INTRODUCTION
Survival in persons with HIV infection has dramatically improved following the introduction of highly active anti-retroviral therapy (HAART). As HIV-infected individuals live longer, some chronic conditions, such as premature coronary artery disease (CAD), have emerged as a long-term concern in this population. The responsible factors are not well-defined. Any chronic infection may increase circulating cytokines and other inflammatory mediators that enhance the development and progression of atherosclerosis. HIV itself could directly infect, damage, and cause proliferation of human arterial smooth muscle cells [1]. In addition, the antiretroviral therapy (ART) used to treat HIV infection may have adverse effects, including promoting atherosclerosis, though the findings in this regard are not consistent [2–5].
We have reported cross-sectional data demonstrating high rates of subclinical CAD in HIV-infected African Americans residing in Baltimore, Maryland [6–8]. Since African Americans have the highest overall CAD mortality rate of any ethnic group in the United States [9], it is critically important to estimate the incidence of subclinical coronary atherosclerosis and investigate whether ART or other factors influence the development of subclinical CAD in HIV-infected African Americans.
The aim of the present study was to estimate the incidence of subclinical CAD and investigate the risk factors for the development of subclinical coronary atherosclerosis in HIV-infected African Americans.
SUBJECTS AND METHODS
Study participants
Between August 2003 and September 2010, 188 HIV-infected African Americans without known, or symptoms of, coronary artery disease were consecutively enrolled in a prospective study investigating the incidence of and the risk factors for cardiac CT-defined subclinical CAD in Baltimore, Maryland.
Subclinical CAD was defined as the presence of coronary artery calcium (CAC) and/or coronary plaque by cardiac CT. Inclusion criteria were age between 25 and 60 years, HIV positivity (determined by ELISA and confirmed by Western blot test), and African American race (self-designated). Exclusion criteria were (1) any evidence of clinical CAD (All the study participants in this study are patients at the Johns Hopkins HIV-clinic. We have access to their medical records, including history of ECG abnormalities and any cardiovascular diagnoses or symptoms, including chest pain or heart failure symptoms.. Self-reported information was not used for defining CAD), (2) any symptoms believed to be related to CAD, (3) any evidence of renal insufficiency, (4) known allergy to the contrast used for the CT, and (5) pregnancy. During the baseline visit, each subject was interviewed to obtain information on sociodemographic characteristics, cardiovascular risk (including cigarette smoking and alcohol use), illicit drug-use behaviors, medical history, and all medications used. Cocaine use was defined as chronic use of cocaine by any route for at least 6 months, administered at least four times a month. Information about the frequency (how many times a day in the past week, in the past month), patterns/forms of cocaine (speedball, crack, etc.), administration mode (injection, smoking, etc.), and duration of cocaine use was collected. Information about use of other drugs, such as opiates, benzodiazepines, or methamphetamine, was also collected.
A medical chart review was used to confirm information on medical history and medications that was provided by the subjects. Each subject also had a physical examination and several tests were performed: namely, a fasting lipid profile, vitamin D, high-sensitivity C-reactive protein (hs-CRP) test, 64-slice multidetector computed tomography (MDCT) for CAC, and CT coronary angiography (contrast-enhanced). The study participants underwent reexaminations and interviews approximately 2 years later.
Of the 188 participants at baseline, 69 were diagnosed as having subclinical CAD and 119 were free of subclinical CAD. These 119 without subclinical CASD were included in this study.
The Committee on Human Research at the Johns Hopkins School of Medicine approved the study protocol, and all study participants provided written informed consent. All procedures used in this study were in accordance with institutional guidelines.
Blood pressure measurement
Sitting systolic and diastolic blood pressures (SBP and DBP) were measured twice with a standard mercury sphygmomanometer. A nurse at the clinic measured the study participant’s arm circumference and applied a correctly sized cuff. The participant sat quietly for 5 minutes, and then the nurse obtained the SBP and DBP. A second measurement was obtained 3 minutes later, and the average of the two readings is reported.
Measurement of lipids
Venous blood samples were obtained after an overnight fast from a large antecubital vein. Serum was separated by centrifugation (2000 g for 15 minutes at 4°C) and stored at 75°C until assayed. Serum lipid variables, including total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, and LDL cholesterol levels, were directly determined with an analyzer (Hitachi 747 analyzer; Roche, Englewood, NJ).
Vitamin D measurement
Sera were collected, centrifuged and stored at −70C until analyzed. Serum 25-OH vitamin D was determined by a direct, competitive chemiluminescence immunoassay (DiaSorin, Stillwater, Minn) [10]. The level of detection for 25-OH vitamin D was <4 ng/ml. This method accurately measures both D2 and D3 together and is reported as a total 25 (OH) vitamin D. The reference range is 32–100 ng / ml. This study identifies vitamin D deficiency according to the Framingham Offspring Study as serum 25 (OH) vitamin D <10 ng/mL.
Coronary CT angiography with a 64 slice Siemens MDCT scanner
A noncontrast MDCT scan was performed on a Sensation 64 Cardiac Siemens Medical Solutions scanner (Erlangen, Germany) to determine the coronary artery calcium score with a sequential scan of 3-mm slices with prospective ECG triggering, 30 × 0.6-mm detector collimation, and tube current 135 mAs at 120 kV. Subsequently, coronary CT angiography (CTA) was performed on the same equipment using 80 mL of iso-osmolar contrast agent (320 mg iodine/mL) injected at 4–5 mL/s. Imaging was performed with retrospective ECG-gating, 32 × 0.6-mm detector collimation with flying focal spot to give effective detector collimation of 64 × 0.6 mm, 330 ms gantry rotation, 850 mAs and 120 kV. Subsequently, 0.75-mm-thick axial slices were reconstructed at 0.4-mm intervals with B25 kernel using a half-scan reconstruction algorithm with resulting temporal resolution of 165–185 ms. Ten reconstructions were done through the cardiac cycle at 10% increments in the R-R interval. If needed, patients were medicated with metoprolol prior to the scan to achieve a heart rate <65 beats per minute.
CAC score, volume, and mass were measured on a workstation (Leonardo, Syngo, Siemens Medical Solutions, Malvern, PA). Regions of interest were placed over each of the coronary arteries with a threshold for pixels of greater than 130 HU for determining calcified plaque. Coronary vessels were assessed for patency and stenoses using 3D visualization tools after the axial images were reviewed for determination of anatomy, quality of the study, and appearance of the vessels. The Agatston method was used to signify development of incident CAC.
One reviewer (E.K.F), blinded to the participants’ risk factor profiles, independently evaluated the contrast-enhanced MDCT scans by examining the axial slices, curved multiplanar reformations, and thin-slab maximum intensity projections. The coronary artery tree was segmented according to the modified American Heart Association classification, and the segments were investigated for plaque and luminal narrowing. The coronary arteries were divided into proximal, mid, and distal segments, with each segment investigated for luminal narrowing. Plaques were classified as calcified or noncalcified, and the degree of stenosis was classified as less than, equal to, or greater than 50% diameter stenosis. Diameter stenosis ≥50% was defined as significant coronary stenosis. This cut-off has been used in prior studies as well [11].
Statistical analysis
Lengthof follow-up was calculated as the time elapsed from the baseline to the second CT examination. Incidencerate was calculated by dividing the number of newly diagnosed cases by theperson-years of follow-up. The 95% confidence interval for the incidence was also calculated. ARTs were categorized on the basis of exposure to four classes—nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), and other ARTs.
Statistical analysis was performed with SAS (version 9.2, SAS Institute, Cary, NC). All continuous parameters were summarized by medians and interquartile ranges (IQRs), and all categorical parameters were summarized as proportions. To compare between-group differences, the nonparametric Wilcoxon two-sample test was used for continuous variables and the Fisher’s exact test was used for categorical variables. The Framingham risk score was calculated to estimate the CAD risk [12].
Survival analysis was used to identify the risk factors for the development of subclinical coronary atherosclerosis. Kaplan-Meier method was used to estimate the survivor functions, and the log-rank test was used to test the equality of survivor functions. Univariate Cox’s proportional hazards regression models were first fitted to evaluate the crude association between the development of coronary plaques and each individual factor—age, sex, total cholesterol, HDL-C, LDL-C, triglycerides, vitamin D, hsCRP, cigarette smoking, alcohol use, glucose level, history of diabetes, and history of hypertension, SBP, DBP, body mass index, baseline CD4 cell count, baseline HIV RNA quantification, duration of each ART drug use, duration of ART use for each ART class (nucleoside reverse-transcriptase inhibitors (NRTI), non-nucleoside reverse-transcriptase inhibitors (NNRTI), protease inhibitors (PI), fusion inhibitors (FI) or integrase inhibitors), duration of any ART use, cocaine or other illicit drug use, and the Framingham risk score.
Those factors that were significant at the p ≤0.20 level in the univariate models were put into an initial multivariate Cox’s proportional hazards regression model to identify the factors that were independently associated with the development of subclinical CAD. Those variables that ceased to make significant contributions to the models were eliminated in a stagewise manner, yielding a final model. The p-values reported are two-sided. A p-value <0.05 indicated statistical significance.
RESULTS
General characteristics
Among the 119 study participants included in this study, subclinical CAD was detected in 14 during the follow-up visit. Of the 14 patients with incident CAD, 9 developed incident CAC, 4 developed incident plaque, and one developed incident CAC and plaque. The general and clinical characteristics of the study participants, with or without incident subclinical CAD, are presented in Table 1. Compared with those who remained free of subclinical CAD, those who developed subclinical CAD were more likely to have been male (p=0.001) and have a lower vitamin D (serum 25(OH) D) level (p=0.029). None of the participants experienced a cardiovascular event during the follow-up period.
Table 1.
Characteristics of Study Participants, by the Development of Subclinical CAD*
| Characteristic | Total | Subclinical CAD | p-value | |
|---|---|---|---|---|
| (N = 119) | No (N = 105) | Yes (N = 14) | ||
| Age (year) | 45 (41–49) | 45 (41–49) | 46 (45–51) | 0.32 |
| Male (%) | 52.1 | 46.7 | 92.9 | 0.001 |
| Family history of CAD (%) | 21.9 | 22.9 | 14.3 | 0.45 |
| Diabetes (%) | 7.6 | 8.6 | 0.0 | 0. 25 |
| Hypertension (%) | 19.3 | 20.0 | 14.3 | 0.61 |
| Cocaine use (%) | 78.2 | 79.1 | 71.4 | 0.52 |
| Cigarette smoking (%) | 81.5 | 81.9 | 78.6 | 0.76 |
| Alcohol use (%) | 81.5 | 81.0 | 85.7 | 0.67 |
| Years of HIV infection | 15.3 (9.2–19.5) | 15.2 (10.0–19.5) | 15.7 (7.3–17.8) | 0.63 |
| hsCRP ≥2 mg/dL (%) | 43.7 | 42.9 | 50.0 | 0.61 |
| hsCRP (mg/dL) | 1.4 (0.7–4.7) | 1.4 (0.7–4.6) | 1.9 (0.7–5.9) | 0.54 |
| Serum 25(OH)D (ng/mL) | 17 (11–24) | 19 (11–26) | 12 (9–17) | 0.029 |
| Systolic BP (mm Hg) | 119 (109–130) | 119 (109–130) | 119 (108–124) | 0.80 |
| Diastolic BP (mm Hg) | 75 (68–83) | 76 (68–84) | 71 (66–77) | 0.06 |
| Glucose (mg/dL) | 85 (78–94) | 85 (78–94) | 91 (80–100) | 0.52 |
| BMI | 25.4 (22.4–29.2) | 25.5 (22.4–29.2) | 24.8 (22.4–28.4) | 0.56 |
| Waist-to hip ratio | 0.88 (0.83–0.92) | 0.87 (0.83–0.91) | 0.91 (0.82–0.93) | 0.61 |
| Baseline CD4 (cells/mm3) | 325(205–527) | 325 (193–528) | 297 (222–379) | 0.64 |
| Baseline viral load (copies/mL) | 12000(977–66658) | 12000(716–97015) | 14628(1431–44000) | 0.86 |
| eGFR (mL/min/1.73 m2) | 103 (85–120) | 104 (84–120) | 100 (90–120) | 0.90 |
| Total cholesterol (mg/dL) | 162(137–185) | 163 (137–185) | 160 (137–184) | 0.90 |
| LDL-C (mg/dL) | 80 (62–104) | 80 (59–104) | 80 (65–113) | 0.49 |
| HDL-C (mg/dL) | 52 (43–64) | 51(43–66) | 55 (45–60) | 0.87 |
| Triglycerides (mg/dL) | 98 (78–152) | 98 (79–155) | 103 (72–120) | 0.51 |
| NRTI use (month) | 36.0 (0.4–72.0) | 36.0 (5.0–72.0) | 3.5 (0.0–60.0) | 0.09 |
| NNRTI use (month) | 0 (0.0–20.9) | 0.0 (0.0–17.9) | 0.0 (0.0–36.0) | 0.73 |
| PI use (month) | 24.0 (0.0–70.0) | 29.0 (0.0–72.0) | 3.9 (0.0–60.0) | 0.46 |
| ART use (month) | 45.0 (14.0–99.1) | 45.0 (17.9–96.0) | 54.0 (5.0–101.0) | 0.96 |
| Framingham risk score | 3.0 (2.0–6.0) | 3.0 (2.0–6.0) | 4.5 (3.0–8.0) | 0.13 |
| Framingham score <10.0 (%) | 91.6 | 92.4 | 85.7 | 0.40 |
Median (interquartile range) for continuous variables, proportion (%) for categorical variables.
Abbreviations: CD4, CD4cell count; viral load, HIV RNA quantification; hsCRP = high-sensitivity C-reactive protein; BP = blood pressure; glucose = fasting glucose; BMI = body mass index (kg/m2); eGFR= estimated glomerular filtration rate ((mL/min/1.73 m2); LDL-C = low density lipoprotein cholesterol; HDL-C = high density lipoprotein cholesterol; serum 25(OH)D = 25-hydroxyvitamin D;ART = antiretroviral therapy; Framingham score = Framingham risk score; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
According to the Framingham risk score algorithm, 109 (91.6%) of the 119 participants (54 of the 62 men and 55 of the 57 women) had low risk of CAD; 12 (85.7%) of the 14 participants who developed subclinical CAD had low risk of CAD [12].
Incidence of subclinical CAD
The total sum of person-years (PYs) of follow-up was 284.4. The mean follow-up time was 2.39±1.26 years. Subclinical CAD was detected in 14 of these 119 on the second cardiac CT, yielding an overall incidence of 4.92 per 100 PYs (95% CI: 2.69–8.26).
Association between vitamin D deficiency and time to the development of subclinical CAD
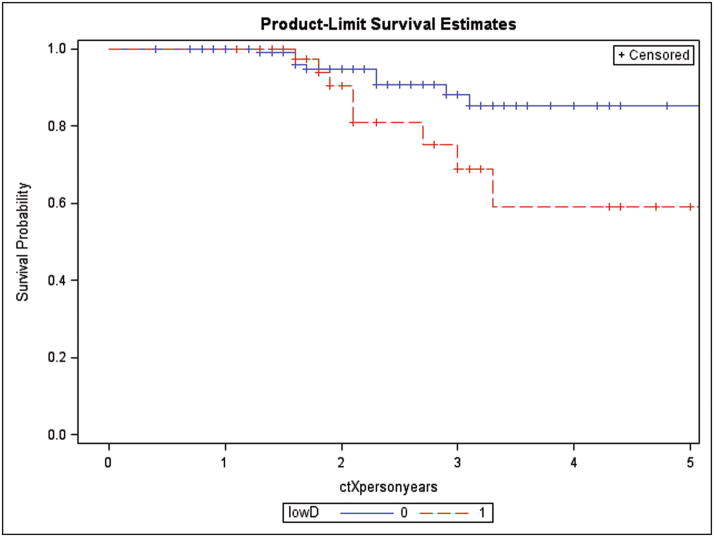
Kaplan-Meier curves of time to the development of subclinical CAD by vitamin D deficiency status are presented in Figure 1. According to the log-rank test, time to the development of subclinical CAD in those who were vitamin D deficient was significantly shorter than that in those who were not vitamin D deficient. (p=0.024).
Figure 1.
Kaplan-Meier subclinical CAD-free survival curve by vitamin D deficiency status. The survival curves by vitamin D deficiency status were statistically different (log-rank test, p value=0.0245).
Factors associated with the development of subclinical CAD
By univariate Cox’s proportional hazards regression analyses, male sex, serum 25(OH)D level, vitamin D deficiency, baseline CD4 count, total serum cholesterol, LDL-cholesterol, and Framingham risk score were associated with the development of subclinical CAD at ≤0.20 level.
The final Cox’s regression model indicated that male sex (adjusted HR: 18.1 [95% CI: 2.18–149.9]) and vitamin D deficiency (adjusted HR: 9.37 [95% CI: 2.23, 39.4]) were independently associated with subclinical CAD (Table 2).
Table 2.
Demographic, Laboratory, and Clinical Factors in Relation to the Risk of Development of Subclinical CAD, Proportional Hazards Regression Analysis*,§
| Variable | Subclinical CAD | |
|---|---|---|
| Crude HR (95% CI) | Adjusted HR (95% CI) | |
| Age (year) | 1.03(0.93,1.14) | |
| Sex | ||
| Female | 1.00 | 1.00 |
| Male | 9.30 (1.21–71.3) | 18.1 (2.18–149.9) |
| Cigarette smoking | ||
| Never | 1.00 | |
| Ever | 0.55 (0.15–2.03) | |
| Alcohol use | ||
| No | 1.00 | |
| Yes | 3.86 (0.23–1.54) | |
| Cocaine use | ||
| Never | 1.00 | |
| Ever | 0.80(0.24–2.61) | |
| Diabetes | ||
| No | 1.00 | |
| Yes | 0.00 (0.00 not estimatable) | |
| Hypertension | ||
| No | 1.00 | |
| Yes | 0.44 (0.10–2.03) | |
| Years of HIV infection | ||
| <15 years | 1.00 | |
| ≥15 years | 0.71 (0.24–2.14) | |
| eGFR (ml/min/1.73 m2) | 0.99 (0.98–1.02) | |
| hsCRP (mg/dL) | 1.05 (0.97,1.14) | |
| Serum 25(OH)D (ng/mL) | 0.87 (0.77–0.98) | |
| Vitamin D deficiency | 3.66 (1.11–12.1) | 9.37 (2.23–39.4) |
| Systolic BP (mm Hg) | 1.00 (0.97–1.04) | |
| Diastolic BP (mm Hg) | 0.98 (0.94–1.03) | |
| Glucose (mg/dL) | 0.99 (0.97–1.02) | |
| BMI (kg/m2) | 0.97 (0.89–1.06) | |
| Waist-to-hip ratio | 4.20 (0.05–357.4) | |
| Baseline CD4 count (cells/mm3) | 1.00 (0.99–1.00) | |
| Baseline viral load (copies/mL) | 0.90 (0.72–1.12) | |
| Total cholesterol (mg/dL) | 1.12 (1.00–1.03) | |
| LDL-C (mg/dL) | 1.02 (1.00–1.03) | |
| HDL-C (mg/dL) | 1.00 (0.97–1.04) | |
| Triglycerides (mg/dL) | 1.00 (0.99–1.01) | |
| NRTI use (month) | ||
| Combivir | 0.76 (0.17–3.45) | |
| Epzicom | 0.49 (0.06–3.82) | |
| Trizivir | 2.43 (0.29–20.0) | |
| Zerit | 0.55 (0.07–4.26) | |
| Truvada | 0.46 (0.14–1.50) | |
| NNRTI use (month) | ||
| Sustiva | 1.46 (0.45–4.73) | |
| PI use (month) | ||
| Kaletra | 0.86 (0.19–3.95) | |
| Lexiva | 0.93 (012–7.22) | |
| Norvir | 0.69 (0.23–2.09) | |
| Reyataz | 0.51 (0.13–1.93) | |
| Other ARTs use (month) | ||
| Isentress | 0.50 (006–3.86) | |
| Duration of NRTI use (month) | 0.99 (0.98–1.00) | |
| Duration of NNRTI use (month) | 1.00 (0.99–1.01) | |
| Duration of PI use (month) | 1.00 (0.99–1.01) | |
| Duration of ART use (month) | 1.00 (0.99–1.01) | |
| Framingham score | 1.17 (1.02–1.34) | |
Abbreviations: HR, hazards ratio; CD4, CD4cell count; viral load, HIV RNA quantification; eGFR=estimated Glomerular Filtration Rate; hsCRP = high-sensitivity C-reactive protein; BP = blood pressure; glucose = fasting glucose; BMI = body mass index (kg/m2); LDL-C = low density lipoprotein cholesterol; HDL-C = high density lipoprotein cholesterol; serum 25(OH)D = 25-dihydroxyvitamin D; vitamin D deficiency = serum 25(OH)D <10 ng/mL; Framingham score = Framingham risk score; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
The initial multivariate proportional hazards model included age, sex, cigarette smoking, Serum 25(OH)D, vitamin D deficiency, systolic blood pressure, diastolic blood pressure, glucose, LDL, triglycerides, total cholesterol, and Framingham risk score.
DISCUSSION
Ours may be the first study to investigate the incidence of and the risk factors for subclinical CAD in HIV-infected African Americans. The key strength of the this report is that the study participants were cardiac CT-proved subclinical CAD-free participants. The first objective of this study was to estimate the incidence rate of subclinical CAD in HIV-infected African Americans. Although the prevalence of subclinical coronary atherosclerosis has been reported in HIV-infected African Americans, the incidence of subclinical CAD has not. Our findings demonstrate that the overall incidence of subclinical CAD in a population of HIV-infected African Americans is provocatively high—4.92 per 100 PY (95% CI: 2.69–8.26) —despite the fact that the participants in this study were at low risk according to the Framingham risk score.
The National Heart Lung and Blood Institute sponsored several studies in which the incidence of clinical CAD was estimated in the United States [13]. For example, the Atherosclerosis Risk in Communities (ARIC) study reported that the average age-adjusted CAD incidence rates per 1,000 person-years were 1.06 and .51, in African American men and women aged 45 to 64 years, respectively [13].Nevertheless, the incidence of subclinical CAD in African American population has not been reported. Since African Americans have the highest overall CAD mortality rate of any ethnic group in the United States, and acute myocardial infarction or sudden cardiac death is often the first clinical manifestation of CAD in up to 50% of patients, early detection of subclinical disease, before the clinical CAD occurs, and the early identification of the risk factors of subclinical CAD may be critical in this population [9,14,15].
The second objective of this study was to identify the risk factors of subclinical CAD in HIV-infected African Americans. We found that male sex was independently associated with development of subclinical CAD. Although it has been reported that males are at a higher risk of developing CAD in general populations [16], this study suggests that the risk associated with the development of subclinical CAD in HIV-infected African American men is far greater than that in HIV-infected African American women: male sex was associated with a 18-fold increased risk of developing subclinical CAD as compared with female sex. Further studies are needed to examine the magnitude of relative risk of developing subclinical CAD of men and women. Although one’s sex is not a modifiable risk factor, this finding is useful in identifying those who might benefit from intensive primary prevention/intervention.
The present study also suggests that vitamin D deficiency is significantly associated with the development of subclinical CAD in HIV-infected African Americans. The effects of vitamin D deficiency on clinical CAD in general populations have been widely investigated. A recent published study analyzed a large electronic medical records database to determine the prevalence of vitamin D deficiency and the relation of vitamin D levels to prevalent and incident CV risk factors and diseases in a general healthcare population [17]. This study revealed that vitamin D deficiency was significantly associated with increases in the prevalence of diabetes, hypertension, hyperlipidemia, and peripheral vascular disease, and that vitamin D levels were also highly associated with coronary artery disease, myocardial infarction, heart failure, and stroke, as well as with incident death, heart failure, coronary artery disease/myocardial infarction, and stroke [17]. The Framingham Offspring Study, which evaluated 1,739 study participants without prior cardiovascular disease, reported that vitamin D deficiency (defined as 25[OH] vitamin D <10 ng/ml) was associated with an increased risk for developing a first cardiovascular event after 5 years of follow-up as compared with subjects with 25(OH) vitamin D levels >15 ng/ml (hazard ratio: 1.80, 95% CI: 1.05–3.08) [18]. A recent study of ours suggests that vitamin D deficiency (adjusted OR=2.18, 95% CI: 1.07–4.43) is independently associated with the presence of significant coronary stenosis after controlling for traditional risk factors in cocaine users [19].
According to the present study, vitamin D deficiency is associated with a nine-fold increased risk of developing subclinical CAD. This finding has not been reported before and suggests new approaches to prevention of CAD and opportunities to investigate the mechanisms responsible for the development of subclinical CAD.
The mechanisms explaining the association between vitamin D deficiency and the development of subclinical coronary disease are unclear. It has been reported that low circulating 25(OH) D levels are associated with several risk factors for CAD, such as obesity, diabetes, hypertension and dyslipidemia, and inflammatory markers, such as, C-reactive protein and IL-6 [20–23]. A large cohort study of 3,258 patients scheduled for coronary angiography with a median follow-up period of 7.7 years found that low 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels are independently associated with all-cause and cardiovascular mortality [24]. Nevertheless, elevating these risk factors by low vitamin D levels may not be sufficient to explain why vitamin D deficiency is associated with the rapid development of coronary atherosclerosis.
The mechanisms that contribute to vitamin D deficiency and early atherosclerosis remain unclear. Several studies demonstrate that vitamin D modulates the immune system through multiple mechanisms. In fact, some of these mechanisms, such as dendritic cell (DC) activation, induction of T cell responses, and cytokine production, are associated with early stages of atherosclerosis [25,26]. Therefore, the severely depressed levels of vitamin D in HIV patients with subclinical CAD noted in our study may be a reflection of an up-regulation of immune responses and inflammation.
Activated vitamin D (1,25(OH)2 D) and its analogues have been reported to inhibit proliferation of mitogen-activated vascular smooth muscle cell proliferation in vitro [27–30] and to inhibit ET-induced activation of Cdk2 activity in neonatal rat vascular smooth muscle cells [30]. Recently, it was shown that 1,25 dihydroxyvitamin D’s (1,25(OH)2D) inhibits vascular smooth muscle cell proliferation through a cell division cycle 25 homolog A-dependent mechanism, suggesting that this hormone may prove useful in the management of disorders characterized by aberrant proliferation of vascular smooth muscle cells in the vascular wall [31]. Further studies are certainly warranted.
This study found no evidence suggesting CD4 count, HIV viral load, ART use, or cocaine use were significantly associated with subclinical CAD. A study with a larger sample size is needed to examine the effects of CD4 count, HIV vital load, and cocaine use on the development of subclinical CAD.
According to the Framingham risk score algorithm, 109 (91.6%) of the 119 participants (54 of the 62 men and 55 of the 57 women) in this study were at low risk of CAD, and 12 (85.7%) of the 14 participants who developed subclinical CAD were at low risk of CAD. Thus, the conventional preventive strategies to reduce traditional risk factors may not be effective in this population.
To our best knowledge, there are only two published studies evaluating the effect of vitamin D supplementation on surrogate markers of CAD. One examined the effect of 16-week vitamin D supplementation on flow-mediated dilation (FMD) in overweight African American adults [32]. The other trial was performed in African American adolescents aged 14 to 18. The outcome variables were adiposity and arterial stiffness [33].
We recognize several limitations to the present study. First, the participants were not a random sample of African Americans with HIV infection, and therefore the results may not be generalizable to other populations. Second, since a large majority of participants were cigarette smokers and/or cocaine users, the effects of cigarette smoking or cocaine use on subclinical CAD or the joint effects of vitamin D deficiency and cigarette smoking or cocaine use on subclinical CAD could not be fully evaluated, either individually or combined. Third, the sample size was small and the follow-up time was short. Thus, the confidence intervals of the hazards ratios are quite wide, indicating great uncertainty in the point estimate of hazards ratios. Due to the small sample size and the low number of end-points, the generalizability of the findings is quite limited. The study was underpowered to fully evaluate risk factors that may be associated with the development of incident CAD. Fourth, since this study was performed in African Americans living in inner-city Baltimore, the effects of some socioeconomic variables on subclinical CAD could not be completely controlled for. Fifth, we only had one reviewer for the CAC results. Also, since 8 of 9 participants developed CAC < 10, our study may suffer from interscan variation in CAC. It has been reported that the largest score variation in CAC occurred in the left main coronary artery [34]. No CAC in our study was identified in the left main coronary artery. Lastly, since the baseline data in this prospective study were not collected at the time of diagnosis of HIV infection, the effect of HIV infection and ART use on vitamin D levels could not be evaluated.
Despite its limitations, this study provides a disturbing estimate of the incidence of subclinical CAD in HIV-infected African Americans. This high incidence of subclinical CAD may highlight the urgent need for an identification of high-risk groups in African Americans with HIV infection.
Although this study suggests vitamin D deficiency is an independent risk factor for subclinical CAD, Further studies with a larger sample size should be conducted to validate our findings. Since there is not adequate data to support Vitamin D supplementation to reduce cardiovascular risk in HIV infected individuals, well-designed randomized clinical trials may be urgently needed to examine the safety and efficacy of vitamin D supplementation in HIV-infected persons who are vitamin D deficient.
Acknowledgments
The study was supported by grants from the National Institute on Drug Abuse, National Institutes of Health (NIH R01-DA 12777, DA15020 and DA25524). We thank the study participants for their contributions.
References
- 1.Eugenin EA, Morgello S, Klotman ME, Mosoian A, Lento PA, Berman JW, Schecter AD. Human Immunodeficiency Virus (HIV) Infects Human Arterial Smooth Muscle Cells in Vivo and in Vitro: Implications for the Pathogenesis of HIV-Mediated Vascular Disease. Am J Pathol. 2008;172(4):1100–11. doi: 10.2353/ajpath.2008.070457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbaro G. Heart and HAART: Two sides of the coin for HIV-associated cardiology issues. World J Cardiol. 2010 Mar 26;2(3):53–7. doi: 10.4330/wjc.v2.i3.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348:702–10. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 4.The Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination Antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2004. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 5.The DAD Study Group. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 6.Lai S, Lai H, Vlahov D, et al. HIV-1 infection, cocaine, and coronary calcification. Arch Intern Med. 2005;165:690–695. doi: 10.1001/archinte.165.6.690. [DOI] [PubMed] [Google Scholar]
- 7.Lai S, Fishman EK, Lai H, Moore R, Cofrancesco J, Pannu H, Tong WJ, Du JF, Bartlett J. Long-term cocaine use and antiretroviral therapy are associated with silent coronary artery disease in cardiovascularly asymptomatic African Americans with HIV infection. Clin Infect Dis. 2008 Jan 14; doi: 10.1086/526782. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai S, Bartlett J, Lai H, Moore R, Cofrancesco J, Pannu H, Tong W, Gao PJ, Meng W, Sun H, Fishman EK. Long-term combination antiretroviral therapy Is associated with the risk of coronary plaques in African Americans with HIV Infection. AIDS Patient Care STDs. 2009 Oct;23(10):815–24. doi: 10.1089/apc.2009.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferdinand KC. Coronary heart disease and lipid-modifying treatment in African American patients. Am Heart J. 2004;147:774–782. doi: 10.1016/j.ahj.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Ersfeld DL, Rao DS, Body JJ, Sackrison JL, Jr, Miller AB, Parikh N, Eskridge TL, Polinske A, Olson GT, MacFarlane GD. Analytical and clinical validation of the 25(OH) vitamin D assay for the Liaison automated analyzer. Clin Biochem. 2004;37:867–874. doi: 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008 Nov 27;359(22):2324–36. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 12.Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 13.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011 Feb 1;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. Epub 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 15.Anand DV, Lim E, Raval U, Lipkin D, Lahiri A. Prevalence of silent myocardial ischemia in asymptomatic individuals with subclinical atherosclerosis detected by electron beam tomography. J Nucl Cardiol. 2004 Jul-Aug;11(4):450–7. doi: 10.1016/j.nuclcard.2004.06.125. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PW, Castelli WP, Kannel WB. Coronary risk prediction in adults (the Framingham Heart Study) Am J Cardiol. 1987 May 29;59(14):91G–94G. doi: 10.1016/0002-9149(87)90165-2. [DOI] [PubMed] [Google Scholar]
- 17.May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, Lappé DL, Muhlestein JB Intermountain Heart Collaborative (IHC) Study Group. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010 Oct 1;106(7):963–8. doi: 10.1016/j.amjcard.2010.05.027. Epub 2010 Aug 11. [DOI] [PubMed] [Google Scholar]
- 18.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai H, Fishman EK, Gerstenblith G, Brinker JA, Tong W, Bhatia S, Detrick B, Lai S. Vitamin D deficiency is associated with significant coronary stenoses in asymptomatic African American chronic cocaine users. Int J Cardiol. 2011 Feb 2; doi: 10.1016/j.ijcard.2011.01.032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 21.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: Data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 22.Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352:709–10. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 23.Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92:39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008 Jun 23;168(12):1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 25.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006 Mar 24;311(5768):1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 26.Overton ET, Yin MT. The rapidly evolvoing research on vitamin D among HIV-infected populations. Curr Infect Dis Rep. 2011;13:83–93. doi: 10.1007/s11908-010-0144-x. [DOI] [PubMed] [Google Scholar]
- 27.Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13(6 Pt 2):954–959. doi: 10.1161/01.hyp.13.6.954. [DOI] [PubMed] [Google Scholar]
- 28.Mitsuhashi T, Morris RC, Jr, Ives HE. 1,25-Dihydroxyvitamin D3 modulates growth of vascular smooth muscle cells. J Clin Invest. 1991;87(6):1889–1895. doi: 10.1172/JCI115213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu-Wong JR, Nakane M, Ma J, Ruan X, Kroeger PE. Effects of Vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis. 2006;186(1):20–28. doi: 10.1016/j.atherosclerosis.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 30.Chen S, Law CS, Gardner DG. Vitamin D-dependent suppression of endothelin-induced vascular smooth muscle cell proliferation through inhibition of CDK2 activity. J Steroid Biochem Mol Biol. 2010;118(3):135–141. doi: 10.1016/j.jsbmb.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen S, Law CS, Grigsby CL, Olsen K, Gardner DG. A role for the cell cycle phosphatase Cdc25a in vitamin D-dependent inhibition of adult rat vascular smooth muscle cell proliferation. J Steroid Biochem Mol Biol. 2010 Nov;122(5):326–32. doi: 10.1016/j.jsbmb.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris RA, Pedersen-White J, Guo DH, Stallmann-Jorgensen IS, Keeton D, Huang Y, Shah Y, Zhu H, Dong Y. Vitamin d(3) supplementation for 16 weeks improves flow-mediated dilation in overweight african-american adults. Am J Hypertens. 2011 May;24(5):557–62. doi: 10.1038/ajh.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong Y, Stallmann-Jorgensen IS, Pollock NK, Harris RA, Keeton D, Huang Y, Li K, Bassali R, Guo DH, Thomas J, Pierce GL, White J, Holick MF, Zhu H. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab. 2010 Oct;95(10):4584–91. doi: 10.1210/jc.2010-0606. [DOI] [PubMed] [Google Scholar]
- 34.Yoon HC, Goldin JG, Greaser LE, 3rd, Sayre J, Fonarow GC. Interscan variation in coronary artery calcium quantification in a large asymptomatic patient population. AJR Am J Roentgenol. 2000 Mar;174(3):803–9. doi: 10.2214/ajr.174.3.1740803. [DOI] [PubMed] [Google Scholar]