Abstract
Objective
Very low birth weight (VLBW) preterm infants are at risk for impaired cerebral autoregulation with pressure passive blood flow. Fluctuations in cerebral perfusion may occur in infants with a hemodynamically significant patent ductus arteriosus (hsPDA), especially during ductal closure. Our goal was to compare cerebral autoregulation using near-infrared spectroscopy (NIRS) in VLBW infants treated for a hsPDA.
Study design
This prospective observational study enrolled 28 VLBW infants with a hsPDA diagnosed by echocardiogram and 12 control VLBW infants without a hsPDA. NIRS cerebral monitoring was applied during conservative treatment, indomethacin, or surgical ligation. A cerebral pressure passivity index (PPI) was calculated and PPI differences were compared using a mixed effects regression model. Cranial ultrasound and MRI data were also assessed.
Results
Infants with surgically ligated PDAs were more likely to have had a greater PPI within two hours following ligation compared with those treated with conservative management (p=0.04) or indomethacin (p=0.0007). These differences resolved six hours following treatment.
Conclusions
Cerebral autoregulation was better preserved after indomethacin treatment of a hsPDA compared with surgical ligation. Infants requiring surgical hsPDA ligation may be at increased risk for cerebral pressure passivity in the six hours following surgery.
Keywords: near-infrared spectroscopy, neonate, pressure passivity, cerebral oxygenation
Persistence of a hemodynamically significant patent ductus arteriosus (hsPDA) in preterm very low birth weight (VLBW) infants may increase the potential for cerebral injury due to left-to-right shunting of blood through the hsPDA and alterations in cerebral perfusion. Preterm infants with a hsPDA have lower regional cerebral oxygenation levels (1) and have a higher risk for intraventricular hemorrhage (IVH) and cerebral white matter injury (2, 3). Changes in cerebral perfusion pressure during ductal shunting and after ductal closure may predispose these infants to injury. Intact cerebral autoregulation maintains cerebral blood flow despite fluctuations in cerebral perfusion pressure, however preterm infants are more susceptible to altered cerebral autoregulation (4, 5). We previously found that preterm VLBW infants with a hsPDA treated with surgical ligation were more likely to have a >20% increase in cerebral oxygen saturation levels (6). Although this change may be due to a more severe left-to-right shunt at baseline, the impact of a sudden increase in cerebral oxygenation after hsPDA treatment in a preterm infant remains unclear. We hypothesized that infants treated with surgical ligation would also have greater impairment of cerebral autoregulation compared with those treated with indomethacin.
Growing controversy over the optimal management of a hsPDA (7, 8) underscores the need to investigate how treatment of a hsPDA affects cerebral autoregulation and brain injury. Treatment options include conservative management of symptoms while awaiting spontaneous hsPDA closure, active pharmacologic treatment with a prostaglandin synthesis inhibitor such as indomethacin or ibuprofen, or surgical ligation. Treatment decisions are influenced by echocardiographic characteristics of the duct, clinical status of the infant, and potential side effects of therapy. Conservative management of a hsPDA may result in prolonged exposure to fluctuating or reduced cerebral blood flow due to ongoing left-to-right ductal shunting. Surgical hsPDA ligation has been associated with higher rates of neurosensory deficits (9), neurodevelopmental impairment, or death (10). Indomethacin use may decrease brain blood flow (11-13), but reduce rates of IVH and white matter injury (14, 15). It is unclear whether periods of cerebral pressure passivity due to impaired autoregulation may contribute to changes in cerebral perfusion with these different treatment strategies.
Impaired cerebral autoregulation can be assessed with continuous arterial blood pressure monitoring in conjunction with NIRS (near-infrared spectroscopy) monitoring of cerebral oxygenation. As a non-invasive, bedside technology, NIRS measures regional cerebral oxygenation (rSO2) levels as a surrogate for cerebral blood flow (16-18). The degree of correlation between mean arterial blood pressure (MAP) and rSO2 determines cerebral pressure passivity and loss of autoregulation (4, 5). The aim of this prospective observational study was to compare the cerebral autoregulatory capacity of preterm VLBW infants treated for a hsPDA with conservative management, indomethacin, or surgical ligation. Associations between cerebral pressure passivity and abnormal neuroimaging outcomes were also investigated.
Methods
VLBW preterm infants with birth weight 401-1500 g were screened for eligibility at the time an initial echocardiogram for clinical suspicion of a hsPDA. Hemodynamic significance of a PDA was determined by a cardiologist based on size (moderate to large) as well as the presence of left-to-right shunting through the ductus or retrograde or absent diastolic aortic flow. Exclusion criteria were birth asphyxia, congenital or chromosomal anomalies, congenital infection, altered skin integrity precluding placement of NIRS sensors, or decision not to provide full intensive care support. Informed written consent was obtained from parents of the infants and the Stanford Human Research Protection Program approved the study.
Perinatal and demographic data were collected and a baseline cranial ultrasound obtained prior to initiation of hsPDA treatment if not previously obtained within 48 h. A PDA score (19) was calculated to measure clinical severity of the hsPDA, but was not utilized in treatment decisions. This prospective observational study included the following hsPDA treatment strategies made at the discretion of the attending neonatologist within 24 hours of the echocardiogram: (1) conservative management--fluid restriction, diuretics, maintaining higher hematocrit levels, and/or delivering higher pulmonary distending pressures to minimize congestive heart failure while awaiting spontaneous ductal closure; (2) indomethacin treatment—0.2 mg/kg intravenously every 12 hours for three doses; and (3) surgical ligation—performed as a primary procedure or after failed indomethacin closure.
Treatment with indomethacin was initiated for infants with clinical instability including feeding intolerance, respiratory compromise requiring significant mechanical ventilation support, or hypotension requiring pressors. Treatment with surgical ligation was performed for the above factors in addition to hydrocortisone use, history of necrotizing enterocolitis, or unlikely to respond to indomethacin based on chronologic age.
A NIRS neonatal cerebral sensor (INVOS 5100, Covidien Corp., Boulder, CO) was applied to the central forehead at least 2 h prior to treatment with conservative management, indomethacin, or surgical ligation and left in place for a minimum of 24 h after treatment. Simultaneous continuous monitoring of MAP was achieved through an umbilical arterial catheter or peripheral arterial line already in place. Anesthesia during surgical ligation was with fentanyl, ketamine, and rocuronium, and morphine was used for post-operative pain control. A separate control group of preterm VLBW infants were enrolled if they had no echocardiographic evidence of a hsPDA. These control infants were studied with NIRS monitoring for 24 h.
Outcome Measures
Impairment of autoregulation was measured by a pressure passivity index (PPI) over the entire study period. The PPI was calculated by determining the percentage of 20 minute intervals with concordance between MAP and rSO2 (Pearson correlation coefficient r>0.5). Maximum concordance levels (r) over study intervals were also determined. Greater concordance is seen when cerebral autoregulation is impaired and cerebral blood flow becomes pressure-passive. (4, 5)
A secondary outcome was abnormal neuroimaging as defined by worsening cranial ultrasound or magnetic resonance imaging (MRI) compared with baseline. Cranial ultrasound abnormalities included Grade 3 or 4 IVH, periventricular leukomalacia, increased echogenicities, or ventricular dilation. MRI abnormalities at 36-42 wks post-menstrual age included white matter signal abnormalities, ventricular dilation, cystic abnormalities, or reduction in white matter volume.
Data Analysis
Simultaneously collected NIRS and MAP data with a sampling frequency of 0.2 Hz were downloaded to a personal computer for subsequent analysis. Data processing eliminated periods of time during arterial blood gas sampling or movement artifact associated with loss of signal, spikes, or non-physiologic MAP or rSO2 values. Data analysis was also limited to time periods without significant fluctuations in systemic oxygen saturation or pCO2 levels which could disturb cerebral oxygenation independent of cerebral perfusion pressure. None of the infants were transfused withblood over the study period and none had glucose levels <40 mg/dl. Eliminated data represented <10% of total study time. Sequential 20-minute epochs of uninterrupted data were utilized to determine correlation coefficients and PPI over the entire study period as described above with SAS v. 9.1 software (SAS Institute, Cary, NC). Baseline PPI was calculated using 8 hours of continuous NIRS and MAP data prior to hsPDA treatment. If fewer than 8 hours of data were available prior to intervention, the baseline PPI was calculated from a minimum of 2 hours of data collection.
Statistics
Given non-parametric data, patient characteristics and PPI between hsPDA treatment groups were compared using Fisher exact test for categorical variables and Kruskal-Wallis test for continuous variables with step-down Bonferroni correction for multiple comparisons. Logistic regression analysis was used to assess neuroimaging outcomes between groups and adjusted for gestational age, birth weight, and sex. PPI and patient variables were further analyzed with multivariate regression. Mixed effects models were used to compare PPI over time for each group at discrete time points between 2-24 hours after treatment. All statistical analyses were performed with SAS software (v. 9.1).
Results
Six patients received conservative management, 12 received indomethacin, and 10 received surgical ligation. A control group of 12 preterm infants without a hsPDA also were studied. Two subjects had NIRS studies repeated during surgical ligation after failed indomethacin. NIRS data were analyzed separately by treatment group. No subjects died prior to hospital discharge and none received prophylactic indomethacin.
No significant difference in perinatal or neonatal variables between hsPDA treatment groups existed (Table I) except that chronologic age at hsPDA diagnosis was higher in the conservatively managed group (6 days) when compared with the ligation group or the indomethacin group (3 days, p=0.03). Average age of the control group was 5 ± 3 days, and ranged from 2 to 11 days. Detailed echocardiographic characteristics of each treatment group demonstrated a larger ductal diameter in the ligation group (2 mm) compared with the conservative treatment group (1.4 mm, p=0.02) and the indomethacin group (1.5 mm, p=0.01), but no other significant differences (Table II). Mean gestational age for all subjects was 26 ± 1 weeks and mean birth weight was 830 ± 162 g. All infants were intubated and mechanically ventilated for respiratory support. Previously published findings have also shown that compared with other treatment groups, the surgically ligated patients also had a significant increase in rSO2 after ductal closure (63 ± 4% to 72 ± 3%, p=0.02) with a corresponding, non-significant increase in MAP (35.8 ± 5 mm Hg to 40.5 ± 6 mm Hg) (6).
Table 1.
Perinatal and Neonatal Variables by hsPDA Treatment Group
| Characteristic | Control (N=12) |
Conservative (N=6) |
Indomethacin (N=12) |
Ligation (N=10) |
|---|---|---|---|---|
| Birth weight (g) | 840 ± 168 | 775 ± 148 | 846 ± 169 | 830 ± 170 |
| Gestational age (wks) | 26 ± 1 | 26 ± 2 | 27 ± 1 | 26 ± 1 |
| Male sex, n (%) | 8 (67) | 4 (67) | 4 (33) | 5 (50) |
| Antenatal steroids, n (%) | 10 (83) | 5 (83) | 8 (67) | 8 (80) |
| Antenatal indomethacin, n (%) | 2 (17) | 2 (33) | 3 (25) | 3 (30) |
| Apgar score <4 at 1 min, n (%) | 6 (50) | 3 (50) | 2 (20) | 1 (10) |
| Apgar score <4 at 5 min, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 (10) |
| PDA score, n (%) C3 (Moderate) C4 (Severe) |
n/a n/a |
4 (67) 2 (33) |
6 (50) 6 (50) |
4 (40) 6 (60) |
| Age at PDA diagnosis (days)* | n/a | 6 ± 3 | 3 ± 2 | 3 ± 2 |
| Surfactant, n (%) | 11 (92) | 6 (100) | 11 (92) | 9 (90) |
| Dopamine, n (%) | 4 (33) | 6 (100) | 7 (58) | 8 (80) |
| Postnatal hydrocortisone, n (%) | 0 (0) | 2 (33) | 1 (8) | 1 (10) |
| pCO2 (mm Hg) | 48 ± 7 | 47 ± 8 | 44 ± 9 | 52 ± 8 |
| Hematocrit (%) | 35.2 ± 3 | 37.4 ± 4 | 36.7 ± 3 | 35.4 ± 6 |
| Baseline MAP, (mm Hg) | 38.9 ± 5 | 34.3 ± 3 | 36.3 ± 4 | 35.8 ± 5 |
| Post-treatment MAP, (mm Hg) | 39.1 ± 6 | 34.8 ± 4 | 38.2 ± 3 | 40.5 ± 6 |
Plus-minus values are means ± SD.
PDA score from McNamara and Sehgal, Arch Dis Child Fetal Neonatal Ed. 2007;92:F424-427.
MAP= mean arterial blood pressure. Post-treatment MAP obtained 24 h after PDA treatment.
p=0.03 for difference in age at PDA diagnosis between conservatively managed and ligation groups or between conservatively managed and indomethacin groups.
Table 2.
Echocardiographic Characteristics of Treatment Groups
| Characteristic | Conservative (N=6) |
Indomethacin (N=12) |
Ligation (N=10) |
|---|---|---|---|
| Internal ductal diameter (mm)* | 1.5 ± 0.3 | 1.4 ± 0.2 | 2 ± 0.4 |
| Left atrial to aortic dimension (LA:Ao) ratio |
1.3 ± 0.1 | 1.6 ± 0.4 | 1.6 ± 0.2 |
| Left atrial enlargement, n (%) | 2 (33) | 5 (42) | 6 (60) |
| Mitral regurgitation, n (%) | 0 (0) | 3 (25) | 2 (20) |
| Holodiastolic retrograde abdominal aorta flow, n (%) |
3 (50) | 6 (50) | 4 (40) |
| Abdominal aorta reverse to forward flow ratio |
0.4 ± 0.2 | 0.3 ± 0.1 | 0.6 ± 0.4 |
Plus-minus values are means ± SD.
p=0.02 for difference in ductal diameter between Conservative and Ligation groups and p=0.01 for Indomethacin versus Ligation groups
Cerebral Pressure Passivity Comparisons
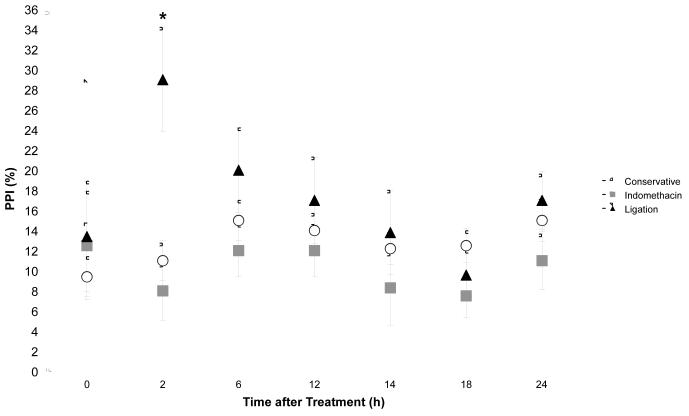
The degree of concordance between simultaneous NIRS and MAP tracings determines the degree of impairment of cerebral autoregulatory capacity (Figure 1). There was no difference in maximum concordance levels between treatment groups. However, PPI was significantly greater in the first 2 hours following surgical ligation compared with control (p=0.04) and indomethacin treatment (p=0.0007) (Table II). This difference disappeared by six hours after treatment. Two hours after each indomethacin dose, PPI was also less than baseline with a slow return to baseline levels (Figure 2).
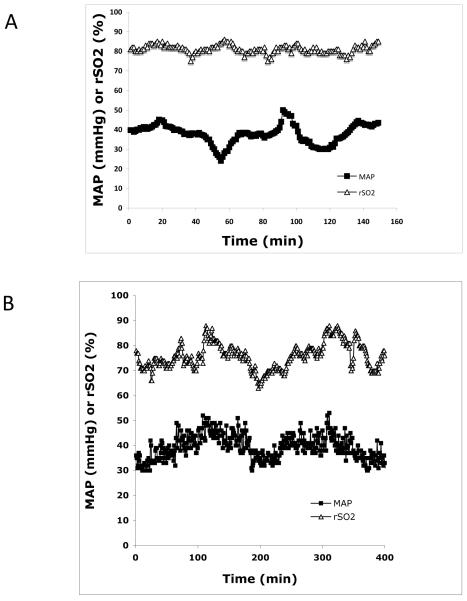
Figure 1. Changes in Cerebral Autoregulatory Capacity.
(A) Simultaneous rSO2 and MAP tracing from a 26-week gestation infant during surgical ligation of a hsPDA. Despite fluctuations in MAP, rSO2 remains relatively stable, indicating intact cerebral autoregulation (r=−0.43). (B) The same preterm infant 2 hours after ligation demonstrates concordance between MAP and rSO2, indicating a loss of cerebral autoregulation (r=0.82).
Figure 2. Change in PPI after hsPDA Treatment.
Average PPI is transiently increased following surgical ligation compared with conservative management (p=0.04) and decreased after indomethacin (p=0.0007). Differences in PPI become non-significant 6 hours after treatment in all groups.
Baseline PPI differences were not statistically significant between treatment groups, although the control group without a hsPDA had the lowest PPI (5 ± 2%). After univariate analysis, greater baseline PPI was correlated with hydrocortisone use (p=0.003), with a trend towards significance for dopamine use (p=0.07) and lower 5 minute Apgar (p=0.06). However, after multivariate analysis, none of these variables remained significantly associated with baseline PPI.
Average PPI over the entire study period was also not significantly different between treatment and control groups, but with univariate regression was associated with hydrocortisone use (p<0.0001), dopamine use (p=0.04), lower pCO2 level (p=0.03), hematocrit (p=0.02), and lower 5 minute Apgar score (p=0.04). After multivariate regression analysis, dopamine use (p=0.05) and lower 5 minute Apgar score (p=0.02) remained associated with average PPI. Dopamine use was also associated with maximum concordance level (p=0.01), independent of PDA treatment strategy.
Neuroimaging
All patients had a MRI and/ or follow-up cranial ultrasound studies done prior to discharge or hospital transfer, and 10 (25%) developed worsening neuroimaging findings compared with baseline. Increased abnormalities were seen in 27% (3 of 11) who were treated with indomethacin alone, 40% (4 of 10) who were surgically ligated after failed indomethacin closure, and 60% (3 of 5) who received primary surgical ligation. However, there was no statistically significant association between the development of neuroimaging abnormalities and either PPI, maximum concordance level, or type of hsPDA treatment.
Discussion
We examined cerebral autoregulatory capacity with NIRS monitoring in preterm infants with a hsPDA receiving different treatment strategies. Preterm infants are at high risk for alterations in cerebral blood flow due to changing hemodynamics from both ductal shunting and ductal closure. Infants who had their hsPDA surgically ligated were more likely to have had longer periods of cerebral pressure passivity for up to 6 h post-operatively compared with those who received indomethacin or those who were conservatively managed. Duration of cerebral pressure passivity was not associated with subsequent neuroimaging abnormalities, although the small sample size may have limited our ability to detect a change.
Our findings confirm prior studies demonstrating impaired cerebral autoregulation in preterm infants using NIRS monitoring (4, 5, 20, 21). Other methods of measuring cerebral autoregulation with transcranial Doppler ultrasound or xenon-133 clearance also support these findings (22, 23). Soul et al found a prevalence of cerebral pressure passivity (PPI) during 20% of study monitoring time. We found an average baseline PPI of between 9-13% in all subjects prior to treatment, with a transiently greater PPI up to 29% in those receiving surgical ligation of their hsPDA. Although not statistically significant, control infants without a hsPDA had a lower PPI with mean of 5% (ranging from 0-13%). Lower PPI values overall in this study may be attributable to the use of the different mathematical technique of transfer function analysis to calculate pressure passivity in Soul’s study. However, even if our study underestimated the prevalence of pressure passivity overall, the comparison of PPI between hsPDA treatment groups remains valid.
We found a low 5 minute Apgar score to be significantly associated with average PPI (p=0.02), substantiating other studies demonstrating greater pressure passivity in sicker infants (4, 22, 24). Use of dopamine in our study was also associated with maximum concordance (p=0.01) and average PPI (p=0.05). This finding is similar to the impairment of cerebral autoregulation seen with the use of inotropic medications (5, 25). It remains unclear whether inotropic medications themselves or the condition of systemic hypotension in a preterm infant lead to the risk for cerebral pressure passivity. However, dopamine use and hypotension in preterm infants were associated with increased mortality, neuroimaging abnormalities, and impaired neurodevelopment (26, 27), potentially due in part to altered cerebral autoregulation. hsPDA was associated with IVH and cerebral white matter injury (28-31), but the role of impaired cerebral autoregulation in the development of these brain abnormalities remains unclear. We did not find an association between PPI and worsening neuroimaging abnormalities in our small cohort of preterm infants treated for a hsPDA. However, other investigators have failed to find an association between PPI and IVH in preterm infants (4, 5, 32). In contrast, others have found that the magnitude of cerebral pressure passivity rather than its prevalence was associated with early IVH (20, 21). Discrepancies in timing of neuroimaging and methods of defining autoregulatory impairment may account for these differing results.
There has been limited study of cerebral autoregulation following PDA ligation. Post-operative pressure passivity may be attributed to a surgical stress response or effects of anesthesia. However, previous studies have shown that both fentanyl and ketamine administration in preterm infants do not alter cerebral autoregulation (33, 34). A single study found disturbed autoregulation 6h after open cardiac surgery (18). A sudden increase in cerebral blood flow has been documented by transcranial Doppler ultrasound studies following PDA ligations (35, 36). In conjunction with impaired autoregulation, injury to the vulnerable germinal matrix may occur after PDA ligations. A decrease in cerebral oxygenation during the first hours after ductal ligation with normalization by 24 h has been reported (37) and may be further evidence of impaired autoregulation. Even though we did not measure left ventricular output after surgical ligation, others have found that 27% of infants developed cardiovascular decompensation with hypotension within 12 hours of surgical ligation (38). These post-operative hemodynamic fluctuations further predispose preterm infants to alterations in cerebral perfusion given impaired autoregulation.
Although rates of neuroradiographic brain injury did not reach statistical significance in our study, infants primarily treated with surgical ligation were more likely to have had worsening neuroimaging abnormalities compared with those who were ligated after receiving indomethacin or those who received indomethacin alone. Impaired cerebral autoregulation may contribute to the increased risk of ensuing neurosensory or neurodevelopmental impairment after surgical hsPDA ligation (9, 10). Our findings suggest that in the immediate post-operative period following hsPDA ligation, fluctuations in cerebral perfusion should be minimized. Cerebral blood flow may be influenced by changes in systemic perfusion, pCO2, and glucose levels. However, maintaining stable MAP may not always reflect adequate systemic blood flow or cerebral blood flow (39). NIRS monitoring to ensure stability of cerebral oxygenation may be of benefit after surgical ligation, given the high risk for impaired cerebral autoregulation.
We found that after indomethacin treatment, there was a trend for PPI to be transiently less than baseline, which did not reach statistical significance. Due to its vasoconstrictive properties, indomethacin may decrease CO2 reactivity in the brain (40) and reset the brain’s autoregulatory capacity. Indomethacin exposure has been associated with decreased white matter injury by MRI in preterm infants (3) and decreased severe IVH (14, 15). The cerebral-protective effects of indomethacin may be partially explained by preserved cerebral autoregulation.
We recognize several limitations of this observational study. Infants were not randomly assigned to type of hsPDA treatment, therefore inherent differences between groups may exist. The surgical ligation group has the potential of being more critically ill prior to intervention and predisposed to abnormal cerebral monitoring. This group had a larger ductal diameter. Also, although not statistically significant, this group had more subjects with a severe clinical PDA score and a greater increase in MAP after ligation, suggesting a more hemodynamically significant PDA with clinical instability. The ligation group also had a higher incidence of new cerebral abnormalities on neuroimaging, again suggesting a sicker group of infants. The severity of illness and impact of a more significant hemodynamic shunt may explain cerebral pressure passivity differences, especially in surgically ligated patients. However, baseline and 24 h PPI values did not appear significantly different between groups. The only PPI differences occurred until 6 h after treatment, suggesting the potential contribution of a treatment effect on PPI.
Our research may have benefited from other more precise mathematical techniques to define loss of cerebral autoregulation, such as dynamic time warping to compare time series or transfer function analysis of coherence (32). However, we utilized simple linear correlation given the discrete five-second sampling interval of our NIRS device rather than waveform data. Previous literature supports use of this technique (24). As cerebral autoregulation is a dynamic process, our study also had the advantage of a longer time period of analysis. We used 24 h to detect pressure passivity compared with the <6 hours time blocks used in previous autoregulation studies (5, 21, 22).
We also may have underestimated the extent of cerebral pressure passivity. Patients with decreased MAP variability, particularly those in the indomethacin and conservative treatment groups, would potentially have limited detection of a pressure passive state (32). Moreover, even though <10% of total study time eliminated data due to significant hypoxia and hypercarbia may have represented significant periods of impaired cerebral autoregulation that were not captured. Developmental changes resulting in maturation of cerebral autoregulatory capacity may contribute further to a lower PPI for older subjects, especially those in the conservatively managed group.
Table 3.
Comparison of Treatment Groups by Pressure Passivity Index (PPI)
| Measure | Control (N=12) |
Conservative (N=6) |
Indomethacin (N=12) |
Ligation (N=10) |
|---|---|---|---|---|
| Baseline rSO2 (%) | 74 ± 3 | 68 ± 4 | 68 ± 3 | 63 ± 4 |
| Baseline PPI (%) | 5 ± 2 | 9 ± 2 | 13 ± 5 | 13 ± 6 |
| 2 h PPI (%) | n/a | 11 ± 3 | 8 ± 4 | 29 ± 2 |
| Δ 2 h PPI (%)* | 2 ± 3 | −5 ± 4 | 16 ± 3 | |
| 6 h PPI (%) | n/a | 15 ± 3 | 12 ± 3 | 20 ± 2 |
| Δ 6 h PPI (%) | 6 ± 3 | −0.8 ± 3 | 7 ± 2 | |
| 12 h PPI (%) | n/a | 14 ± 2 | 12 ± 4 | 17 ± 3 |
| Δ 12 h PPI (%) | 5 ± 2 | 0.9 ± 3 | 4 ± 2 | |
| 24 h PPI (%) | n/a | 15 ± 2 | 11 ± 3 | 17 ± 2 |
| Δ 24 h PPI (%) | 6 ± 2 | -2 ± 3 | 4 ± 1 | |
Plus-minus values are means ± standard error of the mean.
PPI values are averages over the time interval.
Difference between conservative management and ligation groups (p=0.04), and difference between indomethacin and ligation groups (p=0.0007).
Acknowledgments
We thank Elisabeth Merkel for her assistance with data collection and Anthea Buchin and Alec Barlow for their assistance with data processing.
Supported by the Lucile Packard Foundation for Children’s Health and the Stanford NIH/NCRR CTSA (grant KL2 RR025743).
Abbreviations
- hsPDA
Hemodynamically significant patent ductus arteriosus
- IVH
Intraventricular hemorrhage
- MAP
Mean arterial blood pressure
- MRI
Magnetic resonance imaging
- NIRS
Near-infrared spectroscopy
- PPI
Pressure passivity index
- rSO2
Regional cerebral oxygen saturation
- VLBW
Very low birth weight
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Lemmers PM, Toet MC, van Bel F. Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics. 2008;121:142–7. doi: 10.1542/peds.2007-0925. [DOI] [PubMed] [Google Scholar]
- 2.Evans N, Kluckow M. Early ductal shunting and intraventricular haemorrhage in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;75:F183–6. doi: 10.1136/fn.75.3.f183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller SP, Mayer EE, Clyman RI, Glidden DV, Hamrick SE, Barkovich AJ. Prolonged indomethacin exposure is associated with decreased white matter injury detected with magnetic resonance imaging in premature newborns at 24 to 28 weeks' gestation at birth. Pediatrics. 2006;117:1626–31. doi: 10.1542/peds.2005-1767. [DOI] [PubMed] [Google Scholar]
- 4.Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61:467–73. doi: 10.1203/pdr.0b013e31803237f6. [DOI] [PubMed] [Google Scholar]
- 5.Wong FY, Leung TS, Austin T, Wilkinson M, Meek JH, Wyatt JS, et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics. 2008;121:e604–11. doi: 10.1542/peds.2007-1487. [DOI] [PubMed] [Google Scholar]
- 6.Chock VY, Ramamoorthy C, Van Meurs KP. Cerebral oxygenation during different treatment strategies for a patent ductus arteriosus. Neonatology. 2011;100:233–40. doi: 10.1159/000325149. [DOI] [PubMed] [Google Scholar]
- 7.Benitz WE. Treatment of persistent patent ductus arteriosus in preterm infants: time to accept the null hypothesis? J Perinatol. 2010;30:241–52. doi: 10.1038/jp.2010.3. [DOI] [PubMed] [Google Scholar]
- 8.Laughon M, Bose C, Clark R. Treatment strategies to prevent or close a patent ductus arteriosus in preterm infants and outcomes. J Perinatol. 2007;27:164–70. doi: 10.1038/sj.jp.7211662. [DOI] [PubMed] [Google Scholar]
- 9.Kabra NS, Schmidt B, Roberts RS, Doyle LW, Papile L, Fanaroff A. Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: results from the Trial of Indomethacin Prophylaxis in Preterms. J Pediatr. 2007;150:229–34. 34–e1. doi: 10.1016/j.jpeds.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 10.Chorne N, Leonard C, Piecuch R, Clyman RI. Patent ductus arteriosus and its treatment as risk factors for neonatal and neurodevelopmental morbidity. Pediatrics. 2007;119:1165–74. doi: 10.1542/peds.2006-3124. [DOI] [PubMed] [Google Scholar]
- 11.Evans DH, Levene MI, Archer LN. The effect of indomethacin on cerebral blood-flow velocity in premature infants. Dev Med Child Neurol. 1987;29:776–82. doi: 10.1111/j.1469-8749.1987.tb08823.x. [DOI] [PubMed] [Google Scholar]
- 12.Pryds O, Greisen G, Johansen KH. Indomethacin and cerebral blood flow in premature infants treated for patent ductus arteriosus. Eur J Pediatr. 1988;147:315–6. doi: 10.1007/BF00442705. [DOI] [PubMed] [Google Scholar]
- 13.Van Bel F, Van de Bor M, Stijnen T, Baan J, Ruys JH. Cerebral blood flow velocity changes in preterm infants after a single dose of indomethacin: duration of its effect. Pediatrics. 1989;84:802–7. [PubMed] [Google Scholar]
- 14.Ment LR, Oh W, Ehrenkranz RA, Philip AG, Vohr B, Allan W, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics. 1994;93:543–50. [PubMed] [Google Scholar]
- 15.Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344:1966–72. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- 16.Rais-Bahrami K, Rivera O, Short BL. Validation of a noninvasive neonatal optical cerebral oximeter in veno-venous ECMO patients with a cephalad catheter. J Perinatol. 2006;26:628–35. doi: 10.1038/sj.jp.7211573. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji M, duPlessis A, Taylor G, Crocker R, Volpe JJ. Near infrared spectroscopy detects cerebral ischemia during hypotension in piglets. Pediatr Res. 1998;44:591–5. doi: 10.1203/00006450-199810000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Bassan H, Gauvreau K, Newburger JW, Tsuji M, Limperopoulos C, Soul JS, et al. Identification of pressure passive cerebral perfusion and its mediators after infant cardiac surgery. Pediatr Res. 2005;57:35–41. doi: 10.1203/01.PDR.0000147576.84092.F9. [DOI] [PubMed] [Google Scholar]
- 19.McNamara PJ, Sehgal A. Towards rational management of the patent ductus arteriosus: the need for disease staging. Arch Dis Child Fetal Neonatal Ed. 2007;92:F424–7. doi: 10.1136/adc.2007.118117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Leary H, Gregas MC, Limperopoulos C, Zaretskaya I, Bassan H, Soul JS, et al. Elevated cerebral pressure passivity is associated with prematurity-related intracranial hemorrhage. Pediatrics. 2009;124:302–9. doi: 10.1542/peds.2008-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuji M, Saul JP, du Plessis A, Eichenwald E, Sobh J, Crocker R, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000;106:625–32. doi: 10.1542/peds.106.4.625. [DOI] [PubMed] [Google Scholar]
- 22.Boylan GB, Young K, Panerai RB, Rennie JM, Evans DH. Dynamic cerebral autoregulation in sick newborn infants. Pediatr Res. 2000;48:12–7. doi: 10.1203/00006450-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Pryds O, Greisen G, Lou H, Friis-Hansen B. Heterogeneity of cerebral vasoreactivity in preterm infants supported by mechanical ventilation. J Pediatr. 1989;115:638–45. doi: 10.1016/s0022-3476(89)80301-4. [DOI] [PubMed] [Google Scholar]
- 24.Lemmers PM, Toet M, van Schelven LJ, van Bel F. Cerebral oxygenation and cerebral oxygen extraction in the preterm infant: the impact of respiratory distress syndrome. Exp Brain Res. 2006;173:458–67. doi: 10.1007/s00221-006-0388-8. [DOI] [PubMed] [Google Scholar]
- 25.Munro MJ, Walker AM, Barfield CP. Hypotensive extremely low birth weight infants have reduced cerebral blood flow. Pediatrics. 2004;114:1591–6. doi: 10.1542/peds.2004-1073. [DOI] [PubMed] [Google Scholar]
- 26.Fanaroff JM, Wilson-Costello DE, Newman NS, Montpetite MM, Fanaroff AA. Treated hypotension is associated with neonatal morbidity and hearing loss in extremely low birth weight infants. Pediatrics. 2006;117:1131–5. doi: 10.1542/peds.2005-1230. [DOI] [PubMed] [Google Scholar]
- 27.Low JA, Froese AB, Galbraith RS, Smith JT, Sauerbrei EE, Derrick EJ. The association between preterm newborn hypotension and hypoxemia and outcome during the first year. Acta Paediatr. 1993;82:433–7. doi: 10.1111/j.1651-2227.1993.tb12717.x. [DOI] [PubMed] [Google Scholar]
- 28.Jim WT, Chiu NC, Chen MR, Hung HY, Kao HA, Hsu CH, et al. Cerebral hemodynamic change and intraventricular hemorrhage in very low birth weight infants with patent ductus arteriosus. Ultrasound Med Biol. 2005;31:197–202. doi: 10.1016/j.ultrasmedbio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Perlman JM, Rollins N, Burns D, Risser R. Relationship between periventricular intraparenchymal echodensities and germinal matrix-intraventricular hemorrhage in the very low birth weight neonate. Pediatrics. 1993;91:474–80. [PubMed] [Google Scholar]
- 30.Pladys P, Beuchee A, Wodey E, Treguier C, Lassel L, Betremieux P. Patent ductus arteriosus and cystic periventricular leucomalacia in preterm infants. Acta Paediatr. 2001;90:309–15. [PubMed] [Google Scholar]
- 31.Shortland DB, Gibson NA, Levene MI, Archer LN, Evans DH, Shaw DE. Patent ductus arteriosus and cerebral circulation in preterm infants. Dev Med Child Neurol. 1990;32:386–93. doi: 10.1111/j.1469-8749.1990.tb16957.x. [DOI] [PubMed] [Google Scholar]
- 32.Hahn GH, Christensen KB, Leung TS, Greisen G. Precision of coherence analysis to detect cerebral autoregulation by near-infrared spectroscopy in preterm infants. J Biomed Opt. 2010;15:037002. doi: 10.1117/1.3426323. [DOI] [PubMed] [Google Scholar]
- 33.Hamon I, Hascoet JM, Debbiche A, Vert P. Effects of fentanyl administration on general and cerebral haemodynamics in sick newborn infants. Acta Paediatr. 1996;85:361–5. doi: 10.1111/j.1651-2227.1996.tb14033.x. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt A, Ryding E, Akeson J. Racemic ketamine does not abolish cerebrovascular autoregulation in the pig. Acta Anaesthesiol Scand. 2003;47:569–75. doi: 10.1034/j.1399-6576.2003.00089.x. [DOI] [PubMed] [Google Scholar]
- 35.Lundell BP, Sonesson SE, Cotton RB. Ductus closure in preterm infants. Effects on cerebral hemodynamics. Acta Paediatr Scand Suppl. 1986;329:140–7. doi: 10.1111/j.1651-2227.1986.tb10401.x. [DOI] [PubMed] [Google Scholar]
- 36.Sonesson SE, Lundell BP, Herin P. Changes in intracranial arterial blood flow velocities during surgical ligation of the patent ductus arteriosus. Acta Paediatr Scand. 1986;75:36–42. doi: 10.1111/j.1651-2227.1986.tb10154.x. [DOI] [PubMed] [Google Scholar]
- 37.Lemmers PM, Molenschot MC, Evens J, Toet MC, van Bel F. Is cerebral oxygen supply compromised in preterm infants undergoing surgical closure for patent ductus arteriosus? Arch Dis Child Fetal Neonatal Ed. 2010;95:F429–34. doi: 10.1136/adc.2009.180117. [DOI] [PubMed] [Google Scholar]
- 38.Teixeira LS, Shivananda SP, Stephens D, Van Arsdell G, McNamara PJ. Postoperative cardiorespiratory instability following ligation of the preterm ductus arteriosus is related to early need for intervention. J Perinatol. 2008;28:803–10. doi: 10.1038/jp.2008.101. [DOI] [PubMed] [Google Scholar]
- 39.Evans N. Assessment and support of the preterm circulation. Early Hum Dev. 2006;82:803–10. doi: 10.1016/j.earlhumdev.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Levene MI, Shortland D, Gibson N, Evans DH. Carbon dioxide reactivity of the cerebral circulation in extremely premature infants: effects of postnatal age and indomethacin. Pediatr Res. 1988;24:175–9. doi: 10.1203/00006450-198808000-00007. [DOI] [PubMed] [Google Scholar]