Abstract
Nutritional management is essential for patients with inborn errors of metabolism, such as urea cycle disorders (UCDs). Lack of appetite is common in these patients and can lead to underconsumption of calories, catabolism, and subsequently loss of metabolic control. The etiology of anorexia in these patients is largely unexplored. The neuroendocrine hormone peptide tyrosine tyrosine (PYY), secreted postprandially from endocrine cells of the ileum and colon, induces feelings of satiety and decreases food intake. While plasma PYY levels have been characterized in a number of populations, they have not been examined in UCD patients. In a retrospective study, plasma PYY concentrations were measured in UCD (n=42) patients and controls (n=28) via an ELISA to determine if levels of this anorexigenic hormone are altered in this patient population. Median PYY levels were significantly higher in UCD patients compared to controls (p=3.5×10−5). Body mass index was significantly associated with increased PYY levels in controls (p=0.02), while UCD diagnosis subtype was associated with PYY levels (p=1×10−3) in cases. Median PYY levels were significantly lower in ornithine carbamoyltransferase deficient patients compared with all other UCD subtypes (p=9×10−3), but significantly higher compared to controls (p=1.6×10−3). Overall, this study demonstrates that UCD cases have increased PYY levels compared to controls, suggesting that regulation of PYY may be altered in these patients. These observations may lead to a better understanding of the development of anorexia in UCD patients.
Keywords: urea cycle disorders, appetite, nitrogen, PYY, N-acetylglutamate synthase
1.0 Introduction
Nutrition is the foundation of both the acute and chronic management of patients with inborn errors of amino acid metabolism, such as urea cycle disorders (UCD) [1]. Feeding problems, including lack of appetite, are common in UCD patients [2] . Poor appetite can lead to reduced caloric intake resulting in catabolism and subsequently, a loss of metabolic control [3,1]. While historically attributed to hyperammonemia, the etiology of anorexia observed in UCD patients remains poorly understood.
Studies report that levels of appetite regulating hormones, such as ghrelin, may be altered in some patients with inborn errors of metabolism [4,5]. The neuroendocrine hormone peptide tyrosine tyrosine (PYY), secreted from the L cells of the ileum and colon in response to ingested nutrients, plays a role in appetite regulation as well, inducing satiety postprandially [6]. This peptide also inhibits gastric acid secretion, delays gastric emptying, and slows gut motility, rendering it a mediator of the ileal brake [7,8]. Studies have shown that administering exogenous PYY decreases food intake in mouse and human [9,10]. As a result, this peptide has garnered much attention in recent years as a potential therapeutic target for obesity.
PYY is widely studied for its role in energy homeostasis, and consequently circulating levels have been characterized in multiple healthy and diseased populations. Plasma PYY levels are higher in infants [11,12], compared to children [13,14] and adults [15,16,17], and increased PYY levels are thought to contribute to the weight loss observed in patients who have undergone gastric bypass surgery [18,19]. Additionally, multiple studies report increased PYY levels in individuals with anorexia nervosa, suggesting PYY may play a role in the pathogenesis of this disease [20,21].
PYY levels have not been previously examined in patients with UCDs, a population in which anorexia is commonly observed. To determine if levels of this anorexigenic hormone are altered in this group we measured plasma PYY concentrations in UCD patients and controls. Furthermore, we explored the relationship between PYY levels and a number of demographic and epidemiological variables among UCD patients versus controls.
2.0 Materials and Methods
2.1 Study Populations
De-identified surplus plasma samples from infant, child, and adult urea cycle disorder (UCD; n=66) patients undergoing clinical testing were collected from the Vanderbilt Pathology Lab. UCD diagnoses included: carbamoyl-phosphate synthase 1 (CPS1; MIM# 237300), ornithine carbamoyltransferase (OTC; MIM# 311250), argininosuccinate synthase 1 (ASS1; MIM# 215700), and argininosuccinate lyase (ASL; MIM# 207900) deficiencies. A subset of the patients had multiple plasma samples available for study; the most recent sample was used in the analysis. Given that plasma PYY levels are increased in infants compared with children and adults [11,12] samples from UCD patients less than one year of age were excluded from analysis. The control group consisted of fasted, normal weight, and obese children ages 7-11 years from a previously published prospective study examining PYY levels in prepubertal children [13]. Surplus plasma samples from these subjects were retrieved for analysis in the present study.
Relevant demographic and clinical patient data were gathered for this study, including age, sex, race/ethnicity, and body mass index (BMI). This study was approved by the Institutional Review Board at Vanderbilt University Medical Center (IRB# 081080).
2.2 Plasma PYY determination
Total PYY was measured for all samples by ELISA (Millipore, EZHPYYT66K). This assay measures human PYY1-36 and PYY3-36, and does not cross-react with neuropeptide Y or pancreatic polypeptide. Samples were measured in duplicate. If the difference between duplicate results of a sample was greater than 15% coefficient of variation (CV), the sample was assayed again in duplicate. Because surplus plasma samples were collected for this study, a proportion of the samples did not have an adequate volume available to perform the assay multiple times. Therefore, if the % CV was greater than 15 for duplicate results of a sample and the sample could not be assayed again, it was excluded from the analysis.
2.3 Statistical Analysis
Statistical analyses were performed using STATA 10.1. Data are summarized as median and interquartile range (IQR). Sex and race differences between groups were determined by Fisher’s exact test. Age, BMI, and plasma PYY concentrations were not normally distributed; therefore a Wilcoxon rank-sum test was used to test for between-group differences in these variables.
To test for differences in PYY levels by UCD diagnosis subtype, the patients were stratified by ornithine carbamoyltransferase deficiency (OTCD, n=28) and compared to all other UCD subtypes (CPS1 (n=2), ASS1 (n=8) and ASL (n=4)). A Kruskal-Wallis test was used to test for differences in PYY levels among controls, OTCD patients, and patients with all other subtypes of UCDs. A Wilcoxon rank-sum test was used to perform pair-wise comparisons between groups. P-values were not adjusted for multiple testing and were considered significant at p ≤ 0.05. Additionally, linear regressions were performed to identify variables significantly associated with log-transformed PYY levels in both UCD patients and controls.
3.0 Results
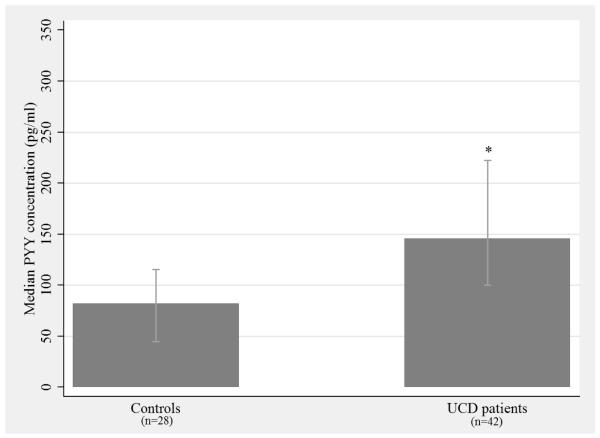
Study population characteristics are given in Table 1. Overall, sex differed across study groups (p = 5×10−4) as the control group was mostly male (82%) compared with the UCD group (38%). There was no difference in the percent of European Americans between controls (68%) and cases (82%). Neither the distributions of age, nor the distributions of BMI were significantly different between UCD cases and controls. As illustrated by Figure 1, median plasma PYY levels were significantly higher in UCD patients (146 pg/mL) compared to controls (82 pg/mL, p = 3.5×10−5).
Table 1.
Study Population Characteristics
| Controls (n=28) |
UCD patients (n=42) |
p-value | |
|---|---|---|---|
| % Male | 82 | 38 | <5.0×10−4* |
| % European American | 68 | 82 | 0.25* |
| Median Age, years (IQR) |
9.7 (2.1) |
9.5 (13) |
0.98** |
| Median BMI kg/m2(IQR) |
22.9 (10.1) |
18.8 (6.7) |
0.14** |
Abbreviations: Body mass index (BMI), Interquartile range (IQR).
Fisher’s exact test
Wilcoxon ranksum test
Figure 1. Median plasma PYY concentration in UCD patients compared with controls.
Median plasma PYY concentrations (pg/mL) are reported; bars represent the IQR for each group (control IQR = 70.6 and UCD IQR = 122.0). A Wilcoxon rank-sum test was performed to determine if the distributions of PYY levels are significantly different between UCD patients and controls. * p = 3.5 × 10−5
PYY = peptide tyrosine tyrosine, UCD = urea cycle disorder, IQR = interquartile range
We also identified variables significantly associated with PYY levels in UCD cases and controls (Table 2). Age, sex, and race/ethnicity were not significantly associated with PYY levels in either the UCD group or the controls. BMI was significantly associated with increased PYY levels (β = 0.47; p = 0.02) in the control group, while diagnosis (β = 0.319; p = 5×10−4) was significantly associated with PYY levels in the UCD group.
Table 2.
Association results of log-transformed PYY levels for Controls and UCD patients
|
Controls (n=28) |
UCD patients (n=42) |
|||||
|---|---|---|---|---|---|---|
| Variable | β | 95% CI | p-value | β | 95% CI | p-value |
| Sex | −0.50 | −1.17, 0.17 |
0.14 | 0.05 | −0.39, 0.48 | 0.84 |
| Race | −0.29 | −0.76, 0.18 |
0.22 | −0.14 | −0.41, 0.13 | 0.30 |
| Age | −0.04 | −0.24, 0.16 |
0.71 | −0.01 | −0.03, 0.004 |
0.13 |
| BMI | 0.05 | 0.01, 0.83 |
0.02 | −0.01 | −0.04, 0.03 | 0.59 |
| Diagnosis | -- | -- | -- | 0.32 | 0.15, 0.49 | 1.00×10−3 |
UCD diagnosis includes: CPS1, OTC, ASS1, and ASL deficiencies.
Abbreviations: Body mass index (BMI)
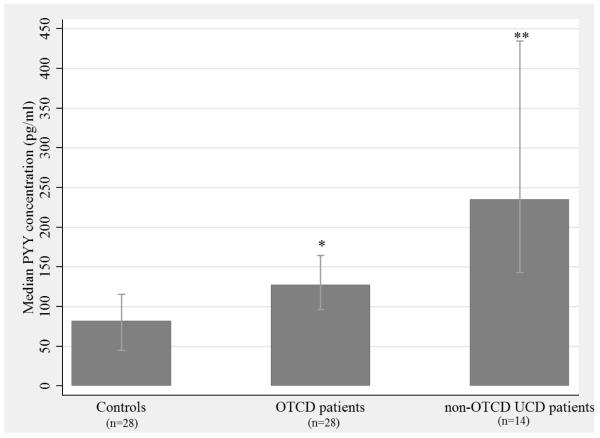
Ornithine transcarbamylase deficiency (OCTD) is the most common UCD subtype, and it differs from other UCD subtypes in that it is an X-linked, partially dominant disorder with highly variable clinical phenotypes [22]. Consequently, we stratified the UCD cases based on OTCD status to compare PYY levels between patients with OTCD and those with other UCD subtypes. While the median PYY level in the OTCD group (128 pg/mL) was significantly higher compared to controls (Figure 2; p = 1.6×10−3), it was significantly lower compared to all other UCD diagnoses (Figure 2; p = 9×10−3). As expected, the median PYY level in the non-OTCD UCD patients (235 pg/mL) was significantly higher than that of controls (Figure 2; p = 1×10−4).
Figure 2. Median plasma PYY levels in OTCD patients and all other UCD patients compared with controls.
Median plasma PYY concentrations (pg/mL) are reported; the bars represent the IQR for each group (control IQR = 70.6, OTCD IQR =68.1, other UCDs IQR =292.0). A Kruskal-Wallis test was performed to determine if median PYY levels differ across the three groups (p = 1×10−4), and a Wilcoxon rank-sum test was performed for pair-wise comparisons. *OTC vs controls (p = 1.6 × 10−3), **OTC vs other UCDs (p = 9 × 10−3)
PYY = peptide tyrosine tyrosine, OTCD = ornithine transcarbamylase deficiency, non-OTCD UCDs = urea cycle disorders other than ornithine transcarbamylase deficiency (includes CPS1, ASS1 & ASL deficiencies), IQR = interquartile range
4.0 Discussion & Conclusions
In this study, we determined that PYY levels were significantly increased in UCD patients compared to a control group. It is unclear why PYY levels are increased in UCD patients, but one possible explanation relates to control of nitrogen intake and co-regulation of the genes for PYY and N-acetylglutamate synthase (NAGS). NAGS supplies the co-factor for CPS1, the first and rate-limiting step of the urea cycle. The genes for PYY and NAGS are divergently transcribed, an arrangement consistent with coordinate regulation [23,24]. In such a model, PYY and NAGS are both upregulated in response to dietary protein; PYY induces satiety, while NAGS is critical for urea cycle function [25,26,16]. Thus, co-regulation of PYY and NAGS is a potential mechanism linking suppression of nitrogen intake via PYY to processing of waste nitrogen through the urea cycle, which in UCD patients could result in prolonged satiation manifesting as a lack of appetite. Additional studies are needed to confirm this hypothesis.
Among our UCD patients, diagnosis subtype was associated with PYY levels. Patients with OTCD had significantly lower PYY levels compared with patients of all other UCD diagnoses; however PYY levels in OTCD patients were significantly higher than controls. These findings may relate to the clinical variability of OTCD and the inclusion of carrier females in the OTCD study population. Urea cycle function is less compromised in OTCD carrier females than in patients with other UCD diagnoses and this could explain why PYY levels are lower in this group.
Increased body mass index (BMI) was significantly associated with increased PYY levels in the control group but not in the UCD group. The reasons for this are unclear, however the relationship between BMI and PYY is inconsistent in the literature with some studies showing a negative correlation [17,10], some a positive correlation [27], and still others no correlation at all [28]. The control group, which was ascertained from a previously published study, includes both normal weight and obese children [13]. In comparison, there were few patients within the UCD (n=8) group that were overweight or obese (BMI ≥ 25). Small sample size and a relative lack of overweight and obese patients in the UCD patient group may contribute to our inability to detect an association of BMI with PYY levels in this group.
This is the first study to examine PYY levels in UCD patients. Due to the retrospective nature of this study, there are some limitations. First, cases and controls were not matched for demographic variables such as age or sex, since controls were drawn from a previous study [13]. Additionally, we did not have access to fasting status for UCD patients. Minimally, these patients were fasted for two hours prior to sample collection whereas control subjects were fasted overnight. While this is a potential confounder, we believe, it is unlikely to drive the significant effect to the degree observed in our UCD patients given that plasma PYY levels typically peak approximately 60 minutes after eating[29,10], and nearly half of our UCD cases have PYY levels greater than peak levels reported by Hill et al in a study of diurnal variation of PYY[30]. Despite these limitations, this study reveals that regulation of PYY may be altered in UCD patients, and increased levels of PYY may contribute to the anorexia often observed in these patients.
In conclusion, we demonstrate that PYY levels are significantly elevated in our UCD patients compared with controls. Factors associated with PYY levels include BMI in the control group and diagnosis subtype in the UCD patient group, an association that has not been previously reported. Larger, prospective studies are needed to gain a better understanding of how appetite regulating hormones such as PYY impact the nutritional management of UCD patients.
Highlights.
First study examining plasma PYY levels in urea cycle disorder patients
PYY levels are increased in urea cycle disorder patients compared to controls.
PYY levels are associated with urea cycle disorder subtype.
Propose a gene regulatory mechanism linking appetite regulation and the urea cycle.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) grant 9U54HD061221, the Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources, NIH, and the O’Malley Family Foundation Grant.
Authors confirm independence from the sponsors; the content of this article has not been influenced by the sponsors.
We would like to thank Shelia Dawling, Maxine Perlen and Diane Combs of Vanderbilt Pathology Laboratory Services for their assistance in sample collection.
All authors have made substantial contributions and had final approval of the conceptions, drafting, and final version of this manuscript. SM was involved in study conception and design, carried out the assays, performed statistical analysis and data interpretation, drafted and revised the manuscript. TWB, the research study coordinator, gathered relevant demographic and clinical patient data for this study and contributed to study design. She also handled the IRB submissions for this project. LD assisted with the statistical analysis plan and critical manuscript revision. JL provided control samples and was involved in critically revising the manuscript. DM contributed to study design and critical manuscript revision. The Urea Cycle Disorders Consortium contributed samples as well as scientific review and concept design. DCC assisted with statistical analysis and interpretation of data. She was also involved in drafting and critically revising the manuscript. M. Summar was involved in study conception, design, and manuscript revision.
Abbreviations
- ASL
Argininosuccinate lyase
- ASS1
Argininosuccinate synthase 1
- BMI
Body mass index
- CPS1
Carbamoyl-phosphate synthase 1
- NAGS
N-acetylglutamate synthase
- OTC
Ornithine carbamoyltransferase
- OTCD
Ornithine carbamoyltransferase deficiency
- PYY
Peptide tyrosine tyrosine
- UCD
Urea cycle disorder(s)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6.0 Conflict of interest statement The authors declare no potential conflicts of interest
7.0 Details of ethics approval This study was approved by the Institutional Review Board at Vanderbilt University Medical Center (IRB# 081080).
References
- [1].Mofidi S, Kronn D. Emergency Management of Inherited Metabolic Disorders. Topics in Clinical Nutrition. :24374–384. [Google Scholar]
- [2].Pediatric Nutrition in Chronic Diseases and Developmental Disorders Second.
- [3].Lee B, Singh RH, Rhead WJ, Sniderman KL, Smith W, Summar ML. Considerations in the difficult-to-manage urea cycle disorder patient. Crit Care Clin. :21S19–S25. doi: 10.1016/j.ccc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- [4].Schulpis KH, Papassotiriou I, Vounatsou M, Karikas GA, Tsakiris S, Chrousos GP. Morning preprandial plasma ghrelin and catecholamine concentrations in patients with phenylketonuria and normal controls: evidence for catecholamine-mediated ghrelin regulation. J.Clin.Endocrinol.Metab. :893983–3987. doi: 10.1210/jc.2004-0311. [DOI] [PubMed] [Google Scholar]
- [5].Schulpis KH, Papakonstantinou ED, Tzamouranis J. Plasma leptin concentrations in phenylketonuric patients. Horm.Res. :5332–35. doi: 10.1159/000023510. [DOI] [PubMed] [Google Scholar]
- [6].Vincent RP, Le Roux CW. The satiety hormone peptide YY as a regulator of appetite. J.Clin.Pathol. :61548–552. doi: 10.1136/jcp.2007.048488. [DOI] [PubMed] [Google Scholar]
- [7].Leiter AB, Toder A, Wolfe HJ, Taylor IL, Cooperman S, Mandel G, Goodman RH. Peptide YY. Structure of the precursor and expression in exocrine pancreas. J.Biol.Chem. :26212984–12988. [PubMed] [Google Scholar]
- [8].Korner J, Leibel RL. To eat or not to eat - how the gut talks to the brain. N.Engl.J.Med. :349926–928. doi: 10.1056/NEJMp038114. [DOI] [PubMed] [Google Scholar]
- [9].Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. :418650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- [10].Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N.Engl.J.Med. :349941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- [11].Adrian TE, Smith HA, Calvert SA, Aynsley-Green A, Bloom SR. Elevated plasma peptide YY in human neonates and infants. Pediatr.Res. :201225–1227. doi: 10.1203/00006450-198612000-00007. [DOI] [PubMed] [Google Scholar]
- [12].Siahanidou T, Mandyla H, Vounatsou M, Anagnostakis D, Papassotiriou I, Chrousos GP. Circulating peptide YY concentrations are higher in preterm than full-term infants and correlate negatively with body weight and positively with serum ghrelin concentrations. Clin.Chem. :512131–2137. doi: 10.1373/clinchem.2005.054908. [DOI] [PubMed] [Google Scholar]
- [13].Lomenick JP, Melguizo MS, Mitchell SL, Summar ML, Anderson JW. Effects of meals high in carbohydrate, protein, and fat on ghrelin and peptide YY secretion in prepubertal children. J.Clin.Endocrinol.Metab. :944463–4471. doi: 10.1210/jc.2009-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roth CL, Enriori PJ, Harz K, Woelfle J, Cowley MA, Reinehr T. Peptide YY is a regulator of energy homeostasis in obese children before and after weight loss. J.Clin.Endocrinol.Metab. :906386–6391. doi: 10.1210/jc.2005-1357. [DOI] [PubMed] [Google Scholar]
- [15].El KD, El-Rassi R, Azar S, Hwalla N. Postprandial ghrelin and PYY responses of male subjects on low carbohydrate meals to varied balancing proportions of proteins and fats. Eur.J.Nutr. :49493–500. doi: 10.1007/s00394-010-0108-9. [DOI] [PubMed] [Google Scholar]
- [16].Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. :4223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- [17].Le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. :1473–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- [18].Le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann.Surg. :243108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pournaras DJ, Osborne A, Hawkins SC, Mahon D, Ghatei MA, Bloom SR, Welbourn R, Le Roux CW. The gut hormone response following Roux-en-Y gastric bypass: cross-sectional and prospective study. Obes.Surg. :2056–60. doi: 10.1007/s11695-009-9989-1. [DOI] [PubMed] [Google Scholar]
- [20].Lawson EA, Klibanski A. Endocrine abnormalities in anorexia nervosa. Nat.Clin.Pract.Endocrinol.Metab. :4407–414. doi: 10.1038/ncpendmet0872. [DOI] [PubMed] [Google Scholar]
- [21].Misra M, Miller KK, Tsai P, Gallagher K, Lin A, Lee N, Herzog DB, Klibanski A. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J.Clin.Endocrinol.Metab. :911027–1033. doi: 10.1210/jc.2005-1878. [DOI] [PubMed] [Google Scholar]
- [22].McCullough BA, Yudkoff M, Batshaw ML, Wilson JM, Raper SE, Tuchman M. Genotype spectrum of ornithine transcarbamylase deficiency: correlation with the clinical and biochemical phenotype. Am.J.Med.Genet. :93313–319. doi: 10.1002/1096-8628(20000814)93:4<313::aid-ajmg11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- [23].Adachi N, Lieber MR. Bidirectional gene organization: a common architectural feature of the human genome. Cell. :109807–809. doi: 10.1016/s0092-8674(02)00758-4. [DOI] [PubMed] [Google Scholar]
- [24].Trinklein ND, Aldred SF, Hartman SJ, Schroeder DI, Otillar RP, Myers RM. An abundance of bidirectional promoters in the human genome. Genome Res. :1462–66. doi: 10.1101/gr.1982804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Colombo JP, Pfister U, Cervantes H. The regulation of N-acetylglutamate synthetase in rat liver by protein intake. Biochem.Biophys.Res.Commun. :1721239–1245. doi: 10.1016/0006-291x(90)91582-d. [DOI] [PubMed] [Google Scholar]
- [26].Morris SM., Jr Regulation of enzymes of the urea cycle and arginine metabolism. Annu.Rev.Nutr. :2287–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- [27].Lomenick JP, Clasey JL, Anderson JW. Meal-related changes in ghrelin, peptide YY, and appetite in normal weight and overweight children. Obesity.(Silver.Spring) :16547–552. doi: 10.1038/oby.2007.129. [DOI] [PubMed] [Google Scholar]
- [28].Kim BJ, Carlson OD, Jang HJ, Elahi D, Berry C, Egan JM. Peptide YY is secreted after oral glucose administration in a gender-specific manner. J.Clin.Endocrinol.Metab. :906665–6671. doi: 10.1210/jc.2005-0409. [DOI] [PubMed] [Google Scholar]
- [29].Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. :891070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- [30].Hill BR, De Souza MJ, Williams NI. Characterization of the diurnal rhythm of peptide YY and its association with energy balance parameters in normal-weight premenopausal women. Am.J.Physiol Endocrinol.Metab. :301E409–E415. doi: 10.1152/ajpendo.00171.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]