Abstract
The α proteobacter Rhodobacter sphaeroides accumulates two cytochrome c oxidases (CcO) in its cytoplasmic membrane during aerobic growth: a mitochondrial-like aa3-type CcO containing a di-copper CuA center and mono-copper CuB, plus a cbb3-type CcO that contains CuB but lacks CuA. Three copper chaperones are located in the periplasm of R. sphaeroides, PCuAC, PrrC (Sco) and Cox11. Cox11 is required to assemble CuB of the aa3-type but not the cbb3-type CcO. PrrC is homologous to mitochondrial Sco1; Sco proteins are implicated in CuA assembly in mitochondria and bacteria, and with CuB assembly of the cbb3-type CcO. PCuAC is present in many bacteria, but not mitochondria. PCuAC of Thermus thermophilus metallates a CuA center in vitro, but its in vivo function has not been explored. Here, the extent of copper center assembly in the aa3- and cbb3-type CcOs of R. sphaeroides has been examined in strains lacking PCuAC, PrrC, or both. The absence of either chaperone strongly lowers the accumulation of both CcOs in the cells grown in low concentrations of Cu2+. The absence of PrrC has a greater effect than the absence of PCuAC and PCuAC appears to function upstream of PrrC. Analysis of purified aa3-type CcO shows that PrrC has a greater effect on the assembly of its CuA than does PCuAC, and both chaperones have a lesser but significant effect on the assembly of its CuB even though Cox11 is present. Scenarios for the cellular roles of PCuAC and PrrC are considered. The results are most consistent with a role for PrrC in the capture and delivery of copper to CuA of the aa3-type CcO and to CuB of the cbb3-type CcO, while the predominant role of PCuAC may be to capture and deliver copper to PrrC and Cox11.
Keywords: cbb3-type cytochrome c oxidase, aa3-type cytochrome c oxidase, copper chaperone, copper center assembly, CuA, Sco protein
During aerobic growth in the laboratory the α proteobacter Rhodobacter sphaeroides accumulates two cytochrome c oxidases (CcOs) in the cytoplasmic membrane. One is an aa3-type CcO with high similarity to mitochondrial CcO [1–5] while the other is a cbb3-type CcO, evolutionarily distant from the aa3-type CcO [6, 7]. The aa3-type CcO contains two heme A and two copper centers [4, 5, 8]. The di-copper CuA center in subunit II accepts electrons from cytochrome c and transfers them to low-spin heme a in subunit I. In R. sphaeroides and other α proteobacteria, both soluble cytochromes c as well as membrane-anchored cytochrome cy transfer electrons to CuA [9–14]. The two coppers of CuA are bound by two copper-bridging cysteines, two histidines, one methionine and a backbone carbonyl group. From heme a, electrons flow to the buried heme a3-CuB site in subunit I, where O2 is reduced to water [15]. The mono-copper CuB center, ubiquitous and structurally conserved in the heme-Cu oxidase superfamily, is composed of three histidines that bind the single copper near the five-coordinate iron of the heme of the active site [4, 5, 16]. Cbb3-type CcOs, widespread in proteobacteria, contain a subunit I with metal centers similar to all members of the heme-Cu oxidase superfamily, including an O2 reduction site composed of five-coordinate heme b3 plus CuB [6, 17, 18]. However, the cbb3-type CcO lacks CuA [6]. Instead of a subunit II like that of the aa3-type CcO, the cbb3-type CcO contains two subunits with extramembrane domains that bind c-type cytochromes and extend into the periplasm [17, 19]. These two cytochrome c subunits function to accept electrons from soluble cytochromes c or from membrane-bound cytochrome cy [20].
R. sphaeroides also contains several CcO-specific assembly proteins with homologs in other bacteria and/or mitochondria. Cox10 and Cox15 participate in the synthesis of heme A, and possibly its insertion, in bacteria and mitochondria [21, 22]. Surf1 enhances the insertion of the active site heme in bacteria and mitochondria [22–24]. Proteins encoded by the ccoGHIS operon, including the copper transporter CcoI, have been implicated in the assembly of the cbb3-type CcO in other α proteobacters [25–28]. The roles of three periplasmic copper chaperones - Sco, Cox11 and PCuAC - are discussed below.
The Sco protein copper chaperones are present in mitochondria and widespread in bacterial species. Sco proteins are anchored in the membrane by a single transmembrane helix while a thioredoxin-like extramembrane domain extends into the inter-membrane space or the bacterial periplasm to bind a single copper, using two cysteines and one histidine [29, 30]. Human and yeast mitochondria contain two Sco proteins [30–32]. Both Sco1 and Sco2 have been implicated in the assembly of CuA in mitochondria on the basis of CcO deficiency when one or both Sco proteins are deleted or mutated, plus demonstrations of interactions between Sco1, Sco2 and subunit II of CcO [30, 33–35]. Bacterial Sco proteins have also been implicated in the assembly of CuA. The deletion of Sco from B. subtilus strongly reduces the expression of a caa3-type CcO containing CuA but not the expression of a quinol oxidase that lacks CuA [36]. Similarly, the deletion of the gene for Sco in Bradyrhizobium japonicum decreases the accumulation of its aa3-type CcO that contains CuA, but not the cbb3-type CcO that lacks CuA [37]. The single Sco protein present in R. sphaeroides (termed PrrC) binds Cu(II) and Ni(II) [38], similar to human Sco1 [31]. PrrC has disulfide reductase activity [39], a finding which has stimulated discussion about whether the role of bacterial Sco proteins in CuA assembly might be restricted to the reduction of the cysteines of the apo-CuA center. In fact, a soluble form of Sco of Thermus thermophilus was found to reduce the disulfide of Thermus apo-CuA in vitro, but it could not metallate the reduced apo-CuA center [40].
Sco proteins have also been implicated in the assembly of CuB. The membrane-bound copper chaperone Cox11 is absolutely required for the assembly of the CuB center in the aa3-type CcOs of α proteobacters and mitochondria [30, 41, 42]. However, Cox11 is not required for the insertion of CuB into the cbb3-type CcOs [37, 41, 42]. Rhodobacter capsulatus and Pseudomonas aeruginosa strains lacking their Sco proteins exhibit decreased accumulation of active cbb3-type CcO, but increasing the concentration of exogenous copper restores the synthesis of active enzyme [43–45]. This indicates a role for Sco in the delivery of copper to the CuB center of the cbb3-type CcO in these species. In contrast, the deletion of Sco from B. japonicum does not affect the accumulation of its cbb3-type CcO [37].
Many bacteria contain a periplasmic copper chaperone of the PCuAC family. In these proteins, a cupredoxin-like fold binds a single Cu(I) via methionine and histidine side chains [46]. Unlike Sco and Cox11, PCuAC of R. sphaeroides and other α proteobacteria may not be tethered in the cytoplasmic membrane since computer analysis indicates that the predicted hydrophobic sequence at the N-terminus appears more like a signal sequence than a transmembrane helix. The hydrophilic domain of R. sphaeroides PCuAC is 53–55% similar to its orthologs in Deinococcus radiodurans and Thermus thermophilus, for which solution structures have been determined [40, 46]. PCuAC was originally suggested as a candidate for a functional analog of mitochondrial Cox17 in bacteria [46]; in mitochondria, Cox17 is soluble in the intermembrane space where it transfers copper to membrane-bound Sco and Cox11 [47]. Later, the same group performed NMR experiments of copper transfer and protein interaction to show that, in vitro, a recombinant form of Thermus PCuAC inserted copper into the CuA site of soluble subunit II of the Thermus ba3-type CcO [40]. One problem in extrapolating this result to a universal mechanism for CuA assembly is that PCuAC is not present in mitochondria.
It has also been suggested that PCuAC may deliver copper to the CuB center of the cbb3-type CcO of B. japonicum [37]. In a strain of B. japonicum lacking one of its two predicted PCuAC proteins, total CcO activity decreased but the cbb3-type CcO was not assayed independently [48].
With the demonstration that PCuAC delivers copper to a CuA site in vitro [40], plus the suggestion that PCuAC may be responsible for the assembly of CuB of a cbb3-type CcO [37], there exists a need to explore the role of PCuAC in the cell. For example, to what extent is PCuAC required for the assembly of either the aa3-type CcO or the cbb3-type CcO? If both PCuAC and Sco participate in the assembly of the copper centers of these two oxidases, are their functions unique or redundant? These questions and others have been examined in R. sphaeroides, a bacterium that has proven useful for elucidating functions of CcO assembly proteins also present in mitochondria, such as Cox11 and Surf1 [23, 41]. This is partly due to the evolutionary relationship between R. sphaeroides and mitochondria [49, 50] and also because this bacterium tends to accumulate partially assembled CcO forms that can be purified and analyzed [23, 41, 51, 52]. The experiments presented here take advantage of the additional feature that R. sphaeroides accumulates both the aa3-type and the cbb3-type CcO during aerobic growth [1, 6, 20]. Therefore, the assembly of these two evolutionarily distant oxidases can be compared in the same cellular environment, i.e. with the same complement of copper chaperones and concentration of copper. The results show that PCuAC has a significant effect on the assembly of CuA of the aa3-type CcO and CuB of the cbb3-type CcO. However, PrrC (R. sphaeroides Sco) has an even greater effect and PCuAC appears to function upstream of PrrC rather than as a redundant copper delivery pathway.
Material and Methods
Bacterial growth. R. sphaeroides strains were grown in Sistrom's media A [53] supplemented with 1 µg/ml tetracycline, 50 µg/ml spectinomycin and streptomycin and 25 µg/ml kanamycin, when necessary. Batches of ten-fold concentrated media were prepared using Nanopure water, without the addition of copper. Copper was added to the media just before cell growth, when desired, by the addition of CuSO4 from a stock solution. Glass and plastic containers used in the formulation and storage of concentrated media were bathed in 100 µM EDTA and rinsed with Nanopure water before use. Metal analysis of the media by inductively coupled plasma optical emission spectroscopy (ICP-OES) indicated that the final (1X) media contained <50 nM copper when no additional CuSO4 was added. For each growth, cells were taken from frozen stocks and grown on Sistrom's agar, containing the final desired copper concentration, for three days at 30 C. A heavy loop of cells from these plates was used to innoculate 100 ml of media in a 500 ml Erlenmeyer flask for overnight growth at 32 C with rapid shaking. 10 ml of these cultures were used to inoculate 600 ml of media in 2L baffled flasks for growth at 32 C with rapid shaking. Cells were grown to late exponential phase (O.D.660 nm = 1.0–1.2), harvested by centrifugation at 4 C and frozen at −80 C. Flasks used for copper-deficient growth were kept separate. All of the flasks used for cell growth were prepared for re-use by rinsing them with Nanopure water after cell harvest followed by autoclaving. This procedure allows the previous cell culture to deplete the glass of copper.
Construction of a plasmid to express PCuAC. A derivative of the broad host range vector PBBR1MCS-3 (tetracycline resistance) [54] was prepared with the gene for R. sphaeroides PCuAC under the control of the R. sphaeroides promoter for coxI, the gene for subunit I of the aa3-type CcO, as follows. First, a 1010 bp fragment of genomic DNA was isolated from R. sphaeroides 2.4.1 by PCR using primers that created an NdeI restriction site at the ATG of the PCuAC gene (NdeIfwd 5’- GCCAAATCACACAGTCAGGAGAGACAtATGACCCCG-3’) and a SacI restriction site in the 3’ non-coding region of the gene (SacIrev 5’- GGCGGCTGCCAAGGGAGCtCGCGGGACCG-3’). After purification, this fragment was further restricted with NdeI and SacI to remove the primer extensions. In order to prepare the host plasmid, a KpnI-SacI fragment containing the gene for subunit III (coxIII) 3' to the coxI promoter was excised from pJG211, a derivative of PBBR1MCS-2 created previously [55]; the KpnI-SacI fragment was then cloned into the multiple cloning site of pBBR1MCS-3 [54]. This new derivative of pBBR1MCS-3 was restricted with NdeI and SacI to release coxIII and the NdeI-SacI DNA containing the gene for PCuAC was inserted. The final product, named pPCuAC, contains the gene for PCuAC under the control of the coxI promoter; pPCuAC was transformed into E. coli S-17 for conjugation into R.sphaeroides by established procedures [56].
Inactivation of the genomic gene for PCuAC. Starting with pPCuAC, a PstI site and a BamHI site were introduced into the gene for PCuAC using the QuikChange mutagenesis system (Agilent). A 300 bp DNA fragment completely internal to the gene was removed and cloned into the multiple cloning site of pKNOCK-Km [57] using its PstI and BamHI restriction sites to create pJG245. pJG245 was transformed into E. coli S-17, conjugated into R. sphaeroides 2.4.1 and colonies resistant to 50 µg/ml kanamycin were selected. Genomic DNA was extracted from several of these colonies, PCR was performed using the primers presented in the previous section, and the resulting fragments were cloned into the TOPO 2.0 vector (Invitrogen). PCR analysis and DNA sequencing confirmed the interruption of the gene for PCuAC by pJG245 and the absence of a normal gene in the genome. One of these strains was retained as R. sphaeroides ΔPCuAC.
R. sphaeroides PRRC4, which contains a silent deletion of the gene for PrrC [58], was obtained from the laboratory of Prof. Sam Kaplan, U.T. Health Sciences, Houston. pJG245 was also used to inactivate the gene for PCuAC in R. sphaeroides PRRC4 to create ΔPrrC-ΔPCuAC.
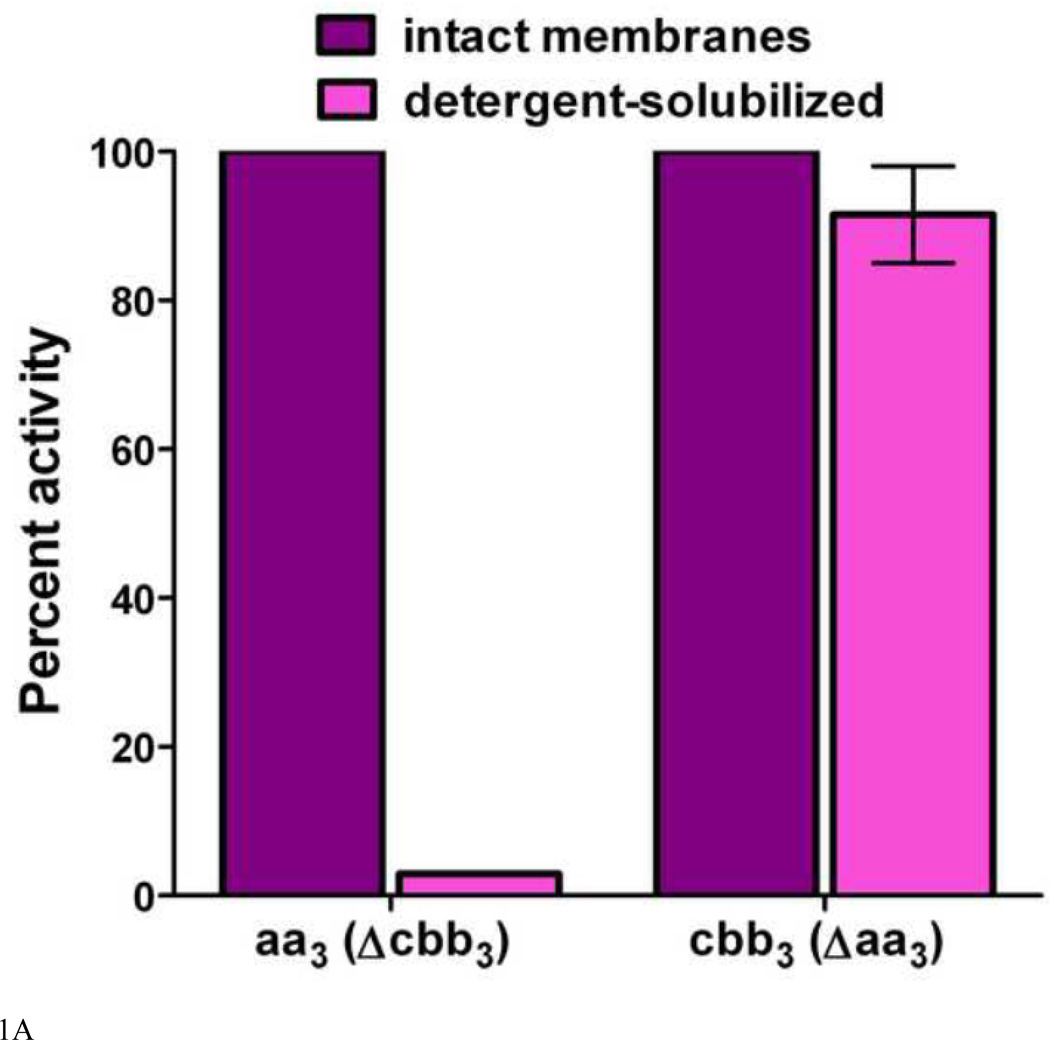
Activity assays. The activity of purified aa3-type CcO was measured as previously described [59]. Simultaneous measurements of the activity of the aa3-type CcO and the cbb3-type CcO in purified cytoplasmic membranes were performed as O2 consumption assays using a Clark-type O2 electrode (Yellow Springs) and a YSI 5300 dissolved O2 monitor at 25 C. The 1.7 ml reaction mixture contained 50 mM Tris-HCl, 75 mM KCl, pH 7.2, 5 µM CCCP, 0.8 µg/ml valinomycin and 0.1–0.3 mg intact, purified cytoplasmic membranes (measured as total membrane protein). O2 consumption was initiated by the addition of ascorbic acid to 3 mM and TMPD to 0.3 mM. After the consumption of all of the O2 in the reaction cuvette, dodecyl maltoside was added to a final concentration of 0.1% to solubilize the membranes. After two minutes, re-purified soybean phospholipids [60], sonicated into a stock solution of 40 mg/ml lipid in 10 mM Tris-HCl, pH 7.0 plus 1.0% dodecyl maltoside, were added to a final concentration of 0.5 mg/ml lipid. O2 was returned to the reaction cuvette by blowing humidified 100% O2 over the top of the solution until the O2 concentration in the reaction cell equaled that of air-saturated buffer (i.e. the concentration of O2 at the beginning of the experiment). The rate of ascorbate/TMPD-driven O2 consumption was further measured until most of the O2 in the cuvette was consumed. A representative O2 electrode tracing of this assay is shown in Figure 1B and discussed further in Results.
Figure 1.
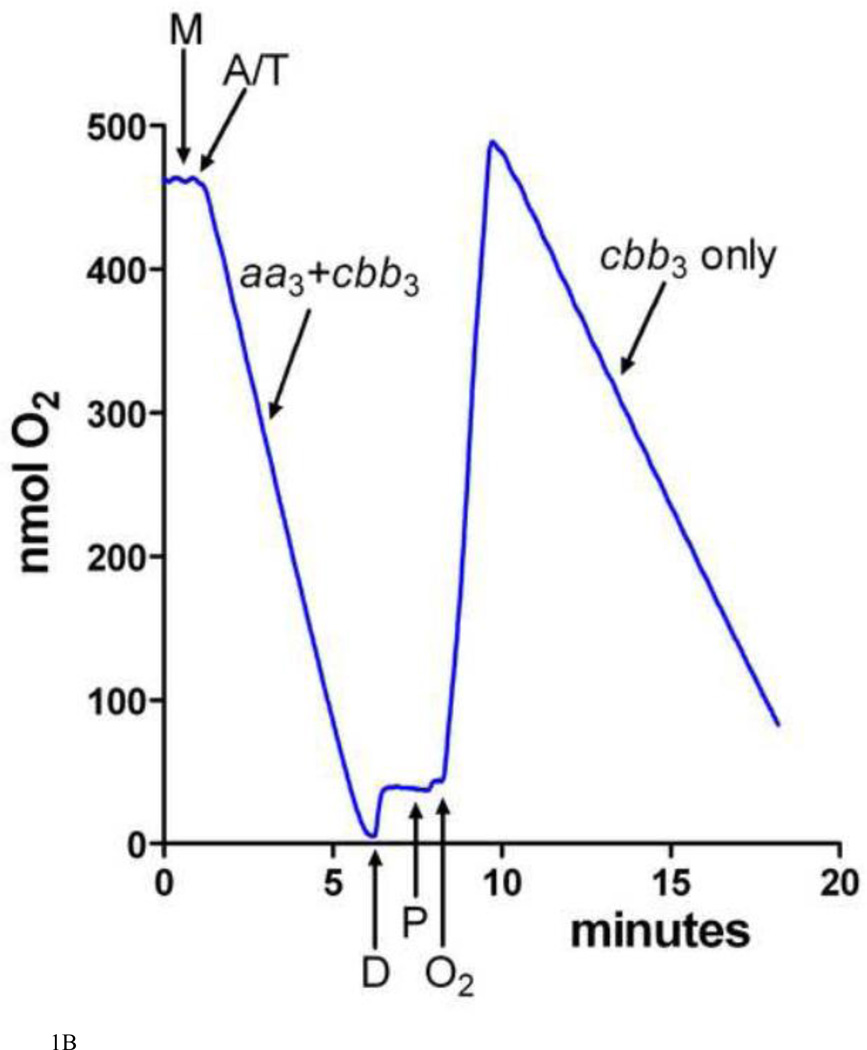
A. The effect of dodecyl maltoside on the TMPD oxidase activity of the aa3-type CcO and the cbb3-type CcO in purified cytoplasmic membranes. The activity of the aa3-type CcO was assayed in membranes isolated from R. sphaeroides CBB3Δ, which lacks the structural genes for the cbb3-type CcO [77]. The activity of the cbb3-type CcO was assayed in membranes isolated from R. sphaeroides YZ200, which lacks the coxII-III operon for the aa3-type CcO [78]. The activities of the intact membranes are set to 100% and the assays were performed as described in Methods. The TMPD oxidase activity of the aa3-type CcO is lost when detergent disrupts its interaction with membrane-bound cytochrome cy, but the TMPD oxidase activity of the cbb3-type CcO is retained. Error is standard deviation. B. A representative O2 electrode tracing of the assay for the TMPD oxidase activity of the aa3-type and cbb3-type CcOs. See Methods and Results for details. Intact, purified cytoplasmic membranes (M) are added to the reaction cell and O2 consumption is initiated by the addition of ascorbate and TMPD (A/T). The first register measures total CcO activity (aa3 plus cbb3). After all O2 has been consumed, dodecyl maltoside (D) is added to solubilize the membranes and thereby disrupt the interaction of cytochrome cy with the aa3-type CcO. After two minutes, soybean phospholipids are added (P) followed by humidified O2, which restores O2 consumption activity. In the second register, only the activity of the cbb3-type CcO is measured.
Copper content measurements. Purified CcO samples were incubated in 20 mM Tris-HCl, pH 7.4 (prepared in low-metal Nanopure water) containing 1.0 mM EDTA for 5 minutes and then the EDTA plus any other low molecular weight species were removed by a series of washes in 20 mM-Tris HCl, pH 7.4, in an ultrafiltration device with a 50 kDa cutoff membrane until the EDTA concentration was calculated to be <0.01 µM. Samples containing 3.5 ml of 4 µM protein were injected into a Spectro Genesis ICP-OES spectrometer to simultaneously measure the concentrations of copper at 324.754 nm and sulfur at 180.731 nm. Each analysis yields the average of three successive determinations and each sample was analyzed two-three times. The element standards used to develop the regression lines were purchased from Inorganic Ventures. The concentration of CcO was obtained by dividing the sulfur concentration by the sum of cysteines and methionines in R. sphaeroides CcO (54).
Other. Cytoplasmic membranes were purified as in Hosler et al [1]. The aa3-type CcO was purified by Ni-affinity chromatography [41] followed by FPLC anion exchange chromatography on DEAE-5PW (Toso-Haas) [51]. The concentrations of total membrane protein were determined using the BioRad DC Protein Assay system.
Results
Measuring the accumulation of fully assembled aa3 and cbb3 CcOs in the cytoplasmic membranes of R. sphaeroides cells. In order to determine the effect of PrrC and PCuAC on the assembly of the aa3-type and cbb3-type CcOs of R. sphaeroidesit is necessary to assess the accumulation of the active, and therefore fully assembled, protein complexes in the cytoplasmic membranes of various strains. Visible spectroscopy has often been used to measure the accumulation of the aa3-type CcO due to its unique ± band absorbance ~605 nm. However, several studies have demonstrated the ability of R. sphaeroidescells to insert incompletely assembled, inactive aa3-type CcO complexes into the membrane [23, 41, 51, 52]. Many of these partially assembled forms contain heme a and thereby absorb in the ± band region. Thus, in a population of the aa3-type CcO containing partially and fully assembled forms it is difficult to parse the fraction of fully assembled CcO by visible spectroscopy. Visible spectroscopy of the cbb3-type CcO in the intact membrane is confounded by absorbance signals arising from other b-and c-type cytochromes.
In this study, ascorbate/TMPD-driven O2 consumption has been used to assess the accumulation of fully assembled aa3-type and cbb3-type CcO in purified cytoplasmic membranes. Both CcO types efficiently oxidize TMPD as long as a cytochrome c is present [61, 62]. Soluble cytochromes care removed during the purification of the cytoplasmic membranes, but membrane-bound cytochrome cy is present [20]. The cytochrome cy:aa3 complex in the intact membrane catalyzes rapid TMPD oxidation, but disruption of the membrane with low levels of dodecyl maltoside stops electron flow from ascorbate/TMPD (Figure 1A). In contrast, the cbb3-type CcO contains extramembrane c-type cytochromes that allow this enzyme to oxidize TMPD in both its membrane-bound and detergent-solubilized forms (Figure 1A). An oxygen electrode tracing of the assay, as described in Methods, is shown in Figure 1B. Ascorbate/TMPD-driven O2 consumption by the intact membrane reflects the activity of both CcOs, while after dissolution of the membrane by dodecyl maltoside only the cbb3-type CcO is capable of oxidizing TMPD. The activity of the aa3-type oxidase is obtained by subtraction. The detergent to membrane protein ratio is maintained within experimentally determined limits to be sure that the cytochrome cy:aa3 complex is fully dissociated. The amount of cytochrome cy is sufficient to catalyze high rates of TMPD oxidation by the aa3-type CcO in membranes isolated from wild-type cells grown in copper-sufficient media (Figure 2A). Therefore, the content of cytochrome cy is unlikely to be rate limiting for samples where the rates of TMPD oxidation are slower. Because the purified intact membranes may contain sealed vesicles, uncoupler (CCCP plus valinomycin) is added to prevent the formation of any membrane potential that could slow CcO activity. The membrane permeability of TMPD ensures its access to both sides of a vesicle and the use of saturating concentrations of ascorbate and TMPD avoids the partitioning of electrons when both CcOs are active.
Figure 2.
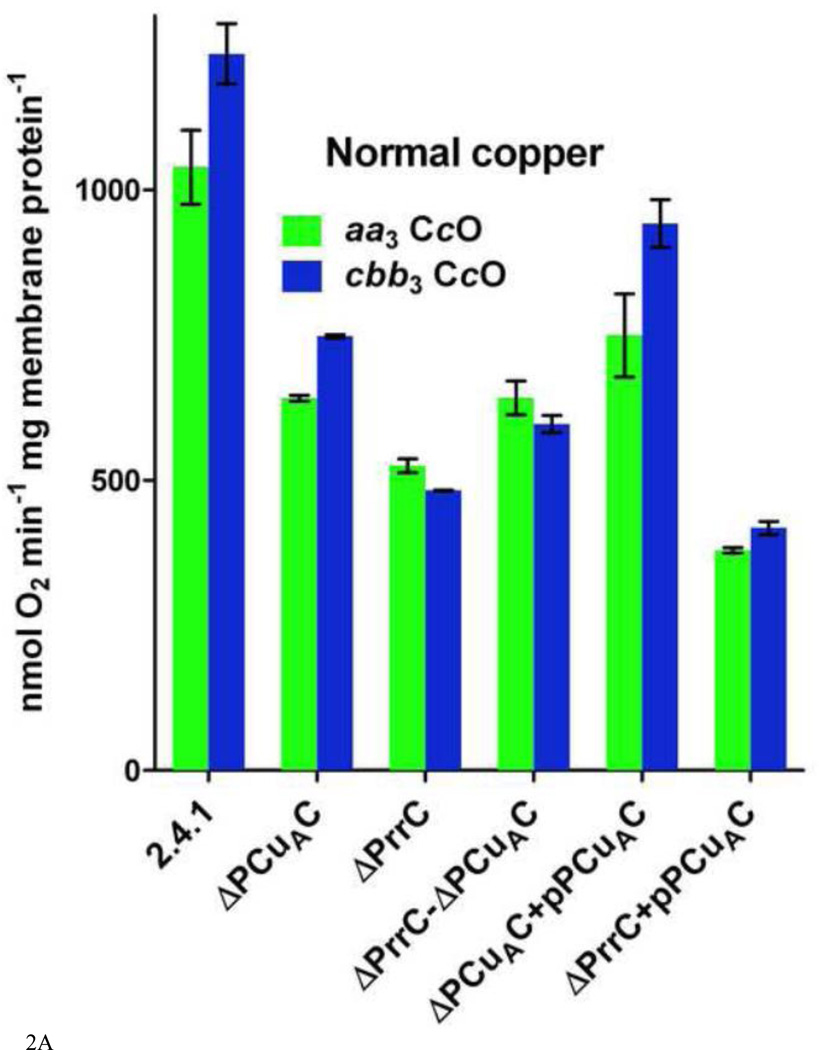
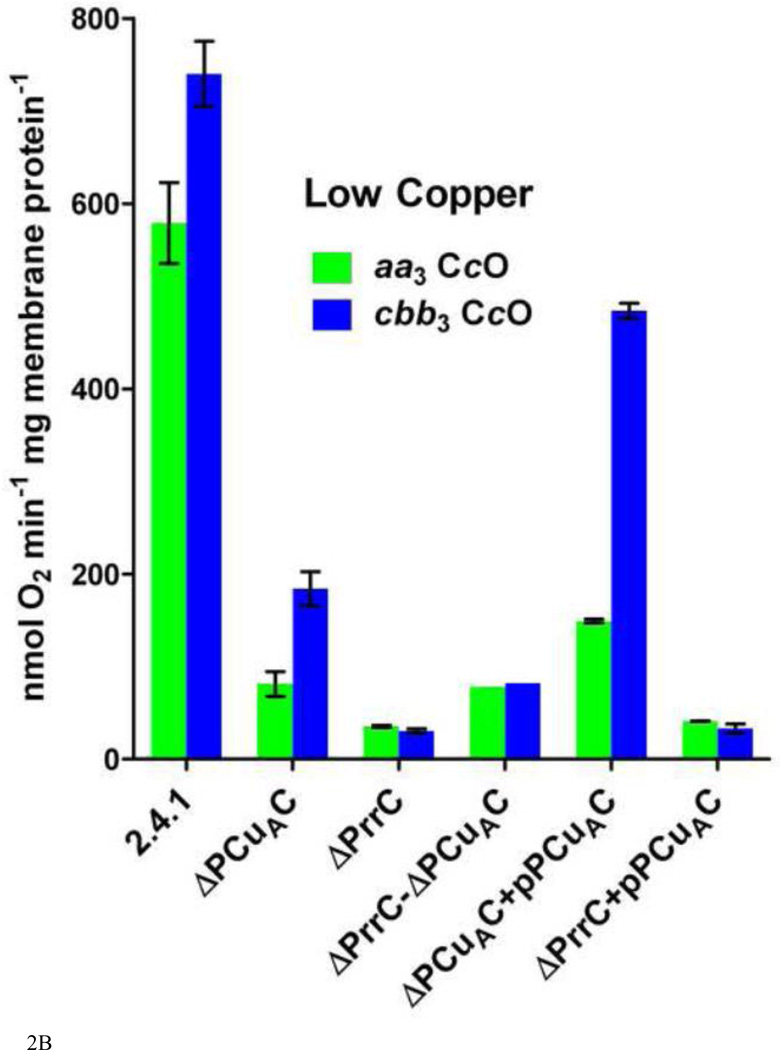
The activities of the aa3-type and cbb3-type CcOs in cytoplasmic membranes purified from wild-type R. sphaeroides cells and from strains containing different amounts of the copper chaperones PrrC (Sco) and PCuAC. Assays were performed as described in Methods and Results. A. Oxidase activities in membranes isolated from cells grown in 1.6 µM Cu2+ B. Oxidase activities in membranes isolated from cells grown in <50 nM Cu2+ Error is standard deviation.
The ability of R. sphaeroidesto express multiple terminal oxidases [63] also assists assays of the accumulation of the two cytochrome c oxidases. A 94–96% reduction in the content of the cytochrome c oxidases (shown below) has little effect on the growth rate of the cells (data not shown), even though aerobic growth of R. sphaeroides is dependent upon a functional terminal oxidase. The likely reason is the expression of a quinol oxidase with homology to the heme bb-type (non-copper) quinol oxidase of Pseudomonas aeruginosa [64]. Carbon-monoxide difference spectra (not shown) of membranes isolated from R. sphaeroides cells lacking the cbb3-type CcO [65] are consistent with expression of the heme bb-type quinol oxidase during aerobic growth. Characterization of this enzyme from Pseudomonas nautica indicates that it has no TMPD oxidase activity [66], consistent with our observations.
The effect of PrrC and PCuAC deletions on CcO accumulation in the membrane. The activity measurements using purified cytoplasmic membranes show that wild-type R. sphaeroides cells (strain 2.4.1), containing normal levels of PrrC and PCuAC, accumulate both the aa3-type and the cbb3-type oxidases during aerobic growth (Figure 2A). A derivative of R. sphaeroides2.4.1 (wild-type) that lacks PrrC (PRRC4 or ΔPrrC) was obtained from the laboratory of Sam Kaplan, UT Health Sciences, Houston [58]. R. sphaeroides ΔPCuAC was created by inactivating the gene for PCuAC in R. sphaeroides 2.4.1 using a pKNOCK construct as described in Methods. A strain lacking both chaperones (ΔPrrC-ΔPCuAC) was created by using the same pKNOCK construct to inactivate the gene for PCuAC in R. sphaeroides PRRC4. In cells grown aerobically in media containing 1.6 µM Cu2+, the absence of PrrC leads to a 50% decrease in the content of fully-assembled aa3-type CcO and a 60% loss of fully-assembled cbb3-type CcO (Figure 2A). The absence of PCuAC leads to a 40% decrease in both oxidases. These decreases imply that both PrrC and PCuAC play a role in the assembly of the aa3-type CcO and the cbb3-type CcO. However, the continued accumulation of significant amounts of both oxidases in the absence of either or both chaperones indicates the existence of multiple pathways for copper delivery.
The R. sphaeroides strains lacking PrrC and PCuAC were then grown in copper-deficient media, as described in Methods. With a concentration of <50 nM Cu2+ in the media, the amount of free copper in the periplasmic space of each cell is vanishingly small. Nevertheless, R. sphaeroides 2.4.1 grows as rapidly in media containing <50 nM Cu2+ as it does in media containing 1.6 µM Cu2+ (data not shown). In wild-type cells grown in low copper, the amounts of fully-assembled aa3-type CcO and cbb3-type CcO in the cell membrane each decrease by ~40% (Figure 2A,B). Assuming an initial Cu2+ concentration of 50 nM, simple calculations indicate that by the time of cell harvest essentially all of the copper initially present in the media has been captured and incorporated into aa3-type and the cbb3-type heme-Cu oxidases. In order to accomplish this, the cells must be using systems with a high affinity for copper and with a high efficiency for the transfer of captured copper to the three copper binding sites in the two heme-Cu oxidases. Therefore, the extent of copper center assembly in the copper chaperone mutants grown in copper-deficient media should best reveal the capability of each chaperone to contribute to the assembly of each center.
In cells grown in low copper media, the absence of PrrC (ΔPrrC) has a strong effect on the assembly of both heme-Cu oxidases. ΔPrrC accumulates fully assembled aa3-type and cbb3-type CcO to only 6% and 4%, respectively, of their levels in normal cells grown in low copper (Figure 2B). The absence of PCuAC has a significant but less detrimental effect on oxidase assembly. Accumulation of the fully assembled aa3-type CcO in ΔPCuAC cells is 14% that of wild-type cells grown in low copper media while the accumulation of active cbb3-type CcO is 25% that of wild-type (Figure 2B). While the reduced accumulations of active aa3-type CcO could be due to decreased CuA or CuB assembly, or both, the decreases in active cbb3-type CcO should only reflect impaired assembly of its CuB center.
A significant question is whether PrrC and PCuAC have parallel, redundant roles in the assembly of the copper centers or more separate functions. Two tests were performed to explore this question. First, a strain lacking both PrrC and PCuAC was prepared. If the role of PCuAC in the assembly of either oxidase is redundant to that of PrrC it would be expected that removing both chaperones would have a greater effect on the accumulation of the fully assembled oxidase than removing PrrC or PCuAC individually. In fact, the assembly and accumulation of the aa3-type CcO and the cbb3-type CcO in cells lacking both PrrC and PCuAC is no less than in cells lacking only PrrC or only PCuAC (Figure 2). This suggests separate functions for the two chaperones. However, for unknown reasons the double deletion strain accumulates slightly greater amounts of both oxidases under both normal copper and low copper growth conditions. This clouds the interpretation of the result. As a second test, an expression plasmid for PCuAC was created (pPCuAC; see Methods) in order to vary the in vivo ratio of PCuAC to PrrC. The introduction of pPCuAC into the copper-limited ΔPCuAC strain increases the accumulation of the aa3-type CcO from 14% to 26%, compared to wild-type, and the synthesis of the cbb3-type CcO from 25% of wild-type to 65% (Figure 2B). The incomplete restoration of CcO assembly suggests that the plasmid-borne expression of PCuAC is less efficient than the expression of PCuAC from the genome. Nonetheless, the introduction of pPCuAC has an obvious effect. Therefore, it is significant that using pPCuAC to increase the amount of PCuAC in cells lacking PrrC fails to enhance the assembly of the aa3-type CcO and yields but a slight increase in the accumulation of the cbb3-type CcO, from 4% to 8% (Figure 2B). The results suggest that PCuAC cannot compensate for the absence of PrrC in the synthesis of the aa3-type CcO or the cbb3-type CcO, i.e. the in vivorole of the two chaperones is not redundant. The results obtained using the same strains grown in normal copper (Figure 2A) support this conclusion.
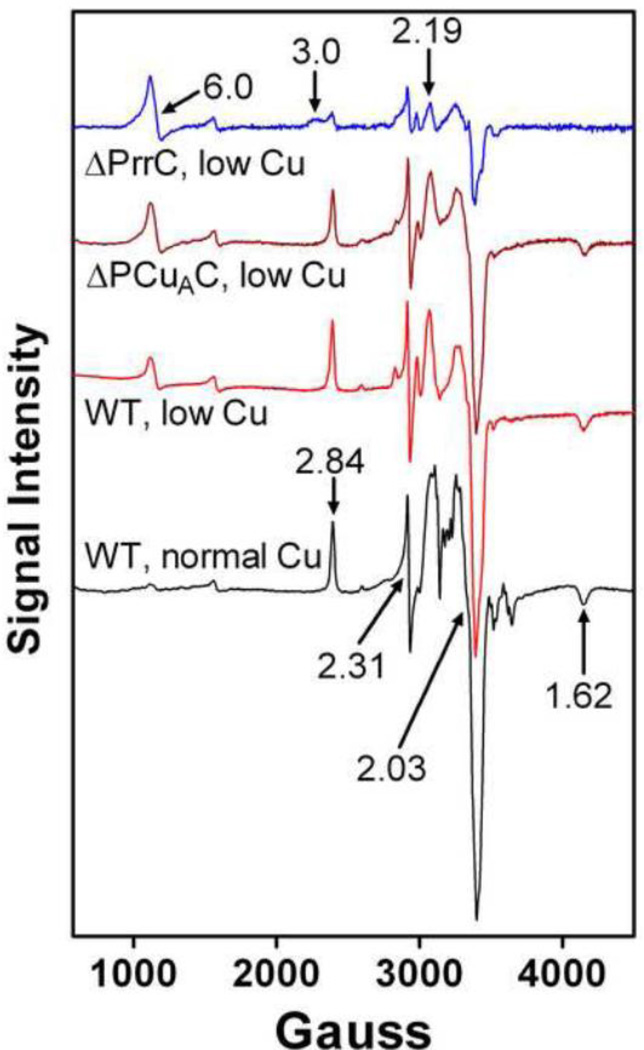
The effect of PCuAC and PrrC deletions on the CuA and CuB centers of the aa3-type CcO. Unlike the cbb3-type CcO which contains a single copper as CuB, decreased accumulation of active aa3-type CcO in the chaperone deletion strains could result from impaired assembly of CuB or the di-copper CuA center, or both. In order to explore these possibilities, the aa3-type CcO was purified from wild-type R. sphaeroides cells, ΔPCuAC and ΔPrrC and examined by X-band EPR spectroscopy and metal analysis. Figure 3 shows the EPR spectra of four purified CcO samples, all normalized using the content of six-coordinate heme a as reported by α-band absorbance at 604–605 nm. The presence of heme a is a constant feature in both fully-assembled and partially-assembled aa3-type CcO forms in R. sphaeroides, except for the apo-oxidase complex [23, 41, 51, 52]. As shown in previous studies [23, 41, 51], relative changes in the content of CuA can be assessed using the peak-to-trough amplitude of the g = 2.03 signal for CuA in X-band EPR spectra of oxidized CcO. The population of the aa3-type CcO purified from wild-type cells grown in copper-depleted media shows ~30% less CuA than CcO isolated from wild-type cells grown in copper-sufficient media. This indicates that the lower content of active aa3-type CcO in membranes of wild-type cells grown in low copper (Figure 2) is mostly due to the decreased assembly of CuA. The result also confirms that copper is a limiting reagent for R. sphaeroides cells grown in low copper media. The absence of PCuAC further lowers the CuA content of purified aa3-type CcO by approximately 15%. Assembly of the aa3-type CcO in the presence of PCuAC but the absence of PrrC has a more pronounced effect; the CuA content is reduced to ~20% of that present in the aa3-type CcO isolated from wild-type cells grown in 1.6 µM Cu2+ or ~30% of the CuA content of wild-type cells grown in low copper. Protein gels (not shown) indicate that the loss of CuA is not accompanied by a decrease in the content of subunit II. Unlike CuA, there are no direct signals for CuB in the X-band EPR spectrum. There are, however, two heme-specific transitions that are qualitatively characteristic for CcO lacking CuB, as described previously for CcO that assembles in the absence of functional Cox11 [41, 42]. In normal, oxidized CcO, heme a3 and CuB are strongly spin-coupled and therefore EPR silent [67]. When CuB is reduced to Cu1+ spin coupling is lost and an axial signal indicative of high-spin heme appears at g ~6 [68]. The same is true for the aa3-type CcO of R. sphaeroides that lacks CuB but contains all of the other metal centers (termed ΔCox11 or ΔCuB) [41]. However, in ΔCuB the intensity of the high-spin heme signal at g ~6 is always less than an equivalent concentration of a high-spin heme standard and the shape of the signal can vary (data not shown). Recent resonance Raman spectra of an R. sphaeroides CcO form lacking CuB shows the presence of significant amounts of low-spin, six-coordinate heme a3 (Rousseau, unpublished data). From this result it follows that structural heterogeneity of heme a3 in CcO molecules lacking CuB apparently leads to a mixture of low-spin and high-spin heme a3, which accounts for the observed variability in the g ~6 signal. Thus, the increasing amplitudes of the g ~6 signal in the EPR spectra of ΔPCuAC and ΔPrrC (Figure 3) reflect the loss of CuB but it is difficult to use this signal to quantify the extent of the loss.
Figure 3.
EPR spectra of purified aa3-type CcO assembled in wild-type cells grown in 1.6 µM Cu2+ or <50 nM Cu2+ (low Cu), and in cells lacking PCuAC or PrrC and grown in <50 nM Cu2+. The spectra were recorded at X band using a Bruker (Billerica, MA) EMX spectrometer. Each spectrum is an average of ten scans taken at 10 K using 25–50 µM CcO. The spectra were recorded using a microwave power of 2 mW at 9.38 GHz. The sweep time was 160 s, and the time constant was 83 ms. The amplitudes of the spectra were normalized by the heme a content of the samples as described in Results.
Another CuB-related signal arises from low-spin heme a, which is close to heme a3 in subunit I. Heme a of normal CcO shows signals at g = 2.84, 2.31 and 1.62. In the absence of CuB, the signals are altered, e.g. the g = 2.84 signal shifts to g = 3 and becomes more broad, probably due to multiple orientations of heme a. The broad g = 3.0 signal is most evident in the EPR spectrum of CcO isolated from ΔPrrC cells (Figure 3) indicating that this protein contains less CuB than CcO isolated from ΔPCuAC or wild-type cells.
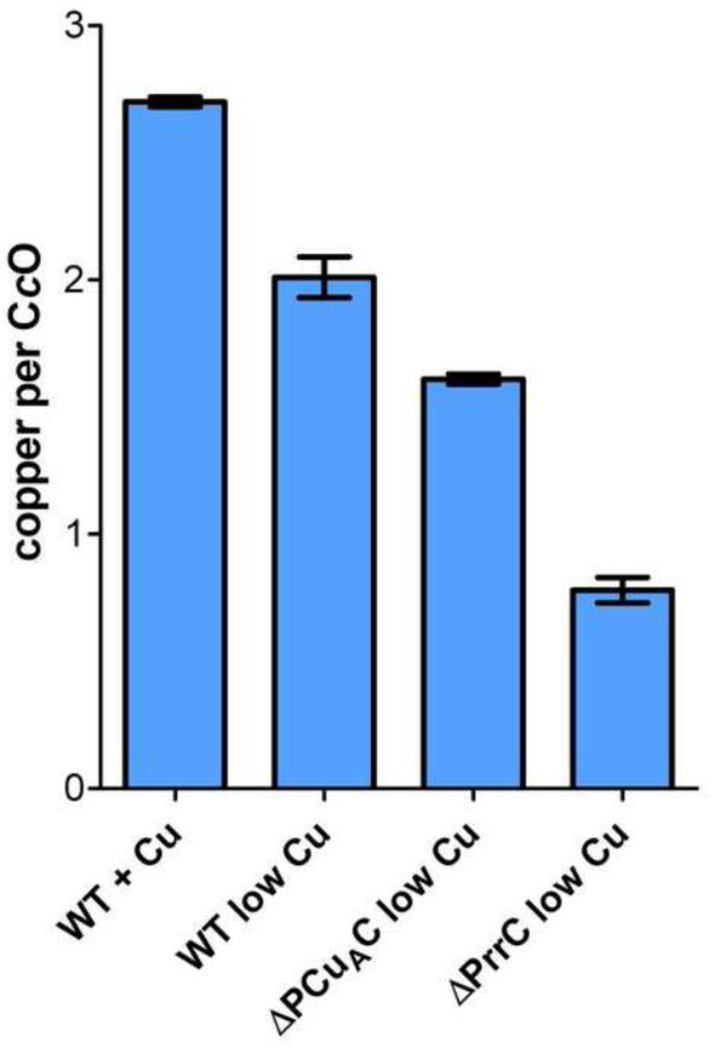
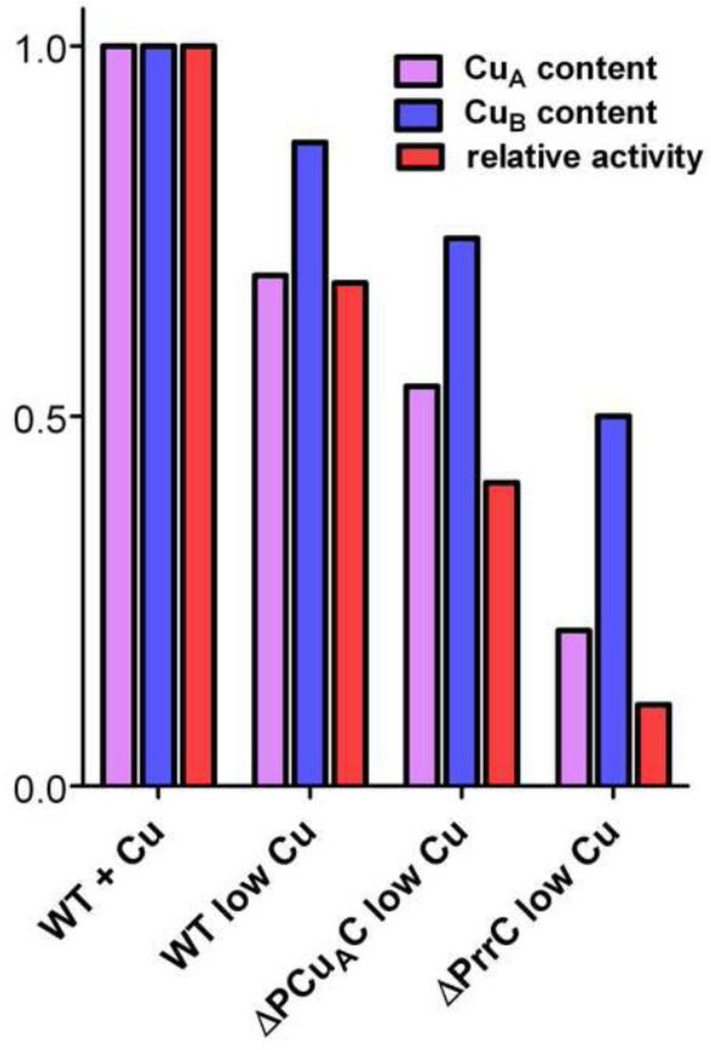
Quantitative estimates of the relative amounts of CuA and CuB present in the samples of purified aa3-type CcO are possible if the total amount of copper is also known. The total copper content of the samples was determined using ICP-OES (Figure 4); the concentration of CcO was simultaneously determined from the sulfur content. The copper per CcO value for the complex isolated from wild-type cells grown in copper-sufficient media is 2.7, within 10% of the value of 3.0 expected for absolutely pure, fully-assembled CcO. Protein gels (not shown) indicate that the error can be attributed to additional sulfur from small amounts of contaminating protein, and that the level of protein contamination is similar in all of the samples. The total copper content of the aa3-type CcO purified from ΔPCuAC is ~60% that of the copper present in wild-type cells grown in 1.6 µM Cu2+, while the aa3-type CcO isolated from ΔPrrC contains 29% of the normal amount of copper (Figure 4). The fractional amounts of CuA (Figure 5) present in the aa3-type CcOs isolated from wild-type R. sphaeroides, ΔPCuAC and ΔPrrC are estimated from the g = 2.03 signals of the EPR spectra of Figure 3, as discussed above. In order to estimate the amount of CuB, the amount of copper present as CuA is subtracted from the total amount of copper given by the data of Figure 4. The calculations assume two coppers per CuA because studies of the reconstitution of CuA sites show that the normal set of CuA ligands will bind two coppers, but not one [69–71]. The results compiled in Figure 5 show that the absence of PCuAC or PrrC affects the assembly of both CuA and CuB, but the absence of either chaperone has a greater effect on the assembly of CuA than CuB. Also, the absence of PrrC has a greater effect on the assembly of CuA and CuB than does the absence of PCuAC.
Figure 4.
Copper content per CcO of the aa3-type CcO samples used in Figure 3. The number of coppers per CcO was determined by ICP-OES as described in Methods. Error is standard deviation.
Figure 5.
The relative CuA content, CuB content and O2 reduction activity of purified aa3-type CcO assembled in wild-type cells grown in 1.6 µM Cu2+ or <50 nM Cu2+ (low Cu), and in cells lacking PCuAC or PrrC and grown in <50 nM Cu2+. The fractional content of CuA, relative to wild-type cells grown in 1.6 mM Cu2+, is taken from the amplitudes of the g = 2.03 signal in Figure 3. The fractional content of CuB is estimated as described in Results. The relative activities are taken from the TNmax values for cytochrome c-driven O2 reduction measured at pH 6.5 as described in Varanasi and Hosler [59]. The TNmax for the wild-type aa3-type CcO grown in 1.6 mM Cu2+ (100%) is 1717 ± 41 e− sec−1.
The decreased content of CuB in the aa3-type CcOs of ΔPrrC and ΔPCuAC could be an indirect consequence of impaired assembly of CuA if the CuB center is destabilized by the absence of CuA. In other words, the loss of CuB may only occur in those CcO molecules that already lack CuA. However, previous work shows that the reverse interaction does not take place, i.e. the absence of CuB does not destabilize CuA [41]. Moreover, the presence of a stable CcO form that lacks CuA but not CuB can be deduced from the observation that the CcO sample purified from wild-type R. sphaeroides cells grown in low copper shows a 30% loss of CuA but only a 10% loss of CuB (Figure 5). The decreased contents of CuB (Figure 5) could also arise from the formation of ΔCuB, i.e. CcO lacking only CuB, in the absence of PCuAC or PrrC. Measurements of the O2 reduction activities of the CcO forms (Figure 5) support the latter hypothesis. For the CcOs isolated from ΔPCuAC and ΔPrrC, the activities of the purified CcO populations indicate the presence of greater amounts of inactive CcO than would be predicted if CuB is absent only from those CcO molecules that already lack CuA. In fact, for CcO isolated from ΔPrrC, the measured activity fits well to that predicted assuming separate populations of CcO that are inactive because they lack either CuA or CuB.
Discussion
Both PCuAC and PrrC enhance the assembly of the aa3-type CcO, particularly when exogenous copper levels are low (<50 nM Cu2+), a situation in which the cell’s more efficient pathways for copper capture and delivery should predominate. Under low copper conditions, the absence of PrrC decreases the accumulation of fully-assembled aa3-type CcO in the bacterial membrane by up to 94% while the absence of PCuAC decreases its accumulation by up to 86% (Figure 2B). The effect of deleting PrrC is similar to results obtained using strains of Bacillus subtilus [36] and Bradyrhizobium japonicum [37] that lack their Sco proteins, as well as the losses of mitochondrial CcO in eukaryotic cells lacking a functional Sco1 [30]. Increasing the exogenous copper level from <50 nM to 1.6 µM partially restores the assembly of the aa3-type CcO in the absence of PrrC in R. sphaeroides (Figure 2A); similar results have been reported for strains of B. subtilus and B. japonicum lacking Sco1 [36, 37], and in human cells lacking fully functional Sco2 [72, 73]. The chaperone associated with CuB assembly in the aa3-type CcO, Cox11, is present in R. sphaeroides ΔPrrC and ΔPCuAC. Accordingly, normal assembly of CuB takes place in ΔPrrC and ΔPCuAC when they are grown in copper-sufficient media (data not shown). As for CuA assembly, the greater concentration of Cu2+ may facilitate its self-assembly or it may drive the metallation of CuA by a chaperone with a low efficiency of copper delivery to CuA. The presence of sufficient exogenous copper may be responsible for the finding that the deletion of PCuAC in B. japonicum has no effect on the CcO activity of aerobically-grown cells [48].
PrrC and PCuAC also enhance the assembly of the cbb3-type CcO, especially in cells grown in limited amounts of copper. In the absence of PrrC, the accumulation of active cbb3-type CcO is reduced by 96% while in the absence of PCuAC its accumulation decreases by 75% (Figure 2B). Once again, increasing the exogenous copper concentration ~thirty-fold partially compensates for the absence of these chaperones. The results obtained for PrrC agree with earlier reports showing that the deletion of the Sco proteins of R. capsulatus and Pseudomonas aeruginosa lowers the accumulation of active cbb3-type CcO in these bacteria [43–45]. In apparent contradiction, the deletion of Sco1 of B. japonicum has little effect on the accumulation of its cbb3-type CcO [37], but it is not clear if exogenous copper concentrations were limiting. A previous study of R. sphaeroides PRRC4 (ΔPrrC) reported no large loss of total CcO activity (aa3 plus cbb3) in this strain. This is actually consistent with the results reported here as the cells of the previous study were grown in the presence of 1.6 µM Cu2+ [58]. Examination of the requirement for a copper chaperone under conditions where the metal is a limiting reagent appears necessary for concluding whether or not the chaperone contributes to metal center assembly.
Cellular roles of PCuAC and PrrC in the assembly of the CuB center of the aa3-type CcO. In studies in B. subtilus, B. japonicum and mitochondria, the decreased accumulation of the aa3-type CcO in the absence of Sco has been attributed to impaired assembly of the CuA center [30, 36, 37]. The results of this study show that the absence of PrrC, and to a lesser extent PCuAC, impairs the assembly of both CuA and CuB of the aa3-type CcO (Figure 5). The role of PrrC, in particular, can be described as specific since in the absence of PrrC some CcO assembles with CuA but without CuB. Nonetheless, CuB assembly requires Cox11, which is present in all of the strains used in these experiments, and neither PrrC nor PCuAC can assemble CuB in the absence of Cox11. One possible explanation of these findings is that PrrC delivers copper to Cox11 more efficiently than Cox11 self-metallates in the presence of low exogenous Cu2+ concentrations.
Cellular roles of PCuAC and PrrC in the assembly of CuA of the aa3-type CcO. The question of how Sco and PCuAC participate in the assembly of CuA has previously been addressed using an in vitro system by Abriata et al [40]. A soluble form of PCuAC of Thermus thermophilus delivered both coppers to the ligands of CuA in a soluble form of subunit II of the ba3-type CcO of T. thermophilus. In the same study, a soluble form of Sco of T. thermophilus was incapable of delivering copper into apo-CuA but it could provide the disulfide reductase activity necessary to reduce the cysteines of the CuA center prior to copper delivery.
In this study, the analyses of purified aa3-type oxidase show that the absence of PrrC or PCuAC primarily impairs the assembly of CuA, with PrrC having the greater effect. The assembly of CuB is also affected, as discussed above, but to a lesser extent. In addition to the analyses of purified CcO, the measurements of the accumulation of active aa3-type CcO in the cytoplasmic membranes of different strains of R. sphaeroides (Figure 2) can be used to explore how Sco (PrrC) and PCuAC participate in the assembly of CuA in the cell. This is possible because 1) the activity of the aa3-type CcO depends upon the successful assembly of both CuB and CuA, and 2) the major effect of deleting either chaperone is impairment of the assembly of CuA.
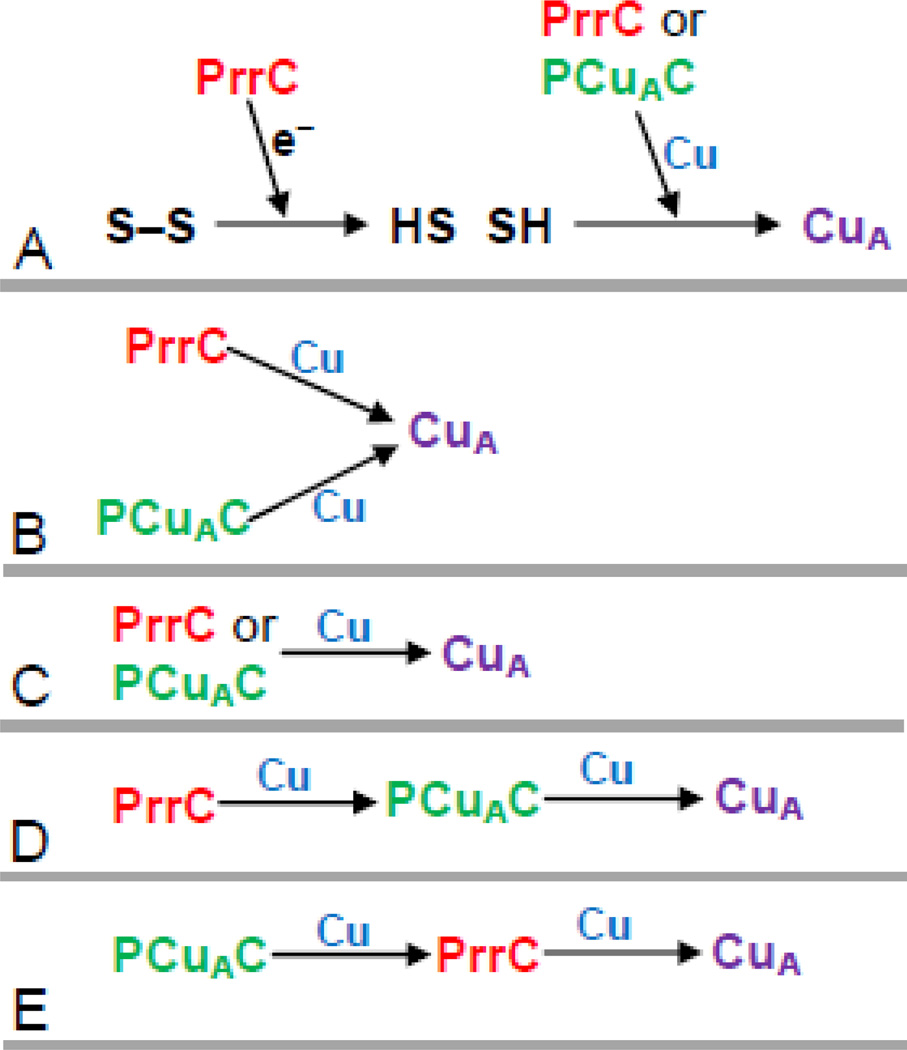
Several scenarios of the cellular roles of the two copper chaperones in the process of CuA assembly can be envisioned for discussion (Figure 6). Only one of these, Scenario E, is fully consistent with the results of this study. PrrC has been shown to have disulfide reductase activity [39]. Extrapolating from the results of Abriata et al [40], Scenario A of Figure 6 explains the strong effect of the deletion of PrrC by positing that PrrC does not deliver copper but rather functions as the predominant disulfide reductase in the periplasm that prepares the cysteines of the nascent CuA center for copper binding. However, Scenario A is not consistent with results presented here and elsewhere. First, PrrC is not required for increased levels of CuA assembly in ΔPrrC cells grown in higher copper, even though the requirement for PrrC as the predominant disulfide reductase should remain. Second, B. subtilus and B. japonicum also assemble CuA without Sco, given sufficient copper [36, 37]. Third, both B. japonicum and R. sphaeroides contain a separate membrane-bound disulfide reductase (tlpA) that has been implicated in the assembly of the aa3-type CcO [74]. Scenario B of Figure 6 posits that PCuAC and PrrC each deliver one copper to CuA. In this case, the removal of either chaperone from the cell should have the same effect on the accumulation of active enzyme. However, the observation is that the removal of PrrC has a significantly greater effect than the removal of PCuAC. In Scenario C, PrrC and PCuAC are independently capable of delivering both coppers to CuA. The greater effect of PrrC on CuA assembly does not rule out this possibility, e.g. PrrC could be present in significantly greater amounts than PCuAC. However, Scenario C does predict that removing both PrrC and PCuAC should impair the assembly of the aa3-type oxidase more than removing either chaperone alone. This is not observed; the double deletion of PrrC and PCuAC shows no greater loss of the aa3-type CcO than either of the separate deletions. Moreover, increasing the PCuAC population in the cell membrane fails to enhance the assembly of CuA if PrrC is absent (ΔPrrC + pPCuAC; Figure 2B). This is inconsistent with a significant role for PCuAC in directly delivering both coppers to CuA. The failure of increased expression of PCuAC to enhance CcO assembly in cells lacking PrrC also disfavors Scenario D, in which PCuAC delivers both coppers to CuA while the (observed) stronger requirement for PrrC is postulated to derive from its ability to drive the assembly process by efficiently metallating PCuAC. Scenario D is further disfavored by the evolutionary relationship between R. sphaeroides and mitochondria. Mitochondria do not contain a homolog of PCuAC, but PrrC is homologous to mitochondrial Sco1 (the primary sequence of the extramembrane domain of PrrC is >50% similar to that of human Sco1). The catalytic cores of the aa3-type CcO of R. sphaeroides and mitochondrial CcO are highly similar, and mitochondria contain homologs of other bacterial assembly proteins required for assembly of the catalytic core (Cox11, Surf1, Cox10, Cox15). These homologies make it likely that the assembly process for CuA in R. sphaeroides will be fundamentally similar to that of mitochondria. Scenario E, in which PrrC (Sco) delivers both coppers to CuA, is consistent with the all of the data of this study, as well as with previous observations in B. subtilus, B. japonicum and mitochondria [30, 36, 37]. PCuAC is posited to supply copper to PrrC, based on the finding that its presence does enhance the assembly of CuA. This assignment is equivalent to stating that PCuAC plays a role in copper homeostasis, with the explicit recognition that copper transfer at low copper concentrations must require protein-protein interaction. In Scenario E, the role of PCuAC is similar to that of Cox17 of mitochondria, as previously proposed [46]. A homolog of Cox17 is not present in bacteria.
Figure 6.
Possible schemes for the functions of PCuAC and PrrC in the assembly of CuA of the aa3-type CcO. Explanations are provided in the text. In Scheme A the sulfurs (S) are those of the two cysteine ligands of CuA.
As yet, there exists no obvious way to reconcile Scenario E of Figure 6 with the in vitro CuA assembly study of Abriata et al [40] in which T. thermophilus Sco does not deliver copper to soluble T. thermophilus CuA. Interestingly, T. thermophilus lacks Cox11, indicating that its system for the assembly of CuB of its ba3-type CcO differs from those bacteria that synthesize a mitochondrial-like aa3-type CcO. Thus, T. thermophilus may also have different requirements for the assembly of CuA of the ba3-type CcO. As stated above, the similarities between the aa3-type CcOs of R. sphaeroides and mitochondria, along with the retention of Cox11 and Sco in both, make it likely that their CuA and CuB assembly pathways share basic commonality.
The roles of PrrC and PCuAC in the assembly of CuB of the cbb3-type CcO. The roles of PCuAC and PrrC in the assembly of the CuB center of the cbb3-type CcO appear remarkably similar to their roles in the assembly of the CuA center of the aa3-type CcO. The removal of either PrrC or PCuAC decreases the assembly of CuB in the cbb3-type CcO, using the amount of active oxidase as a measure of the extent of CuB assembly. The removal of PrrC has a greater effect than the removal of PCuAC and increasing the amount of PCuAC in the cell membrane enhances the assembly of CuB only if PrrC is present. Once again, the most straightforward conclusion is that PrrC delivers copper to the CuB center and that this method of assembly predominates when exogenous copper levels are low. PCuAC enhances the assembly of CuB, most likely by facilitating the delivery of copper to PrrC as proposed above. This proposed role for PrrC in R. sphaeroides is consistent with the previous proposals for the roles of the Sco proteins of R. capsulatus and P. aeruginosa in the assembly of their cbb3-type CcOs [43, 44]. Moreover, Ekici et al [28] report physical interactions between R. capsulatus Sco (SenC) and subunits of its cbb3-type CcO. Further, a putative Cu-ATPase, CcoI, has been shown to be required for the assembly of the cbb3-type CcO in several a proteobacteria [28]. It seems likely that CcoI and the bacterial Sco proteins, including PrrC, cooperate in the assembly of CuB of this oxidase.
Gene regulation by PrrC. Sco proteins have been argued to play a role in gene regulation [43, 58] leading to the consideration that the reduced accumulation of the aa3-type and cbb3-type oxidases observed in R. sphaeroides strains lacking PrrC could result from down-regulated expression of the apo-proteins of the terminal oxidases. Direct gene expression experiments are not included. However, the isolation and analysis of partially-assembled forms of the aa3-type CcO from ΔPrrC cells grown in low copper shows directly that the loss of active CcO is due to decreased assembly of CuA and CuB (Figure 5), and not to decreased expression of the apo-proteins. Consistent with this, the finding that increased levels of copper restore significant levels of both oxidases even in the absence of PrrC (Figure 2A and ref. [58]) argues that the absence of PrrC is not responsible for the low accumulation of both oxidases in cells grown in low copper. The deletion of the Sco homolog (SenC) of R. capsulatus does cause a two-fold decrease in the expression of subunit I of its cbb3-type CcO [43]. However, this decrease in expression was shown to be a secondary result of a larger decrease in CcO accumulation due to impaired assembly of CuB in the absence of SenC, since the expression of the cbb3-type CcO in R. capsulatus is partly controlled by a signal transduction system that responds to the level of its activity [75, 76].
Highlights.
The presence of Sco strongly enhances the assembly of CuA of aa3-type CcO.
The presence of Sco strongly enhances the assembly of CuB of cbb3-type CcO.
The Cu chaperone PCuAC enhances assembly but requires the presence of Sco.
Acknowledgements
Supported by National Institutes of Health Grant GM 56824 (J.P.H.) and National Science Foundation MCB-0843537 (A.L.)
Abbreviations
- CCCP
carbonylcyanide m-chlorophenylhydrazone
- CcO
cytochrome c oxidase
- ICP-OES
inductively coupled plasma optical emission spectroscopy
- TMPD
N,N,N',N'-tetramethyl-p-phenylenediamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hosler JP, Fetter J, Tecklenburg MM, Espe M, Lerma C, Ferguson-Miller S. Cytochrome aa3 of Rhodobacter sphaeroides as a model for mitochondrial cytochrome c oxidase. Purification, kinetics, proton pumping, and spectral analysis. J. Biol. Chem. 1992;267:24264–24272. [PubMed] [Google Scholar]
- 2.Cao J, Hosler J, Shapleigh J, Revzin A, Ferguson-Miller S. Cytochrome aa3 of Rhodobacter sphaeroides as a model for mitochondrial cytochrome c oxidase. The coxII/coxIII operon codes for structural and assembly proteins homologous to those in yeast. J. Biol. Chem. 1992;267:24273–24278. [PubMed] [Google Scholar]
- 3.Shapleigh JP, Gennis RB. Cloning, sequencing, and deletion from the chromosome of the gene encoding subunit I of the aa3-type cytochrome c oxidase of Rhodobacter sphaeroides. Mol. Microbiol. 1992;6:635–642. doi: 10.1111/j.1365-2958.1992.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 4.Svensson-Ek M, Abramson J, Larsson G, Tornroth S, Brzezinski P, Iwata S. The X-ray crystal structures of wild-type and EQ(I-286) mutant cytochrome c oxidases from Rhodobacter sphaeroides. J. Mol. Biol. 2002;321:329–339. doi: 10.1016/s0022-2836(02)00619-8. [DOI] [PubMed] [Google Scholar]
- 5.Qin L, Hiser C, Mulichak A, Garavito RM, Ferguson-Miller S. Identification of conserved lipid/detergent-binding sites in a high-resolution structure of the membrane protein cytochrome c oxidase. Proc. Natl. Acad. Sci. USA. 2006;103:16117–16122. doi: 10.1073/pnas.0606149103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Horsman JA, Berry E, Shapleigh JP, Alben JO, Gennis RB. A novel cytochrome c oxidase from Rhodobacter sphaeroides that lacks CuA. Biochemistry. 1994;33:3113–3119. doi: 10.1021/bi00176a046. [DOI] [PubMed] [Google Scholar]
- 7.Hemp J, Robinson DE, Ganesan KB, Martinez TJ, Kelleher NL, Gennis RB. Evolutionary Migration of a Post-Translationally Modified Active-Site Residue in the Proton-Pumping Heme-Copper Oxygen Reductases. Biochemistry. 2006;45:15405–15410. doi: 10.1021/bi062026u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosler JP, Ferguson-Miller S, Calhoun MW, Thomas JW, Hill J, Lemieux L, Ma J, Georgiou C, Fetter J, Shapleigh J, Tecklenburg MJ, Babcock GT, Gennis RB. Insight into the active-site structure and function of cytochrome oxidase by analysis of site-directed mutants of bacterial cytochrome aa3 and cytochrome bo. J. Bioenerg. Biomembr. 1993;25:121–136. doi: 10.1007/BF00762854. [DOI] [PubMed] [Google Scholar]
- 9.Zhen Y, Hoganson CW, Babcock GT, Ferguson-Miller S. Definition of the interaction domain for cytochrome c on cytochrome c oxidase. I. Biochemical, spectral, and kinetic characterization of surface mutants in subunit II of Rhodobacter sphaeroides cytochrome aa3. J. Biol. Chem. 1999;274:38032–38041. doi: 10.1074/jbc.274.53.38032. [DOI] [PubMed] [Google Scholar]
- 10.Wang K, Zhen Y, Sadoski R, Grinnell S, Geren L, Ferguson-Miller S, Durham B, Millett F. Definition of the interaction domain for cytochrome c on cytochrome c oxidase. II. Rapid kinetic analysis of electron transfer from cytochrome c to Rhodobacter sphaeroides cytochrome oxidase surface mutants. J. Biol. Chem. 1999;274:38042–38050. doi: 10.1074/jbc.274.53.38042. [DOI] [PubMed] [Google Scholar]
- 11.Otten MF, van der Oost J, Reijnders WN, Westerhoff HV, Ludwig B, Van Spanning RJ. Cytochromes c550 c552, and c1 in the electron transport network of Paracoccus denitrificans: redundant or subtly different in function? J. Bacteriol. 2001;183:7017–7026. doi: 10.1128/JB.183.24.7017-7026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rios-Velazquez C, Cox RL, Donohue TJ. Characterization of Rhodobacter sphaeroides cytochrome c2 proteins with altered heme attachment sites. Arch. Biochem. Biophys. 2001;389:234–244. doi: 10.1006/abbi.2001.2330. [DOI] [PubMed] [Google Scholar]
- 13.Myllykallio H, Jenney FE, Jr, Moomaw CR, Slaughter CA, Daldal F. Cytochrome cy of Rhodobacter capsulatus is attached to the cytoplasmic membrane by an uncleaved signal sequence-like anchor. J. Bacteriol. 1997;179:2623–2631. doi: 10.1128/jb.179.8.2623-2631.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drosou V, Malatesta F, Ludwig B. Mutations in the docking site for cytochrome c on the Paracoccus heme aa3 oxidase. Eur. J. Biochem. 2002;269:2980–2988. doi: 10.1046/j.1432-1033.2002.02979.x. [DOI] [PubMed] [Google Scholar]
- 15.Brzezinski P, Reimann J, Adelroth P. Molecular architecture of the proton diode of cytochrome c oxidase. Biochem. Soc. Trans. 2008;36:1169–1174. doi: 10.1042/BST0361169. [DOI] [PubMed] [Google Scholar]
- 16.Kaila VR, Johansson MP, Sundholm D, Laakkonen L, Wikstrom M. The chemistry of the CuB site in cytochrome c oxidase and the importance of its unique His-Tyr bond. Biochim. Biophys. Acta. 2009 doi: 10.1016/j.bbabio.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Sharma V, Puustinen A, Wikstrom M, Laakkonen L. Sequence analysis of the cbb3 oxidases and an atomic model for the Rhodobacter sphaeroides enzyme. Biochemistry. 2006;45:5754–5765. doi: 10.1021/bi060169a. [DOI] [PubMed] [Google Scholar]
- 18.Hemp J, Christian C, Barquera B, Gennis RB, Martinez TJ. Helix switching of a key active-site residue in the cytochrome cbb3 oxidases. Biochemistry. 2005;44:10766–10775. doi: 10.1021/bi050464f. [DOI] [PubMed] [Google Scholar]
- 19.Buschmann S, Warkentin E, Xie H, Langer JD, Ermler U, Michel H. The structure of cbb3 cytochrome oxidase provides insights into proton pumping. Science. 2010;329:327–330. doi: 10.1126/science.1187303. [DOI] [PubMed] [Google Scholar]
- 20.Daldal F, Mandaci S, Winterstein C, Myllykallio H, Duyck K, Zannoni D. Mobile cytochrome c2 and membrane-anchored cytochrome cy are both efficient electron donors to the cbb3- and aa3-type cytochrome c oxidases during respiratory growth of Rhodobacter sphaeroides. J. Bacteriol. 2001;183:2013–2024. doi: 10.1128/JB.183.6.2013-2024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown BM, Wang Z, Brown KR, Cricco JA, Hegg EL. Heme O synthase and heme A synthase from Bacillus subtilis and Rhodobacter sphaeroides interact in Escherichia coli. Biochemistry. 2004;43:13541–13548. doi: 10.1021/bi048469k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soto IC, Fontanesi F, Liu J, Barrientos A. Biogenesis and assembly of eukaryotic cytochrome c oxidase catalytic core. Biochim. Biophys. Acta. 2011 doi: 10.1016/j.bbabio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith D, Gray J, Mitchell L, Antholine WE, Hosler JP. Assembly of cytochrome c oxidase in the absence of assembly protein Surf1p leads to loss of the active site heme. J. Biol. Chem. 2005;280:17652–17656. doi: 10.1074/jbc.C500061200. [DOI] [PubMed] [Google Scholar]
- 24.Hannappel A, Bundschuh FA, Ludwig B. Role of Surf1 in heme recruitment for bacterial COX biogenesis. Biochim. Biophys. Acta. 2011 doi: 10.1016/j.bbabio.2011.09.007. in press. [DOI] [PubMed] [Google Scholar]
- 25.Kulajta C, Thumfart JO, Haid S, Daldal F, Koch HG. Multi-step assembly pathway of the cbb3-type cytochrome c oxidase complex. J. Mol. Biol. 2006;355:989–1004. doi: 10.1016/j.jmb.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 26.Pawlik G, Kulajta C, Sachelaru I, Schroder S, Waidner B, Hellwig P, Daldal F, Koch HG. The putative assembly factor CcoH is stably associated with the cbb3-type cytochrome oxidase. J. Bacteriol. 2010;192:6378–6389. doi: 10.1128/JB.00988-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preisig O, Zufferey R, Hennecke H. The Bradyrhizobium japonicum fixGHIS genes are required for the formation of the high-affinity cbb3-type cytochrome oxidase. Arch. Microbiol. 1996;165:297–305. doi: 10.1007/s002030050330. [DOI] [PubMed] [Google Scholar]
- 28.Ekici S, Pawlik G, Lohmeyer E, Koch HG, Daldal F. Biogenesis of cbb3-type cytochrome c oxidase in Rhodobacter capsulatus. Biochim. Biophys. Acta. 2011 doi: 10.1016/j.bbabio.2011.10.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banci L, Bertini I, Cavallaro G, Ciofi-Baffoni S. Seeking the determinants of the elusive functions of Sco proteins. Febs J. 2011;278:2244–2262. doi: 10.1111/j.1742-4658.2011.08141.x. [DOI] [PubMed] [Google Scholar]
- 30.Cobine PA, Pierrel F, Winge DR. Copper trafficking to the mitochondrion and assembly of copper metalloenzymes. Biochim. Biophys. Acta. 2006;1763:759–772. doi: 10.1016/j.bbamcr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Banci L, Bertini I, Calderone V, Ciofi-Baffoni S, Mangani S, Martinelli M, Palumaa P, Wang S. A hint for the function of human Sco1 from different structures. Proc. Natl. Acad. Sci. USA. 2006;103:8595–8600. doi: 10.1073/pnas.0601375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horng YC, Leary SC, Cobine PA, Young FB, George GN, Shoubridge EA, Winge DR. Human Sco1 and Sco2 function as copper-binding proteins. J. Biol. Chem. 2005;280:34113–34122. doi: 10.1074/jbc.M506801200. [DOI] [PubMed] [Google Scholar]
- 33.Leary SC, Sasarman F, Nishimura T, Shoubridge EA. Human SCO2 is required for the synthesis of CO II and as a thiol-disulphide oxidoreductase for SCO1. Hum. Mol. Genet. 2009;18:2230–2240. doi: 10.1093/hmg/ddp158. [DOI] [PubMed] [Google Scholar]
- 34.Lode A, Kuschel M, Paret C, Rodel G. Mitochondrial copper metabolism in yeast: interaction between Sco1p and Cox2p. FEBS Lett. 2000;485:19–24. doi: 10.1016/s0014-5793(00)02176-1. [DOI] [PubMed] [Google Scholar]
- 35.Lode A, Paret C, Rodel G. Molecular characterization of Saccharomyces cerevisiae Sco2p reveals a high degree of redundancy with Sco1p. Yeast. 2002;19:909–922. doi: 10.1002/yea.883. [DOI] [PubMed] [Google Scholar]
- 36.Mattatall NR, Jazairi J, Hill BC. Characterization of YpmQ, an accessory protein required for the expression of cytochrome c oxidase in Bacillus subtilis. J. Biol. Chem. 2000;275:28802–28809. doi: 10.1074/jbc.M002741200. [DOI] [PubMed] [Google Scholar]
- 37.Buhler D, Rossmann R, Landolt S, Balsiger S, Fischer HM, Hennecke H. Disparate pathways for the biogenesis of cytochrome oxidases in Bradyrhizobium japonicum. J. Biol. Chem. 2010;285:15704–15713. doi: 10.1074/jbc.M109.085217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McEwan AG, Lewin A, Davy SL, Boetzel R, Leech A, Walker D, Wood T, Moore GR. PrrC from Rhodobacter sphaeroides, a homologue of eukaryotic Sco proteins, is a copper-binding protein and may have a thiol-disulfide oxidoreductase activity. FEBS Lett. 2002;518:10–16. doi: 10.1016/s0014-5793(02)02532-2. [DOI] [PubMed] [Google Scholar]
- 39.Badrick AC, Hamilton AJ, Bernhardt PV, Jones CE, Kappler U, Jennings MP, McEwan AG. PrrC, a Sco homologue from Rhodobacter sphaeroides, possesses thiol-disulfide oxidoreductase activity. FEBS Lett. 2007;581:4663–4667. doi: 10.1016/j.febslet.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 40.Abriata LA, Banci L, Bertini I, Ciofi-Baffoni S, Gkazonis P, Spyroulias GA, Vila AJ, Wang S. Mechanism of CuA assembly. Nat Chem Biol. 2008;4:599–601. doi: 10.1038/nchembio.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hiser L, Di Valentin M, Hamer AG, Hosler JP. Cox11p is required for stable formation of the CuB and magnesium centers of cytochrome c oxidase. J. Biol. Chem. 2000;275:619–623. doi: 10.1074/jbc.275.1.619. [DOI] [PubMed] [Google Scholar]
- 42.Thompson AK, Smith D, Gray J, Carr HS, Liu A, Winge DR, Hosler JP. Mutagenic analysis of Cox11 of Rhodobacter sphaeroides : insights into the assembly of CuB of cytochrome c oxidase. Biochemistry. 2010;49:5651–5661. doi: 10.1021/bi1003876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swem DL, Swem LR, Setterdahl A, Bauer CE. Involvement of SenC in assembly of cytochrome c oxidase in Rhodobacter capsulatus. J. Bacteriol. 2005;187:8081–8087. doi: 10.1128/JB.187.23.8081-8087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frangipani E, Haas D. Copper acquisition by the SenC protein regulates aerobic respiration in Pseudomonas aeruginosa PAO1. FEMS Microbiol. Lett. 2009;298:234–240. doi: 10.1111/j.1574-6968.2009.01726.x. [DOI] [PubMed] [Google Scholar]
- 45.Borsetti F, Tremaroli V, Michelacci F, Borghese R, Winterstein C, Daldal F, Zannoni D. Tellurite effects on Rhodobacter capsulatus cell viability and superoxide dismutase activity under oxidative stress conditions. Res. Microbiol. 2005;156:807–813. doi: 10.1016/j.resmic.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Banci L, Bertini I, Ciofi-Baffoni S, Katsari E, Katsaros N, Kubicek K, Mangani S. A copper(I) protein possibly involved in the assembly of CuA center of bacterial cytochrome c oxidase. Proc. Natl. Acad. Sci. USA. 2005;102:3994–3999. doi: 10.1073/pnas.0406150102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horng YC, Cobine PA, Maxfield AB, Carr HS, Winge DR. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome c oxidase. J. Biol. Chem. 2004;279:35334–35340. doi: 10.1074/jbc.M404747200. [DOI] [PubMed] [Google Scholar]
- 48.Arunothayanan H, Nomura M, Hamaguchi R, Itakura M, Minamisawa K, Tajima S. Copper metallochaperones are required for the assembly of bacteroid cytochrome c oxidase which is functioning for nitrogen fixation in soybean nodules. Plant Cell Physiol. 2010;51:1242–1246. doi: 10.1093/pcp/pcq079. [DOI] [PubMed] [Google Scholar]
- 49.Yang D, Oyaizu Y, Oyaizu H, Olsen GJ, Woese CR. Mitochrondrial Origins. Proc. Natl. Acad. Sci. USA. 1985;82:4443–4447. doi: 10.1073/pnas.82.13.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whatley FR. The establishment of mitochondria: Paracoccus and Rhodopseudomonas. Ann. N. Y. Acad. Sci. 1981;361:330–340. doi: 10.1111/j.1749-6632.1981.tb46529.x. [DOI] [PubMed] [Google Scholar]
- 51.Bratton MR, Hiser L, Antholine WE, Hoganson C, Hosler JP. Identification of the structural subunits required for formation of the metal centers in subunit I of cytochrome c oxidase of Rhodobacter sphaeroides. Biochemistry. 2000;39:12989–12995. doi: 10.1021/bi0003083. [DOI] [PubMed] [Google Scholar]
- 52.Hiser L, Hosler JP. Heme A is not essential for assembly of the subunits of cytochrome c oxidase of Rhodobacter sphaeroides. J. Biol. Chem. 2001;276:45403–45407. doi: 10.1074/jbc.M107016200. [DOI] [PubMed] [Google Scholar]
- 53.Sistrom WR. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J. Gen. Microbiol. 1960;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- 54.Kovach ME, Phillips RW, Elzer PH, Roop RM, II, Peterson KM. pBBR1MCS: A Broad-Host-Range Cloning Vector. Biotechniques. 1994;16:800–802. [PubMed] [Google Scholar]
- 55.Varanasi L, Mills D, Murphree A, Gray J, Purser C, Baker R, Hosler J. Altering conserved lipid binding sites in cytochrome c oxidase of Rhodobacter sphaeroides perturbs the interaction between subunits I and III and promotes suicide inactivation of the enzyme. Biochemistry. 2006;45:14896–14907. doi: 10.1021/bi061390q. [DOI] [PubMed] [Google Scholar]
- 56.Donohue T, Kaplan S. Genetic Techniques in Rhodospirillaceae. Methods Enzymol. 1991;204:459–485. doi: 10.1016/0076-6879(91)04024-i. [DOI] [PubMed] [Google Scholar]
- 57.Alexeyev MF. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. Biotechniques. 1999;26:824–826. 828. doi: 10.2144/99265bm05. [DOI] [PubMed] [Google Scholar]
- 58.Eraso JM, Kaplan S. From redox flow to gene regulation: role of the PrrC protein of Rhodobacter sphaeroides 2.4.1. Biochemistry. 2000;39:2052–2062. doi: 10.1021/bi9923858. [DOI] [PubMed] [Google Scholar]
- 59.Varanasi L, Hosler J. Alternative initial proton acceptors for the D pathway of Rhodobacter sphaeroides cytochrome c oxidase. Biochemistry. 2011;50:2820–2828. doi: 10.1021/bi102002v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sone N, Yoshida M, Hirata H, Kagawa Y. Asolectin purification procedure. J. Biochem. (Tokyo) 1977;81:519–528. doi: 10.1093/oxfordjournals.jbchem.a131485. [DOI] [PubMed] [Google Scholar]
- 61.Nicholls P, Hildebrandt V, Hill BC, Nicholls F, Wrigglesworth JM. Pathways of cytochrome c oxidation by soluble and membrane-bound cytochrome aa3. Can. J. Biochem. 1980;58:969–977. doi: 10.1139/o80-132. [DOI] [PubMed] [Google Scholar]
- 62.Preisig O, Zufferey R, Thony-Meyer L, Appleby CA, Hennecke H. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J. Bacteriol. 1996;178:1532–1538. doi: 10.1128/jb.178.6.1532-1538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackenzie C, Choudhary M, Larimer FW, Predki PF, Stilwagen S, Armitage JP, Barber RD, Donohue TJ, Hosler JP, Newman JE, Shapleigh JP, Sockett RE, Zeilstra-Ryalls J, Kaplan S. The home stretch, a first analysis of the nearly completed genome of Rhodobacter sphaeroides 2.4.1. Photosynth Res. 2001;70:19–41. doi: 10.1023/A:1013831823701. [DOI] [PubMed] [Google Scholar]
- 64.Mouncey NJ, Gak E, Choudhary M, Oh J, Kaplan S. Respiratory pathways of Rhodobacter sphaeroides 2.4.1(T): identification and characterization of genes encoding quinol oxidases. FEMS Microbiol. Lett. 2000;192:205–210. doi: 10.1111/j.1574-6968.2000.tb09383.x. [DOI] [PubMed] [Google Scholar]
- 65.Oh JI, Kaplan S. The cbb3 terminal oxidase of Rhodobacter sphaeroides 2.4.1: structural and functional implications for the regulation of spectral complex formation. Biochemistry. 1999;38:2688–2696. doi: 10.1021/bi9825100. [DOI] [PubMed] [Google Scholar]
- 66.Arnaud S, Malatesta F, Guigliarelli B, Gayda JP, Bertrand P, Miraglio R, Denis M. Purification and characterization of the oxidase from the marine bacterium Pseudomonas nautica 617. Eur. J. Biochem. 1991;198:349–356. doi: 10.1111/j.1432-1033.1991.tb16022.x. [DOI] [PubMed] [Google Scholar]
- 67.Hunter DJ, Moody AJ, Rich PR, Ingledew WJ. EPR spectroscopy of Escherichia coli cytochrome bo which lacks CuB. FEBS Lett. 1997;412:43–47. doi: 10.1016/s0014-5793(97)00735-7. [DOI] [PubMed] [Google Scholar]
- 68.Aasa R, Albracht PJ, Falk KE, Lanne B, Vanngard T. EPR signals from cytochrome c oxidase. Biochim. Biophys. Acta. 1976;422:260–272. doi: 10.1016/0005-2744(76)90137-6. [DOI] [PubMed] [Google Scholar]
- 69.Lappalainen P, Aasa R, Malmstrom BG, Saraste M. Soluble CuA-binding domain from the Paracoccus cytochrome c oxidase. J. Biol. Chem. 1993;268:26416–26421. [PubMed] [Google Scholar]
- 70.van der Oost J, Lappalainen P, Musacchio A, Warne A, Lemieux L, Rumbley J, Gennis RB, Aasa R, Pascher T, Malmstrom BG, et al. Restoration of a lost metal-binding site: construction of two different copper sites into a subunit of the E. coli cytochrome o quinol oxidase complex. EMBO J. 1992;11:3209–3217. doi: 10.1002/j.1460-2075.1992.tb05398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zickermann V, Wittershagen A, Kolbesen BO, Ludwig B. Transformation of the CuA redox site in cytochrome c oxidase into a mononuclear copper center. Biochemistry. 1997;36:3232–3236. doi: 10.1021/bi962040e. [DOI] [PubMed] [Google Scholar]
- 72.Jaksch M, Paret C, Stucka R, Horn N, Muller-Hocker J, Horvath R, Trepesch N, Stecker G, Freisinger P, Thirion C, Muller J, Lunkwitz R, Rodel G, Shoubridge EA, Lochmuller H. Cytochrome c oxidase deficiency due to mutations in SCO2, encoding a mitochondrial copper-binding protein, is rescued by copper in human myoblasts. Hum. Mol. Genet. 2001;10:3025–3035. doi: 10.1093/hmg/10.26.3025. [DOI] [PubMed] [Google Scholar]
- 73.Salviati L, Hernandez-Rosa E, Walker WF, Sacconi S, DiMauro S, Schon EA, Davidson MM. Copper supplementation restores cytochrome c oxidase activity in cultured cells from patients with SCO2 mutations. Biochem. J. 2002;363:321–327. doi: 10.1042/0264-6021:3630321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Loferer H, Bott M, Hennecke H. Bradyrhizobium japonicum TlpA, a novel membrane-anchored thioredoxin-like protein involved in the biogenesis of cytochrome aa3 and development of symbiosis. EMBO J. 1993;12:3373–3383. doi: 10.1002/j.1460-2075.1993.tb06011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elsen S, Swem LR, Swem DL, Bauer CE. RegB/RegA, a highly conserved redox-responding global two-component regulatory system. Microbiol. Mol. Biol. Rev. 2004;68:263–279. doi: 10.1128/MMBR.68.2.263-279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim YJ, Ko IJ, Lee JM, Kang HY, Kim YM, Kaplan S, Oh JI. Dominant role of the cbb3 oxidase in regulation of photosynthesis gene expression through the PrrBA system in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 2007;189:5617–5625. doi: 10.1128/JB.00443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oh JI, Kaplan S. Oxygen adaptation. The role of the CcoQ subunit of the cbb3 cytochrome c oxidase of Rhodobacter sphaeroides 2.4.1. J. Biol. Chem. 2002;277:16220–16228. doi: 10.1074/jbc.M200198200. [DOI] [PubMed] [Google Scholar]
- 78.Zhen Y, Qian J, Follmann K, Hayward T, Nilsson T, Dahn M, Hilmi Y, Hamer AG, Hosler JP, Ferguson-Miller S. Overexpression and purification of cytochrome c oxidase from Rhodobacter sphaeroides. Prot. Express. Purif. 1998;13:326–336. doi: 10.1006/prep.1998.0903. [DOI] [PubMed] [Google Scholar]