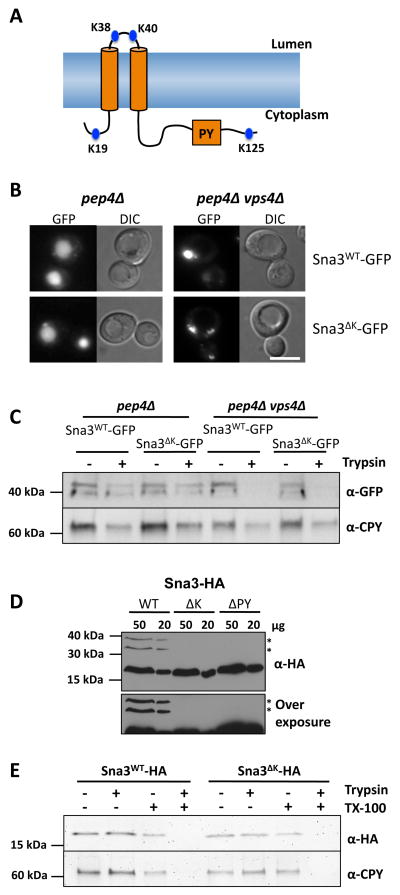
Figure 1. Sna3 lacking cytosolic lysine residues can traffic to the vacuole.
A) Schematic representation of Sna3 depicting its two predicted transmembrane domains and the relative location of its PY motif and lysine residues.
B) Localization of Sna3-GFP or Sna3ΔK-GFP in pep4Δ and pep4Δ vps4Δ cells Bar = 5 μm.
C) Post-nuclear supernatants were prepared from cells in A and treated in the absence of presence of 25 μg /ml trypsin for 15 min at 22°C before addition of protease inhibitors. Samples were then subjected to immunoblot analysis using antibodies against GFP and CPY.
D) Lysates from wild-type cells expressing versions of Sna3 (wild-type, ΔK and ΔPY) tagged with a C terminal 2 x HA epitope were prepared for iummunoblot analysis using anti-HA antibodies. High (50 μg) and low (20 μg) levels of lysates are depicted (upper panel). * indicates ubiquitinated species. An over-exposure better illustrating ubiquitinated bands is shown in lower panel.
E) Trypsin protection experiments with post-nuclear supernatants from pep4Δ cells expressing HA-tagged Sna3 and Sna3ΔK. Samples were treated with or without trypsin and in the presence or absence of Triton X-100.