Abstract
The process in which ubiquitin (Ub) conjugation is required for trafficking of integral membrane proteins into multivesicular bodies (MVBs) and eventual degradation in the lumen of lysosomes/vacuoles is well-defined. However, Ub-independent pathways into MVBs are less understood. To better understand this process, we have further characterized the membrane protein Sna3, the prototypical Ub-independent cargo protein sorted through the MVB pathway in yeast. We show that Sna3 trafficking to the vacuole is critically dependent on Rsp5 ligase activity and ubiquitination. We find Sna3 undergoes Ub-dependent MVB sorting either by becoming ubiquitinated itself or associating with other ubiquitinated membrane protein substrates. In addition, our functional studies support a role for Sna3 as an adaptor protein that recruits Rsp5 to cargo such as the methionine transporter Mup1, resulting in efficient Mup1 delivery to the vacuole.
Keywords: Ubiquitin, Endosome, Multivesicular body, Lysosome, Vacuole
INTRODUCTION
The levels of cell surface membrane proteins are determined in large part by their trafficking to, and degradation in, lysosomes. Integral membrane proteins destined for degradation are modified by the attachment of ubiquitin (Ub), which serves as a signal for internalization and subsequent entry into intralumenal vesicles (ILVs) that accumulate within late endosomes/multivesicular bodies (MVBs). The actions of Ub ligases and deubiquitinating enzymes, that modify proteins by adding or removing Ub respectively, dictate the fate of proteins as they pass through the endosomal system (1, 2). Ubiquitinated cargo proteins that maintain a Ub signal are transported to the late endosome where the sequential action of the Endosomal Sorting Complexes Required for Transport (ESCRT) machinery incorporate ubiquitinated cargo proteins into MVB ILVs (3). Many of the ESCRT subunits contain Ub-binding domains (UBDs), thus forming a system designed to process and sort ubiquitinated cargo (4). MVBs eventually fuse with lysosomes (or in yeast, the vacuole), thereby delivering the ILV-encapsulated cargo for degradation in the lysosomal lumen.
The major ligase responsible for ubiquitination of integral membrane proteins at the plasma membrane in yeast is Rsp5 (5), which is homologous to mammalian Nedd4 (6). The tryptophan-tryptophan (WW) domains within Rsp5 and its homologs have been shown to directly interact with a PPXY (PY) motif (7, 8) found within some MVB cargo proteins (9, 10). However, many membrane proteins lack a PY motif and rely on interaction with adaptor proteins to recruit Rsp5 and facilitate their ubiquitination (11–14), creating a regulatory mechanism for Rsp5 specificity. Many of these Rsp5 adaptors are themselves ubiquitinated, and integral membrane Rsp5 adaptors, such as Bsd2, also become sorted into the MVB pathway (15).
One cargo protein of particular interest to trafficking studies is Sna3, a small membrane protein that is sorted efficiently into the MVB pathway and accumulates in the vacuole lumen (16). Many studies have proposed that Sna3 does not use a Ub-dependent sorting mechanism (16–19). Studies have also revealed that Sna3 contains a PY motif that mediates interaction with the WW domains of Rsp5; and mutational studies disrupting this interaction have shown it to be essential for Sna3 sorting to the vacuole (17–19). However, mutation of the cytosolic lysine residues within Sna3 does not perturb its vacuolar sorting, suggesting Sna3 follows an Ub-independent route to the vacuole (16). In addition, cells carrying particular mutant alleles of RSP5 that have attenuated Rsp5 Ub-ligase activity appear to sort Sna3 normally through the MVB pathway to the vacuole lumen (17–20). These data support a model whereby Sna3 accesses a Ub-independent MVB sorting route via physical association with Rsp5, which in turn associates with the ESCRT apparatus (17, 18, 20, 21). This model, however, has not been fully verified since other experiments indicate that Sna3 may require ubiquitination, at least to some extent, for efficient MVB sorting (18, 19).
Parallels between an Ub-independent MVB sorting mechanism for Sna3 and cargoes in other systems have been drawn, since mutation of the cytoplasmic lysine residues within several mammalian cargo proteins is not sufficient to fully disrupt their lysosomal entry. These include: the GPCR murine δ opioid receptor (DOR); the transferrin receptor, TfR2; and the CD4 receptor, (22–24). Where tested, each lysineless mutant cargo follows an ESCRT-dependent route into MVBs (22–25). However, the exact nature of an ESCRT-dependent MVB sorting route that does not use Ub as a sorting signal has not been clarified yet.
In this study we investigate the role ubiquitination plays in Sna3 trafficking and address unresolved questions regarding this process. We find ubiquitination does control the sorting of Sna3 into the MVB pathway. We find that Sna3 behaves as an Rsp5 adaptor protein that contributes to the downregulation of the methionine transporter Mup1. In addition, our results indicate that sorting of Sna3 into the MVB pathway relies on ubiquitination at some level-whether of Sna3 itself or of substrate proteins with which Sna3 associates.
RESULTS
Sna3 without lysine residues is sorted into the MVB pathway
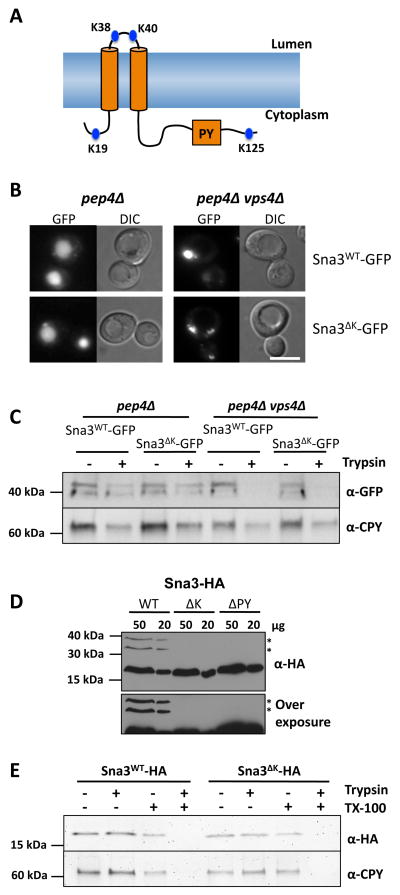
Sna3 containing a GFP tag readily localizes to the interior of the vacuole by following a sorting route into the intralumenal vesicles of MVBs (16). Sna3 has 4 lysine residues, 2 of which are predicted to be cytosolic (Figure 1A), and previous studies have shown that eliminating these lysine residues within Sna3 (in the context of a Sna3-GFP fusion) does not perturb localization to the vacuole lumen (16–18, 21). These data support the idea that Sna3 need not be ubiquitinated for entry into the MVB pathway. These observations are confirmed in Figure 1B, showing that both GFP tagged wild-type Sna3 and Sna3 lacking its cytosolic lysines (Sna3ΔK) were correctly sorted into the vacuolar lumen. In contrast, Sna3-GFP sorting into the vacuole lumen was blocked in vps4Δ cells. Vps4 is an AAA-ATPase that is essential for MVB sorting and regulates the assembly of ESCRTs onto endosomes. Loss of Vps4 causes an accumulation of MVB cargoes on the limiting membrane of enlarged endosomes (26). These results confirm earlier demonstrations that Sna3ΔK-GFP still follows an ESCRT-dependent route into the MVB pathway and vacuolar lumen (16, 20).
Figure 1. Sna3 lacking cytosolic lysine residues can traffic to the vacuole.
A) Schematic representation of Sna3 depicting its two predicted transmembrane domains and the relative location of its PY motif and lysine residues.
B) Localization of Sna3-GFP or Sna3ΔK-GFP in pep4Δ and pep4Δ vps4Δ cells Bar = 5 μm.
C) Post-nuclear supernatants were prepared from cells in A and treated in the absence of presence of 25 μg /ml trypsin for 15 min at 22°C before addition of protease inhibitors. Samples were then subjected to immunoblot analysis using antibodies against GFP and CPY.
D) Lysates from wild-type cells expressing versions of Sna3 (wild-type, ΔK and ΔPY) tagged with a C terminal 2 x HA epitope were prepared for iummunoblot analysis using anti-HA antibodies. High (50 μg) and low (20 μg) levels of lysates are depicted (upper panel). * indicates ubiquitinated species. An over-exposure better illustrating ubiquitinated bands is shown in lower panel.
E) Trypsin protection experiments with post-nuclear supernatants from pep4Δ cells expressing HA-tagged Sna3 and Sna3ΔK. Samples were treated with or without trypsin and in the presence or absence of Triton X-100.
We also used a protease-protection biochemical assay, to confirm the delivery of Sna3 into intralumenal vesicles (which are protected from proteases by the vacuolar limiting membrane; Figure 1C). These experiments were carried out with membranes from Sna3-GFP expressing cells lacking the vacuolar hydrolase Pep4, which would otherwise degrade the intralumenal vesicles within the vacuole (27). Cell membrane fractions were incubated with trypsin, denatured with 5% SDS, and subjected to western blotting with anti-GFP antibodies. This revealed that ~50% of Sna3-GFP was resistant to trypsin digestion. Immunoblotting for carboxypeptidase Y (CPY), a soluble intralumenal vacuolar hydrolase, gave a similar profile, with ~50% of the protein protected from trypsin. Thus, the “latent” population of Sna3-GFP supports the microscopy result demonstrating its localization to the vacuolar lumen. The sensitivity of a portion of Sna3-GFP to trypsin digestion is likely due to rupture of some of the vacuoles during the isolation of cell membranes since CPY, a well-established marker for the vacuolar lumen, had the same digestion profile. Importantly, all of the Sna3-GFP within membranes from vps4Δ mutant cells was sensitive to trypsin treatment, consistent with the accumulation of Sna3-GFP on the limiting membranes of endosomes and vacuoles.
The Sna3ΔK-GFP protein sorts normally to the vacuole lumen, however, the fused GFP retains surface-exposed lysines (15 in the GFP variant used in this study). Although eliminating the lysines within Sna3 dramatically reduces ubiquitination of Sna3-GFP, the GFP moiety has the capacity to become ubiquitinated and it is conceivable that this low level could be driving MVB sorting (19). To circumvent this problem, we fused two HA epitopes (YPYDVPDYA, which do not contain ubiquitinatable lysines) to the C-terminus of Sna3 and Sna3ΔK. Immunoblot analysis confirmed that the high molecular weight bands, corresponding to ubiquitinated forms of Sna3, were not readily formed by Sna3ΔK (Figure 1D). We next assessed the distribution of Sna3-HA and Sna3ΔK-HA by trypsin protection, and found that both were protected equally well (Figure 1E), indicating that lysine ubiquitination is not required for entry of Sna3 into the MVB pathway. As a further control we performed the protease treatment in the presence of detergent, thereby disrupting the lipid membranes and allowing the trypsin to access proteins in the vacuole. Addition of Triton X-100 had no effect on overall Sna3 levels but led to the complete digestion of both Sna3 and CPY when trypsin is subsequently added. These findings support the model that Sna3 does not require its lysines to undergo ubiquitination for MVB sorting (17, 18, 21). However, they conflict with previous observations that used immunofluorescence techniques to demonstrate that HA-tagged Sna3ΔK is not localized to the vacuolar lumen (19). One potential explanation for this discrepancy is that under the conditions used in the previous studies, the intravacuolar Sna3-HA may not have been accessible for indirect immunofluorescence detection; in yeast, this procedure typically requires harsh fixation and extraction methods (28).
Sna3 sorting through the MVB pathway is dependent on both Rsp5 activity and ubiquitination
While the above studies clearly indicate that ubiquitination of lysine residues within Sna3 are not required for correct MVB sorting, there are several caveats to concluding that Sna3 strictly uses a different signal than Ub for its sorting. For instance, proteins can undergo ubiquitination of cysteine, serine, or threonine residues under some conditions (29). In addition, proteins can be ubiquitinated on their N-terminus (30) and the Sna3-associated Ub-ligase Rsp5, in particular, has been shown to be capable of ubiquitinating the N-terminus of Sna3 at least in vitro (31).
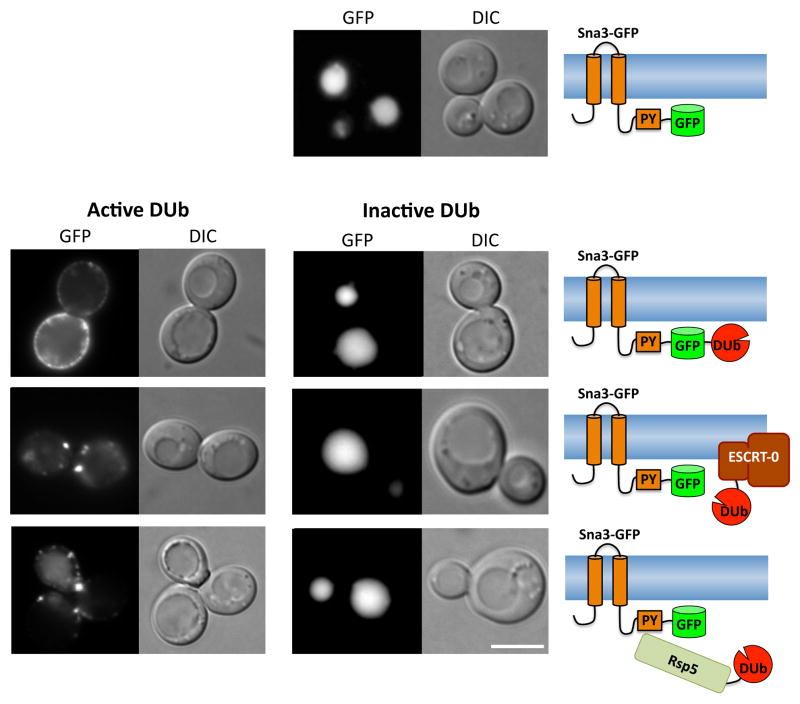
To test whether Sna3 sorting is completely independent of Ub, we used a recently described technique whereby the catalytic domain of a deubiquitinating enzyme can be covalently attached to a protein of interest to make it resistant to ubiquitination (32). In addition, when the DUb domain is attached to the ESCRT machinery, it can block the entry into the MVB of cargo that requires cellular ubiquitination but not cargo that is fused to Ub so that the C-terminal specific DUb peptidase domain cannot remove it. Finally, when fused to the Ub-ligase Rsp5, the DUb domain dominantly inhibits Rsp5-mediated ubiquitination and cargo trafficking by acting as an antagonistic Rsp5 substrate-specific DUb. Figure 2 shows that all of these DUb fusions blocked Sna3-GFP MVB sorting, suggesting that Sna3 does rely on Ub as a sorting determinant. Sna3-GFP fused to the catalytic domain of yeast Ubp7 (Sna3-GFP-Ubp7) failed to localize to the vacuole and was instead found largely at the plasma membrane. This effect was alleviated when the catalytic cysteine residue within the Ubp7 DUb domain was mutated to serine. Thus, the block imposed by the Ubp7 catalytic domain on Sna3-GFP sorting is a consequence of deubiquitination rather than of steric hindrance or the ability of the Ubp7 domain to interact with other yeast proteins.
Figure 2. The ubiquitination of Sna3 is required for sorting to the vacuole.
A) Localization of Sna3-GFP wild-type cells in log phase.
B) Localization in wild-type cells of Sna3-GFP fused to catalytic domain of Ubp7 (left) or the same domain rendered catalytically inactive (right).
C) Localization of Sna3-GFP in wild-type cells co-expressing Hse1 fused to the catalytic domain of the UL36 DUb (left) or the same domain rendered catalytically inactive (right).
D) Localization of Sna3-GFP in wild-type cells co-expressing Rsp5 fused to active (left) or inactive (right) catalytic domain of Ubp7. Expression of Rsp5-DUb and Hse1-DUb proteins was under the copper-inducible control for the CUP1 promoter. Cells were grown in 100 μM CuSo4 for 6 hrs prior to imaging. Schematic representations of how each of the deubiquitinating enzyme fusions are utilized in each experiment are depicted on the right.
MVB sorting of Sna3-GFP was also dramatically blocked in cells expressing Hse1-DUb indicating that Hse1-DUb is capable of removing Ub from the Sna3 cargo, effectively blocking its MVB sorting. The fact that Hse1 fused to catalytically inactive DUb had no effect on Sna3 sorting confirmed that the dominant negative effect of Hse1-DUb on Sna3 sorting is due to deubiquitinating activity, presumably directed towards Sna3 or a Sna3 complex of proteins. Finally, expressing a dominant-negative Rsp5-DUb also disrupted the sorting of Sna3-GFP. In contrast, an Rsp5-DUb wherein the DUb domain is catalytically inactive did not perturb Sna3-GFP sorting into the vacuole. Previous studies have shown that blocks in the sorting of ubiquitinated cargo imposed by DUb fusions with both ESCRT subunits or Rsp5 can be relieved using cargo translationally fused to ubiquitin; thus demonstrating that the MVB sorting pathway itself remains operational under these conditions (32). If Sna3 were to use an Ub-independent sorting signal, then these proteins should not have blocked its transport to the MVB pathway. Taken together, our data strongly suggest that Ub serves as a sorting signal for the entry of Sna3 into the MVB pathway and for its accumulation within the vacuole lumen.
The finding that Sna3 sorting is disrupted by a fusion of Rsp5 with a DUb, supports previous studies that demonstrate that the interaction between Rsp5 and Sna3 facilitates the ubiquitination of Sna3 (17–19, 21). However, it is unclear whether the catalytic activity of Rsp5 is required or whether the association with Rsp5 is sufficient for correct Sna3 sorting (17, 18, 21). Sna3 sorting has been monitored in the context of various rsp5 mutant alleles that have attenuated ligase activity due to point mutations in the HECT domain (Figure 3A). Most of these Rsp5 mutants had little or no effect on the MVB sorting of Sna3 (17, 18, 21). However, how well these mutations blocked Rsp5 activity is unclear, a critical problem for Sna3 since it directly binds Rsp5 and qualifies as a privileged substrate that could potentially be ubiquitinated at a low level by even a severely compromised Rsp5. Using the recently solved structure of Rsp5 (33), we found these mutations map to residues buried in the Rsp5 HECT domain (Fig. 3A) making it difficult to predict how they would diminish ligase activity, while preserving the structural integrity of Rsp5. Therefore, we used an Rsp5 mutant in which the catalytic cysteine that holds the thioeseter-linked Ub prior to ubiquitin conjugation is changed to alanine. Such a conservative change of this surface exposed residue renders HECT domains completely catalytically inactive, while still preserving their overall structure and in the case of Rsp5, does not alter the its level of expression (34). Figure 3B shows that the sorting of Sna3-GFP is disrupted in cells lacking Rsp5 and can be rescued by reintroduction of Rsp5, a finding consistent with previous results (17). However, catalytically inactive Rsp5 with the C777A mutation was unable to facilitate Sna3 sorting to the vacuole. These data suggest that the ligase function of Rsp5 is required for Sna3 to enter the MVB pathway.
Figure 3. Ligase activity of Rsp5 is essential for MVB sorting of Sna3.
A) Point mutations of Rsp5 previously used in studies on Sna3 trafficking were mapped on to the Rsp5 HECT domain crystal structure (PDB: 3OLM). The N-lobe is colored cyan and the C-lobe is magenta. Mutant residues that did not affect Sna3 sorting are labeled in blue (G555D; (17, 20, 21), G707D, G747E, G731I and P784T (18)) and L733, that blocks vacuolar entry of Sna3-GFP when mutated to serine (21), is labeled in green. The C777A mutant that renders Rsp5 inactive for ligase activity is labeled in red. Two orientations of the molecular surface are depicted on the right with the same labeling of residues.
B) Sna3-GFP localization in rsp5Δ cells and rsp5Δ cells expressing wild-type or catalytically inactive (C777A) Rsp5.
Sna3 has a functional role as an Rsp5 adaptor protein for Mup1
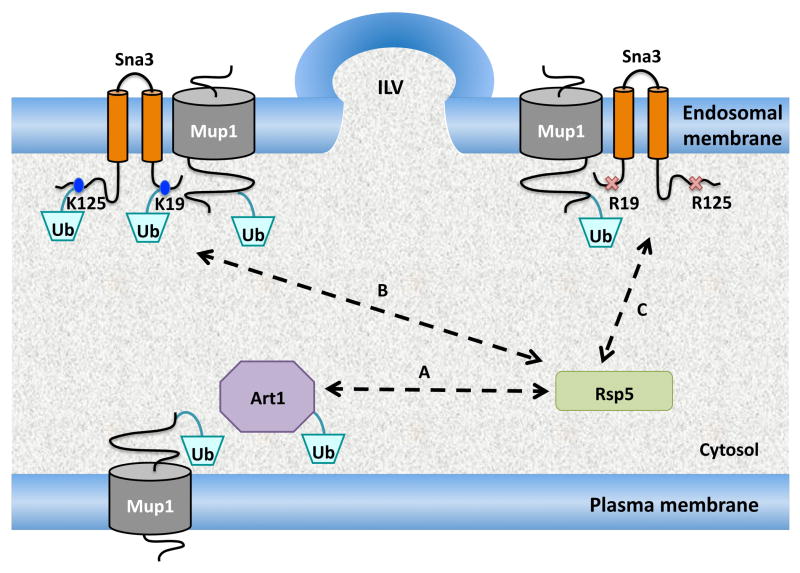
The data described above indicated that either Sna3 was ubiquitinated to produce a sorting signal for its entry into MVBs, or a Sna3 associated protein carried the Ub signal. Although ubiquitinated Sna3-HA was readily detected (Figure 1B), we could not detect ubiquitinated species when the lysine residues were altered to arginine (Sna3ΔK-HA). Although our experiments cannot exclude the possibility that Sna3 is ubiquitinated on lysines at a low level or from non-lysine ubiquitination, our data lend support to a model whereby an ubiquitinated Sna3-associated protein works as an MVB sorting signal. Such a model was proposed previously (35), although clear examples are lacking. This model is presented in Figure 4 and further rationalized by predicting that Sna3 may be acting as an adaptor protein for the Rsp5 ligase. Recently, a number of Rsp5 adaptor proteins have been identified; these proteins contain at least one PY motif that allows them to associate with Rsp5 and other protein interaction domains that allow them to associate with a variety of substrate proteins. For instance, Bsd2 is an integral membrane protein with PY motifs that allows it to act as a substrate adaptor for the metal transporter Smf1 (15). Likewise, cytosolic proteins such as Bul1 and the arrestin-related trafficking adaptors (ARTs) also work as PY motif-containing adaptors for Rsp5 (11, 36–38). Sna3 contains an Rsp5-binding PY motif that is required for its MVB sorting (17–19). Thus, Sna3 may act as an Rsp5 substrate adaptor perhaps like Bsd2, which also contains two transmembrane domains. Sna3 is very abundant in intraluminal vesicles (16) and is transcriptionally activated during late log phase (39). Given that this is when nutrient conditions are suboptimal, it suggests Sna3 may serve particular functions.
Figure 4. Model for Sna3 function and sorting.
A) Rsp5 is recruited to Mup1 in response to high levels of methionine through interaction with the Art1 adaptor protein. This association with Rsp5 results in the ubiquitination of Art1.
B) Mup1 is also internalized in response to nutrient stress, where Rsp5 can be recruited to Mup1 via Sna3, which can act as a complementary adaptor protein. There is partial redundancy between Art1 and Sna3, each of which contributes to MVB sorting. Sna3 can also be ubiquitinated by Rsp5.
C) A lysineless version of Sna3 (cytosolic lysine to arginine mutations), which can still associate with both Rsp5 and Mup1, fosters ubiquitination of Mup1 and uses Mup1~Ub to enter the MVB pathway.
To test the model that Sna3 functions as an Rsp5 adaptor, we sought to find potential membrane proteins that could potentially be targets for this Sna3 action. A global analysis of protein interactions using a DHFR biomolecular complementation assay, which is particularly suited for probing interactions between membrane proteins, demonstrated that Sna3 interacts with a variety of membrane proteins including the methionine transporter, Mup1 (40). One of the major Rsp5 adaptors for Mup1 is Art1/Ldb19, a protein that is required for methionine-induced ubiquitination and downregulation of Mup1 in cells grown in early/mid log phase (11). We hypothesized that Sna3 might also contribute to the downregulation and MVB sorting of Mup1.
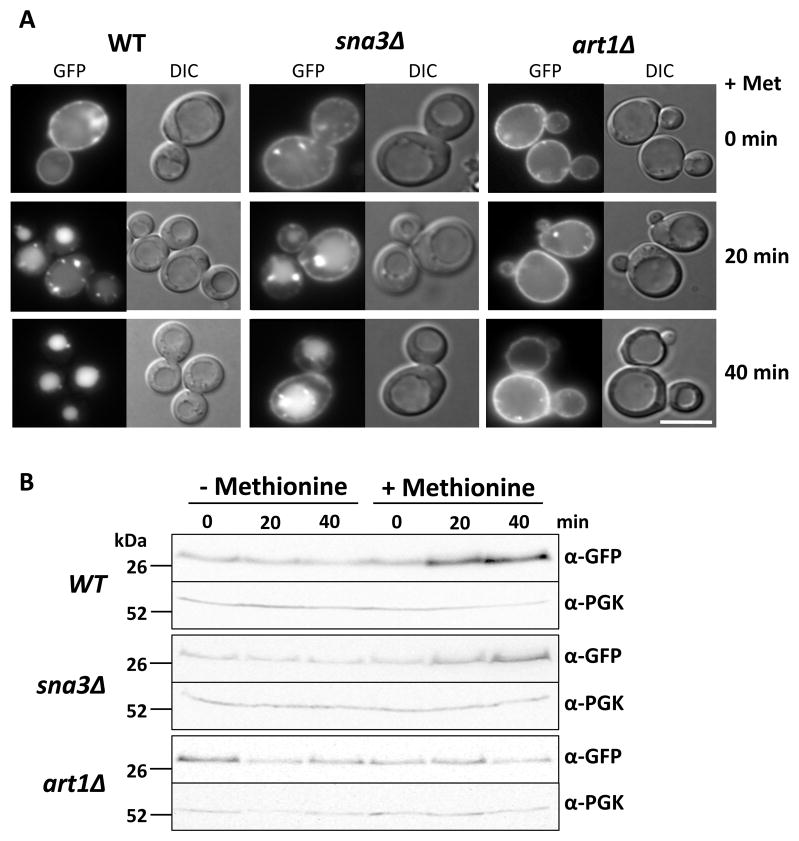
In cells grown to log-phase in the absence of methionine where Mup1-GFP is found at the plasma membrane (Figure 5) or in mup1Δ cells (Supplementary Figure S1), Sna3-GFP is localized to the vacuole showing that trafficking of each protein is not solely dependent on the other. To test whether Sna3 can assist with Mup1 sorting to the vacuole, we monitored the internalization of Mup1-GFP induced upon addition of methionine at different time points in wild-type, art1Δ, and sna3Δ cells. In wild-type cells, Mup1-GFP is largely found in the vacuole as early as 20 minutes after methionine addition. As previously demonstrated, however, in cells lacking Art1, Mup1 internalization is dramatically reduced (11), even 40 minutes after methionine addition (Figure 5A). Notably, the sna3Δ mutant cells also showed a defect in Mup1 trafficking, although not as severe as that in the art1Δ cells. Despite the fact that sna3Δ cells could traffic some Mup1-GFP to the vacuole after methionine was added, significant amounts of Mup1-GFP remained at the cell surface 40 minutes later.
Figure 5. Sna3 is an adaptor protein for the methionine transporter Mup1.
A) Wild-type, sna3Δ and art1Δ cells transformed with a plasmid expressing Mup1-GFP were grown to an optical density OD600 = 1.0 in minimal media. Mup1-GFP internalization was then induced by addition of 20 μg/ml methionine.
B) Cells were harvested at given time points after methionine addition, lysed and subjected to SDS-PAGE and immunoblot analysis using anti-GFP (upper panels) and anti-PGK (lower panels) antibodies.
When Mup1-GFP is delivered to the vacuole interior, the full-length protein is proteolyzed, generating a protease-resistant fragment of GFP. This processing of Mup1 provided an additional way to follow the kinetics of Mup1 downregulation on sna3Δ cells (Figure 5B). Full-length Mup1-GFP contains 13 membrane-spanning domains and even optimized protocols produced aggregated Mup1 (Supplementary Figure S2), which meant full-length Mup1-GFP was difficult to consistently analyze and quantify by SDS-PAGE. However, extraction and detection of the vacuole-processed soluble GFP (vpGFP) allowed a measure of how much Mup1-GFP was being targeted to the vacuole. In wild-type cells, methionine addition resulted in a dramatic increase in Mup1-GFP derived vpGFP, whereas, no increase was observed in art1Δ cells. The sna3Δ cells were also defective, producing vpGFP less rapidly than wild-type, an effect most obvious from comparison at the 20 minute time-point. These data demonstrate that Sna3 can contribute to the downregulation and MVB sorting of Mup1.
Delivery of plasma membrane proteins to the vacuole/lysosome can be induced by various mechanisms, such as: ligand binding to pheromone receptors (41); substrate interaction with transporters (11, 42); nutrient starvation (43) and toxic stress (44). In an effort to induce Mup1 downregulation through a different mechanism, we stressed cells by growing them just past a late log phase, at which time nutrients would be limiting. Microarray experiments have shown that these growth conditions also induce expression of SNA3 (39). In the absence of methionine, Mup1 was localized to the vacuole under these conditions in wild-type cells (Figure 6A). Interestingly, Mup1-GFP was also localized to the vacuole in art1Δ cells, demonstrating that Art1 is not required for all modes of Mup1 downregulation. In contrast, Mup1-GFP targeting to the vacuole was defective in cells lacking Sna3. The sorting defect in sna3Δ cells was corrected by reintroduction of plasmid expressed Sna3-HA or Sna3ΔK-HA without lysine residues. However, sorting was not restored by Sna3ΔPY-HA, lacking the ability of binding Rsp5 (Figure 6B). These findings support the hypothesis that Sna3 not only acts as an Rsp5 adaptor protein for Mup1, but also that its role is distinct from that of Art1.
Figure 6. Mup1 internalization is induced at high cell density.
Wild-type cells expressing Mup1-GFP were grown in minimal media and GFP localization was assessed at OD600 = 1.0 (upper panels) and then at OD600 = 2.0 (lower panels).
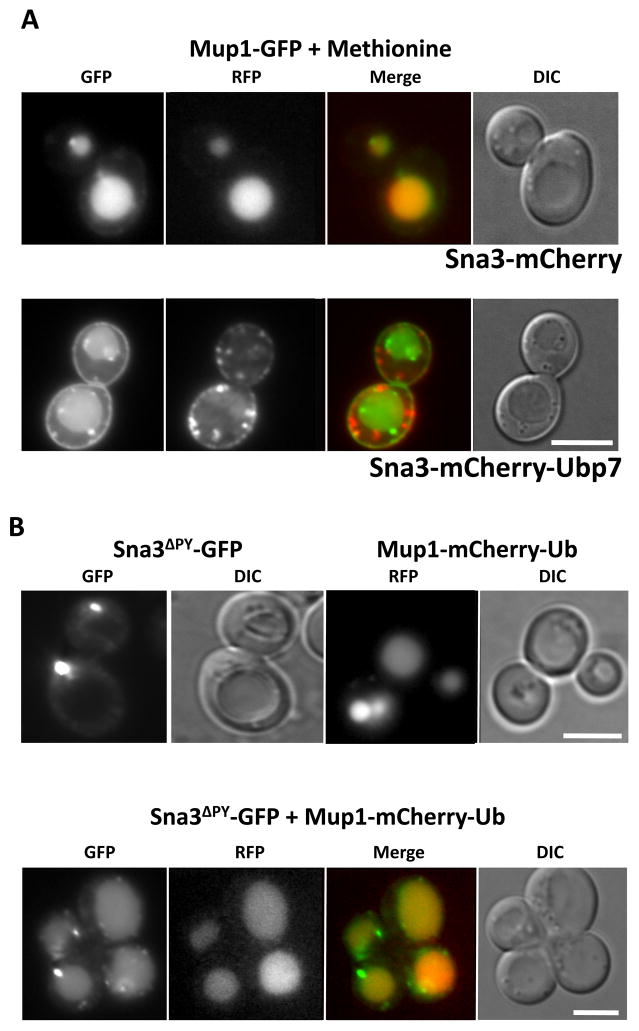
As an additional test, we exploited our observation that a Sna3-DUb fusion protein no longer localizes to the vacuole (Figure 2). We reasoned that if Sna3 is a bona fide Rsp5 Ub-ligase adaptor protein for Mup1, the expression of Sna3-DUb should disrupt the Ub-dependent sorting of target proteins such as Mup1 into the MVB pathway. We co-expressed either Sna3-mCherry or Sna3-mCherry-Ubp7 with Mup1-GFP, and induced downregulation of cell surface Mup1-GFP with a 40 min treatment of methionine. As expected, Sna3-mCherry and Mup1-GFP were found exclusively in the vacuolar interior. However, expressing Sna3-mCherry-Ubp7 dramatically reduced the amount of Mup1-GFP delivered into the vacuole (Figure 7A). Mup1 labeling at the plasma membrane after methionine addition was more pronounced in cells expressing Sna3-GFP-Ubp7 than in sna3Δ cells (Figure 5), presumably because the DUb fusion dominantly acts to deubiquitinate Mup1 regardless of whether Sna3 or another mechanism, such as Art1 recruitment of Rsp5, was responsible for its ubiquitination. We found that in addition to methionine inducing downregulation of Mup1, Sna3-mCherry-Ubp7 also blocked MVB sorting of Mup1-GFP in response to high cell density (Supplementary Figure S3). Collectively, these data support a role for Sna3 as an Rsp5 adaptor for Mup1.
Figure 7. The interdependence between Mup1 and Sna3 for MVB sorting.
A) Mup1-GFP (green) was co-expressed in wild-type cells with either Sna3-mCherry (red) of Sna3-mCherry fused to the catalytic domain of Ubp7 (Sna3-mCherry-Ubp7).
B) Localization in wild-type cells of Sna3-GFP mutant lacking its Rsp5-binding PY motif (Sna3ΔPY-GFP: upper left) or Mup1-mCherry-Ub (upper right). Lower panels show wild-type cells co-expressing Sna3ΔPY-GFP and Mup1-mCherry-Ub. Mup1-mCherry-Ub was induced under the CUP1 promoter by addition of 100 μM CuSO4 to the growth media.
Sna3 can be sorted into the MVB pathway by its substrate
According to our model (Figure 4), Sna3 associates with substrates such as Mup1 and uses its PY motif to bind Rsp5, which in turn ubiquitinates either Sna3 or Mup1 or both proteins. Previous studies have shown that loss of the PY motif within Sna3 (Sna3ΔPY) blocks the ubiquitination and MVB sorting of Sna3 and instead causes it to localize to puncta (2–3 larger puncta as well as numerous smaller puncta (17–19). We also found that Sna3ΔPY-GFP did not localize to the vacuole interior (Figure 7B) and that ubiquitination of Sna3ΔPY-HA is blocked (Figure 1D). Thus, failure of Sna3ΔPY to sort to the MVB is explained in our model (Figure 4) as a consequence of loss of Ub from either Sna3 or its substrate protein. This model predicts that restoring ubiquitination of the substrate will also restore MVB sorting of Sna3ΔPY. To test this, we expressed Mup1-mCherry-Ub under control of the CUP1 promoter, with the addition of copper allowing high levels of protein expression. In addition to the in-frame fusion of Ub, Mup1 internalization was further favored by adding methionine to the growth media. Mup1-mCherry-Ub was exclusively found inside the vacuole. Moreover, expression of Mup1-mCherry-Ub was sufficient to also induce MVB sorting of Sna3ΔPY-GFP (Figure 7B). These data support the idea of a “piggyback” mechanism for sorting of Sna3 and its possible substrates.
DISCUSSION
Previous studies provided evidence both for and against the hypothesis that Sna3 requires lysine ubiquitination for its MVB sorting (16–19, 21). To date, this discrepancy could be explained by constructs expressing Sna3 tagged with lysine-containing GFP that could act as acceptors for ubiquitination (17–19). By using a biochemical approach with versions of Sna3 tagged either with or without lysine residues, we have confirmed that the lysine residues within Sna3 are not essential for its correct sorting through the MVB pathway (Figure 1).
However, in contrast to previous studies that find ubiquitination superfluous for Sna3 MVB sorting, our data support the model that Ub does serve a critical role in Sna3 trafficking. This was most effectively shown by following Sna3 that was tagged with a DUb catalytic domain that confers the ability to deubiquitinate proteins to which it is attached (32). Additionally, expressing the ESCRT-0 subunit Hse1 or the ubiquitin ligase Rsp5 as a fusion with a DUb enzyme also disrupted Sna3 trafficking, implicating Ub as the sorting signal for ESCRT mediated sorting of Sna3 into the vacuole (Figure 2). Previous data in support of a Ub-independent sorting mechanism for Sna3 also included the observation that sorting was not perturbed in doa4Δ cells, which have reduced levels of Ub and ubiquitination (16). However, a more in-depth investigation using different strains and growing cells to different optical densities found that Sna3-GFP could not localize to the vacuole in some doa4Δ cells (19), supporting the view that ubiquitination was somehow important. Another indication that ubiquitination might play a role in this process was a kinetic delay observed in vacuolar entry of Sna3 lacking lysine residues compared to wild-type (18).
A Ub-independent MVB sorting mechanism was also supported by the observation that mutations that compromised Rsp5 activity did not block Sna3 sorting (17–20). However, Sna3 binds directly to Rsp5, likely giving Sna3 a privileged status as a substrate that might be effective even when Rsp5 activity is compromised. Thus, even the use of Rsp5 mutants with residual ligase activity might be enough to support Sna3 sorting. The only previously used rsp5 HECT domain mutant that did not support Sna3 sorting was L733A (21), which could either have less ligase activity or might instead have compromised structure. We find that direct inactivation of Rsp5 via mutation of its catalytic cysteine, while still preserving the overall structure of Rsp5, blocks Sna3 sorting and phenocopies the rsp5Δ phenotype. This result correlates with our observation that Rsp5 fused to a DUb causes missorting of Sna3 (Figure 2). Collectively, these findings are not consistent with the view that direct protein:protein interactions via Rsp5 are what drives an apparent Ub-independent sorting route into MVBs. In addition, these results agree with previous findings showing that proper MVB sorting of Sna3ΔK-GFP in cells with reduced Rsp5 activity is severely perturbed (18).
Our data (Figure 7) also agree with previous observations that mutation of the Rsp5-binding PY motif within Sna3 ablates vacuolar localization (17–19). Yet, we were able to force Sna3ΔPY-GFP into the lumen of the vacuole by over-expressing one of its proposed substrate partners, Mup1, fused to Ub. This finding demonstrates that association with ubiquitinated proteins is sufficient for Sna3ΔPY to be processed by the ESCRT machinery and incorporated into ILVs. The idea that Sna3 can use either its own lysine ubiquitination or piggyback with other ubiquitinated proteins for MVB sorting explains previous results showing Sna3 lacking its lysine residues fails to sort correctly in mutants with reduced Rsp5 activity, even though each of those mutant conditions alone does not block Sna3 sorting (18). Moreover, our data underscore the possibility that a wide variety of proteins may boost their MVB sorting efficiency by associating with other ubiquitinated proteins.
Similar Ub-independent routes into MVBs have been proposed in mammalian cells (22, 23, 35, 45, 46). Although it is important to remember that there may be other ESCRT-independent ILV pathways such as generation of exosomes, the relevant issue for our studies is whether sorting along an ESCRT-dependent lysosomal degradative pathway into MVB ILVs occurs without Ub as a sorting signal. Our data suggest that the basis of using Sna3 as an example of Ub-independent MVB sorting and applying that analogy to pathways in higher eukaryotes may be inappropriate. Likewise, the idea that an Ub-independent mechanism for Sna3 sorting fulfills a unique requirement for formation of ILVs themselves, as proposed (47), now seems unlikely.
Studies on Sna3 have thus far been focused on its ability to serve as an MVB cargo. Here we define a role for Sna3 as an Rsp5 adaptor for at least one substrate, the methionine transporter Mup1. This function overlaps with other Rsp5 adaptors such as Art1, which are required together for optimal methionine-induced delivery of Mup1 to the vacuole. A further test of this adaptor model might be to analyze the ubiquitination status of Mup1 in sna3Δ cells. However, in log phase cells, Sna3 only contributes to the overall efficiency of Mup1 trafficking, in contrast to the necessary role Art1 plays (11). Indeed we speculate that Sna3 may play its major role at later steps in the endocytic pathway after Art1 initiates Ub-dependent internalization from the plasma membrane. Regardless of exactly when Sna3 acts, it is likely to have a modest effect on the overall levels of ubiquitinated Mup1. Mup1 is known to be ubiquitinated by other proteins such as Art1, which could operate in a variety of compartments other than where Sna3 may act. Experimentally, we found it impractical to measure such subtle differences because Mup1 readily oligomerizes once solubilized (Supplemental Figure 2). Nonetheless, the idea that Sna3 is an Rsp5 adaptor is further supported by the block of Mup1 trafficking to the vacuole caused by expression of Sna3-fused to a DUb as well as the ability of “pre-ubiquitinated” Mup1 (Mup1-mCherry-Ub) to pull Sna3ΔPY into the MVB pathway (Figure 7).
Collectively, our work indicates that Sna3 has a function as an Rsp5 adaptor protein that facilitates the ubiquitination of Mup1 as well as perhaps a variety of other membrane proteins. Our data demonstrate that the quondam example of Sna3 conforms to the canonical mode whereby Ub serves as the sorting signal for ESCRT dependent MVB sorting. Moreover, it is the first to indicate that Sna3 works as an Rsp5 adaptor. Sna3 itself has been shown to be a very abundant protein within MVB ILVs suggesting that it may facilitate ubiquitination and sorting of a wide variety of proteins besides Mup1 and the few other membrane proteins identified in interaction screens. We speculate that the abundance of Sna3, its possible wide client range, and its status as a privileged substrate for ubiquitination by Rsp5 may help trap other ubiquitinated proteins into a membrane subdomain where they can be largely deubiquitinated by Doa4 (thus recycling some Ub from vacuolar degradation) while collectively remaining a ubiquitinated complex of proteins sufficient for completion of sorting into MVB ILVs.
Materials and Methods
Reagents
General chemicals and antibodies against GFP, CPY, PGK and the Hemagglutinin (HA) epitope sequence YPYDVPDYA were used as previously described (32). Yeast strains and plasmids used are listed in Tables 1 and 2 respectively.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| BY4742 | MATα his3Δ0 leu2Δ0 lys2Δ0 ura3Δ0 | (51) |
| BY4742 YOR322C | MATα art1Δ:: Kanr his3Δ0 leu2Δ0 lys2Δ0 ura3Δ0 | (52) |
| yPL3661 | MATα pep4Δ::LEU2 his3Δ0 lys2Δ0 ura3Δ0 | This study |
| BY4742 YJL151C | MATα sna3Δ:: Kanr his3Δ0 leu2Δ0 lys2Δ0 ura3Δ0 | (52) |
| yPL1781 | MATα vps4Δ:: NEO, pep4-Δ1137, ura3-52, his-Δ200, leu2-3,112, lys2-801, trp1-Δ901, suc2-Δ9 | This study |
| yPL4111 | MATα ura3-52, leu2-3,112, ade2, his3-11, trp1-1, can1-100 rsp5Δ::LEU2, MGA2(1-732) :: 13myc-HIS3MX | This study |
Table 2.
Plasmids used in this study
| Plasmid | Description | Reference |
|---|---|---|
| pRS313 | Low copy yeast shuttle plasmid containing HIS3 marker | (53) |
| pRS315 | Low copy yeast shuttle plasmid containing LEU2 marker | (53) |
| pRS316 | Low copy yeast shuttle plasmid containing URA3 marker | (53) |
| Figure 1 | ||
| pPL3667 | pRS316 expressing Sna3-GFP from SNA3 promoter | This study |
| pPL2279 | pRS316 expressing Sna3ΔK-GFP from SNA3 promoter. Sna3 (K19R K125R) | This study |
| pPL4618 | pRS316 expressing Sna3-2xHA from SNA3 promoter | This study |
| pPL4619 | pRS316 expressing Sna3ΔK-2xHA from SNA3 promoter. Sna3 (K19R K125R) | This study |
| Figure 2 | ||
| pPL2089 | pRS315 expressing Sna3-GFP from SNA3 promoter | This study |
| pPL3886 | pRS316 expressing Sna3-GFP-Ubp7 from SNA3 promoter. Ubp7 residues 561–1071 | This study |
| pPL3904 | pRS316 expressing Sna3-GFP-ubp7* from SNA3 promoter. pPL3904 with Upb7 C618S mutation | This study |
| pPL3874 | pRS313 expressing Hse1-Ubp7 from CUP1 promoter. Ubp7 residues 561–1071 | This study |
| pPL4141 | pRS315 expressing Hse1-ul36* from CUP1 promoter. Inactive UL36 catalytic domain | (32) |
| pPL3742 | pRS316 expressing Rsp5-Ubp7-3xHA from CUP1 promoter. Active Ubp7 catalytic domain | (32) |
| pPL4502 | pRS316 expressing Rsp5-ubp7*-3xHA from CUP1 promoter. Inactive Ubp7 catalytic domain | (32) |
| Figure 3 | ||
| pPL4708 | pRS315 expressing Sna3-GFP (pPL2089) from SNA3 promoter converted to ADE2 | This study |
| pPL4772 | pPL4708 expressing HA-Rsp5C777A from RSP5 promoter. | This study |
| pRS416-RSP5 | pRS416 expressing Rsp5 from RSP5 promoter. | (54) |
| Figure 5/6 | ||
| pCHL642 | pRS416 expressing Mup1-GFP from MUP1 promoter | (11) |
| pPL4621 | pRS316 expressing Sna3ΔPY-2xHA from SNA3 promoter. Sna3 P106PAY mutated to G106GAA | This study |
| Figure 7 | ||
| pPL4146 | pRS315 expressing Mup1-GFP from CUP1 promoter | (32) |
| pPL4064 | pRS316 expressing Sna3-mCherry from SNA3 promoter | This study |
| pPL4066 | pRS316 expressing Sna3-mCherry-Ubp7 from SNA3 promoter. Ubp7 residues 561–1071 | This study |
| pPL4566 | pRS316 expressing Sna3ΔPY-GFP from SNA3 promoter. P106PAY mutated to G106GAA | This study |
| pPL4568 | pRS316 expressing Mup1-mCherry-Ub from CUP1 promoter | This study |
Cell Culture
Yeast cultures were grown in standard minimal media supplemented with appropriate amino acids. Cells were generally grown overnight and then resuspended in fresh media and grown to mid log phase. If a specific cell density was required, optical density at 600 nm was monitored before cells harvesting (OD600 = 1.0, or 2.0) and further processing. Expression of proteins under the CUP1 promoter was induced by the addition of 100 μM CuSO4 to the media. Mup1 internalization was induced by the aseptic addition of 20 μg/ml methionine to the media. To obtain the rsp5Δ strain used in Figure 3 we circumvented the lethal requirement of Rsp5 by genetic modification of MGA2, which encodes one of the functionally redundant transcription factors required for the essential activation of the OLE1 gene (48). We integrated the myc-HIS3 sequence at basepair 2196 of MGA2 to produce a truncated version of Mga2 lacking its TMD, which can function without proteasome processing (49, 50). The plasmid encoding wild-type Rsp5 was then expelled from this strain using 5-FOA.
Fluorescence microscopy
Cells were grown to a mid-log phase in minimal media before harvesting and resuspension in 100 mM Tris.HCl (pH =8.0) buffer containing 0.2% (w/v) NaN3 and 0.2% (w/v) NaF. Cells expressing GFP or mCherry fluorescent fusion proteins were then spotted onto a glass plate and viewed with an epifluorescence microscope (BX60; Olympus) with a 100× objective lens with NA 1.4. Cells were viewed through either differential interference contrast (DIC) optics or appropriate fluorescence filters at room temperature and images were captured with a cooled charge-coupled device camera (Orca R2; Hamamatsu Photonics) using iVision-Mac software (Biovision Technology). Digital processing of images was performed in PhotoShop (version CS4; Adobe).
Trypsin protection Assay
Overnight cell cultures were used to inoculate 100 ml minimal media, which was grown over night to an OD600 = 1.0. Cells were then harvested and incubated in 100 mM Tris pH 9.0 buffer, containing 50 mM DTT for 5 min at room temperature. Cells were harvested again and resuspended in 1.2 M sorbitol, 50 mM KPO4 pH 7.5, 0.05% (w/v) NaN3, 0.05% NaFl, before addition of 100 μl zymolase and incubation at 30°C for 90 min. Spheroplasts were then layered onto a 5 ml solution of 1.2 M sucrose, 50 mM KPO4 pH 7.5, 0.05% (w/v) sodium azide, 0.05% sodium fluoride in a 15 ml conical tube. Samples were centrifuged at 1,000 g for 10 minutes, and supernatant discarded. Samples were then resuspended in 1 ml ice cold intracellular lysis buffer (ILB; 100 mM KAc, 50 mM KCl, 200 mM sorbitol, 20 mM PIPES pH 6.8) containing a protease inhibitor cocktail tablet (Roche) and stored on ice for 10 min. Sample was then centrifuged at 400 g on a tabletop centrifuge at 4°C for 10 min. The supernatant was then transferred to a fresh Beckman ultrafuge tube and subjected to high speed centrifugation at 40,000 rpm for 30 minutes. The pellet was resuspended in ILB lacking protease inhibitors and the relative protein concentration was determined using a BCA assay. Samples were normalized to equal protein concentrations and then separated into separated tubes and treated as indicated with ILB alone, 25 μg/ml trypsin, and Triton X-100 at a final concentration of 0.2% (v/v). Samples were incubated at room temperature for 30 min before an equal volume of 8M Urea, 5% SDS, 50mM Tris pH 6.8 containing protease inhibitors was added to each tube and the samples were resolved by SDS-PAGE. SDS-PAGE and immunoblot analysis was performed as described previously (32).
Supplementary Material
Supplementary Figure S1. GFP versions of Mup1 and Sna3 (including mutants: ΔK and ΔPY) were expressed in mup1Δ cells and grown to log-phase before imaging localization of tagged proteins.
Supplementary Figure S2. Wild-type cells were grown to mid log phase in minimal media before addition of 20 μg/ml methionine. Samples were extracted from cultures and at 0, 20 and 40 min after methionine addition, harvested and resuspended in freshly made SDS/Urea sample buffer. Samples were resolved by SDS-PAGE followed by a 1 hr incubation of the gel in transfer buffer containing 0.05% (w/v) SDS. Gels were then subjected to a 3 hr transfer in buffer containing SDS, before immunoblot analysis as described. Upper panel shows α-GFP immunoblot, where the insoluble proteins aggregates that were unable to migrate through the gel can be observed at the top. The membrane was then stripped and re-probed with α-PGK antibodies as a loading control (lower panel).
Supplementary Figure S3. Wild-type cells expressing Mup1-GFP were grown beyond log phase to an OD600 = 2.0 before imaging. Mup1-GFP containing cells were either co-expressing Sna3-mCherry (upper panels) or Sna3-mCherry-Ubp7 (lower panels). A merged image depicts the co-localization of these two fluorescently labeled proteins.
Acknowledgments
This work was supported by NIH R01GM58202 to RCP. We thank David Katzmann for helpful discussion.
References
- 1.d’Azzo A, Bongiovanni A, Nastasi T. E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic. 2005;6(6):429–441. doi: 10.1111/j.1600-0854.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- 2.Komander D, Clague MJ, Urbe S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10(8):550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 3.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458(7237):445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 4.Shields SB, Piper RC. How Ubiquitin Functions with ESCRTs. Traffic. doi: 10.1111/j.1600-0854.2011.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauwers E, Erpapazoglou Z, Haguenauer-Tsapis R, Andre B. The ubiquitin code of yeast permease trafficking. Trends Cell Biol. 2010;20(4):196–204. doi: 10.1016/j.tcb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009;10(6):398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 7.Chen HI, Einbond A, Kwak SJ, Linn H, Koepf E, Peterson S, Kelly JW, Sudol M. Characterization of the WW domain of human yes-associated protein and its polyproline-containing ligands. J Biol Chem. 1997;272(27):17070–17077. doi: 10.1074/jbc.272.27.17070. [DOI] [PubMed] [Google Scholar]
- 8.Sudol M, Chen HI, Bougeret C, Einbond A, Bork P. Characterization of a novel protein-binding module--the WW domain. FEBS Lett. 1995;369(1):67–71. doi: 10.1016/0014-5793(95)00550-s. [DOI] [PubMed] [Google Scholar]
- 9.Schild L, Lu Y, Gautschi I, Schneeberger E, Lifton RP, Rossier BC. Identification of a PY motif in the epithelial Na channel subunits as a target sequence for mutations causing channel activation found in Liddle syndrome. EMBO J. 1996;15(10):2381–2387. [PMC free article] [PubMed] [Google Scholar]
- 10.Staub O, Rotin D. Role of ubiquitylation in cellular membrane transport. Physiol Rev. 2006;86(2):669–707. doi: 10.1152/physrev.00020.2005. [DOI] [PubMed] [Google Scholar]
- 11.Lin CH, MacGurn JA, Chu T, Stefan CJ, Emr SD. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell. 2008;135(4):714–725. doi: 10.1016/j.cell.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Nikko E, Pelham HR. Arrestin-mediated endocytosis of yeast plasma membrane transporters. Traffic. 2009;10(12):1856–1867. doi: 10.1111/j.1600-0854.2009.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikko E, Sullivan JA, Pelham HR. Arrestin-like proteins mediate ubiquitination and endocytosis of the yeast metal transporter Smf1. EMBO Rep. 2008;9(12):1216–1221. doi: 10.1038/embor.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan JA, Lewis MJ, Nikko E, Pelham HR. Multiple interactions drive adaptor-mediated recruitment of the ubiquitin ligase rsp5 to membrane proteins in vivo and in vitro. Mol Biol Cell. 2007;18(7):2429–2440. doi: 10.1091/mbc.E07-01-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hettema EH, Valdez-Taubas J, Pelham HR. Bsd2 binds the ubiquitin ligase Rsp5 and mediates the ubiquitination of transmembrane proteins. EMBO J. 2004;23(6):1279–1288. doi: 10.1038/sj.emboj.7600137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reggiori F, Pelham HR. Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. EMBO J. 2001;20(18):5176–5186. doi: 10.1093/emboj/20.18.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNatt MW, McKittrick I, West M, Odorizzi G. Direct binding to Rsp5 mediates ubiquitin-independent sorting of Sna3 via the multivesicular body pathway. Mol Biol Cell. 2007;18(2):697–706. doi: 10.1091/mbc.E06-08-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oestreich AJ, Aboian M, Lee J, Azmi I, Payne J, Issaka R, Davies BA, Katzmann DJ. Characterization of multiple multivesicular body sorting determinants within Sna3: a role for the ubiquitin ligase Rsp5. Mol Biol Cell. 2007;18(2):707–720. doi: 10.1091/mbc.E06-08-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stawiecka-Mirota M, Pokrzywa W, Morvan J, Zoladek T, Haguenauer-Tsapis R, Urban-Grimal D, Morsomme P. Targeting of Sna3p to the endosomal pathway depends on its interaction with Rsp5p and multivesicular body sorting on its ubiquitylation. Traffic. 2007;8(9):1280–1296. doi: 10.1111/j.1600-0854.2007.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katzmann DJ, Sarkar S, Chu T, Audhya A, Emr SD. Multivesicular body sorting: ubiquitin ligase Rsp5 is required for the modification and sorting of carboxypeptidase S. Mol Biol Cell. 2004;15(2):468–480. doi: 10.1091/mbc.E03-07-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson H, Bonifacino JS. Direct binding to Rsp5p regulates ubiquitination-independent vacuolar transport of Sna3p. Mol Biol Cell. 2007;18(5):1781–1789. doi: 10.1091/mbc.E06-10-0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Wang J, Meyers KR, Enns CA. Transferrin-directed internalization and cycling of transferrin receptor 2. Traffic. 2009;10(10):1488–1501. doi: 10.1111/j.1600-0854.2009.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.daSilva LL, Sougrat R, Burgos PV, Janvier K, Mattera R, Bonifacino JS. Human immunodeficiency virus type 1 Nef protein targets CD4 to the multivesicular body pathway. J Virol. 2009;83(13):6578–6590. doi: 10.1128/JVI.00548-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanowitz M, Von Zastrow M. Ubiquitination-independent trafficking of G protein-coupled receptors to lysosomes. J Biol Chem. 2002;277(52):50219–50222. doi: 10.1074/jbc.C200536200. [DOI] [PubMed] [Google Scholar]
- 25.Hislop JN, Marley A, Von Zastrow M. Role of mammalian vacuolar protein-sorting proteins in endocytic trafficking of a non-ubiquitinated G protein-coupled receptor to lysosomes. J Biol Chem. 2004;279(21):22522–22531. doi: 10.1074/jbc.M311062200. [DOI] [PubMed] [Google Scholar]
- 26.Babst M, Wendland B, Estepa EJ, Emr SD. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 1998;17(11):2982–2993. doi: 10.1093/emboj/17.11.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baba M, Osumi M, Scott SV, Klionsky DJ, Ohsumi Y. Two distinct pathways for targeting proteins from the cytoplasm to the vacuole/lysosome. J Cell Biol. 1997;139(7):1687–1695. doi: 10.1083/jcb.139.7.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silver P. Indirect immunofluorescence labeling in the yeast Saccharomyces cerevisiae. Cold Spring Harb Protoc 2009. 2009;(11):pdb prot5317. doi: 10.1101/pdb.prot5317. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Herr RA, Hansen TH. Ubiquitination of Substrates by Esterification. Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciechanover A, Ben-Saadon R. N-terminal ubiquitination: more protein substrates join in. Trends Cell Biol. 2004;14(3):103–106. doi: 10.1016/j.tcb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Kim HC, Huibregtse JM. Polyubiquitination by HECT E3s and the determinants of chain type specificity. Mol Cell Biol. 2009;29(12):3307–3318. doi: 10.1128/MCB.00240-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stringer DK, Piper RC. A single ubiquitin is sufficient for cargo protein entry into MVBs in the absence of ESCRT ubiquitination. J Cell Biol. 2011;192(2):229–242. doi: 10.1083/jcb.201008121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HC, Steffen AM, Oldham ML, Chen J, Huibregtse JM. Structure and function of a HECT domain ubiquitin-binding site. EMBO Rep. 2011;12(4):334–341. doi: 10.1038/embor.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamadurai HB, Souphron J, Scott DC, Duda DM, Miller DJ, Stringer D, Piper RC, Schulman BA. Insights into ubiquitin transfer cascades from a structure of a UbcH5B approximately ubiquitin-HECT(NEDD4L) complex. Mol Cell. 2009;36(6):1095–1102. doi: 10.1016/j.molcel.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanyaloglu AC, von Zastrow M. A novel sorting sequence in the beta2-adrenergic receptor switches recycling from default to the Hrs-dependent mechanism. J Biol Chem. 2007;282(5):3095–3104. doi: 10.1074/jbc.M605398200. [DOI] [PubMed] [Google Scholar]
- 36.Helliwell SB, Losko S, Kaiser CA. Components of a ubiquitin ligase complex specify polyubiquitination and intracellular trafficking of the general amino acid permease. J Cell Biol. 2001;153(4):649–662. doi: 10.1083/jcb.153.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soetens O, De Craene JO, Andre B. Ubiquitin is required for sorting to the vacuole of the yeast general amino acid permease, Gap1. J Biol Chem. 2001;276(47):43949–43957. doi: 10.1074/jbc.M102945200. [DOI] [PubMed] [Google Scholar]
- 38.Yashiroda H, Kaida D, Toh-e A, Kikuchi Y. The PY-motif of Bul1 protein is essential for growth of Saccharomyces cerevisiae under various stress conditions. Gene. 1998;225(1–2):39–46. doi: 10.1016/s0378-1119(98)00535-6. [DOI] [PubMed] [Google Scholar]
- 39.Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11(12):4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarassov K, Messier V, Landry CR, Radinovic S, Serna Molina MM, Shames I, Malitskaya Y, Vogel J, Bussey H, Michnick SW. An in vivo map of the yeast protein interactome. Science. 2008;320(5882):1465–1470. doi: 10.1126/science.1153878. [DOI] [PubMed] [Google Scholar]
- 41.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 42.Paiva S, Vieira N, Nondier I, Haguenauer-Tsapis R, Casal M, Urban-Grimal D. Glucose-induced ubiquitylation and endocytosis of the yeast Jen1 transporter: role of lysine 63-linked ubiquitin chains. J Biol Chem. 2009;284(29):19228–19236. doi: 10.1074/jbc.M109.008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beck T, Schmidt A, Hall MN. Starvation induces vacuolar targeting and degradation of the tryptophan permease in yeast. J Cell Biol. 1999;146(6):1227–1238. doi: 10.1083/jcb.146.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galan JM, Haguenauer-Tsapis R. Ubiquitin lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J. 1997;16(19):5847–5854. doi: 10.1093/emboj/16.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henry AG, White IJ, Marsh M, von Zastrow M, Hislop JN. The role of ubiquitination in lysosomal trafficking of delta-opioid receptors. Traffic. 2010;12(2):170–184. doi: 10.1111/j.1600-0854.2010.01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamashita Y, Kojima K, Tsukahara T, Agawa H, Yamada K, Amano Y, Kurotori N, Tanaka N, Sugamura K, Takeshita T. Ubiquitin-independent binding of Hrs mediates endosomal sorting of the interleukin-2 receptor beta-chain. J Cell Sci. 2008;121(Pt 10):1727–1738. doi: 10.1242/jcs.024455. [DOI] [PubMed] [Google Scholar]
- 47.Nickerson DP, Russell MR, Odorizzi G. A concentric circle model of multivesicular body cargo sorting. EMBO Rep. 2007;8(7):644–650. doi: 10.1038/sj.embor.7401004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang F, Chen Y, Kweon DH, Kim CS, Shin YK. The four-helix bundle of the neuronal target membrane SNARE complex is neither disordered in the middle nor uncoiled at the C-terminal region. J Biol Chem. 2002;277(27):24294–24298. doi: 10.1074/jbc.M201200200. [DOI] [PubMed] [Google Scholar]
- 49.Chellappa R, Kandasamy P, Oh CS, Jiang Y, Vemula M, Martin CE. The membrane proteins, Spt23p and Mga2p, play distinct roles in the activation of Saccharomyces cerevisiae OLE1 gene expression. Fatty acid-mediated regulation of Mga2p activity is independent of its proteolytic processing into a soluble transcription activator. J Biol Chem. 2001;276(47):43548–43556. doi: 10.1074/jbc.M107845200. [DOI] [PubMed] [Google Scholar]
- 50.Hoppe T, Matuschewski K, Rape M, Schlenker S, Ulrich HD, Jentsch S. Activation of a membrane-bound transcription factor by regulated ubiquitin/proteasome-dependent processing. Cell. 2000;102(5):577–586. doi: 10.1016/s0092-8674(00)00080-5. [DOI] [PubMed] [Google Scholar]
- 51.Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14(2):115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 52.Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, Chu AM, Connelly C, Davis K, Dietrich F, Dow SW, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285(5429):901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 53.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang G, Yang J, Huibregtse JM. Functional domains of the Rsp5 ubiquitin-protein ligase. Mol Cell Biol. 1999;19(1):342–352. doi: 10.1128/mcb.19.1.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. GFP versions of Mup1 and Sna3 (including mutants: ΔK and ΔPY) were expressed in mup1Δ cells and grown to log-phase before imaging localization of tagged proteins.
Supplementary Figure S2. Wild-type cells were grown to mid log phase in minimal media before addition of 20 μg/ml methionine. Samples were extracted from cultures and at 0, 20 and 40 min after methionine addition, harvested and resuspended in freshly made SDS/Urea sample buffer. Samples were resolved by SDS-PAGE followed by a 1 hr incubation of the gel in transfer buffer containing 0.05% (w/v) SDS. Gels were then subjected to a 3 hr transfer in buffer containing SDS, before immunoblot analysis as described. Upper panel shows α-GFP immunoblot, where the insoluble proteins aggregates that were unable to migrate through the gel can be observed at the top. The membrane was then stripped and re-probed with α-PGK antibodies as a loading control (lower panel).
Supplementary Figure S3. Wild-type cells expressing Mup1-GFP were grown beyond log phase to an OD600 = 2.0 before imaging. Mup1-GFP containing cells were either co-expressing Sna3-mCherry (upper panels) or Sna3-mCherry-Ubp7 (lower panels). A merged image depicts the co-localization of these two fluorescently labeled proteins.