Abstract
Temporal organization of nutrient and energy metabolism is important for maintaining homeostasis in mammals. Autophagy is a conserved cellular pathway that is activated in response to nutrient limitation, resulting in the degradation of cytoplasmic components and the release of amino acids and other nutrients. Recently, we reported that autophagy exhibits robust circadian rhythm in mouse liver, accompanied by cyclic induction of genes involved in various steps of autophagy. Rhythmic activation of physiological autophagy appears to be regulated by transcription factor C/EBPβ, which is sufficient and required for nutritional and circadian regulation of autophagy gene expression. These findings provide new insight into transcriptional control of autophagy and reveal a potentially important role of autophagy cycles in metabolic homeostasis.
To adapt to the light/dark cycles on the earth, organisms have evolved various strategies to coordinate their behavior and metabolism. In mammals, many physiological processes exhibit circadian rhythm, including blood pressure, hormonal secretion as well as nutrient and energy metabolism. In the 1970s, several electron microscopy studies by Pfeifer and colleagues demonstrated that the abundance of autophagic vacuoles varies according to the time of day in several rat tissues. However, whether physiological autophagy is rhythmic and how cyclic autophagy activation is orchestrated remained unknown.
To determine whether autophagy is rhythmically activated during the light/dark cycle, we examined molecular markers of autophagy and performed electron microscopy. Immunoblotting analyses of tissue lysates harvested at different time points indicate that protein levels of LC3-I/LC3-II and p62 are rhythmic in the liver, skeletal muscle, heart, and to a lesser extent in kidney. Whereas the relative abundance of LC3-I and LC3-II is a useful marker for autophagy under certain conditions, their steady-state levels do not provide an accurate assessment of autophagy flux. To further clarify whether autophagy activation is rhythmic, we performed autophagy flux measurements in mice injected with a single dose of saline or leupeptin, a lysosomal protease inhibitor. These studies clearly demonstrate that the rate of LC3-I to LC3-II conversion peaks at noon and decreases to lower levels in the dark phase. These findings are consistent with our electron microscopy data, in which we found that autophagosomes are most abundant in the afternoon, rapidly decrease at night, and their numbers rise again throughout the light phase. Together, these studies demonstrate that autophagy activity, as revealed by LC3-I to LC3-II flux and autophagosome formation, is highly rhythmic in the liver.
Transcriptional regulation of the autophagy gene program is emerging as an important mechanism that transduces physiological signals to autophagy. In fact, mRNA levels of genes whose products are involved in autophagosome formation (Ulk1, LC3B, Gabarapl1), mitophagy (Bnip3), and lysosomal degradation (Ctsl and Atp6v1d) are highly rhythmic in the liver. It is interesting to note that not all autophagy genes are regulated at the transcriptional level. To identify factors that control the program of autophagy gene expression, we examined a set of transcription factors and cofactors known to regulate the mammalian clock and/or hepatic starvation response. These functional analyses uncovered C/EBPβ as a potent activator of autophagy gene expression. C/EBPβ also stimulates the expression of a number of lysosomal genes, particularly subunits of the vacuolar-type H+-ATPase, which is responsible for lysosomal acidification. We further demonstrated that C/EBPβ is sufficient to stimulate autophagic protein degradation in cultured primary hepatocytes. C/EBPβ regulates its target genes through direct chromatin occupancy, as revealed by chromatin-immunoprecipitation assays. As such, C/EBPβ is a novel component of the transcriptional network that governs the autophagy gene program.
C/EBPβ itself is highly responsive to nutritional and circadian signals. Its expression is induced following starvation in the liver. Further, C/EBPβ mRNA and protein levels exhibit robust diurnal rhythm. Circadian regulation of C/EBPβ requires a functional tissue clock. Liver-specific deficiency of Bmal1, a critical component of the molecular clock, nearly abolishes the diurnal regulation of C/EBPβ. Remarkably, the rhythmic expression of autophagy genes, such as Ulk1, Bnip3, Gabarapl1 and ATP6v1d, is also significantly diminished. These results strongly suggest that cyclic expression of autophagy genes and autophagy rhythm is under the control of a biological clock in a tissue-autonomous manner. Direct evidence for C/EBPβ in nutritional and circadian regulation of autophagy comes from in vivo RNAi knockdown studies. Using recombinant adenoviral gene delivery into the liver, we found that liver-specific knockdown of C/EBPβ severely blocks the induction of autophagy in response to starvation and circadian signals.
Together, these studies demonstrated that physiological autophagy is rhythmically activated in the liver, a process that appears to be coordinated by transcription factor C/EBPβ (Fig. 1). While it is clear that a hepatic clock is required for rhythmic regulation of C/EBPβ and autophagy genes, how the clock oscillator regulates C/EBPβ is currently unknown. It is likely that C/EBPβ may serve as a link between the clock and downstream physiological output pathways. As pointed out above, while nutrient signaling pathways exert profound effects on autophagy, particularly under acute conditions, transcriptional regulation of the autophagy gene program might play an important role in physiological contexts. In addition to C/EBPβ, recent work has also implicated TFEB and FOXO3a as transcriptional regulators of autophagy genes. It remains unknown how these factors act in concert and crosstalk with the mTOR or AMPK signaling pathways to regulate autophagy activity.
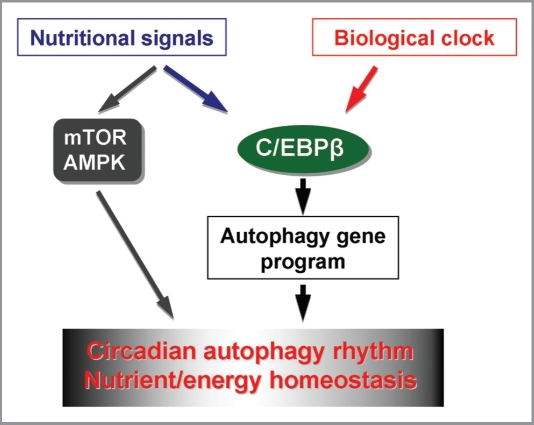
Figure 1.
Transcriptional regulation of circadian autophagy by C/EBPβ, C/EBPβ receives both circadian and nutritional input and coordinates the program of autophagy gene expression and autophagy flux. Rhythmic activation of autophagy may also be modulated by nutrient signaling pathways, such as those regulated by mTOR and AMPK, and contribute to nutrient and energy homeostasis in mammals through light/dark cycles.
Perhaps one of the most interesting unanswered questions is the physiological role of diurnal autophagy cycles in nutrient and energy homeostasis. Rhythmic induction of autophagy at a certain time of the day could serve to provide a steady supply of nutrients throughout the light/dark and feeding cycles. The bulk degradation of cellular components liberates amino acids and lipids for the biosynthesis of critical macromolecules under nutrient-limited periods. In addition, these nutrients could also enter systemic circulation and supply metabolites for organismal energy homeostasis, such as blood glucose control. Recent studies have implicated autophagy in the regulation of hepatic lipid metabolism, adipocyte function, and the pathogenesis of insulin resistance. Whether C/EBPβ participates in the autophagy of lipid droplets in the liver and adipocytes warrants further investigation in the future.
Punctum to: Ma D, Panda S, Lin JD. Temporal orchestration of circadian autophagy rhythm by C/EBPβ. EMBO J. 2011;30:4642–4651. doi: 10.1038/emboj.2011.322.