Abstract
The cellular recycling process of autophagy is emerging as a central player in many of the conserved longevity pathways in C. elegans, but the underlying mechanisms that link autophagy and life span remain unclear. In a recent study, we provided evidence to suggest that autophagy modulates aging through an effect on lipid homeostasis. Specifically, we identified a role for autophagy in a longevity model in which germline removal in C. elegans extends life span. Life-span extension in these animals is achieved, at least in part, through increased expression of the lipase LIPL-4. We found that autophagy and LIPL-4-dependent lipolysis are both upregulated in germline-less animals and work interdependently to prolong life span. While these genetic results lend further support to a growing link between autophagy and lipid metabolism, our findings are the first to suggest a possible molecular mechanism by which autophagy modulates organismal aging.
Keywords: autophagy, aging, lipolysis, lipophagy, TOR, PHA-4, DAF-16, LIPL-4, C. elegans
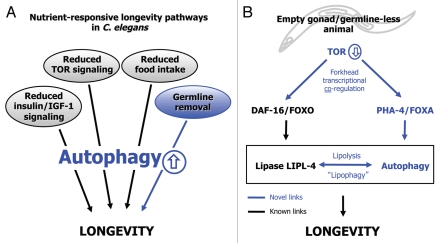
Over the past two decades, the nematode C. elegans has played a central role in identifying the molecular components of several nutrient-sensing signaling pathways that extend life span. The most well-studied example is the conserved insulin/IGF-1 signaling pathway, in which inactivation of the insulin/IGF-1 receptor DAF-2 acts through the FoxO transcription factor DAF-16 to regulate the expression of longevity genes and extend life span. More recent research has focused on understanding the cellular mechanisms by which such pathways modulate life span, and has highlighted a direct role for the cellular recycling process of autophagy in aging. Specifically, long-lived C. elegans mutants with perturbations in nutrientsensing pathways induce autophagy, and require autophagy to live long (Fig. 1A). However, it is not yet known how the autophagy process modulates aging, which presumably occurs through the beneficial turnover of as-yet-unidentified cargo.
Figure 1.
Role of autophagy in nutrient-sensitive longevity pathways in the nematode C. elegans. (A) Multiple conserved longevity pathways extend life span in C. elegans by upregulating autophagy. Highlighted in blue is the germline signaling pathway, in which removal of germline stem cells extends the life span of adult worms. Germline loss can be achieved by laser ablation of germline precursor cells in the worm larvae, or by genetic mutation. (B) Model of the mechanism by the germline signaling pathway extends life span in C. elegans by coordinately regulating LIPL-4-mediated lipolysis and autophagy. These processes are likely regulated by the common upstream regulator TOR through the forkhead transcription factors DAF-16/FoxO and PHA/FoxA. These two transcription factors regulate lipid metabolism and autophagy, respectively. This model is modified from Lapierre et al., Curr Biol 2011.
Removal of the germline represents another nutrient-responsive longevity paradigm, which, similar to the insulin/IGF-1 signaling pathway, relies on DAF-16/FoxO to extend life span in both worms and flies. Interestingly, lipid metabolism is critically required for the long life span of germline-less C. elegans. Germline removal induces the expression of a predicted lipase called LIPL-4 through the transcription factor DAF-16/FoxO, and lipl-4 is required for these animals to live long. Importantly, overexpression of LIPL-4 alone is sufficient to extend life span, a finding that provides one of the first direct links between lipid metabolism and longevity. Because recent reports have suggested that autophagy can hydrolyze lipids through a process termed lipophagy, we investigated whether autophagy might be important for the long life span observed in germline-less mutants, and if so, whether there was a role for lipid metabolism in that process. Indeed, demonstrating an autophagy-lipid homeostasis-longevity connection would not just have an impact upon the longevity field but may also likely have implications for human diseases like metabolic syndrome and diabetes.
We provided evidence for this model (Fig. 1B) in the following ways. We found that germline-deficient animals have increased numbers of autophagic events in their intestines as well as in the hypodermis, as assessed by electron microscopy. These two tissues were specifically examined because they are both sites of fat storage in the worm, and they are tissues in which the lipase LIPL-4 is expressed. Consistent with these observations, we detected increased levels of the autophagy marker LGG-1/LC3 in these same tissues. Interestingly, we discovered that induction of autophagy was likely transcriptionally regulated, because germline-less animals showed upregulated expression of several autophagy genes, including unc-51/ULK1, bec-1/BECN1, and lgg-1/LC3. Induction of these autophagy genes was regulated by the FoxA transcription factor PHA-4, and we next asked if autophagy genes and PHA-4/FoxA were required for the long life span of germline-less animals. We found this was indeed the case. The life span of germline-less, but not wild-type, animals was significantly shortened by feeding worms RNAi of several genes functioning at different steps in the autophagy pathway. From these results, we concluded that increased autophagy is required for germline-less animals to live long.
Having established a role for autophagy in germline-less animals, we next sought to determine if autophagy could be linked to lipid metabolism. Because the lipase LIPL-4 was required for the long life span of germline-less animals, similar to the autophagy genes, we asked if lipolysis and autophagy might be connected, and if so, whether such a link was important for lifespan extension. We used an in vitro lipase assay to show that autophagy genes are important for the increased lipase activity observed in germline-less animals. Conversely, we found that overexpression of LIPL-4 was sufficient to increase autophagic activity. To make the final link to longevity, we asked if long-lived animals overexpressing the lipase LIPL-4 required the activity of autophagy genes. In support of this idea, we found that multiple autophagy genes as well as PHA-4 were required for LIPL-4-overexpressing animals to live long, just like we observed in germline-less animals. Importantly, additional results suggested that the conserved autophagy regulator TOR (Target of Rapamycin) plays an important role in the link between LIPL-4 and autophagy, as TOR likely functions as a common upstream regulator of both LIPL-4 and autophagy in germline-less animals (Fig. 1B). Taken together, these results suggest that autophagy and lipolysis function interdependently to extend the life span of germline-less C. elegans, and further imply that partitioning of lipids through lipophagy is a possible mechanism by which autophagy modulates organismal aging.
As such, our new findings underscore the importance of autophagy for lifespan extension in C. elegans, and provide additional support for autophagy playing an influential role in lipid homeostasis. Our results also raise many interesting questions. It will be important to probe the link between autophagy and lipid metabolism in more detail; for example, by asking how autophagy and the lipase LIPL-4 might function interdependently to affect longevity, and in which tissues these changes may be important for life-span extension. Moreover, it will be critical to examine how autophagy affects lipid metabolism, and how deregulation of autophagy influences lipid profiles, including those of membrane lipids, in long-lived germline-less animals. Another priority will be to investigate this novel link in additional longevity mutants that show increased autophagy levels. These questions constitute important avenues for future research, and the results will no doubt shed light on the fascinating role of autophagy in the aging process.
Acknowledgments
We thank Drs. Hannes Bülow and Anne O'Rourke for comments on the manuscript. MH is supported by two R01 grants from the National Institute of Aging, and AM is supported by a National Science Foundation Research Initiation Award and a National Institute of Aging supplement award. MH and AM are both Ellison Medical Foundation New Scholars in Aging.
Punctum to: Lapierre LR, Gelino S, Melendez A, Hansen M. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr Biol. 2011;21:1507–1514. doi: 10.1016/j.cub.2011.07.042.