Abstract
Intrathecal enzyme replacement therapy is an experimental option to treat central nervous system disease due to lysosomal storage. Previous work shows that MPS I dogs receiving enzyme replacement with recombinant human alpha-L-iduronidase into the cisterna magna showed normal brain glycosaminoglycan (GAG) storage after three or four doses. We analyzed MPS I dogs that received intrathecal enzyme in a previous study using an assay that detects only pathologic GAG (pGAG). To quantify pGAG in MPS I, the assay measures only those GAG which display terminal iduronic acid residues on their non-reducing ends. Mean cortical brain pGAG in six untreated MPS I dogs was 60.9 ± 5.93 pmol per mg wet weight, and was 3.83 ± 2.64 in eight normal or unaffected carrier animals (p<0.001). Intrathecal enzyme replacement significantly reduced pGAG storage in all treated animals. Dogs with low anti-iduronidase antibody titers showed normalization or near-normalization of pGAG in the brain (mean 8.17 ± 6.17, n=7), while in dogs with higher titers, pGAG was reduced but not normal (mean 21.9 ± 6.02, n=4). Intrathecal enzyme therapy also led to a mean 69% reduction in cerebrospinal fluid pGAG (from 83.8 ± 26.3 to 27.2 ± 12.3 pmol/ml CSF). The effect was measurable one month after each dose and did not differ with antibody titer. Prevention of the immune response to enzyme may improve the efficacy of intrathecal enzyme replacement therapy for brain disease due to MPS I.
Keywords: Mucopolysaccharidosis, lysosomal storage disorder, enzyme replacement therapy, Hurler, glycosaminoglycan, immune response
1. Introduction
Mucopolysaccharidosis I (MPS I) is a lysosomal storage disorder that causes accumulation of glycosaminoglycans (GAG) throughout the body and central nervous system, causing disease [1]. Though both hematopoietic stem cell transplantation and intravenous enzyme replacement therapy with recombinant human alpha-L-iduronidase (rhIDU, EC 3.2.1.76) are clinically used for patients, these treatments do not completely address CNS manifestations of MPS I. Intravenous enzyme replacement therapy is hindered by the blood-brain barrier, which prevents at least the bulk of the protein from accessing the CNS. Hematopoietic stem cell transplantation can result in prevention or stabilization of CNS disease in MPS I patients, due mostly to the ability of donor cells to enter the brain, become neurons and glial cells, and secrete enzyme [2–4]. However, patients must be transplanted before significant CNS disease has occurred, and even then learning disabilities may persist [5].
Intrathecal (IT) enzyme replacement therapy administered into the cisterna magna of MPS I dogs normalizes brain total GAG levels [6,7]. The dose of IT rhIDU is small (~1 mg or less), and only three or four injections were needed to achieve this result. Similarly, intra-CSF enzyme replacement therapy has shown promise in animal models of MPS IIIA, MPS VI, Niemann-Pick type A, globoid cell leukodystrophy, neuronal ceroid lipofuscinosis, and fucosidosis [8–13].
Recently, we showed that the effectiveness of intravenous (IV) enzyme replacement therapy on systemic disease is improved in animals with low anti-iduronidase antibody titers [14]. Dogs with low anti-iduronidase antibody levels showed higher iduronidase activity and lower tissue GAG levels than dogs with higher titers, and low-titer animals had improved pathology even in the hard-to-treat renal tubules, synovium, and heart valve. To evaluate whether a similar phenomenon occurs with an immune response against brain-directed enzyme replacement therapy, we analyzed brain and CSF samples from dogs that received IT enzyme with rhIDU in a previous study, some of which received an immunosuppressive regimen designed to prevent the immune response against rhIDU [15]. We used a highly sensitive and specific assay that detects only pathologic GAG (GAG fragments that are left behind due to the deficiency of a specific enzyme), to determine whether antibody levels alter effectiveness of IT enzyme replacement therapy in the brain.
2. Methods
2.1. Sample collection
Experimental subjects consisted of 11 dogs affected with canine MPS I that received monthly IT rhIDU and weekly IV rhIDU as previously described [6]. Dogs received three or four IT rhIDU treatments. Immune tolerance was induced in some animals prior to rhIDU treatment using a 60-day regimen of cyclosporine and azathioprine combined with low doses of IV rhIDU as previously published [15]. Eight normal or carrier animals and six untreated MPS I animals were used as controls. Dogs were housed at the Los Angeles Biomedical Research Institute at Harbor-UCLA or Iowa State University, both AALAC-accredited facilities. Study procedures were approved by each institution’s Animal Care and Use Committee.
CSF was collected pre-treatment, once per month (prior to each IT rhIDU injection), and at necropsy. Animals were sacrificed 48 hours (AD, NI, UM, YE, UR, BD, and CY) or 3 months (CT, CU, EL, ET) following the final IT rhIDU dose. At necropsy, right hemispheres were divided into six coronal sections (numbered rostral-caudal, approximately 1.5 cm thick) and snap frozen at approximately −80ºC. Serum was collected at end-study. Anti-iduronidase IgG levels were determined using ELISA as previously described [14]. This ELISA method does not determine a “positive” titer at a set optical density (OD) value, but rather determines the OD value at a dilution that is in the linear range of the assay. The OD value is multiplied by the dilution factor to determine a concentration in OD units per μl of undiluted serum.
2.2. Glycosaminoglycan evaluations
CSF (50–150 μl) and brain (100–200 mg rostral cortex, from the second coronal section) were coded with a numerical identifier and sent blinded on dry ice to Zacharon Pharmaceuticals Inc. (San Diego, CA) for pathologic GAG (pGAG) analysis using the Sensi-Pro Non-Reducing End (NRE) assay. This assay employs high performance liquid chromatography (HPLC) to quantify the abundance of only those GAG which have accumulated due to reduced activity of a specific lysosomal enzyme, by labeling and measuring the non-reducing ends (NRE) of the GAG fragments [16,17]. In MPS I, the pGAG markers are unique NRE-derived disaccharides that terminate in iduronic acid due to the lysosomal deficiency in alpha-L-iduronidase. Briefly, tissue GAG was extracted and purified by DEAE chromatography, digested with heparin lyases (IBEX Technologies, Montreal, Canada), fluorescently labeled and analyzed by HPLC as previously described [18]. Heparin lyases degrade all forms of heparan sulfate but do not cleave any other GAG class such as chondroitin sulfate, dermatan sulfate or keratan sulfate. Therefore the pGAG markers reported here are exclusively from heparan sulfate accumulation, which is the major pathogenic GAG in the central nervous system in MPS I. The MPS I NRE structures (β-D-idopyranosyluronate-(1→4)-2-N-sulfamino-6-sulfo-2-deoxy-α/β-D-glucopyranoside, I0S6, [17]) were quantified based on standard curves of saturated heparan sulfate derived disaccharides (Iduron, UK). Heparan sulfate standards were analyzed with each set of samples. At the time these samples were assayed, the coefficient of variance of the method was 13%. For comparison with pGAG measurements, tGAG was analyzed using an Alcian blue dye-based assay as previously described [6].
2.3. Statistics
Means and standard deviations were calculated from Excel. Data were graphed using SigmaPlot 11 (Systat Software, Chicago, IL), which also calculated median values for Fig. 1 and error bars for Fig. 3. Samples were analyzed using SYSTAT 12 (Systat Software, Chicago, IL) with either two-tailed student’s t-test or ANOVA with post-hoc Tukey-Kramer (for brain pGAG) or Bonferroni (for CSF) where appropriate. A p-value of less than 0.05 was considered significant. Repeated measures ANOVA was not possible for CSF samples, due to six missing values in the data set (for example, CSF sample volume too low or contaminated by blood).
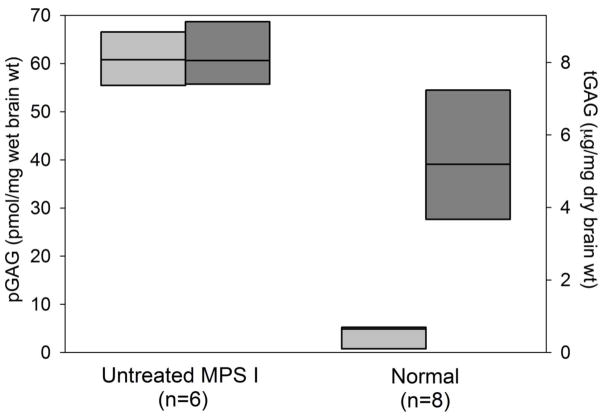
Fig. 1.
Cortical brain measurement of pathologic GAG (pGAG, light gray/left axis) and total GAG (tGAG, dark gray/right axis) in untreated MPS I versus normal or carrier animals. Full data range is shown (bars). The median value is represented by a horizontal line within each bar.
Fig. 3.
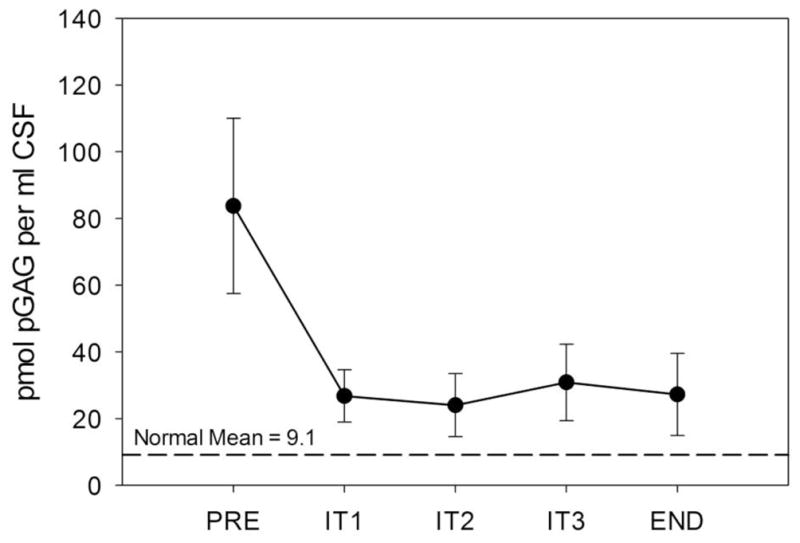
Cerebrospinal fluid pGAG before and after four monthly doses of intrathecal rhIDU in MPS I dogs. Pretreatment value (“PRE”), values at one-month intervals (“IT1” through “IT3”), and necropsy (48 hours after the IT4 treatment, “END”). CSF was sampled immediately prior to each IT injection (which was one month after the previous IT injection). Means and standard error are shown.
3. Results
3.1. Antibodies to iduronidase alter the efficacy of intrathecal enzyme replacement therapy
Study animals, IT rhIDU dosage, serum and CSF anti-iduronidase IgG antibody titers, and cortical brain pGAG reduction are listed in Table 1. MPS I dogs that are naïve to rhIDU (untreated) have no detectable anti-iduronidase antibody titer. Previously, the anti-iduronidase IgG titer cut-off value of 20 OD units per μl undiluted serum was established operationally to define immune tolerance in MPS I dogs [14]. Animals were therefore separated into “Higher-titer” and “Low-titer” groups based on this cut-off value. Animals with serum anti-iduronidase IgG titers less than 20 were placed in the “Low-titer” group. All low-titer animals had received cyclosporine and azathioprine coupled with low-dose IV rhIDU, except for one (EL). EL mounted low anti-iduronidase antibody titers in both serum and CSF despite a lack of immune suppression and adequate exposure to antigen. However, its titers were the highest in the Low-titer group. Serum IgG antibody titers to rhIDU were 302 ± 288 OD units/μl in the Higher-titer group, compared with 5.89 ± 5.66 in the Low-titer group (p=0.019). Anti-iduronidase antibody titers in CSF were 8.04 ± 10.4 in the Higher-titer group, versus 0.047 ± 0.067 in the Low-titer group (p=0.089).
Table 1.
Study animals
| Animal | IT rhIDU dose | Age at End- study (months) | Serum Anti- rhIDU IgG titera | CSF Anti- rhIDU IgG titera | Cortical Brain pGAG (pmol/mg)b |
|---|---|---|---|---|---|
| Higher-titer | |||||
| ETb | 0.46 mg | 13 | 70.4 | 1.92 | 26.9 |
| NI | 1.38 mg | 40 | 83.8 | 23.3 | 20.8 |
| YE | 1.38 mg | 19 | 377 | 0.766 | 13.9 |
| UM | 1.38 mg | 33 | 678 | 6.19 | 26.2 |
| Low-titer | |||||
| CY | 0.46 mg | 15 | 0.627 | 0.077 | 1.45 |
| CTb | 0.46 mg | 18 | 1.05 | 0.000 | 8.24 |
| CUb | 0.46 mg | 18 | 1.36 | 0.011 | 8.69 |
| UR | 1.38 mg | 24 | 5.77 | 0.003 | 4.69 |
| AD | 1.38 mg | 25 | 6.43 | 0.022 | 7.57 |
| BD* | 0.46 mg | 18 | 9.92 | 0.171 | 5.59 |
| ELb | 0.46 mg | 13 | 16.1 | 0.860 | 21.0 |
rhIDU: recombinant human alpha-L-iduronidase
anti-rhIDU titers (OD units/μl) reported are those values obtained at end of study except for BD* (CSF anti-rhIDU obtained prior to IT treatment #3)
sacrificed 3 months after final IT rhIDU treatment
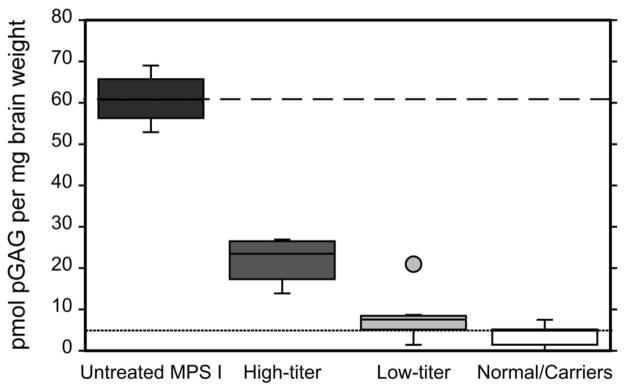
Levels of pGAG were measured in frontal cortical samples of the brain of MPS I and normal (wild-type) or unaffected carrier (heterozygous) dogs. Each blinded sample was analyzed twice and results averaged. The amount of pGAG in untreated MPS I affected animals was 60.9 ± 5.93 (range 52.9–69.0) pmol per mg wet weight, and was 3.83 ± 2.64 (0.04–7.50, p<0.0001) in normal/carrier animals. This was a greater separation between normal /carrier and affected than achieved with measurements of total GAG (tGAG) measured using the Alcian blue dye-based assay (Fig. 1). In MPS I dogs receiving IT rhIDU, the mean cortical brain tGAG in the higher-titer group was 3.33 ± 0.820 μg/mg, and was not significantly different from tGAG in the low-titer group (3.50 ± 0.453, p=0.78; Supplemental Table 1). However, cortical brain pGAG in dogs treated with IT rhIDU showed a clear separation between animals with low anti-iduronidase antibody titers and higher titers (Fig. 2). Mean brain pGAG in low-titer dogs was 8.17 ± 6.17 pmol/mg (range 1.45 to 21.0, p<0.0001 vs. untreated MPS I). Mean brain pGAG in higher-titer animals was 21.9 ± 6.02 pmol/mg (range 13.9 to 26.9, p<0.0001 vs. untreated MPS I). There was a statistically significant difference between low-titer and higher-titer dogs (p=0.002) and between normal/carrier and higher titer dogs (p<0.0001). Brain pGAG in the low titer group was not significantly different from normals/carriers, and was within the range of normal/carrier in three of the seven low-titer animals. There was no systematic variation in cortical brain pGAG levels with age at end-study among animals within each group. Cortical pGAG in dog EL (which had a low titer but was never immunosuppressed) was 21.0, which was more similar to higher-titer animals than low-titer animals. When the statistical analysis was repeated with dog EL treated as an outlier from the Low-titer group (studentized residual 3.2, grey circle, Fig. 2) the mean brain pGAG in this group (n=6) showed greater normalization to 6.04 ± 2.73 compared to untreated MPS I animals, and the p-values for comparison of mean brain pGAG among low-titer, high-titer, and untreated animals remained statistically significant (<0.001).
Fig. 2.
Cortical brain pGAG measured in normal and MPS I dogs. Data for four groups of animals -- untreated MPS I dogs (n=6, black box), higher anti-rhIDU titer dogs (n=4, dark grey box), low anti-rhIDU titer dogs (n=7, light grey box), and normal dogs (n=8, empty box) -- are shown. Mean pGAG is indicated for untreated MPS I dogs (dashed line) and normal dogs (dotted line). In each group capped vertical lines indicate the minimum and maximum data values, while the lower and upper boundaries of each box indicate the 25 and 75 percentiles respectively, and the median is indicated by the horizontal line within each box. The dog EL that had a serum anti-rhIDU IgG titer of 16.1 and brain pGAG 21.0 pmol/mg is represented by the light grey circle above the “Low Titer” column and is not included in the data range represented by the box and capped lines.
3.2. Intrathecal enzyme replacement therapy reduces CSF GAG
CSF samples were taken prior to each intrathecal injection for GAG analysis. Samples were available for five dogs that received monthly intrathecal enzyme replacement therapy (Fig. 3). The blinded experimental samples in this set were run only once due to the limited amount of sample. Pretreatment pGAG averaged 83.8 ± 26.3 pmol/ml CSF. IT rhIDU led to a 41–87% reduction in CSF pGAG (to 27.2 ± 12.3; a mean 69% reduction at end-study). Reduction in pGAG was evident in each animal following each treatment. All post-treatment CSF pGAG levels reached statistical significance versus pre-treatment (p<0.02). CSF pGAG in IT-treated animals did not reach the level found in normal/carrier dogs (9.10 ± 19.4 pmol/ml CSF). There was no difference in CSF pGAG reduction between low-titer (AD, UR) and higher-titer (UM, YE, NI) groups.
4. Discussion
We analyzed samples from MPS I dogs previously treated with IT and IV rhIDU for pathologic GAG (pGAG) storage in the brain cortex and in CSF. Treated dogs that showed a higher anti-iduronidase antibody titer did not have normal pGAG levels in the brain by this assay, while the low-titer dogs that had received a tolerizing regimen had cortical brain pGAG that was normal to near-normal. One dog that did not receive immunosuppressive or tolerizing therapy had an intermediate titer, just below our previously established cut-off of 20 OD units/μl. This dog showed brain pGAG that was similar to higher-titer animals, suggesting the possibility that an anti-iduronidase antibody titer that is at the upper end of what was previously considered tolerant may yet be sufficient to interfere with rhIDU uptake. Previously, we showed that IT rhIDU reduced brain GAG levels to normal levels in nineteen treated animals when measured by Alcian blue dye-binding assay [6]. We were not able to detect a difference between low-titer and higher-titer animals in regards to the effectiveness of IT rhIDU using that method. The Alcian blue assay measures total GAG (tGAG), so that tissues with relatively low levels of storage show little separation in affected versus unaffected animals. In contrast, the assay method used here permits selective quantification of GAG with terminal iduronic acid on their non-reducing ends left by alpha-L-iduronidase deficiency. This gives a very low background signal and a much larger separation between affected and normal levels. In this study, which was focused on the brain, we digested GAG with heparin lyases and measured only pGAG resulting from heparan sulfate accumulation. However, chondroitin and dermatan sulfate could also be measured by this method following digestion as appropriate for those GAG species [17].
The development of therapies for brain disease due to lysosomal storage has been hampered to some extent by the difficulty in finding non-invasive, objective measures of success. CSF sampling prior to each IT rhIDU injection may allow quantitative measurement of storage levels in the CNS and their response to treatment. We had been unable to accurately measure CSF GAG in dogs, due to the low sensitivity of our Alcian blue dye-binding method. In the five animals for which sufficient pre- and post-treatment samples were available, CSF pGAG decreased following the initial IT rhIDU dose, and stayed low throughout the treatment period. The decrease in CSF pGAG was detectable one month after the dose.
GAG storage generally increases with age in MPS. In our study, age at necropsy varied within groups, with several of the oldest dogs in the higher-titer group. However, age did not appear to affect brain pGAG after IT rhIDU treatment. Four dogs (EL, ET, CT, CU) were sacrificed three months after the final IT rhIDU dose; all other animals were studied 48 hours after the last IT injection. Quantitative measurement of brain pGAG in this study also did not show substantial reaccumulation after three months. Interestingly though, hiatus dogs CT and CU had the highest pGAG levels in the low-titer group (8.24 and 8.69, respectively). We had previously found that in these “hiatus” animals there did not appear to be reaccumulation of tGAG quantitatively at the three-month time point, though histopathology did show more storage in cells than in dogs examined at 48 hours [6].
The central nervous system is relatively immune-privileged, but low levels of antibodies to rhIDU were seen in IT-treated animals. Antibodies to enzyme replacement can reduce efficacy by several different mechanisms, including neutralization of activity, enhanced clearance from circulation, and altered distribution and uptake in tissues [19]. Growing evidence points to an improvement in the effectiveness of enzyme replacement therapy when antibody titers are low. In the case of MPS I, antibodies reduce intracellular uptake and alter tissue distribution, favoring tissues with higher reticuloendothelial (RE) content [14]. Preventing this antibody response in dogs improved the effectiveness of IV enzyme replacement in low-RE tissues, including improved tGAG levels and/or histopathology of the lung, myocardium, kidney, synovium and heart valve. The mechanism by which antibodies may reduce effectiveness of rhIDU in the brain in MPS I dogs is not clear. We did not find lower iduronidase activity in brain cortex of dogs with higher titers, though this evaluation was limited by the fact that some animals received a lower rhIDU dose than others (Supplemental Table 1). We did not study cellular and subcellular distribution of enzyme in these animals, to determine the extent of rhIDU uptake into each cell type.
The development of immune tolerance may theoretically enhance the safety profile of CNS therapeutics. IT rhIDU administered to MPS I dogs causes an inflammatory response in the meninges, consisting principally of lymphocytes and plasma cells [6,7]. Similar findings were seen in adult MPS IIIA dogs treated with intra-cisternal recombinant human sulfamidase [20]. IT recombinant human N-acetylgalactosamine 4-sulfatase did not cause meningitis in tolerized MPS VI cats [9], nor IT sulfamidase in tolerized MPS IIIA dogs [21], showing that this response can be prevented. In our experience with MPS I dogs, both low titer and reduced injection frequency may lessen meningeal inflammation due to IT rhIDU, though we did not independently evaluate these factors [6]. In all cases, the inflammatory response in the meninges was not associated with clinical signs or symptoms in the animals, nor was there inflammation in the brain parenchyma. This mirrors findings in MPS I patients receiving weekly IV rhIDU, in whom IgG antibodies to enzyme almost always develop, and do not correlate with infusion reactions [22]. Finally, human subjects receiving IT rhIDU in clinical trials have not developed clinical meningitis, though low levels of leukocytes have been measured in CSF (maximum 37 cells/mm3, our unpublished observation). It is unclear to what extent the observed inflammatory response to IT rhIDU in dogs represents a safety concern.
Several different methods have been used to attain immune tolerance in animals, including pharmacologic, viral, and cell-based approaches [23–27]. A sixty-day regimen of high-dose cyclosporine and azathioprine, along with low-dose IV rhIDU infusions, prevented higher anti-iduronidase antibody responses in MPS I dogs. A clinical trial is underway to assess the ability of this regimen to induce tolerance to IV rhIDU in human MPS I patients (NCT00741338). Our findings underscore the importance of inducing immune tolerance to therapeutic lysosomal enzymes, and that doing so may improve their efficacy in both systemic and CNS compartments.
Supplementary Material
Highlights.
MPS I dogs may develop antibodies against intrathecal enzyme replacement therapy
We studied the brains of these dogs using a pGAG assay
We detected higher pGAG in brains of treated dogs with higher antibody titers
We detected a 69% reduction in pGAG in cerebrospinal fluid of treated dogs
Acknowledgments
We appreciate the technical assistance of Dr. Shinhsin Kan, who helped with sample preparation, and Dr. Peter Christenson, who provided advice on statistical methodology. Support was provided by the National Institutes of Health (NS054242 to PID and NS071774 to BEC), the UCLA Clinical and Translational Science Institute at Harbor-UCLA Medical Center (UL1-TR000124), the Ryan Foundation (NME, PID), the Center for Integrated Animal Genomics/ISU (NME), the State of Iowa Board of Regents Battelle Platform and Infrastructure Grant Programs (NME), and the National MPS Society (BEC, JRB, RGW). Recombinant enzyme was donated by Biomarin Pharmaceutical Inc. (Novato, CA). Additional breeding animals for the Iowa State University colony were provided by grants from the National Institutes of Health to Drs. Mark E. Haskins (RR002512, University of Pennsylvania) and Kathy P. Ponder (DK066448, Washington University St. Louis).
Abbreviations
- rhIDU, EC 3.2.1.76
Recombinant human alpha-L-idurondiase
- MPS
mucopolysaccharidosis
- IT
intrathecal
- GAG
glycosaminoglycan
- pGAG
pathologic GAG
- tGAG
total GAG
- NRE
non-reducing end
- ELISA
enzyme-linked immunosorbent assay
Footnotes
Conflicts of Interest
Brett Crawford, Jillian Brown and Robert Witt are employees of Zacharon Pharmaceuticals, Inc. The Los Angeles Biomedical Research Institute at Harbor-UCLA and the Department of Pediatrics at Harbor-UCLA have a financial interest in rhIDU (formulated as laronidase).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Patricia I. Dickson, Email: pdickson@labiomed.org.
N. Matthew Ellinwood, Email: mellinwo@iastate.edu.
Jillian R. Brown, Email: jbrown@zacharon.com.
Robert G. Witt, Email: robbygwitt@gmail.com.
Steven Q. Le, Email: sqle213@yahoo.com.
Merry B. Passage, Email: mbpassage@yahoo.com.
Moin U. Vera, Email: mvera@labiomed.org.
Brett E. Crawford, Email: bcrawford@zacharon.com.
References
- 1.Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Valle D, Sly WS, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2001. pp. 3421–3452. [Google Scholar]
- 2.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 3.Brazelton TR, Rossi FMV, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 4.Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci USA. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldenhoven M, Boelens J, de Koning TJ. The clinical outcome of Hurler syndrome after stem cell transplantation. Biol Blood Marrow Transpl. 2008;14:485–498. doi: 10.1016/j.bbmt.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Dickson P, McEntee M, Vogler C, Le S, Levy B, Peinovich M, Hanson S, Passage M, Kakkis E. Intrathecal enzyme replacement therapy: successful treatment of brain disease via the cerebrospinal fluid. Mol Genet Metab. 2007;91:61–68. doi: 10.1016/j.ymgme.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakkis E, McEntee M, Vogler C, Le S, Levy B, Belichenko P, Mobley W, Dickson P, Hanson S, Passage M. Intrathecal enzyme replacement therapy reduces lysosomal storage in the brain and meninges of the canine model of MPS I. Mol Genet Metab. 2004;83:163–174. doi: 10.1016/j.ymgme.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Hemsley KM, Beard H, King BM, Hopwood JJ. Effect of high dose, repeated intra-cerebrospinal fluid injection of sulphamidase on neuropathology in mucopolysaccharidosis type IIIA mice. Genes Brain Behav. 2008;7:740–753. doi: 10.1111/j.1601-183X.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- 9.Auclair D, Finnie J, White J, Nielsen T, Fuller M, Kakkis E, Cheng A, O'Neill CA, Hopwood JJ. Repeated intrathecal injections of recombinant human 4-sulphatase remove dural storage in mature mucopolysaccharidosis VI cats primed with a short-course tolerisation regimen. Mol Genet Metab. 2010;99:132–141. doi: 10.1016/j.ymgme.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Lee WC, Tsoi YK, Troendle FJ, DeLucia MW, Ahmed Z, Dicky CA, Dickson DW, Eckman CB. Single-dose intracerebroventricular administration of galactocerebrosidase improves survival in a mouse model of globoid cell leukodystrophy. FASEB J. 2007;21:2520–2527. doi: 10.1096/fj.06-6169com. [DOI] [PubMed] [Google Scholar]
- 11.Chang M, Cooper JD, Sleat DE, Cheng SH, Dodge JC, Passini MA, Lobel P, Davidson BL. Intraventricular enzyme replacement improves disease phenotypes in a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol Ther. 2008;16:649–656. doi: 10.1038/mt.2008.9. [DOI] [PubMed] [Google Scholar]
- 12.Dodge JC, Clarke J, Treleaven CM, Taksir TV, Griffiths DA, Yang W, Fidler JA, Passini MA, Karey KP, Schuchman EH, Cheng SH, Shihabuddin LS. Intracerebroventricular infusion of acid sphingomyelinase corrects CNS manifestations in a mouse model of Niemann-Pick A disease. Exp Neurol. 2009;215:349–357. doi: 10.1016/j.expneurol.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Kondagari GS, King BM, Thomson PC, Williamson P, Clements PR, Fuller M, Hemsley KM, Hopwood JJ, Taylor RM. Treatment of canine fucosidosis by intracisternal enzyme infusion. Exp Neurol. 2011;230:218–226. doi: 10.1016/j.expneurol.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Dickson P, Peinovich M, McEntee M, Lester T, Le S, Krieger A, Manuel H, Jabagat C, Passage M, Kakkis E. Immune tolerance improves the efficacy of enzyme replacement therapy in the canine model of mucopolysaccharidosis I. J Clin Invest. 2008;118:2868–2876. doi: 10.1172/JCI34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kakkis E, Lester T, Yang R, Tanaka C, Anand V, Lemontt J, Peinovich M, Passage M. Successful induction of immune tolerance to enzyme replacement therapy in canine mucopolysaccharidosis I. Proc Natl Acad Sci USA. 2004;101:829–834. doi: 10.1073/pnas.0305480101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence R, Brown JR, Al-Mafraji K, Lamanna WC, Beitel JR, Boons GJ, Esko JD, Crawford BE. Disease-specific non-reducing end carbohydrate biomarkers for mucopolysaccharidoses. Nat Chem Biol. 2012;8:197–204. doi: 10.1038/nchembio.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrence R, Olson SK, Steele RE, Wang L, Warrior R, Cummings RD, Esko JD. Evolutionary differences in glycosaminoglycan fine structure detected by quantitative glycan reductive isotope labeling. J Biol Chem. 2008;283:33674–33684. doi: 10.1074/jbc.M804288200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deakin JA, Lyon M. A simplified and sensitive fluorescent method for disaccharide analysis of both heparan sulfate and chondroitin/dermatan sulfates from biological samples. Glycobiol. 2008;18:483–491. doi: 10.1093/glycob/cwn028. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Lozier J, Johnson G, Kirshner S, Verthelyi D, Pariser A, Shores E, Rosenberg A. Neutralizing antibodies to therapeutic enzymes: considerations for testing, prevention and treatment. Nat Biotech. 2008;26:901–908. doi: 10.1038/nbt.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemsley KM, Norman EJ, Crawley AC, Auclair D, King B, Fuller M, Lang DL, Dean CJ, Jolly RD, Hopwood JJ. Effect of cisternal sulfamidase delivery in MPS IIIA Huntaway dogs - a proof of principle study. Mol Genet Metab. 2009;98:383–392. doi: 10.1016/j.ymgme.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Crawley AC, Marshall N, Beard H, Hassiotis S, Walsh V, King B, Hucker N, Fuller M, Jolly RD, Hopwood JJ, Hemsley KM. Enzyme replacement reduces neuropathology in MPS IIIA dogs. Neurobiol Dis. 2011;43:422–434. doi: 10.1016/j.nbd.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Wraith JE, Clarke LA, Beck M, Kolodny EH, Pastores GM, Muenzer J, Rapoport DM, Berger KI, Swiedler SJ, Kakkis ED, Braakman T, Chadbourne E, Walton-Bowen K, Cox GF. Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human α-L-iduronidase (laronidase) J Pediatr. 2004;144:581–588. doi: 10.1016/j.jpeds.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 23.Joseph A, Munroe K, Housman M, Garman R, Richards S. Immune tolerance induction to enzyme-replacement therapy by co-administration of short-term, low-dose methotrexate in a murine Pompe disease model. Clin Exp Immunol. 2008;152:138–146. doi: 10.1111/j.1365-2249.2008.03602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponder KP, Wang B, Wang P, Ma X, Herati R, Wang B, Cullen K, O'Donnell P, Ellinwood NM, Traas A, Primeau TM, Haskins ME. Mucopolysaccharidosis I cats mount a cytotoxic T lymphocyte response after neonatal gene therapy that can be blocked with CTLA4-Ig. Mol Ther. 2006;14:5–13. doi: 10.1016/j.ymthe.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Raben N, Nagaraju K, Lee A, Lu N, Rivera Y, Jatkar T, Hopwood JJ, Plotz PH. Induction of tolerance to a recombinant human enzyme, acid alpha-glucosidase, in enzyme deficient knockout mice. Transgenic Res. 2003;12:171–178. doi: 10.1023/a:1022998010833. [DOI] [PubMed] [Google Scholar]
- 26.Young KJ, Yang L, Phillips MJ, Zhang L. Donor-lymphocyte infusion induces transplantation tolerance by activating systemic and graft-infiltrating double-negative regulatory T cells. Blood. 2002;100:3408–3414. doi: 10.1182/blood-2002-01-0235. [DOI] [PubMed] [Google Scholar]
- 27.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.