011; Köhler et al., 2011; Petit et al., 2002; Wenger et al., 2008; Wu et al., 2001). Neutropils are then mobilized into the circulation, and replace the neutrophils recruited to the site of injury (Furze and Rankin, 2008).
3.2. Protection and destruction
The complexity of the innate immune system evidently shows that many factors work together in order to provide protection against pathogens and disease. The non-redundant role of CXCR2 in the innate immune system is indicated by its significance in the regulation of neutrophil recruitment and the activation of neutrophil effector functions. Evidence for its non-redundancy was shown in vivo, when Cacalano et al. generated the first germline CXCR2 knockout mice (CXCR2−/−) in 1994 (Cacalano et al., 1994). Characteristics of these mice include neutrophilia and impairment in the recruitment of neutrophils during acute inflammatory conditions (Cacalano et al., 1994). Our laboratory recently described a similar high neutrophil count in the blood of CXCR2−/− mice. However, the CXCR2−/− mice did show a neutrophil response into the brain after 5 days of inflammatory cuprizone-induced demyelination similar to that of wild-type mice (Liu et al., 2010a). Flow cytometric staining of infiltrated cells with Ly6G and CD45, as well as peripheral co-staining of Ly6G and CXCR2, suggested that CXCR2-positive neutrophils were required for extensive demyelination in the corpus callosum of wild-type mice, while the CXCR2−/− mice showed resistance to demyelination. This observation suggests that CXCR2 is necessary for the promotion of selective neutrophil effector functions in the CNS, whereas on the contrary, the CXCR2−/− mice in the study of Cacalano et al. showed no impairment in functions required for peripheral bacterial clearance (Cacalano et al., 1994; Liu et al., 2010a). These observations lead to the hypothesis that CXCR2 exerts varying effector funtions in case of acute versus chronic inflammatory reactions, as well as in peripheral versus CNS environments.
Varying functions of CXCR2 are also suggested in other conditions. For example, CXCR2 is considered to create favorable conditions for wound healing in the periphery through the promotion of neutrophil recruitment (Zaja-Milatovic and Richmond, 2008). But on the other hand, depletion of CXCR2-positive neutrophils in subarachnoid hemorrhage is associated with the prevention of delayed cerebral vasospasm (Provencio et al., 2011). Furthermore, CXCR2 and its ligands are assumed to increase cellular senescence in early tumorigenesis, inducing growth arrest in cells. In the case of late stage tumorigenesis, mutations in tumorous cells create circumstances in which ELR+ chemokines are unable to induce cellular senescence, and instead are implicated to create circumstances favoring metastasis formation (Lazanecc and Richmond, 2010). This versatility sets the effector functions of CXCR2, depending on the circumstances, constantly on the border of protection and destruction.
3.3 Cancer regulation
Involvement of CXCR2 ligands in cancer was described, shortly after the first CXCR2 ligands were defined as molecules important in leukocyte recruitment and functioning. The extraction of CXCL1-3 from tumor-derived human melanoma cells and its involvement in melanoma cell growth, suggested roles for CXCR2 and its ligands in tumor regulation as well (Balentien et al., 1991). Now, CXCR2 and all CXCR2 ligands have been implicated in tumor progression including tumor growth, vessel formation and cancer cell proliferation, as well as in neutrophil recruitment to the tumor microenvironment, in which the neutrophils are also associated with tumor development and growth (Horton et al., 2007; Raman et al., 2011).
Robinson et al. described involvement of CXCR2 in oligodendroglioma proliferation and reported upregulation of CXCL1 in primary brain tumors, showing a CXCR2 signaling role in the induction of tumor growth in the brain (Robinson et al., 2001). CXCL8, along with CXCL1 and possibly other CXC chemokines, are produced in the tumor microenvironment through, among others, nuclear factor-kappa B (NF-κB) activation (Amiri et al., 2006; Martin et al., 2009). And although chemotherapy and radiation are used to conquer cancer, they also produce reactive oxygen species in the tumor microenvironment, which in turn activates NF-κB and thus leads to the production of factors like CXC chemokines involved in tumor progession (Reuter et al., 2010).
This is a double-edged sword situation, in which a risk has to be taken in order to defeat the pathology. Even though many reports describe how CXCR2 and its ligands promote tumorigenesis, it has also been shown that they provide protection in the early stages of tumorigenesis (Lazanecc and Richmond, 2010).
4. Differential roles of CXCR2 in the brain
4.1. Oligodendrocyte progenitor cell proliferation and protection
The expression of CXCR2 on oligodendrocyte progenitor cells (OPCs) in the brain and spinal cord was described, after the discovery of CXCL1 production by spinal cord astrocytes (Glabinski et al., 1997; Robinson et al., 1998; Tsai et al., 2002). Chemokine CXCL1, in combination with platelet derived growth factor (PDGF), stimulated the proliferation of OPCs in the spinal cord in vitro (Robinson et al., 1998). CXCR2 on OPCs in vivo interacted with CXCL1 to arrest the migration of OPCs through the rapid and reversible inhibition of PDGF chemokinesis in a transwell assay. Using slice culture, it could be shown that CXCL1 also increased OPC interaction with extracellular matrix substrates, possibly accounting for migration arrest. In postnatal mice, the absence of CXCR2 led to a reduction in myelin thickness, with an increased G-ratio of radial myelin thickness to axonal perimeter, during spinal cord development (Padovani-Claudio et al., 2006; Tsai et al., 2002). Moreover, Tirotta et al. described that CXCR2 protects striatum OPCs, differentiated in culture from mice at postnatal day 1, from apoptosis after 6 days of pro-apoptotic IFN-γ and CXCL10 treatment (Tirotta et al., 2011). Altogether, this indicates that in the CNS, interaction of CXCL1-CXCR2 possibly prevents OPCs from apoptosis, and controls the positioning and proliferation of OPCs in the developing spinal cord through a regulating action on PDGF.
4.2. Neuroinflammation
Neuroinflammatory reactions in neuropathological diseases such as MS, traumatic brain injury (TBI) and Alzheimer’s disease (AD) are associated with the presence of chemokines and cells bearing chemokine receptors. Evidence for the role of chemokines and its receptors in MS is well described (Holman et al., 2011), but evidence for these factors in TBI and AD is found more recently. Studies in AD and TBI indicated that chemokine receptors may play essential roles in the extent of cortical damage and β-amyloid (Aβ) plaque deposition (Cho et al., 2011; Lee et al., 2010; Semple et al., 2010).
The role of CXCR2 during neuroinflammation specifically, has been studied and well described before in the experimental autoimmune encephalomyelitis (EAE) mouse model for MS (Cardona et al., 2008; Carlson et al., 2009; Ransohoff RM, 2009; Ransohoff RM et al., 2007). More recently, we described the function of CXCR2 in an animal model for MS: the cuprizone-induced demyelination model. In this model, the role of CXCR2 on myeloid and non-myeloid cells in relation to the destruction and repair of myelin was described (Liu et al., 2010a; Liu et al., 2010b).
4.2.1. Multiple Sclerosis
MS is a chronic inflammatory demyelinating disease of the CNS, characterized by infiltrating inflammatory components into the CNS and ultimately failing remyelination. The infiltrating inflammatory cells are mostly considered to be T-cells, B-cells and macrophages from the adaptive immune system. But recently the role of the innate immune system is also considered for the involvement in the onset or progression of MS (Carlson et al., 2008; Chabas et al., 2011; McColl et al., 1998; Weiner, 2009). CXCR2 chemokine CXCL1 has been implicated in EAE, CXCL1 expression by reactive astrocytes has been reported at lesion edges, and measurements of serum levels in MS patients showed upregulation of CXCL8, suggesting a role of the innate immune system, and more specifically CXCR2 in MS (Glabinski et al., 1998; Lund et al., 2004; Omari et al., 2006).
The effect of CXCL1 on neutrophil recruitment into the CNS was shown in a transgenic MBP-CXCL1 mouse model, in which expression of CXCL1 by oligodendrocytes led to neutrophilia in the CNS, behavioral impairment and blood-brain-barrier breakdown (Tani et al., 1996). The inoculation of a neurotropic virus into the CNS, simulating immune-mediated demyelination as in MS, also elevated the expression of CXCR2 ligands, suggesting the recruitment of CXCR2-positive neutrophils into the CNS for virus clearance. Deficiency of CXCR2 led to lessened neutrophil infiltration, but had no effect on virus specific T cell viral clearance (Hosking et al., 2009). Another demyelinating animal model for MS recently used by our laboratory, cuprizone-induced demyelination, showed CXCR2-positive neutrophils in the CNS after 5 days of cuprizone feeding (Liu et al., 2010a). After 3 to 6 weeks of cuprizone feeding, CXCR2+/+ mice showed extensive demyelination, while CXCR2−/− mice were relatively resistant to cuprizone-induced demyelination. Moreover, bone marrow chimeric mice, CXCR2+/− → CXCR2+/+ and CXCR2+/− → CXCR2−/−, revealed equal susceptibility to demyelination, but CXCR2−/− → CXCR2+/+ showed resistance to demyelination (Liu et al., 2010a). To control for the impact of radiation with the bone marrow chimeric mice, as it may influence the migration of myeloid cells into the CNS, also CXCR2+/+ → CXCR2−/− were tested on cuprizone-induced demyelination. These mice showed demyelination as extensively as the CXCR2+/+ mice, indicating that in cuprizone-induced demyelination, CXCR2-positive myeloid cells are essential in the demyelination process.
CXCR2 has also been implicated as an essential element in EAE. Induction of CXCL1 and CXCL2 transcription by CD4-positive T cells was shown to be essential in EAE, and CXCR2-positive neutrophils were able to restore susceptibility for EAE in CXCR2−/− mice (Carlson et al., 2008). The use of a CXCR2 antagonist led to a reduction in EAE score, and the overexpression of CXCL1 by astrocytes in double transgenic mice led to a reduction in inflammation and demyelination in EAE, possibly due to CXCR2 receptor downregulation (Kerstetter et al., 2009; Omari et al., 2009). Moreover, Liu et al. showed similar susceptibility for EAE and cuprizone-induced demyelination in CXCR2+/− → CXCR2+/+ and CXCR2+/− → CXCR2−/− bone marrow chimeric mice (Liu et al., 2010b).
Remyelination was improved in the CXCR2+/− → CXCR2−/− bone marrow chimeras during the recovery phase (Liu et al., 2010b), while also mice treated with a CXCR2 antagonist showed better remyelination in the spinal cord after EAE (Kerstetter et al., 2009), leading to the suggestion that CXCR2 expression on myeloid cells is essential in the damage of myelin during chronic CNS inflammation in animal models for MS, while CXCR2 on non- hematopoietic cells is responsible for the inhibition of myelin repair, which may be in line with CXCR2’s arresting role on OPC proliferation in the developing spinal cord (Liu et al., 2010a; Tsai et al., 2002).
4.2.2. Traumatic brain injury
Several clinical studies reported highly elevated levels of CXCL8 in the cerebrospinal fluid (CSF) following severe TBI (Hayakata et al., 2004; Kossmann et al., 1997; Maier et al., 2001; Whalen et al., 2000), indicating that a neuroinflammatory response might be following injury via CXCR2 leukocyte recruitment. But recently, a more apparent link between CXCL8 and CXCR2 was established in a clinical TBI study with a small subset of patients, where poor patient survival of patients with enlarged contusions after TBI, was correlated with elevated CXCL8 serum concentrations (Rhodes et al., 2009).
Although several clinical studies have indicated CXCL8 as a marker following TBI, little research is performed in TBI animal models. A study in 2006 showed rapid upregulation of CXCR2 ligands in rats, as well as neuronal immunoreactivity of CXCR2 following TBI (Vallès et al., 2006). Unfortunately, immunolocalization of CXCR2 has subsequently been found to be nonspecific (Lindner et al., 2008;2009). Semple et al. performed a TBI simulating experiment in mice recently, resulting in upregulation of G-CSF, CXCL1 and CXCL2 in the CNS at 12–24 hours after brain injury (Semple et al., 2010). Mice deficient for CXCR2 showed elevated levels of CXCL1 and reduced neutrophil infiltration after brain injury. Moreover, a correlating attenuation of neuronal loss and tissue damage was described in CXCR2−/− mice (Semple et al., 2010). This suggests a potentially significant role of CXCR2 following TBI, but further research should reveal the specific role of CXCR2 in TBI, to seriously consider it as a possible therapeutic target in the future.
4.2.3. Alzheimer’s disease
AD is a major cause of dementia and is the most common neurodegenerative disease in the elderly. The neuropathology of AD is associated with extracellular Aβ plaques and intracellular neurofibrillary tangles along with marked neuroinflammation, including microgliosis (Reitz et al., 2011). Recent evidence has implicated pro-inflammatory molecules within the AD brain, and, more specifically, chemokine receptors have been associated with a role in Aβ deposition and tau pathology (Bhaskar et al., 2010; Cho et al., 2011; Fuhrmann et al., 2010; Lee et al., 2010). Despite that, clinical interventions based on these discoveries are not elucidated yet, since several clinical trials have turned out to be controversial (de Jong et al., 2007; Schwartz and Shechter, 2010).
The involvement of CXCR2 in AD remains to be clarified too, since only a few studies on the role of CXCR2 in AD have been performed so far (Bakshi et al., 2011; Xia and Hyman, 2002). The studies imply that CXCR2 may enhance AD pathology and that deficiency in CXCR2 reduces Aβ deposition (Bakshi et al., 2011; Xia and Hyman, 2002).
Another study with mice expressing transgenes encoding mutated human presenilin 1 (PS1), the polypeptide linked to familial forms of AD, showed involvement of CXCR2 ligand CXCL1. Resting microglia showed upregulation of CXCL1, and downregulation of neural progenitor cell proliferation, in mice carrying a PS1 mutation, linking neurogenesis impairment and CXCL1 expression in an AD mouse model (Choi et al., 2008). Moreover, a recent clinical study revealed that CSF CXCL1 was lower in cognitively normal as compared with cognitively impaired individuals (Craig-Shapiro et al., 2011), while it has also been shown that CXCL8 CSF levels are significantly increased in AD patients (Zhang et al., 2008).
Although these data point to a possible harmful role of CXCR2 in AD pathology, too little is known to provide specific conclusions at present. Additional research needs to be performed to decipher the exact role of CXCR2 in this neurodegenerative disease.
5. Conclusion and future perspectives
The significance of CXCR2 and its ligands in multiple diseases and disease models has been described. CXCR2 is a promising potential therapeutic target, since brain penetrant inhibitors and CXCR2 antagonist providing promising results in various clinical trails for Alzheimer’s disease and COPD, respectively (Chapman et al., 2009; Frisardi et al., 2010). The mechanisms of CXCR2 in different disease models and environments have not been totally defined yet, so vigilance should be exercised because of the complex biological role CXCR2 carries out.
The role of CXCR2 in the environment of the periphery and the CNS, and during acute and chronic inflammatory reactions, can be very different. Although CXCR2 exerts this versatile role, development of CXCR2 antagonists is logically based on the harmful role CXCR2 seems to play in the different pathologies described here. But it should be noted that CXCR2 is also very important in the physiology of the body, exemplified by the striking phenotype of CXCR2 germline knockout mice (Cacalano et al., 1994). Moreover, some studies have shown that depletion of CXCR2 or neutrophils after initiation of diseases worsens the outcome (Bai et al, 2010; Hosking et al, 2009). This promiscuity in the functioning of CXCR2 may represent the chemokine receptor as a phenomenon, for which it is hard to create standard inhibitors or blockers.
Despite CXCR2’s versatility, also several similarities in the functioning of CXCR2 can be extracted from the studies performed on CXCR2. For example the observation that CXCR2 and its ligands induce cellular senescence during early tumorigenesis (Lazannec and Richmond, 2010), can also be considered a role of CXCR2 under normal circumstances in the body’s physiology. After all, the function of CXCR2 in the innate immune system is to guard the body from infections and disease. In case of an auto-immune disoder as MS, or in case of late tumorigenesis, CXCR2 possibly exerts an autocrine and paracrine ligand signaling, responsible for the maintenance of high ligand levels and the induction of damage, instead of providing protection. Depletion of neutrophils in this case might worsen the outcome, since eliminating the initial immune response and allowing escape of initial clearance.
Regarding the current knowledge about CXCR2, it is important to keep investing effort into the specific role of CXCR2 in different diseases. Since chronic CXCR2 inhibition could be difficult, especially in the CNS, acute inhibition of CXCR2 in TBI or subarachnoid hemorrhage should be considered. Additional information should provide more evidence, to better mark a therapeutic target in the CXCR2 biology for its future application in medicine.
Acknowledgements
Research in the Ransohoff laboratory is supported by the US National Institutes of Health, the National Multiple Sclerosis Society, the Williams Family Fund for Multiple Sclerosis Research (all to R.M.R.), the Dutch Stichting MS Research and Stichting Nijmeegs Universiteits Fonds (both to M.V.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J. Biol Chem. 1996;271:20545–20550. doi: 10.1074/jbc.271.34.20545. [DOI] [PubMed] [Google Scholar]
- Ahuja SK, Ozcelik T, Milatovitch A, Francke U, Murphy PM. Molecular evolution of the human interleukin-8 receptor gene cluster. Nat. Genet. 1992;2:31–36. doi: 10.1038/ng0992-31. [DOI] [PubMed] [Google Scholar]
- Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu. Rev. Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- Amiri KI, Ha HC, Smulson ME, Richmond A. Differential regulation of CXC ligand 1 transcription in melanoma cell lines by poly(ADP-ribose) polymerase-1. Oncogene. 2006;25:7714–7722. doi: 10.1038/sj.onc.1209751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisowicz A, Bardwell L, Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc. Natl. Acad. Sci. U. S. A. 1987;84:7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M, Loetscher P, Moser B. Interleukin-8 and the chemokine family. Int. J Immunopharmacol. 1995;17:103–108. doi: 10.1016/0192-0561(94)00088-6. [DOI] [PubMed] [Google Scholar]
- Bai F, Kong KF, Dai J, Qian F, Zhang L, Brown CR, Fikrig E, Montgomery RR. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J.Infect.Dis. 2010;202:1804–1812. doi: 10.1086/657416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi P, Margenthaler E, Reed J, Crawford F, Mullan M. Depletion of CXCR2 inhibits gamma-secretase activity and amyloid-β production in a murine model of Alzheimer's disease. Cytokine. 2011;53:163–169. doi: 10.1016/j.cyto.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Balentien E, Mufson BE, Shattuck RL, Derynck R, Richmond A. Effects of MGSA/GRO alpha on melanocyte transformation. Oncogene. 1991;6:1115–1124. [PubMed] [Google Scholar]
- Baugher PJ, Richmond A. The carboxyl-terminal PDZ ligand motif of chemokine receptor CXCR2 modulates post-endocytic sorting and cellular chemotaxis. J. Biol Chem. 2008;283:30868–30878. doi: 10.1074/jbc.M804054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar K, Konerth M, Kokiko-Cochran ON, Cardona A, Ransohoff RM, Lamb BT. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68:19–31. doi: 10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielecki B, Mazurek A, Wolinski P, Glabinski A. Treatment of multiple sclerosis with methylprednisolone and mitoxantrone modulates the expression of CXC chemokine receptors in PBMC. J Clin Immunol. 2008;28:122–130. doi: 10.1007/s10875-007-9142-7. [DOI] [PubMed] [Google Scholar]
- Bordoni R, Thomas G, Richmond A. Growth factor modulation of melanoma growth stimulatory activity mRNA expression in human malignant melanoma cells correlates with cell growth. J. Cell Biochem. 1989;39:421–428. doi: 10.1002/jcb.240390408. [DOI] [PubMed] [Google Scholar]
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Bozic CR, Kolakowski LF, Jr, Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biologic characterization of the murine chemokine KC. J. Immunol. 1995;154:6048–6057. [PubMed] [Google Scholar]
- Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Li M, Liu L, Savarin C, Ransohoff RM. Chemokines in and out of the central nervous system: much more than chemotaxis and inflammation. J Leukoc. Biol. 2008;84:587–594. doi: 10.1189/jlb.1107763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Sasse ME, Liu L, Cardona SM, Mizutani M, Savarin C, Hu T, Ransohoff RM. Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood. 2008;112:256–263. doi: 10.1182/blood-2007-10-118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson T, Kroenke M, Rao P, Lane TE, Segal B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp. Med. 2008;205:811–823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catusse J, Faye P, Loillier B, Cremers B, Franck RM, Luccarini JM, Pruneau D, Paquet JL. Cloning and characterization of guinea pig interleukin-8 receptor. Biochem. Pharmacol. 2003;66:1171–1180. doi: 10.1016/s0006-2952(03)00459-3. [DOI] [PubMed] [Google Scholar]
- Chabas D, Ness J, Belman A, Yeh EA, Kuntz N, Gorman MP, Strober JB, De Kouchkovsky I, McCulloch C, Chitnis T, Rodriguez M, Weinstock-Guttman B, Krupp LB, Waubant E. Younger children with MS have a distinct CSF inflammatory profile at disease onset. Neurology. 2011;74:399–405. doi: 10.1212/WNL.0b013e3181ce5db0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RW, Phillips JE, Hipkin RW, Curran AK, Lundell D, Fine JS. CXCR2 antagonists for the treatment of pulmonary disease. Pharmacol.Ther. 2009;121:55–68. doi: 10.1016/j.pharmthera.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N.Engl.J.Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Cho SH, Sun B, Zhou Y, Kauppinen TM, Halabisky B, Wes P, Ransohoff RM, Gan L. CX3CR1 modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer's disease. J Biol. Chem. 2011 doi: 10.1074/jbc.M111.254268. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Veeraraghavalu K, Lazarov O, Marler S, Ransohoff RM, Ramirez JM, Sisodia SS. Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron. 2008;59:568–580. doi: 10.1016/j.neuron.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho FM, Pinho V, Amaral FA, Sachs D, Costa VV, Rodrigues DH, Vieira AT, Silva TA, Souza DG, Bertini R, Teixeira AL, Teixeira MM. The chemokine receptors CXCR1/CXCR2 modulate antigen-induced arthritis by regulating adhesion of neutrophils to the synovial microvasculature. Arthritis & Rheumatism. 2008;58:2329–2337. doi: 10.1002/art.23622. [DOI] [PubMed] [Google Scholar]
- Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, Virelizier JL, Baggiolini M, Sykes BD, Clark-Lewis I. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997;16:6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong B, Kremer BP, Olde Rikkert MG, Verbeek MM. Current state and future directions of neurochemical biomarkers for Alzheimer's disease. Clin. Chem. Lab. Med. 2007;45:1421–1434. doi: 10.1515/CCLM.2007.320. [DOI] [PubMed] [Google Scholar]
- de Kruijf P, Lim HD, Roumen L, Renjaän VA, Zhao J, Webb ML, Auld DS, Wijkmans JC, Zaman GJ, Smit MJ, de Graaf C, Leurs R. Identification of a novel allosteric binding site in the CXCR2 chemokine receptor. Mol Pharmacol. 2011;80:1108–1118. doi: 10.1124/mol.111.073825. [DOI] [PubMed] [Google Scholar]
- Dunstan CA, Salafranca MN, Adhikari S, Xia Y, Feng L, Harrison JK. Identification of two rat genes orthologous to the human interleukin-8 receptors. J. Biol Chem. 1996;271:32770–32776. doi: 10.1074/jbc.271.51.32770. [DOI] [PubMed] [Google Scholar]
- Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120:2423–2431. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan GH, Lapierre LA, Goldenring JR, Richmond A. Differential regulation of CXCR2 trafficking by Rab GTPases. Blood. 2003;101:2115–2124. doi: 10.1182/blood-2002-07-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Patera AC, Pong-Kennedy A, Deno G, Gonsiorek W, Manfra DJ, Vassileva G, Zeng M, Jackson C, Sullivan L, Sharif-Rodriguez W, Opdenakker G, Van Damme J, Hendrick JA, Lundell D, Lira SA, Hipkin RW. Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. J. Biol Chem. 2007;282:11658–11666. doi: 10.1074/jbc.M607705200. [DOI] [PubMed] [Google Scholar]
- Frisardi V, Solfrizzi V, Imbimbo PB, Capurso C, D'Introno A, Colacicco AM, Vendemiale G, Seripa D, Pilotto A, Capurso A, Panza F. Towards disease-modifying treatment of Alzheimer's disease: drugs targeting beta-amyloid. Curr.Alzheimer Res. 2010;7:40–55. doi: 10.2174/156720510790274400. [DOI] [PubMed] [Google Scholar]
- Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, Haass C, LaFerla FM, Kretzschmar H, Herms J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nat. Neurosci. 2010;13:411–413. doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furze RC, Rankin SM. Neutrophil mobilization and clearance in the bone marrow. Immunology. 2008;125:281–288. doi: 10.1111/j.1365-2567.2008.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin A, Proudfoot AE. Chemokines as targets for therapy. Exp. Cell Res. 2011;317:602–612. doi: 10.1016/j.yexcr.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, Tuohy VK, Ransohoff RM. Expression of chemokines RANTES, MIP-1alpha and GRO-alpha correlates with inflammation in acute experimental autoimmune encephalomyelitis. Neuroimmunomodulation. 1998;5:166–171. doi: 10.1159/000026333. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, Tani M, Strieter RM, Tuohy VK, Ransohoff RM. Synchronous synthesis of alpha- and beta-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental autoimmune encephalomyelitis. Am.J.Pathol. 1997;150:617–630. [PMC free article] [PubMed] [Google Scholar]
- Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25:211–217. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- Haskill S, Peace A, Morris J, Sporn SA, Anisowicz A, Lee SW, Smith T, Martin G, Sager R. Identification of three related human GRO genes encoding cytokine functions. Proc.Natl.Acad.Sci.U.S.A. 1990;87:7732–7736. doi: 10.1073/pnas.87.19.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakata T, Shiozaki T, Tasaki O, Ikegawa H, Inoue Y, Toshiyuki F, Hosotubo H, Kieko F, Yamashita T, Tanaka H, Shimazu T, Sugimoto H. Changes in CSF S100B and cytokine concentrations in early-phase severe traumatic brain injury. Shock. 2004;22:102–107. doi: 10.1097/01.shk.0000131193.80038.f1. [DOI] [PubMed] [Google Scholar]
- Hebert CA, Chuntharapai A, Smith M, Colby T, Kim J, Horuk R. Partial functional mapping of the human interleukin-8 type A receptor. Identification of a major ligand binding domain. J.Biol.Chem. 1993;268:18549–18553. [PubMed] [Google Scholar]
- Hipkin RW, Deno G, Fine J, Sun Y, Wilburn B, Fan X, Gonsiorek W, Wiekowski MT. Cloning and pharmacological characterization of CXCR1 and CXCR2 from Macaca fascicularis. J. Pharmacol. Exp. Ther. 2004;310:291–300. doi: 10.1124/jpet.103.063131. [DOI] [PubMed] [Google Scholar]
- Hollman DW, Klein RS, Ransohoff RM. The blood-brain-barrier, chemokines and multiple sclerosis. Biochim. Biophys. Acta. 2011;1812:220–230. doi: 10.1016/j.bbadis.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1280. [PubMed] [Google Scholar]
- Horton LW, Yu Y, Zaja-Milatovic S, Strieter RM, Richmond A. Opposing roles of murine duffy antigen receptor for chemokine and murine CXC chemokine receptor-2 receptors in murine melanoma tumor growth. Cancer Res. 2007;67:9791–9799. doi: 10.1158/0008-5472.CAN-07-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking MP, Liu L, Ransohoff RM, Lane TE. A protective role for ELR+ chemokines during acute viral encephalomyelitis. PLoS Pathog. 2009:5. doi: 10.1371/journal.ppat.1000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Liang D, Li X, Liu HH, Zhang X, Zheng M, Dill D, Shi X, Qiao Y, Yeomans D, Carvalho B, Angst MS, Clark JD, Peltz G. The role of interleukin-1 in wound biology. Part I: Murine in silico and in vitro experimental analysis. Anesth.Analg. 2010a;111:1525–1533. doi: 10.1213/ANE.0b013e3181f5ef5a. [DOI] [PubMed] [Google Scholar]
- Hu Y, Liang D, Li X, Liu HH, Zhang X, Zheng M, Dill D, Shi X, Qiao Y, Yeomans D, Carvalho B, Angst MS, Clark JD, Peltz G. The role of interleukin-1 in wound biology. Part II: In vivo and human translational studies. Anesth.Analg. 2010b;111:1534–1542. doi: 10.1213/ANE.0b013e3181f691eb. [DOI] [PubMed] [Google Scholar]
- Huber AR, Kunkel SL, Todd RF, 3, Weiss SJ. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- Hughes RO, Rogier DJ, Jacobsen EJ, Walker JK, Macinnes A, Bond BR, Zhang LL, Yu Y, Zheng Y, Rumsey JM, Walgren JL, Curtiss SW, Fobian YM, Heasley SE, Cubbage JW, Moon JB, Brown DL, Acker BA, Maddux TM, Tollefson MB, Mischke BV, Owen DR, Freskos JN, Molyneaux JM, Benson AG, Blevis-Bal RM. Design, synthesis, and biological evaluation of 3-[4-(2-hydroxyethyl)piperazin-1-yl]-7-(6-methoxypyridin-3-yl)-1-(2-propox yethyl)pyrido[3,4-b]pyrazin-2(1H)-one, a potent, orally active, brain penetrant inhibitor of phosphodiesterase 5 (PDE5) J.Med.Chem. 2010;53:2656–2660. doi: 10.1021/jm901781q. [DOI] [PubMed] [Google Scholar]
- Huising MO, Stolte E, Flik G, Savelkoul HF, Verburg-van Kemenade BM. CXC chemokines and leukocyte chemotaxis in common carp (Cyprinus carpio L.) Dev. Comp. Immunol. 2003a;27:875–888. doi: 10.1016/s0145-305x(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Huising MO, Stet RJ, Kruiswijk CP, Savelkoul HF, Verburg-van Kemenade BM. Molecular evolution of CXC chemokines: extant CXC chemokines originate from the CNS. Trends Immunol. 2003b;24:307–313. doi: 10.1016/s1471-4906(03)00120-0. [DOI] [PubMed] [Google Scholar]
- Kerns HM, Jutila MA, Hedges JF. The distinct response of gammadelta T cells to the Nod2 agonist muramyl dipeptide. Cell Immunol. 2009;257:38–43. doi: 10.1016/j.cellimm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter AE, Padovani-Claudio DA, Bai L, Miller RH. Inhibition of CXCR2 signaling promotes recovery in models of multiple sclerosis. Exp.Neurol. 2009;220:44–56. doi: 10.1016/j.expneurol.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knall C, Young S, Nick JA, Buhl AM, Worthen GS, Johnson GL. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J. Biol Chem. 1996;271:2832–2838. doi: 10.1074/jbc.271.5.2832. [DOI] [PubMed] [Google Scholar]
- Köhler A, De Fillipo K, Hasenberg M, van den Brandt C, Nye E, Hosking MP, Lane TE, Männ L, Ransohoff RM, Hauser AE, Winter O, Schraven B, Geiger H, Hogg N, Gunzer M. G-CSF-mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood. 2011;117:4349–4357. doi: 10.1182/blood-2010-09-308387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossmann T, Stahel PF, Lenzlinger PM, Redl H, Dubs RW, Trentz O, Schlag G, Morganti-Kossmann MC. Interleukin-8 released into the cerebrospinal fluid after brain injury is associated with blood-brain barrier dysfunction and nerve growth factor production. J Cereb. Blood Flow Metab. 1997;17:280–289. doi: 10.1097/00004647-199703000-00005. [DOI] [PubMed] [Google Scholar]
- Kraemer S, Lue H, Zernecke A, Kapurniotu A, Andreetto E, Frank R, Lennartz B, Weber C, Bernhagen J. MIF-chemokine receptor interactions in atherogenesis are dependent on an N-loop-based 2-site binding mechanism. FASEB J. 2011;25:894–906. doi: 10.1096/fj.10-168559. [DOI] [PubMed] [Google Scholar]
- Krieger M, Brunner T, Bischoff SC, von Tscharner V, Walz A, Moser B, Baggiolini M, Dahinden CA. Activation of human basophils through the IL-8 receptor. J Immunol. 1992;149:2662–2667. [PubMed] [Google Scholar]
- Lazanecc G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol. Med. 2010;16:133–144. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Horuk R, Richard Y, Bennet GL, Camerato T, Wood WI. Characterization of two high affinity human interleukin-8 receptors. J. Biol Chem. 1992;267:16283–16287. [PubMed] [Google Scholar]
- Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, Lamb BT. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer's disease mouse models. Am. J Pathol. 2010;177:2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Smith E, Stark MA. IL-17A-producing neutrophil-regulatory Tn lymphocytes. Immunol.Res. 2006;34:229–242. doi: 10.1385/IR:34:3:229. [DOI] [PubMed] [Google Scholar]
- Lindner M, Trebst C, Heine S, Skripuletz T, Koutsoudaki PN, Stangel M. The chemokine receptor CXCR2 is differentially regulated on glial cells in vivo but is not required for successful remyelination after cuprizone-induced demyelination. Glia. 2008;56:1104–1113. doi: 10.1002/glia.20682. erratum, 57, 465 (2009) [DOI] [PubMed] [Google Scholar]
- Lippert U, Zachmann K, Henz BM, Neumann C. Human T lymphocytes and mast cells differentially express and regulate extra- and intracellular CXCR1 and CXCR2. Exp. Dermatol. 2004;13:520–525. doi: 10.1111/j.0906-6705.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- Liu L, Belkaldi A, Darnall L, Hu T, Drescher C, Cotleur AC, Padovani-Claudio D, He T, Choi K, Lane TE, Miller RH, Ransohoff RM. CXCR2-positive neutrophils are essential for cuprizone-induced demyelination: relevance to multiple sclerosis. Nat. Neurosci. 2010a;13:319–326. doi: 10.1038/nn.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Darnall L, Hu T, Choi K, Lane TE, Ransohoff RM. Myelin repair is accelerated by inactivating CXCR2 on nonhematopoietic cells. J Neurosci. 2010b;30:9074–9083. doi: 10.1523/JNEUROSCI.1238-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodowski DT, Palczewski K. Chemokine receptors and other G protein-coupled receptors. Curr. Opin. HIV AIDS. 2009;4:88–95. doi: 10.1097/COH.0b013e3283223d8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund BT, Ashikian N, Ta HQ, Chakryan Y, Manoukian K, Groshen S, Gilmore W, Cheema GS, Stohl W, Burnett ME, Ko D, Kachuck NJ, Weiner LP. Increased CXCL8 (IL-8) expression in Multiple Sclerosis. J Neuroimmunol. 2004;155:161–171. doi: 10.1016/j.jneuroim.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Maier B, Schwerdtfeger K, Mautes A, Holanda M, Müller M, Steudel WI, Marzi I. Differential release of interleukines 6, 8, and 10 in cerebrospinal fluid and plasma after traumatic brain injury. Shock. 2001;15:421–426. doi: 10.1097/00024382-200115060-00002. [DOI] [PubMed] [Google Scholar]
- McColl SR, Staykova MA, Wozniak A, Fordham S, Bruce J, Willenborg DO. Treatment with anti-granulocyte antibodies inhibits the effector phase of experimental autoimmune encephalomyelitis. Journal of Immunology. 1998;161:6421–6426. [PubMed] [Google Scholar]
- Monteclaro FS, Charo IF. The amino-terminal extracellular domain of the MCP-1 receptor, but not the RANTES/MIP-1alpha receptor, confers chemokine selectivity. Evidence for a two-step mechanism for MCP-1 receptor activation. J. Biol Chem. 1996;271:19084–19092. doi: 10.1074/jbc.271.32.19084. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–3043. [PubMed] [Google Scholar]
- Murphy PM, Charo IF, Hills R, Horuk R, Matsushima K, Oppenheim JJ. Chemokine receptors. 2011 Accessed on 2011-08-23-IUPHAR database (IUPHAR-DB), http://www.iuphar-db.org/DATABASE/FamilyMenuForward?familyId=14.
- Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–1283. [PubMed] [Google Scholar]
- Nasser MW, Raghuwanshi SK, Malloy KM, Gangavarapu P, Shim JY, Rajarathnam K, Richardson RM. CXCR1 and CXCR2 activation and regulation. Role of aspartate 199 of the second extracellular loop of CXCR2 in CXCL8-mediated rapid receptor internalization. J. Biol Chem. 2007;282:6906–6915. doi: 10.1074/jbc.M610289200. [DOI] [PubMed] [Google Scholar]
- Neel NF, Schutyser E, Sai J, Fan GH, Richmond A. Chemokine receptor internalization and intracellular trafficking. Cytokine Growth Factor Rev. 2005;16:637–658. doi: 10.1016/j.cytogfr.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, van de Veerdonk FL, Kullberg BJ, Van der Meer JW, Joosten LA. The role of NLRs and TLRs in the activation of the inflammasome. Expert.Opin.Biol.Ther. 2008;8:1867–1872. doi: 10.1517/14712590802494212. [DOI] [PubMed] [Google Scholar]
- Oh JW, Schwiebert LM, Benveniste EN. Cytokine regulation of CC and CXC chemokine expression by human astrocytes. J.Neurovirol. 1999;5:82–94. doi: 10.3109/13550289909029749. [DOI] [PubMed] [Google Scholar]
- Omari KM, Lutz SE, Santambrogio L, Lira SA, Raine CS. Neuroprotection and remyelination after autoimmune demyelination in mice that inducibly overexpress CXCL1. Am.J.Pathol. 2009;174:164–176. doi: 10.2353/ajpath.2009.080350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omari KM, John G, Lango R, Raine CS. Role for CXCR2 and CXCL1 on glia in multiple sclerosis. Glia. 2005;53:24–31. doi: 10.1002/glia.20246. [DOI] [PubMed] [Google Scholar]
- Padovani-Claudio DA, Liu LP, Ransohoff RM, Miller RH. Alterations in the oligodendrocyte lineage, myelin and white matter in adult mice lacking the chemokine receptor CXCR2. Glia. 2006;54:471–483. doi: 10.1002/glia.20383. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- Pighetti GM, Rambeaud M. Genome conservation between the bovine and human interleukin-8 receptor complex: improper annotation of bovine interleukin-8 receptor b identified. Vet. Immunol. Immunopathol. 2006;114:335–340. doi: 10.1016/j.vetimm.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Prado GN, Suetomi K, Shumate D, Maxwell C, Ravindran A, Rajarathnam K, Navarro J. Chemokine signaling specificity: essential role for the N-terminal domain of chemokine receptors. Biochemistry. 2007;46:8961–8968. doi: 10.1021/bi7004043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado GN, Thomas KM, Suzuki H, LaRosa GJ, Wilkinson N, Folco E, Navarro J. Molecular characterization of a novel rabbit interleukin-8 receptor isotype. J. Biol Chem. 1994;269:12391–12394. [PubMed] [Google Scholar]
- Proost P, De Wolf-Peeters C, Conings R, Opdenakker G, Billiau A, Van Damme J. Identification of a novel granulocyte chemotactic protein (GCP-2) from human tumor cells. In vitro and in vivo comparison with natural forms of GRO, IP-10, and IL-8. J Immunol. 1993;150:1000–1010. [PubMed] [Google Scholar]
- Provencio JJ, Altay T, Smithason S, Moore SK, Ransohoff RM. Depletion of Ly6G/C(+) cells ameliorates delayed cerebral vasospasm in subarachnoid hemorrhage. J Neuroimmunol. 2011;232:94–100. doi: 10.1016/j.jneuroim.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan L, Rajarathnam K. Ligand selectivity and affinity of chemokine receptor CXCR1. Role of N-terminal domain. J. Biol Chem. 2004;279:30000–30008. doi: 10.1074/jbc.M313883200. [DOI] [PubMed] [Google Scholar]
- Rajagopalan L, Rajarathnam K. Structural basis of chemokine receptor function--a model for binding affinity and ligand selectivity. Biosci. Rep. 2006;26:325–339. doi: 10.1007/s10540-006-9025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman D, Sobolik-Delmaire T, Richmond A. Chemokines in health and disease. Exp.Cell Res. 2011;317:575–589. doi: 10.1016/j.yexcr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman D, Neel NF, Sai J, Mernaugh RL, Ham AJ, Richmond AJ. Characterization of chemokine receptor CXCR2 interacting proteins using a proteomics approach to define the CXCR2 "chemosynapse". Methods Enzymol. 2009;460:315–330. doi: 10.1016/S0076-6879(09)05215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos CD, Fernandes KS, Canetti C, Teixeira MM, Silva JS, Cunha FQ. Neutrophil recruitment in immunized mice depends on MIP-2 inducing the sequential release of MIP-1alpha, TNF-alpha and LTB(4) Eur. J Immunol. 2006;36:2025–2034. doi: 10.1002/eji.200636057. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31:711–721. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Liu L, Cardona AE. Chemokines and chemokine receptors: multipurpose players in neuroinflammation. Int. Rev. Neurobiol. 2007;82:187–204. doi: 10.1016/S0074-7742(07)82010-1. [DOI] [PubMed] [Google Scholar]
- Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic.Biol.Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SJ, Sharkey J, Andrews P. Serum IL-8 and MCP-1 concentration do not identify patients with enlarging contusions after traumatic brain injury. J. Trauma. 2009;66:1591–1597. doi: 10.1097/TA.0b013e31819a0344. [DOI] [PubMed] [Google Scholar]
- Richmond A, Lawson DH, Nixon DW, Stevens JS, Chawla RK. Extraction of a melanoma growth-stimulatory activity from culture medium conditioned by the Hs0294 human melanoma cell line. Cancer Res. 1983;43:2106–2112. [PubMed] [Google Scholar]
- Richmond A, Lawson MA, Nixon DW, Chawla RK. Characterization of autostimulatory and transforming growth factors from human melanoma cells. Cancer Res. 1985;45:6390–6394. [PubMed] [Google Scholar]
- Robinson S, Tani M, Strieter RM, Ransohoff RM, Miller RH. The chemokine growth-regulated oncogene-alpha promotes spinal cord oligodendrocyte precursor proliferation. J Neurosci. 1998;18:10457–10463. doi: 10.1523/JNEUROSCI.18-24-10457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Cohen M, Prayson R, Ransohoff RM, Tabrizi N, Miller RH. Constitutive expression of growth-related oncogene and its receptor in oligodendrogliomas. Neurosurgery. 2001;48:864–873. doi: 10.1097/00006123-200104000-00035. [DOI] [PubMed] [Google Scholar]
- Salanga CL, Handel TM. Chemokine oligomerization and interactions with receptors and glycosaminoglycans: the role of structural dynamics in function. Exp Cell Res. 2011;317:590–601. doi: 10.1016/j.yexcr.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarin-Vuaillat C, Ransohoff RM. Chemokines and chemokine receptors in neurological disease: raise, retain or reduce? Neurotherapeutics. 2007;4:590–601. doi: 10.1016/j.nurt.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Shechter R. Systemic inflammatory cells fight off neurodegenerative disease. Nat. Rev. Neurol. 2010;6:405–410. doi: 10.1038/nrneurol.2010.71. [DOI] [PubMed] [Google Scholar]
- Semple BD, Bye N, Ziebell JM, Morganti-Kossmann MC. Deficiency of the chemokine receptor CXCR2 attenuates neutrophil infiltration and cortical damage following closed head injury. Neurobiol. Dis. 2010;40:394–403. doi: 10.1016/j.nbd.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Singh S, Sing AP, Sharma B, Owen LB, Singh RK. CXCL8 and its cognate receptors in melanoma progression and metastasis. Future Oncol. 2010;6:111–116. doi: 10.2217/fon.09.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani M, Fuentes ME, Peterson JW, Trapp BD, Durham SK, Loy JK, Bravo R, Ransohoff RM, Lira SA. Neutrophil infiltration, glial reaction, and neurological disease in transgenic mice expressing the chemokine N51/KC in oligodendrocytes. J.Clin.Invest. 1996;98:529–539. doi: 10.1172/JCI118821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirotta E, Ransohoff RM, Lane TE. CXCR2 signaling protects oligodendrocyte progenitor cells from IFN-γ/CXCL10-mediated apoptosis. Glia. 2011;59:1518–1528. doi: 10.1002/glia.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Frost E, To V, Robinson S, French-Constant C, Geertman R, Ransohoff RM, Miller RH. The chemokine receptor CXCR2 controls positioning of oligodendrocyte precursors in developing spinal cord by arresting their migration. Cell. 2002;110:373–383. doi: 10.1016/s0092-8674(02)00838-3. [DOI] [PubMed] [Google Scholar]
- Vallès A, Grijpink-Ongering L, de Bree FM, Tuinstra T, Ronken E. Differential regulation of the CXCR2 chemokine network in rat brain trauma: implications for neuroimmune interactions and neuronal survival. Neurobiol. Dis. 2006;22:312–322. doi: 10.1016/j.nbd.2005.11.015. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Vasilescu A, Terashima Y, Enomoto M, Heath S, Poonpiriya V, Gatanaga H, Do H, Diop G, Hirtzig T, Auewarakul P, Lauhakirti D, Sura T, Charneau P, Marullo S, Therwath A, Oka S, Kanegasaki S, Lathrop M, Matsushima K, Zagury JF, Matsuda F. A haplotype of the human CXCR1 gene protective against rapid disease progression in HIV-1+ patients. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3354–3359. doi: 10.1073/pnas.0611670104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira SM, Lemos HP, Grespan R, Napimoga MH, Dal-Secco D, Freitas A, Cunha TM, Verri WA, Jr, Souza-Junior DA, Jamur MC, Fernandes KS, Oliver C, Silva JS, Teixeira MM, Cunha FQ. A crucial role for TNF-alpha in mediating neutrophil influx induced by endogenously generated or exogenous chemokines, KC/CXCL1 and LIX/CXCL5. Br. J Pharmacol. 2009;158:779–789. doi: 10.1111/j.1476-5381.2009.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A, Burgener R, Car B, Baggiolini M, Kunkel SL, Strieter RM. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin 8. J Exp.Med. 1991;174:1355–1362. doi: 10.1084/jem.174.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner HL. The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease? Ann. Neurol. 2009;65:239–248. doi: 10.1002/ana.21640. [DOI] [PubMed] [Google Scholar]
- Wenger RH, Wicki AN, Walz A, Kieffer N, Clemetson KJ. Cloning of cDNA coding for connective tissue activating peptide III from a human platelet-derived lambda gt11 expression library. Blood. 1989;73:1498–1503. [PubMed] [Google Scholar]
- Wengner AM, Pitchford SC, Furze RC, Rankin SM. The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood. 2008;111:42–49. doi: 10.1182/blood-2007-07-099648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen MJ, Carlos TM, Kochanek PM, Wisniewski SR, Bell MJ, Clark RS, DeKosky ST, Marion DW, Adelson PD. Interleukin-8 is increased in cerebrospinal fluid of children with severe head injury. Crit. Care. Med. 2000;28:929–934. doi: 10.1097/00003246-200004000-00003. [DOI] [PubMed] [Google Scholar]
- Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, Hamel DJ, Handel TM, Cherezov V, Steven RC. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. 2010 doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Feng L, Park HT, Havlioglu N, Wen L, Tang H, Bacon KB, Jiang Zh, Zhang Xc, Rao Y. The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature. 2001;410:948–952. doi: 10.1038/35073616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Hyman BT. GROalpha/KC, a chemokine receptor CXCR2 ligand, can be a potent trigger for neuronal ERK1/2 and PI-3 kinase pathways and for tau hyperphosphorylation-a role in Alzheimer's disease? J. Neuroimmunol. 2002;122:55–64. doi: 10.1016/s0165-5728(01)00463-5. [DOI] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Richmond A. CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol Histopathol. 2008;23:1399–1407. doi: 10.14670/hh-23.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Sokal I, Peskind AR, Quinn JF, Jankovic J, Kenney C, Chung KA, Millard SP, Nutt JG, Montine TJ. CSF multianalyte profile distinguishes Alzheimer and Parkinson diseases. Am.J.Clin.Pathol. 2008;129:526–529. doi: 10.1309/W01Y0B808EMEH12L. [DOI] [PMC free article] [PubMed] [Google Scholar]