Abstract
Opiate-abusing individuals are in the top three risk-factor groups for HIV infection. In fact, almost 30% of HIV-infected individuals in the USA are reported to abuse opiates, highlighting the intersection of drugs of abuse with HIV/AIDS. Opiate-abusers are cognitively impaired and suffer from neurological dysfunctions that may lead to high-risk sexual behavior, poor adherence to antiretroviral regimens, and hepatitis-C virus infection. Collectively, these factors may contribute to accelerated HIV CNS disease progression. To understand the role of morphine in disease progression, we sought to determine whether morphine influences HIV-induced inflammation or viral replication in human monocyte-derived macrophages (h-mdms) and MAGI cells infected with HIV and exposed to morphine. Chronic morphine exposure of HIV-infected h-mdms led to significant alterations in secretion of IL-6 and MCP-2. Morphine enhanced IL-6 secretion and blunted MCP-2 secretion from HIV-infected h-mdms. However, exposure of HIV-infected h-mdms to morphine had no effect on TNF-α secretion. Morphine had no effect on later-stages of viral replication in HIV-infected h-mdms. Morphine had a potentially additive effect on the HIV-induced production of IL-6 and delayed HIV-induced MCP-2 production. These results suggest that in HIV-infected opiate abusers enhanced CNS inflammation might result even when HIV disease is controlled.
Keywords: CNS, HIV, Interleukin-6, Monocyte chemoattractant protein-2, Opiates
INTRODUCTION
Substance abuse and human immunodeficiency virus (HIV) infections are interlinked public health concerns of global significance. Opiate-abuse has been ranked as one of the top three risk factors for acquiring HIV infection (Grigoryan, 2010). In fact, approximately 30% of all HIV-infected individuals in the USA have a history of opiate-abuse. Moreover, in North America and Western Europe, 25–50% of all HIV-infected individuals are opiate-abusers. The most disconcerting intersection of the substance abuse and HIV epidemic has unraveled in Eastern Europe and Russia where a huge subset of the opiate-abusing population has been infected by HIV (Cohen, 2010). The incidence of HIV infections is not declining, and continues to increase in the USA. Emerging statistics indicate psychiatric and infection co-morbidities in opiate-abusers. These individuals may endure severe psychological stress, neurological-dysfunction, depression and are at high risk for hepatitis-C virus infection (Turrina, et al., 2001). Lastly, opiate-abuse is associated with high-risk sexual behavior. Taken together, these related factors contribute to onset of acquired immune deficiency syndrome (AIDS)-defining illnesses and failure of antiretroviral treatments, and may thus accelerate HIV disease.
The tremendous complexities of HIV infection of the CNS and its intersection with opiate-abuse have hindered progress towards suppressing HIV replication in the CNS. Despite significant advances in antiretroviral therapy efficacy, HIV replication in the central nervous system (CNS) cannot be inhibited efficiently. As a result, this latent viral reservoir continues to present an obstacle to the goal of finding a cure for HIV/AIDS (Richman, et al., 2009). Reactivation of latent virus can manifest in a broad range of HIV-associated neurocognitive disorders that include HIV-associated dementia (HAD), HIV encephalitis (HIVE), or minor cognitive motor disorder (MCMD) (Bell, et al., 2006). Viral proteins, pro-inflammatory cytokines and chemokines secreted by HIV-infected microglia and perivascular macrophages induce inflammation and oxidative damage (Hu, et al., 2002, Albright, et al., 2003, Hu, et al., 2005, Kramer-Hammerle, et al., 2005) thereby contributing to neurocognitive decline. In addition, communication between glia and neurons may be affected by the CXCR4/CCR5 signaling pathways that govern fundamental processes in the CNS and may thus affect disease-progression (Pitcher, et al., 2010, Abt & Meucci, 2011)
The important role of the inflammatory response in HIV neurodegeneration is well characterized (Gonzalez-Scarano & Baltuch, 1999, Langford & Masliah, 2001, Maes, et al., 2011). However, the mechanisms through which opiates intersect with the inflammatory response have not been as extensively studied. Several studies have addressed the involvement of morphine in conjunction with HIV proteins, such as Tat and gp120, in the activation of murine macrophages (El-Hage, et al., 2006, Bruce-Keller, et al., 2008, Bokhari, et al., 2009). In addition, a genome-wide association study from our laboratory provided insights towards key moieties through which morphine exerts its biochemical effects on human monocyte-derived macrophages (h-mdms) (Dave & Khalili, 2010). In this study, we focused our attention on the effects of morphine on the HIV-induced inflammatory response from h-mdms and on viral replication.
MATERIALS AND METHODS
Morphine exposure and HIV infection of h-mdms
h-mdms were generated by differentiation of PBMCs obtained from healthy, HIV seronegative donors as previously described with several modifications (Dave & Pomerantz, 2004). Dynabeads® CD8 (Invitrogen, Carlsbad, CA) were utilized to deplete CD8+ T cells from PBMCs according to manufacturer’s instructions. For studies with acute morphine exposure, CD8-depleted PBMCs (5 × 106 cells in 10cm petri dishes) were allowed to adhere and differentiate into h-mdms for 10 days prior to morphine (Sigma-Aldrich, St. Louis, MO) exposure and/or HIV infection. For studies with chronic morphine exposure, CD8-depleted PBMCs (1 × 105 in each well of 6–well plate) were utilized. CD8-depleted PBMCs were differentiated in DMEM containing 10% heat-inactivated FBS, 10% heat-inactivated horse-serum, 2mM L-glutamine, 50 U/mL of penicillin G, 50μg streptomycin/mL, and 0.5ng/mL granulocyte-macrophage colony stimulating factor and 0.5ng/mL macrophage colony stimulating factor. Fresh media was added at 3-day intervals. At the end of differentiation, adherent h-mdms were washed three times with media to remove the remainder of non-adherent PBMCs. PBMCs obtained from three different donors were utilized in this study to generate separate sets of h-mdms.
HIV viral stock preparation & infectivity assay
The plasmids pYU-2 and pNL4-3 encoding HIV clones YU-2 and NL4-3 were obtained from the National Institute of Health AIDS Research and Reference Reagents Program (Catalog # 1350; Contributors: Dr. B. Hahn & Dr. G. M. Shaw & Catalog # 114; Contributor: Dr. M. Martin). To prepare HIV YU-2 or NL4-3 viral stocks, 293T cells (ATCC, Manassas, VA) were subjected to lipofectamine-mediated transfection of plasmid pYU-2 or pNL4-3, according to manufacturer’s instructions (Invitrogen, Carlsbad, CA). Briefly, plasmid-lipofectamine complexes were produced in Optimem-I reduced serum medium (Invitrogen). These complexes were added to 293T cells in Optimem-I (70 to 90% confluent) on poly-D-lysine-coated plates (BD Biosciences, Bedford, MA) and incubated for 5h. Subsequently, culture medium was replaced with fresh Optimem-I heat-inactivated serum supplemented medium and transfected cells were incubated for 48h at 37°C, 5% CO2. Cell-free supernatant was harvested and centrifuged at 10,000xg for 10min and filtered through a 0.22 μM pore-size polyvinylidene difluoride membrane. Viral titer was determined by quantitation of the HIV-1 p24 core antigen with an enzyme-linked immunosorbent assay (ELISA) kit (Advanced Bioscience Laboratory, Kensington, MD) (Dave & Pomerantz, 2004).
HIV JR-FL was obtained from the National Institute of Health AIDS Research and Reference Reagents Program (Catalog #: 395; Contributor: Dr. I. S. Y. Chen)]. To prepare HIV JR-FL viral stocks, phytohemagglutinin (PHA)-stimulated CD8-depleted PBMCs were utilized, as previously described (Levy & Shimabukuro, 1985). Briefly, CD8-depleted PBMCs were infected with JR-FL and the cell-culture was expanded at weekly intervals by addition of CD8-depleted PBMCs in complete media. Viral production in cell-free culture supernatants was monitored by HIV p24 ELISA (Advanced Bioscience Laboratory). At approximately 4–5 weeks after initial infection, the virus was harvested by centrifugation and the media was filtered as previously described to isolate virus in cell-free supernatant, and viral titers were determined by HIV p24 ELISA (Advanced Bioscience Laboratory). h-mdms were infected with HIV isolates, YU-2 or JR-FL (multiplicity of infection (MOI) = 0.1pg p24/cell) for 2h. After 2h, cells were washed extensively with PBS to remove input virus. h-mdms were subjected to morphine exposure either in an acute or chronic mode. For acute morphine exposure, h-mdms were treated with 0.1 μM morphine (Sigma-Aldrich) for 24h. Subsequently, the culture medium was replenished with h-mdm differentiation medium. For chronic morphine exposure, 0.1μM morphine (Sigma-Aldrich) was added to h-mdm cultures at 0, 3, 7, 10, 14 and 17 days from the start of the treatment.
The effect of morphine on HIV replication was determined by the multinuclear activation in a galactosidase indicator (MAGI) assay with a HelaCD4-LTR-β-Gal cell line as previously described (Kimpton & Emerman, 1992). The HIV isolate NL4-3 was utilized in this assay.
ELISA
Cell-free culture supernatants obtained from h-mdms subjected to acute or chronic morphine exposure and/or HIV infection were utilized to quantitate secretion of chemokines, cytokines and HIV progeny virions. HIV progeny virion production was monitored by quantitation of p24 core antigen according to manufacturer’s instructions (Advanced Bioscience Laboratory).
A human monocyte chemoattractant protein 2 (MCP-2) DuoSet® ELISA Development kit was utilized to quantitate MCP-2 secretion from h-mdms exposed to morphine and/or infected by HIV infection (R & D Systems, Minneapolis, MN) with the following modifications. Briefly, black, high-binding, flat bottom, polystyrene microplates (360 μL/well) (R & D Systems) were coated with the murine anti-human MCP-2 capture antibody. The ELISA plate was incubated overnight at room temperature (RT) and washed 4x with PBS containing 0.05% Tween-20 as per manufacturer’s suggestions. Subsequently, the ELISA plate was blocked with PBS containing 1% BSA for 1h and washed. To each well of the ELISA plate 100 μL of sample, recombinant human MCP-2 standard (R & D Systems) or positive control was added and incubated for 2h at RT. After washing, 100 μL of biotinylated goat anti-human MCP-2 was added to each well and incubated for 2h at RT. To remove unbound secondary antibody, the plate was washed and 100 μL of streptavidin horseradish peroxidase was added and incubated for 20min. The unbound enzyme was washed away and 100 μL of Glo® reagent mix containing 1 part of Glo® reagent A (stabilized enhanced luminol) and 2 parts of Glo® reagent B (stabilized H2O2) was added and protected from the light. The plate was read in a luminometer after a 5–20min incubation.
Interleukin 6 (IL-6) secretion from h-mdms in response to acute and chronic morphine exposure and/or HIV infection was quantitated by ELISA according to manufacturer’s instructions (Antigenix America, Huntington Station, NY). A Quantikine® human tumor necrosis factor alpha (TNF-α) ELISA kit was utilized to quantitate TNF-α secretion according to manufacturer’s instructions (R & D Systems).
Statistical analysis
Statistically significant differences between control and test groups were determined by Student’s t-test using MS excel program. p values of ≤0.05 were considered statistically significant.
RESULTS
To determine if morphine alters production of inflammatory factors by human macrophages or HIV-induced inflammatory response, h-mdms infected with CNS-derived HIV isolates YU-2 or JR-FL, were exposed to chronic or acute morphine treatments, respectively. To define how morphine alters HIV-induced inflammatory response, we characterized expression of MCP-2, IL-6 and TNF-α since these chemokines and cytokines have been shown to be secreted from h-mdms upon morphine-exposure and/or HIV-infection (Dave & Khalili, 2010). The effects of morphine exposure on viral replication in HIV-infected h-mdms and MAGI cells were also assessed.
Morphine induced inflammation
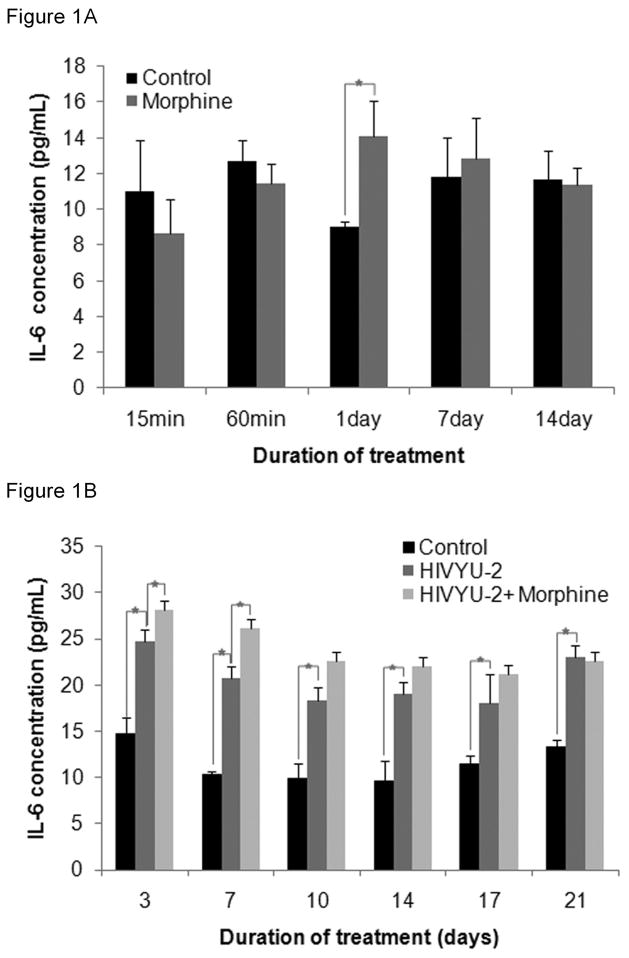
To assess if IL-6 secretion is triggered by HIV is affected by morphine, IL-6 levels were quantified in cell free supernatants from uninfected and HIV-infected h-mdms exposed to chronic morphine treatment. In the HIV uninfected macrophages, 24h after the first dose of 0.1 μM morphine, IL-6 secretion from h-mdms was significantly (1.56 fold) greater than untreated control (p = 0.042). At all other time points, levels of IL-6 secreted from morphine treated h-mdms was not significantly different from control h-mdms (p = 0.262 to 0.840) (Fig 1A).
Figure 1. Chronic morphine exposure increases the quantity of IL-6 secreted from HIV-infected h-mdms.
A: IL-6 secretion was quantified by ELISA in h-mdms subjected to chronic morphine exposure. 0.1 μM morphine was added to h-mdm cultures at 0, 3, 7, 10, and 14 day from the start of morphine treatment. B: h-mdms were infected with HIV and during the course of infection subjected to a chronic morphine exposure paradigm. Il-6 was quantified by ELISA. The mean of three experiments and standard deviations are indicated (*p ≤ 0.05).
As we previously have shown (Dave & Khalili, 2010), IL-6 secretion from HIV-infected h-mdms was significantly greater than uninfected h-mdms. The increase in IL-6 secretion varied from 1.58 to 2.01 fold greater than uninfected h-mdms (p = 0.001 to 0.056). More importantly, the presence of morphine during HIV infection led to significant increases in IL-6 secretion. At 3 and 7 dpi, IL-6 secretion from morphine-treated HIV-infected h-mdms was 1.14 and 1.26 fold greater than HIV-infected h-mdms without morphine (p = 0.018 and 0.012, respectively). Likewise, at other time points, IL-6 secretion was increased in response to morphine treatment of HIV-infected h-mdms and varied from 0.98 to 1.24 fold of HIV-infected h-mdms, although these differences were not statistically significant (p = 0.154 to 0.766) (Fig 1B). These results suggest that morphine may have an additive effect on the HIV-induced production of IL-6 in h-mdms.
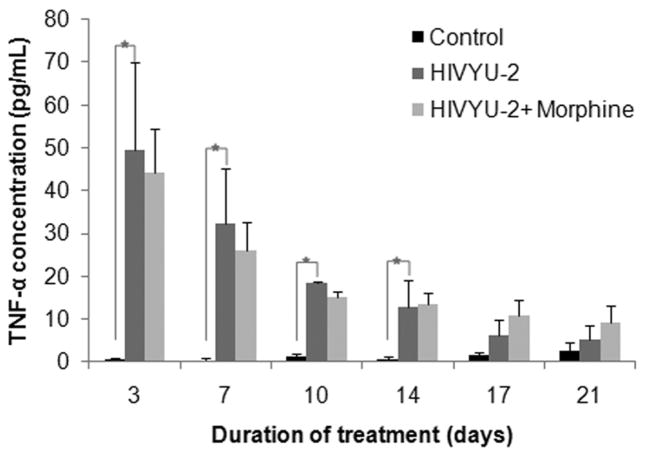
To assess if TNF-α secretion triggered by HIV is affected by morphine, TNF-α levels were quantified in cell free supernatants from uninfected and HIV-infected h-mdms exposed to chronic morphine treatment. During chronic morphine exposure in the absence of HIV infection, h-mdms did not secrete detectable TNF-α (assay detection limit 7.8 pg/mL) (data not shown). TNF-α secretion from HIV-infected h-mdms between day 3 and 14 varied from 93.80 to 13.39 fold greater than control (p = 0.001 to 0.030). After day 14, TNF-α secretion was 1.89 to 3.63 fold greater than control, but was not statistically significant (p = 0.125 to 0.318). When morphine was present in the HIV infected macrophage cultures, TNF-α secretion varied from 0.81 to 1.78 fold change over HIV-infected h-mdms without morphine, but did not achieve statistical significance at any of the time points (p = 0.056 to 0.881) (Fig 2). Hence, HIV infection potently induces TNF-α and morphine did not alter TNF-α production in the HIV-infected or uninfected human macrophages.
Figure 2. Chronic morphine exposure has no effect on TNF-α secretion from HIV-1-infected h-mdms.
h-mdms were infected with HIV and during the course of infection subjected to a chronic morphine exposure. TNF-α secretion was quantified by ELISA. The mean of three experiments and standard deviations are indicated (*p ≤ 0.05).
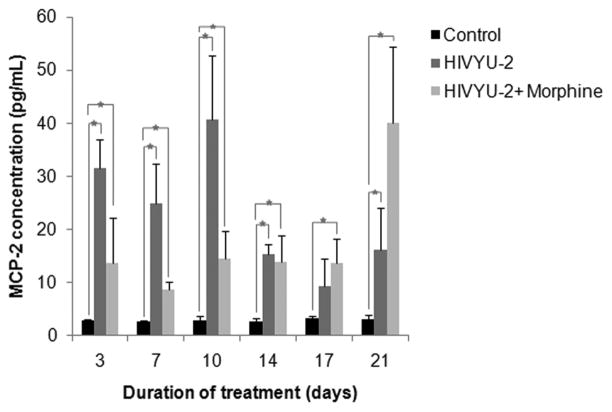
To assess if MCP-2 secretion triggered by HIV is affected by morphine, levels were quantified in cell free supernatants from uninfected and HIV-infected h-mdms exposed to chronic morphine. During chronic morphine exposure, h-mdms secreted low levels of MCP-2 (highest concentration 2.085 ± 0.539 pg/mL) and was not significantly different from control at most of data points (p = 0.116 to 0.225) (data not shown). However, in contrast to macrophages subjected to chronic morphine exposure, HIV-infected h-mdms had significantly higher levels of MCP-2 secretion. MCP-2 secretion from HIV-infected h-mdms varied from 2.97 to 14.46 fold greater than control h-mdms (p = 0.001 to 0.045). However, MCP-2 secretion from morphine-treated HIV-infected h-mdms was not significantly different from HIV-infected h-mdms (p = 0.064 to 0.737) (Fig 3). At day 10 however, MCP-2 secretion from morphine treated, HIV-infected h-mdms was reduced to 35.7% of HIV-infected h-mdms (p = 0.026). These results show that both morphine and HIV lead to induction of MCP-2, but that up until day 14, morphine blunts the HIV-induced macrophage production of MCP-2. Of interest, we observed that macrophages infected with HIV and exposed to morphine for 21 days showed greater production of MCP-2 than the HIV infected macrophages alone. Importantly, the HIV-induced production of MCP-2 at this time point was not significantly altered from earlier time points (compare HIV-induced MCP-2 production at day 14 and 17). Moreover, as shown in Figure 4A, HIV replication as measured by p24 levels at day 21 in morphine treated HIV infected macrophages was not statistically different from either HIV infected cells alone or from HIV infected morphine treated macrophages on day 14 and 17.
Figure 3. Chronic morphine exposure of HIV-infected h-mdms induces MCP-2 secretion.
MCP-2 secretion was quantified by ELISA in h-mdms subjected to chronic morphine exposure. h-mdms were infected with HIV and during the course of infection subjected to a chronic morphine exposure. MCP-2 secretion was quantified by ELISA. The mean of three experiments and standard deviations are indicated (*p ≤ 0.05).
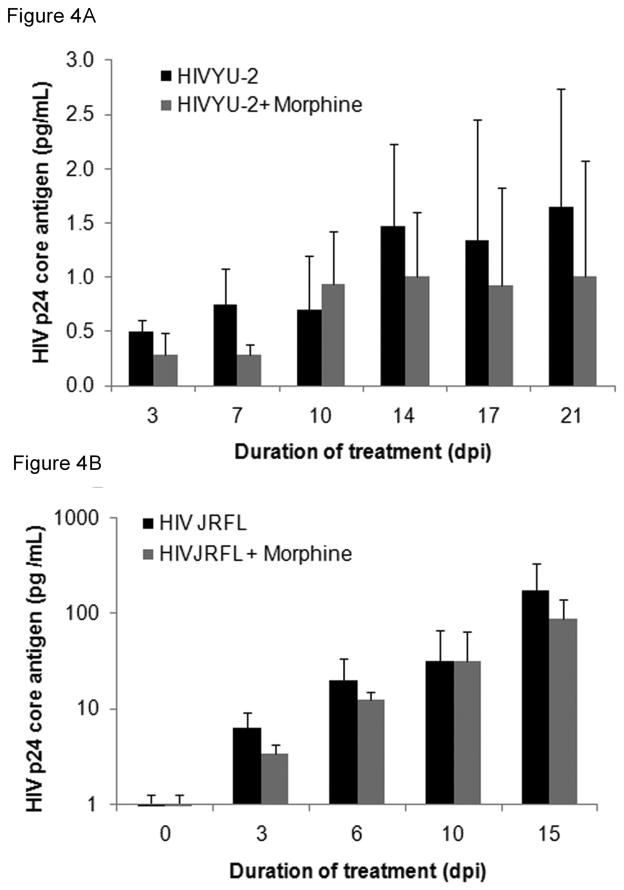
Figure 4. Morphine exposure of h-mdms does not influence HIV replication.
A: HIV progeny virion production was quantified by HIV p24 core antigen ELISA in cell-free culture supernatants from HIV-infected h-mdms subjected to chronic morphine exposure and HIV infection. B: Experiments in this study also measure HIV progeny virion production as in A, except that h-mdms were exposed to morphine only for 24h and then infected with HIV JR-FL. The mean of three experiments and standard deviations are indicated (p ≤ 0.05).
Morphine and HIV replication
Since morphine significantly altered HIV-induced production of inflammatory factors, the role of morphine on HIV replication was assessed. Macrophages were infected with HIV YU-2 and exposed to chronic morphine treatment for 21 days. As the duration of chronic morphine exposure progressed, an increase in HIV p24 concentration was observed (Fig 4A). In both control and morphine exposed h-mdms, HIV p24 core antigen levels increased from 0.504 and 0.285 pg/mL at 3 dpi to 1.644 and 1.008 pg/mL at 21 dpi, respectively. At each of the indicated time points in morphine exposed h-mdms, HIV infection was on an average reduced to 0.71 fold of control infection, but did not achieve statistically significance at any time point (p = 0.133 to 0.645) (Fig 4A). Hence, in these experiments chronic morphine exposure had no significant effect on viral replication in h-mdms.
To ensure that these effects were not specific to YU-2 or chronic morphine exposure, h-mdms were infected with another CNS-derived isolate, HIV JR-FL, and exposed to a 24h acute morphine treatment. At the end of the treatment, both input virus and morphine were washed out and HIV JR-FL progeny virion production was monitored over time (Fig 4B). With increasing duration of infection (0–15 days), HIV-1 p24 concentration in both control and morphine exposed h-mdms increased from 6.22 and 3.44 pg/mL at 3dpi to 171.80 and 87.83 pg/mL at 15dpi, respectively. At each of the indicated time points in morphine exposed macrophages, HIV JR-FL infection ranged from 0.51 to 1.02 fold of control infection, but these changes did not achieve statistical significance (p = 0.186 to 0.984) (Fig 4B). Hence, even acute morphine exposure of h-mdms did not alter HIV replication in h-mdms compared to HIV-infected h-mdms in the absence of morphine.
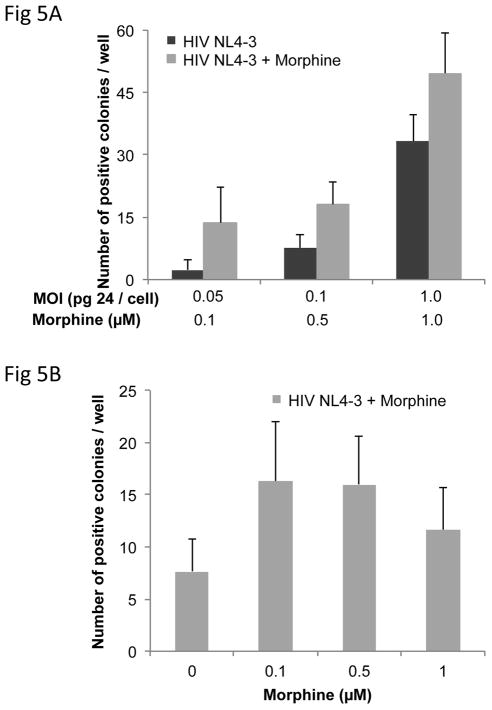
As both chronic and acute morphine exposure of h-mdms did not affect HIV replication of CNS-derived isolates YU-2 and JR-FL, we next utilized the MAGI assay to investigate dose-dependent effects of morphine on HIV replication. The number of positive colonies increased as the MOI in the MAGI assay increased from 0.05 to 1.0 pg p24/cell. The number of positive colonies was not significantly different when a similar infection was performed in presence morphine ranging from 0.1 to 1.0 μM (p = 0.064 to 0.139) (Fig 5A). At MOI of 0.1 pg p24/cell, morphine had no effect on the number of positive colonies generated at any concentration (0.1 to 1.0 μM) (p = 0.101 to 0.941) (Fig 5B). Hence, at all concentrations of morphine utilized, and MOIs of HIV tested, morphine did not alter HIV replication in our system.
Figure 5. Morphine does not affect HIV replication in the MAGI assay.
A: The effect of morphine on HIV NL4-3 viral infectivity was determined in the MAGI assay. HeLaCD4-LTR-β-Gal indicator cells were stained for β-galactosidase enzyme activity with 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal). B: A dose-response relationship to viral infectivity was determined by treating the indicator cells with varying concentrations of morphine at one MOI (0.1 pg p24/cell). The mean of three experiments and standard deviations are indicated (p ≤ 0.05).
In summary, these results show that morphine had an additive effect to the HIV-induced production of IL-6 in human macrophages. On the other hand, morphine blunted the MCP-2 response observed during HIV YU-2 infection of h-mdms. Of note, morphine did not influence the potent HIV induced TNF-α secretion from h-mdms. While morphine influenced HIV-induced inflammatory response, viral replication remained unaffected.
DISCUSSION
These data demonstrate potential mechanism(s) through which opiates may contribute to HIV neurodegenerative disease by showing that in the presence of morphine, HIV-induced production of IL-6 and MCP-2 was altered. As reported by other studies, our results showed that morphine triggered an inflammatory response by inducing secretion of IL-6 (Roy, et al., 1998, El-Hage, et al., 2005, El-Hage, et al., 2008, Turchan-Cholewo, et al., 2009, Dave & Khalili, 2010) and MCP-2 (Dave & Khalili, 2010). TNF-α secretion was found to be exclusively associated with the HIV inflammatory response and morphine had no effects on its production in the presence or absence of HIV.
One of the most striking effects of morphine and HIV-induced production of inflammatory factors was observed with Il-6 secretion. Elevated IL-6 levels are associated with rapid progression to AIDS and manifestation of CNS disease in simian immunodeficiency virus (SIV) macaque models (Mankowski, et al., 2004, Roberts, et al., 2004). IL-6 is secreted upon morphine-exposure from murine macrophages (Roy, et al., 1998), microglia (Turchan-Cholewo, et al., 2009) and astrocytes (El-Hage, et al., 2005, El-Hage, et al., 2008). However, few studies describe morphine-induced IL-6 induction during HIV infection in human primary cell cultures (Dave & Khalili, 2010). In this study, morphine-induced an increase in IL-6 response from HIV infected h-mdms. The level of IL-6 induction was similar to that observed in human neuronal culture system that modeled HIV neurodegeneration (Wang & Gabuzda, 2006) and greater than the response observed in murine macrophages, microglia or astrocytes treated with morphine or HIV viral proteins (Roy, et al., 1998, El-Hage, et al., 2005, Turchan-Cholewo, et al., 2009). Increases in IL-6 secretion have been observed during the acute phase infection in SIV macaque model (Roberts, et al., 2004, Witwer, et al., 2009). In SIV encephalitis model, IL-6 up-regulation is associated with severe neuropathological lesions (Boche, et al., 1999). Thus, the morphine-induced increase in IL-6 secretion may be a contributing factor in accelerating HIV disease in the CNS.
Surprisingly, morphine, significantly affected MCP-2 expression level of HIV-infected macrophages over time. Two important trends were observed as shown in Figure 3. First, in the morphine treated macrophages infected with HIV, the MCP-2 levels detected up to 10 days of morphine exposure were significantly less than the levels produced by HIV infection alone. This observation suggests that during the first 10 days of morphine exposure, macrophages do not respond to HIV infection by producing MCP-2. This effect may be a direct or indirect effect of morphine on MCP-2 production. Second, after 21 days of morphine exposure, the levels of MCP-2 in HIV-infected macrophages spiked dramatically; increasing more than 4 times over levels in HIV infected cultures without morphine. MCP-2 concentrations in morphine treated HIV infected cells at day 21 reached levels detected in HIV-infected macrophages on day 10. These results suggest that morphine is delaying the HIV induction of MCP-2 by approximately 10–11 days, however pathways through which the effects are elicited are unknown. Elevated MCP-2 levels are associated with HIV-infected or activated macrophages and microglia and hence this chemokine is predicted to play an important role in HIV neurodegenerative disease (Wang & Gabuzda, 2006, Dave & Khalili, 2010, Rom, et al., 2010). Our previous study (Dave & Khalili, 2010) is the only group to the best of our knowledge, to report MCP-2 secretion in response to morphine exposure of HIV-infected h-mdms or any other cell type. In addition, studies of MCP-2 from normal and diseased brain are limited. In this study, the level of MCP-2 secreted from h-mdms after morphine-exposure or HIV-infection was similar to the response observed in human neuronal culture system utilized to model HIV neurodegeneration (Wang & Gabuzda, 2006). Elevated levels of MCP-2 have been identified in dendritic cells both in vivo and in vitro in studies utilizing the SIV and HIV Tat, respectively (Izmailova, et al., 2003). MCP-2 is a ligand for CCR-5, and morphine-induced increases in its secretion may lead to cross-talk with the receptor (Yang, et al., 2002). Whether such an interaction may explain the reduced MCP-2 in the presence of HIV infection is a matter of speculation. Nonetheless, MCP-2 is part of larger monocyte chemoattractant protein family including MCP-1 and MCP-3. These molecules play an important role in recruiting and activating CD4+ and CD8+ T cells (Loetscher, et al., 1994). As such, alterations in MCP-2 production may have important implications for disease progression in the CNS.
Neither chronic nor acute morphine exposure of h-mdms affected HIV replication. Since morphine had no effect on HIV replication as measured by p24 and by the MAGI assay, it appears that viral life-cycle is not the cause of alterations in IL-6 or MCP-2. Importantly, it seems that the inflammatory response does not correlate with the level of viral replication as reflected in the distinct kinetic response for each of the individual cytokines studied.
Morphine did not alter HIV-induced secretion of TNF-α from h-mdms. At no time point in this, or in our previous study did we observe any evidence for a role of morphine in TNF-α secretion (Dave & Khalili, 2010). HIV infection induced strong TNF-α response and this observation corroborates previous results (Ghorpade, et al., 2005, Ronaldson & Bendayan, 2006, Yu, et al., 2007, Hoffmann, et al., 2009, Xing, et al., 2009). TNF-α levels decreased as HIV infection progressed. This observation may support a clinical observation involving a study of autopsy cases of patients with HIV encephalitis, where no TNF-α expression was observed from HIV-p24 positive cells. It should be noted that in that study TNF-α expression was detected in CD68-positive macrophage/microglia (Xing, et al., 2009).
In this study an enhancement of HIV replication upon morphine treatment was not observed. An end-step in the viral life-cycle (HIV-1 p24 core antigen in cell-free culture supernatants) was utilized as a measure of the effect of morphine on viral replication. Viral replication kinetics were determined over 15 to 21 days. Our approach is different from previous studies that utilized intermediate steps of viral replication as a means to indicate the effect of morphine on viral dynamics (Guo, et al., 2002, Li, et al., 2003). Of note, this study also contrasts with previous studies that have observed enhancement of infection with HIV R5 isolates in h-mdms treated with morphine (Guo, et al., 2002) and in human neonatal macrophages (Li, et al., 2003). In another study by Steele et al., authors claimed that morphine augments susceptibility of PBMC to HIV infection. However, this study reported only one time point of analysis instead of a kinetic experiment to monitor viral replication (Steele, et al., 2003). Based on our observations from h-mdms, it is unlikely that at physiological concentrations, morphine has a significant effect on replication of CNS-derived isolates of HIV.
Dissecting the kinetics of morphine induced inflammatory responses in HIV infected h-mdms provides insights into how opiates might affect HIV neuropathogenesis. In this context, the present study provides a unique perspective from prior studies in unequivocally demonstrating that morphine does not enhance HIV replication while simultaneously altering the inflammatory response. Our study indicates a commonality of processes between HIV and morphine as reflected in induction of MCP-2 and IL-6 and divergence in the TNF-α response. Distinctive patterns were obvious in the kinetics of the response to each of these cytokines. Further studies of this phenomenon in greater molecular detail would aid in unraveling the mechanism(s) involved and the implications for HIV disease in the CNS in opiate abusers.
Acknowledgments
Acknowledgements are due to National Institute of Drug Abuse for providing financial support to conduct this research through research grant 5PO1DA023860-020003 to Dr. Kamel Khalili and 1R03 DA 031599-01 to Dr. Rajnish S. Dave. In addition, I wish to thank the AIDS Reagent Repository for sharing critical reagents that were utilized to perform these studies. I would also like to thank Drs. T. Dianne Langford and Kamel Khalili for critical reading of the manuscript, advice and support. Finally, I wish to acknowledge the cooperation and advice of all the members of the Center for Neurovirology, Department of Neuroscience, Temple University.
References
- Abt AC, Meucci O. Regulation of Neuronal Ferritin Heavy Chain, A New Player in Opiate-Induced Chemokine Dysfunction. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2011;6:466–476. doi: 10.1007/s11481-011-9278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright AV, Soldan SS, Gonzalez-Scarano F. Pathogenesis of human immunodeficiency virus-induced neurological disease. J Neurovirol. 2003;9:222–227. doi: 10.1080/13550280390194073. [DOI] [PubMed] [Google Scholar]
- Bell JE, Arango JC, Anthony IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006;1:182–191. doi: 10.1007/s11481-006-9018-2. [DOI] [PubMed] [Google Scholar]
- Boche D, Khatissian E, Gray F, Falanga P, Montagnier L, Hurtrel B. Viral load and neuropathology in the SIV model. J Neurovirol. 1999;5:232–240. doi: 10.3109/13550289909015809. [DOI] [PubMed] [Google Scholar]
- Bokhari SM, Yao H, Bethel-Brown C, et al. Morphine enhances Tat-induced activation in murine microglia. J Neurovirol. 2009;15:219–228. doi: 10.1080/13550280902913628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, et al. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56:1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Late for the epidemic: HIV/AIDS in Eastern Europe. Science. 2010;329:160, 162–164. doi: 10.1126/science.329.5988.160. [DOI] [PubMed] [Google Scholar]
- Dave RS, Pomerantz RJ. Antiviral effects of human immunodeficiency virus type 1-specific small interfering RNAs against targets conserved in select neurotropic viral strains. J Virol. 2004;78:13687–13696. doi: 10.1128/JVI.78.24.13687-13696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave RS, Khalili K. Morphine treatment of human monocyte-derived macrophages induces differential miRNA and protein expression: impact on inflammation and oxidative stress in the central nervous system. J Cell Biochem. 2010;110:834–845. doi: 10.1002/jcb.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Bruce-Keller AJ, Yakovleva T, Bazov I, Bakalkin G, Knapp PE, Hauser KF. Morphine exacerbates HIV-1 Tat-induced cytokine production in astrocytes through convergent effects on [Ca(2+)](i), NF-kappaB trafficking and transcription. PLoS ONE. 2008;3:e4093. doi: 10.1371/journal.pone.0004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Wu G, Wang J, et al. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006;53:132–146. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorpade A, Persidsky Y, Swindells S, et al. Neuroinflammatory responses from microglia recovered from HIV-1-infected and seronegative subjects. J Neuroimmunol. 2005;163:145–156. doi: 10.1016/j.jneuroim.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- Grigoryan A, Shouse RL, Durant T, Mastro TD, Espinoza L, Chen M, Kajese T, Wei X, Hall HI. HIV Infection Among Injection-Drug Users--34 States, 2004–2007. JAMA. 2010;303:126–128. [Google Scholar]
- Guo CJ, Li Y, Tian S, Wang X, Douglas SD, Ho WZ. Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta-chemokines and CCR5 receptor. J Investig Med. 2002;50:435–442. doi: 10.1136/jim-50-06-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann O, Zipp F, Weber JR. Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) in central nervous system inflammation. J Mol Med. 2009;87:753–763. doi: 10.1007/s00109-009-0484-x. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine potentiates HIV-1 gp120-induced neuronal apoptosis. The Journal of infectious diseases. 2005;191:886–889. doi: 10.1086/427830. [DOI] [PubMed] [Google Scholar]
- Izmailova E, Bertley FM, Huang Q, Makori N, Miller CJ, Young RA, Aldovini A. HIV-1 Tat reprograms immature dendritic cells to express chemoattractants for activated T cells and macrophages. Nat Med. 2003;9:191–197. doi: 10.1038/nm822. [DOI] [PubMed] [Google Scholar]
- Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Langford D, Masliah E. Crosstalk between components of the blood brain barrier and cells of the CNS in microglial activation in AIDS. Brain Pathol. 2001;11:306–312. doi: 10.1111/j.1750-3639.2001.tb00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JA, Shimabukuro J. Recovery of AIDS-associated retroviruses from patients with AIDS or AIDS-related conditions and from clinically healthy individuals. J Infect Dis. 1985;152:734–738. doi: 10.1093/infdis/152.4.734. [DOI] [PubMed] [Google Scholar]
- Li Y, Merrill JD, Mooney K, et al. Morphine enhances HIV infection of neonatal macrophages. Pediatr Res. 2003;54:282–288. doi: 10.1203/01.PDR.0000074973.83826.4C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. Monocyte chemotactic proteins MCP-1, MCP-2, and MCP-3 are major attractants for human CD4+ and CD8+ T lymphocytes. FASEB J. 1994;8:1055–1060. doi: 10.1096/fasebj.8.13.7926371. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuro Endocrinol Lett. 2011;32:7–24. [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Clements JE, Zink MC. Cerebrospinal fluid markers that predict SIV CNS disease. Journal of neuroimmunology. 2004;157:66–70. doi: 10.1016/j.jneuroim.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Pitcher J, Shimizu S, Burbassi S, Meucci O. Disruption of neuronal CXCR4 function by opioids: preliminary evidence of ferritin heavy chain as a potential etiological agent in neuroAIDS. Journal of neuroimmunology. 2010;224:66–71. doi: 10.1016/j.jneuroim.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- Roberts ES, Burudi EM, Flynn C, et al. Acute SIV infection of the brain leads to upregulation of IL6 and interferon-regulated genes: expression patterns throughout disease progression and impact on neuroAIDS. Journal of neuroimmunology. 2004;157:81–92. doi: 10.1016/j.jneuroim.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Rom S, Rom I, Passiatore G, et al. CCL8/MCP-2 is a target for mir-146a in HIV-1-infected human microglial cells. FASEB J. 2010;24:2292–2300. doi: 10.1096/fj.09-143503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 triggers an inflammatory response in cultured rat astrocytes and regulates the functional expression of P-glycoprotein. Mol Pharmacol. 2006;70:1087–1098. doi: 10.1124/mol.106.025973. [DOI] [PubMed] [Google Scholar]
- Roy S, Cain KJ, Chapin RB, Charboneau RG, Barke RA. Morphine modulates NF kappa B activation in macrophages. Biochemical and biophysical research communications. 1998;245:392–396. doi: 10.1006/bbrc.1998.8415. [DOI] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309:99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga FO, Gupta S, Keller JN, Knapp PE, Hauser KF, Bruce-Keller AJ. Morphine and HIV-Tat increase microglial-free radical production and oxidative stress: possible role in cytokine regulation. Journal of neurochemistry. 2009;108:202–215. doi: 10.1111/j.1471-4159.2008.05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrina C, Fiorazzo A, Turano A, Cacciani P, Regini C, Castelli F, Sacchetti E. Depressive disorders and personality variables in HIV positive and negative intravenous drug-users. J Affect Disord. 2001;65:45–53. doi: 10.1016/s0165-0327(00)00269-x. [DOI] [PubMed] [Google Scholar]
- Wang J, Gabuzda D. Reconstitution of human immunodeficiency virus-induced neurodegeneration using isolated populations of human neurons, astrocytes, and microglia and neuroprotection mediated by insulin-like growth factors. Journal of neurovirology. 2006;12:472–491. doi: 10.1080/13550280601039659. [DOI] [PubMed] [Google Scholar]
- Witwer KW, Gama L, Li M, et al. Coordinated regulation of SIV replication and immune responses in the CNS. PLoS ONE. 2009;4:e8129. doi: 10.1371/journal.pone.0008129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing HQ, Hayakawa H, Izumo K, Kubota R, Gelpi E, Budka H, Izumo S. In vivo expression of proinflammatory cytokines in HIV encephalitis: an analysis of 11 autopsy cases. Neuropathology. 2009;29:433–442. doi: 10.1111/j.1440-1789.2008.00996.x. [DOI] [PubMed] [Google Scholar]
- Yang OO, Garcia-Zepeda EA, Walker BD, Luster AD. Monocyte chemoattractant protein-2 (CC chemokine ligand 8) inhibits replication of human immunodeficiency virus type 1 via CC chemokine receptor 5. J Infect Dis. 2002;185:1174–1178. doi: 10.1086/339678. [DOI] [PubMed] [Google Scholar]
- Yu C, Kastin AJ, Tu H, Waters S, Pan W. TNF activates P-glycoprotein in cerebral microvascular endothelial cells. Cell Physiol Biochem. 2007;20:853–858. doi: 10.1159/000110445. [DOI] [PubMed] [Google Scholar]