Summary
Background
Pseudoxanthoma elasticum (PXE) manifests with cutaneous lesions consisting of yellowish papules coalescing into plaques of inelastic skin. Histopathology demonstrates accumulation of pleiomorphic elastic structures with progressive mineralization. The classic form of PXE is caused by mutations in the ABCC6 gene.
Objectives
A 2-year old patient with PXE of the neck, inguinal folds and lower abdomen, and with extensive tissue mineralization was evaluated for the underlying mutations in candidate genes known to be involved in ectopic mineralization disorders.
Methods
The patient’s genotype was studied by sequencing ABCC6, MGP and ENPP1 genes, encoding proteins which harbor mutations in ectopic mineralization disorders.
Results
No pathogenetic mutations were found in the ABCC6 or MGP genes. Sequencing of ENPP1 disclosed a homozygous missense mutation, p.Y513C, associated with generalized arterial calcification of infancy.
Conclusions
This study demonstrates the presence of the cutaneous features of PXE in a genetically distinct disease, generalized arterial calcification of infancy, and thus expands the spectrum of PXE-related disorders.
Keywords: Pseudoxanthoma elasticum, differential diagnosis, ectopic mineralization disorders, generalized arterial calcification of infancy, heritable skin diseases
Introduction
Pseudoxanthoma elasticum (PXE), a Mendelian autosomal recessive disorder, is characterized by ectopic mineralization of extracellular matrix of connective tissue, clinically affecting the skin, the eyes, and the cardiovascular system.1, 2 The diagnosis of PXE can be challenging to the clinicians due to considerable intra- and interfamilial heterogeneity and late onset of the manifestations. In fact, the diagnosis is often made in teens or later in life. The classic form of PXE is caused by mutations in the ABCC6 gene encoding a transmembrane transporter expressed primarily in the liver and the kidneys. Thus, PXE is considered to be a metabolic disorder with ectopic mineralization of the peripheral tissues.2
The characteristic cutaneous findings in PXE are yellowish papules which develop on the predilection sites, i.e., sides of the neck, antecubital fossae and periumbilical areas. The primary cutaneous lesions coalesce into large cobblestone plaques with considerable cosmetic impact. Histopathology of the skin lesions demonstrates accumulation of fragmented, disordered, and calcified elastic fibers in the mid and deep reticular dermis. Although pseudoxanthoma elasticum in childhood is rare, it can occur. In a retrospective case series of 96 patients with PXE, 15 patients had pediatric onset (before age 15).3 Cutaneous findings resembling those seen in PXE have also been encountered in a number of unrelated conditions (Table 1).4-10 Among them are unrelated heritable disorders, including β-thalassemia and sickle cell anemia, as well as patients with select mutations in the GGCX gene associated with vitamin K-dependent coagulation factor deficiency. The cutaneous features of PXE can also be encountered in patients with metabolic calcification disorders, as a sequela of long-term penicillamine treatment, in patients with eosinophilia-myalgia syndrome, and as a result of occupational exposure to calcium nitrate-containing fertilizers (see Table 1).
Table 1.
Differential diagnosis of PXE-like cutaneous findings
| Disease/Condition | Clinical and genetic associations | References |
|---|---|---|
| Pseudoxanthoma elasticum | Classic findings of yellowish papules and inelastic skin, together with ocular and vascular involvement, due to mutations in the ABCC6 genea) |
2, 12 |
| Vitamin K-dependent coagulation factor deficiency |
Loose and sagging skin with yellowish papules, with bleeding disorder, due to select mutations in the GGCX geneb) |
5-7 |
| Hemoglobinopathies | Cutaneous and ocular findings consistent with PXE in ~25% of patients with β-thalassemia and sickle cell anemiac) |
4, 9, 21 |
| Metabolic calcification disorders | Cutaneous lesions similar to those in PXE in patients with idiopathic hypercalcemia, hyperphosphatemia, tumoral calcinosis and calciphylaxisd) |
1, 22 |
| Drug-induced | Long-term sequela of D-penicillamine treatment, and in a limited number of cases with eosinophilia-myalgia syndrome due to contaminated L-tryptophan ingestione) |
8, 23 |
| Environmental exposure | Percutaneous absorption of salpeter (calcium nitrate) from fertilizersf) | 10 |
| THIS STUDY | ||
| Generalized arterial calcification of infancy |
PXE-like skin findings associated with extensive mineralization of vascular connective tissues due to a homozygous mutation in the ENPP1 gene |
|
>300 mutant alleles of the ABCC6 gene have been disclosed.
In one family, individuals compound heterozygous for one mutation in GGCX and one mutation in ABCC6 were noted, indicating digenic inheritance of PXE.6
The absence of ABCC6 mutations has been documented in some cases.
PXE-like cutaneous findings have been reported in a limited number of patients with each disorder.
Cutaneous findings clinically reminiscent of PXE in association with elastosis perforans serpiginosa have been described in patients undergoing long-term treatment of Wilson’s disease or cystinuria with D-penicillamine; these lesions show elastic fiber abnormalities but no mineralization, and have been dubbed as pseudo-pseudoxanthoma elasticum.
Findings clinically and histologically similar to those in PXE were described in the exposed areas of skin in farmers without systemic manifestations.
Recently, a number of genes have been shown to harbor mutations which result in extensive connective tissue mineralization, particularly in the arterial blood vessels, including ENPP1, NT5E and MGP, which encode powerful anti-mineralization factors, ecto-nucleotide pyrophosphatase/phosphodiesterase 1, CD73, and matrix gla-protein, respectively.11 In this report we describe a two-year old female with extensive soft tissue calcification, including intra-atrial and coronary artery mineral deposits, and with cutaneous findings consistent with PXE.
Materials and methods
The patient was examined at the Dermatology Clinic at Children’s Hospital of Wisconsin, where skin biopsies and blood samples were obtained with appropriate consent forms. For mutation analysis, DNA was isolated from peripheral blood leukocytes with standard procedures. PCR primers were utilized to amplify segments of the ABCC6, ENPP1, and MGP genes.12, 13 The PCR products were sequenced by using an automated sequencer (Applied Biosystems 3730, Foster City, CA). Allelic nucleotide sequence variant frequencies were extracted from the exome variant server and dbSNP (short genetic variations; NCBI) databases (evs.gs.washington.edu/EVS/ and www.ncbi.nlm.nih.gov/projects/SNP/, respectively).
Results
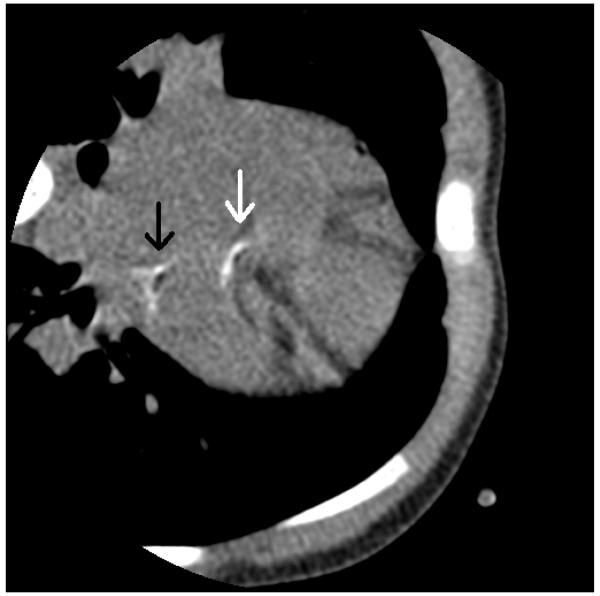
The proband was a two-year old female with developmental delay of unknown etiology. She had extensive past medical history of cardiomyopathy, renovascular hypertension, and hearing loss. The patient presented early in life with labored breathing and was found to be in heart failure. Cardiomyopathy of unknown etiology was diagnosed. Imaging studies revealed calcifications in various areas of her body. Radiographs of the patient’s arms revealed calcifications around the right wrist, and vascular calcifications of both forearms. During work up for hearing loss, CT scan of the temporal bones revealed soft tissue calcification of the cartilaginous components of both earlobes, as well as soft tissues along the lateral margins of the lateral pterygoid muscles. A cardiac CT angiogram with multiplanar and 3-D reformats was performed at the age of 2 years and three months, which revealed calcification of the right atrium, left circumflex artery, and left main coronary artery (Fig. 1). MRI of the brain without contrast was normal. MRA of the brain showed a slightly tortuous basilar artery, and MRA of the neck showed abnormal vertebral arteries, which bifurcate and give rise to many serpentine appearing vessels, with subsequent constitution of codominant, normal caliber vertebral arteries. Echocardiogram showed intra-atrial calcifications. The patient had a normal fundoscopic examination, and specifically, no angioid streaks or peau d’orange features were identified. Laboratory tests, including serum calcium, magnesium, international normalized ratio, potassium and phosphate were within normal limits. Chromosomal microarray did not reveal abnormalities. The patient lived with a foster family and the biologic parents were not available for interview or testing. However, they were known to be related, suggesting the possibility of autosomal recessive mode of inheritance with homozygosity for the disease-causing variant in the proband.
Figure 1.
CT angiogram of the heart prior to contrast. There is abnormal calcification on the left circumflex artery (white arrow) and left main coronary artery (black arrow). There is normal calcification in the sternum and ribs. Photo courtesy of Dr. Sara Arnold at Children’s Hospital of Wisconsin.
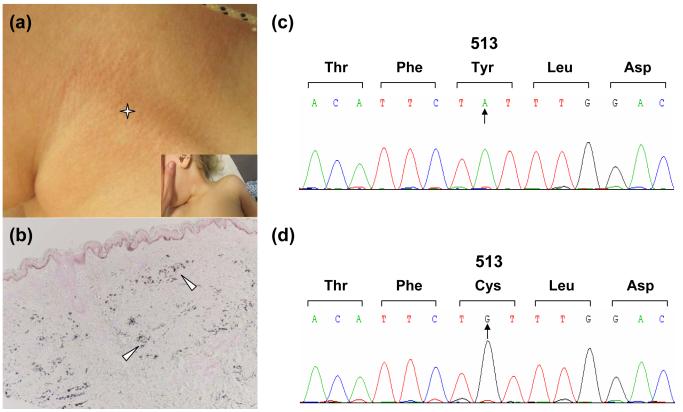
Although the patient presented with abnormal soft tissue calcification and heart failure in infancy, her cardiomyopathy and hypertension consistently improved throughout the first months of life, and therefore the diagnosis of generalized arterial calcification of infancy (GACI) was not initially considered. At 2 years of age, the patient presented to dermatology clinic for evaluation of skin changes on the neck. On physical examination, the patient was an active, healthy appearing female with dysmorphic facial features including low placed, hyperteloric eyes, long protuberant forehead and an unstable gait. The skin examination was notable for pink-yellowish papules coalescing into thin cobblestone plaques distributed circumferentially on the neck and inguinal folds, suggesting the diagnosis of PXE (Fig. 2a). Biopsy was taken from involved skin on the neck. Clumped, irregular elastic fibers were present in the reticular dermis. This abnormality was highlighted with pentachrome stain. Von Kossa stain showed calcifications in the zones of abnormal elastin. (Fig. 2b). Similar skin lesions subsequently appeared on the abdominal area. Based on these observations, the clinical diagnosis of PXE was made, and mutation analysis on the ABCC6 gene was initiated. The initial screening of the coding regions of ABCC6 and the flanking intronic sequences did not reveal pathogenetic mutations. It should be noted that the mutation detection strategy, based on amplification of exons and flanking intronic sequences, does not detect putative mutations in the regulatory elements, in intronic sequences away from the exon-intron borders, or large deletions in the candidate genes.12
Figure 2.
PXE-like cutaneous findings and mutation analysis. The 2-year old patient demonstrates characteristic skin lesions on the side of the neck (a, asterisk), which histopathologically by von Kossa staining reveal ectopic mineralization in the dermis (b, arrowheads). Mutation analysis of the ENPP1 gene reveals a codon for tyrosine in position 513 in control DNA (c), which is replaced in the patient’s DNA by a codon for cysteine (d), confirming the diagnosis of generalized arterial calcification of infancy.
Because the patient had extensive calcifications and earlier than usual onset for PXE, as well as developmental delay, other diagnoses were considered, including the Keutel syndrome caused by mutations in the MGP gene and generalized arterial calcification of infancy (GACI) due to mutations in the ENPP1 gene. First, sequencing of the MGP gene revealed the presence only of a single heterozygous polymorphism (c.379A→A/G; p.T127A). This nonsynonymous polymorphism (rs4236) has a G allelic frequency of 40.1% in the European American population.14 Next, sequencing of the ENPP1 gene revealed two sequence variants. One of them, c.517A→C; p.K173Q, is a frequent polymorphism (rs1044498) in the European American population, with the C allelic frequency of 15.5%.15 In contrast, the second homozygous sequence variant (Fig. 2d), c.1538A→G; p.Y513C, is a pathogenetic mutation that has been previously reported in an Italian patient with GACI, in trans with a p.Y659C mutation.16 Thus, the amino acid tyrosine at position 513 is replaced by cysteine in both alleles (Fig. 2c). These sequence data support the notion that our patient has GACI, a severe disorder of arterial calcification, in this case associated with skin manifestations consistent with PXE.
Discussion
Our study demonstrates cutaneous findings of PXE in a patient with mild GACI. A somewhat similar case has been previously reported17 with clinical features of GACI and PXE due to homozygous D538H mutation in ENPP1. This diagnosis expands the differential diagnosis of PXE, and highlights the clinical relationship of these two conditions. These findings also raise the question of the diagnosis of neonatal PXE made in previous reports on the basis of cutaneous findings and histopathology only. LeBoulanger et al presented a case of an infant who died after his second myocardial infarction at age 15 months.18 Autopsy demonstrated calcifications of the endocardium, with extensive calcifications of the coronary arteries as well as medium sized arteries and the aorta. Interestingly, the patient’s 28 year old brother developed PXE, and testing led to the discovery of two missense mutations in the ABCC6 gene. This case suggested a correlation between PXE and GACI.18 GACI manifests with extensive mineralization which in many cases develops prenatally and often results in early demise of the newborn, within a few hours or days.19, 20 The majority of affected patients do not survive beyond the first six months of life, although prolonged survival has been recognized in some cases even up to 21 years of age. Although our proband does not have the extensive vascular calcification described in many cases in the literature, she likely has a milder form of GACI which is not typically reported in the literature. This may represent an ascertainment bias, as only the more severe cases come to medical attention and receive diagnostic testing. GACI is caused by mutations in the ENPP1 gene which encodes an ecto-nucleotide pyrophosphatase/phosphodiesterase 1, a type II transmembrane glycoprotein that forms disulfide bonded homodimers.11 This protein catalyzes the hydrolysis of ATP to AMP and pyrophosphate, the latter being a powerful inhibitor of hydroxyapatite crystal growth. In the absence of functional ENPP1 gene product, pyrophosphate levels are reduced allowing mineralization to take place in peripheral tissues.
ENPP1 gene mutations have also been shown to result in hypophosphatemic rickets with hyperphosphaturia, a finding associated with improved survival of GACI patients beyond infancy.16 It should be noted that the serum phosphate levels in our patient were within normal limits, and therefore, in the absence of clinical findings for rickets, additional testing, such as the ratio of phosphorus tubular maximum phosphate reabsorption to glomerular filtration rate (TmP/GFR) has not been undertaken. Also, treatment with bisphosphonates, inorganic pyrophosphate analogs which block conversion of calcium phosphate to hydroxyapatite, have been suggested to reduce vascular calcification in GACI with improved survival.16 Thus, it may be worth considering bisphosphonate treatment to prevent calcification of the blood vessels in these patients after side effects and patient’s morbidity has been carefully considered. However, given the mild presentation and lack of progression in our patient, bisphosphonate treatment was not initiated at this time, particularly since there is no human data to guide therapy in patients with GACI.
In summary, this study demonstrates the presence of cutaneous features of PXE in a patient with GACI due to a homozygous mutation in the ENPP1 gene. The reasons for associated clinical findings, such as developmental delay, deafness, dysmorphy and unstable gait, remain unspecified. This finding extends the differential diagnosis of cutaneous PXE to include other ectopic calcification disorders and highlights the potential relation of PXE and GACI. Collectively, these findings also attest to the existence of an intricate mineralization/anti-mineralization network, and mutations in different genes can lead to similar pathophysiologic consequences manifesting with ectopic mineralization of connective tissue, including the skin.
What’s already known about this topic?
PXE manifests with characteristic cutaneous findings with histopathologically demonstrable mineralization of pleiomorphic elastic structures in the skin.
The classic form of PXE is caused by mutations in the ABCC6 gene.
Cutaneous findings of PXE have been encountered in unrelated both heritable and acquired conditions.
What does this study add?
A 2-year old patient with developmental delay and extensive mineralization demonstrated PXE of the neck .
Sequence analysis revealed a homozygous pathogenetic mutation, p.Y513C, in the ENPP1 gene consistent with the diagnosis of generalized arterial calcification of infancy.
This finding extends the differential diagnosis of PXE cutaneous manifestations.
Acknowledgements
We thank the family for participation. Carol Kelly assisted in preparation of this manuscript. These studies were supported by NIH/NIAMS grants R01 AR28450 and R01 AR55225 (JU). Dr. Li is the recipient of a Research Career Development Award from Dermatology Foundation.
Funding sources: NIH/NIAMS grants R01 AR28450 and R01 AR55225 (JU). Research Career Development Award from Dermatology Foundation (QL).
Footnotes
Conflicts of interest: None declared
References
- 1.Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol. 1988;6:1–159. doi: 10.1016/0738-081x(88)90003-x. [DOI] [PubMed] [Google Scholar]
- 2.Uitto J, Li Q, Jiang Q. Pseudoxanthoma elasticum: molecular genetics and putative pathomechanisms. J Invest Dermatol. 2010;130:661–70. doi: 10.1038/jid.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naouri M, Boisseau C, Bonicel P, et al. Manifestations of pseudoxanthoma elasticum in childhood. Br J Dermatol. 2009;161:635–9. doi: 10.1111/j.1365-2133.2009.09298.x. [DOI] [PubMed] [Google Scholar]
- 4.Fabbri E, Forni GL, Guerrini G, Borgna-Pignatti C. Pseudoxanthoma-elasticum-like syndrome and thalassemia: an update. Dermatol Online J. 2009;15:7. [PubMed] [Google Scholar]
- 5.Vanakker OM, Martin L, Gheduzzi D, et al. Pseudoxanthoma elasticum-like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity. J Invest Dermatol. 2007;127:581–7. doi: 10.1038/sj.jid.5700610. [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Grange DK, Armstrong NL, et al. Mutations in the GGCX and ABCC6 genes in a family with pseudoxanthoma elasticum-like phenotypes. J Invest Dermatol. 2009;129:553–63. doi: 10.1038/jid.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Schurgers LJ, Smith AC, et al. Co-existent pseudoxanthoma elasticum and vitamin K-dependent coagulation factor deficiency: compound heterozygosity for mutations in the GGCX gene. Am J Pathol. 2009;174:534–40. doi: 10.2353/ajpath.2009.080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bécuwe C, Dalle S, Ronger-Savlé S, et al. Elastosis perforans serpiginosa associated with pseudo-pseudoxanthoma elasticum during treatment of Wilson’s disease with penicillamine. Dermatology. 2005;210:60–3. doi: 10.1159/000081487. [DOI] [PubMed] [Google Scholar]
- 9.Baccarani-Contri M, Bacchelli B, Boraldi F, et al. Characterization of pseudoxanthoma elasticum-like lesions in the skin of patients with β-thalassemia. J Am Acad Dermatol. 2001;44:33–9. doi: 10.1067/mjd.2001.110045. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen AO, Christensen OB, Hentzer B, et al. Salpeter-induced dermal changes electron-microscopically indistinguishable from pseudoxanthoma elasticum. Acta Derm Venereol. 1978;58:323–7. [PubMed] [Google Scholar]
- 11.Rutsch F, Nitschke Y, Terkeltaub R. Genetics in arterial calcification. Pieces of a puzzle and cogs in a wheel. Circ Res. 2011;109:578–92. doi: 10.1161/CIRCRESAHA.111.247965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfendner EG, Vanakker OM, Terry SF, et al. Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J Med Genet. 2007;44:621–8. doi: 10.1136/jmg.2007.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito T, Shimizu Y, Hori M, et al. A patient with hypophosphatemic rickets and ossification of posterior longitudinal ligament caused by a novel homozygous mutation in ENPP1 gene. Bone. 2011;49:913–6. doi: 10.1016/j.bone.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Misra D, Booth SL, Crosier MD, et al. Matrix Gla protein polymorphism, but not concentrations, is associated with radiographic hand osteoarthritis. J Rheumatol. 2011;38:1960–5. doi: 10.3899/jrheum.100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stolerman ES, Manning AK, McAteer JB, et al. Haplotype structure of the ENPP1 gene and nominal association of the K121W missense single nucleotide polymorphism with glycemic traits in the Framingham heart study. Diabetes. 2008;57:1971–7. doi: 10.2337/db08-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutsch F, Böyer P, Nitschke Y, et al. Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet. 2008;1:133–40. doi: 10.1161/CIRCGENETICS.108.797704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baujat G, Hadj-Rabia S, Le Merrer M, et al. The D538H mutation in ENPP1 is associated with generalized arterial calcification of infancy and pseudoxanthoma elasticum. The American Society of Human Genetics Meeting; Washington, DC. November 2-6, 2010; Abstract 849/F. [Google Scholar]
- 18.Le Boulanger G, Labreze C, Croue A, et al. An unusual severe vascular case of pseudoxanthoma elasticum presenting as generalized arterial calcification of infancy. Am J Med Genet Part A. 152A:118–123. doi: 10.1002/ajmg.a.33162. [DOI] [PubMed] [Google Scholar]
- 19.Ruf N, Uhlenberg B, Terkeltaub R, et al. The mutational spectrum of ENPP1 as arising after the analysis of 23 unrelated patients with generalized arterial calcification of infancy (GACI) Hum Mutat. 2005;25:98. doi: 10.1002/humu.9297. [DOI] [PubMed] [Google Scholar]
- 20.Dlamini N, Splitt M, Durkan A, et al. Generalized arterial calcification of infancy: phenotypic spectrum among three siblings including one case without obvious arterial calcifications. Am J Med Genet Part A. 2009;149A:456–60. doi: 10.1002/ajmg.a.32646. [DOI] [PubMed] [Google Scholar]
- 21.Hamlin N, Beck K, Bacchelli B, et al. Acquired pseudoxanthoma elasticum-like syndrome in beta-thalassaemia patients. Br J Haematol. 2003;122:852–4. doi: 10.1046/j.1365-2141.2003.04484.x. [DOI] [PubMed] [Google Scholar]
- 22.Sprecher E. Familial tumoral calcinosis: from characterization of a rare phenotype to the pathogenesis of ectopic calcification. J Invest Dermatol. 2010;130:652–60. doi: 10.1038/jid.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mainetti C, Schmied E, Masouyé I, et al. L-tryptophan-induced eosinophilia-myalgia syndrome. I. Report of two cases with pseudoxanthoma elasticum-like skin changes. Dermatologica. 1991;183:57–61. [PubMed] [Google Scholar]