Abstract
The homeostasis of copper (Cu) in the cerebrospinal fluid (CSF) is partially regulated by the Cu transporter-1 (CTR1) and divalent metal transporter-1 (DMT1) at the blood-CSF barrier (BCB) in the choroid plexus. Data from human and animal studies suggest an increased Cu concentration in blood, CSF, and brains following in vivo manganese (Mn) exposure. This study was designed to investigate the relative role of CTR1 and DMT1 in Cu transport under normal or Mn-exposed conditions using an immortalized choroidal Z310 cell line. Mn exposure in vitro resulted in an increased cellular 64Cu uptake and the up-regulation of both CTR1 and DMT1. Knocking down CTR1 by siRNA counteracted the Mn-induced increase of 64Cu uptake, while knocking down DMT1 siRNA resulted in an increased cellular 64Cu uptake in Mn-exposed cells. To distinguish the roles of CTR1 and DMT1 in Cu transport, the Z310 cell-based tetracycline (Tet)-inducible CTR1 and DMT1 expression cell lines were developed, namely iZCTR1 and iZDMT1 cells, respectively. In iZCTR1 cells, Tet induction led to a robust increase (25 fold) of 64Cu uptake with the time course corresponding to the increased CTR1. Induction of DMT1 by Tet in iZDMT1 cells, however, resulted in only a slight increase of 64Cu uptake in contrast to a substantial increase in DMT1 mRNA and protein expression. These data indicate that CTR1, but not DMT1, plays an essential role in transporting Cu by the BCB in the choroid plexus. Mn-induced cellular overload of Cu at the BCB is due, primarily, to Mn-induced over-expression of CTR1.
Keywords: manganese, copper, Cu transporter 1 (CTR1), divalent metal transporter 1 (DMT1), choroid plexus, iZCTR1, iZDMT1
Introduction
The relationship between manganese (Mn) intoxication and Parkinsonism has long been recognized (Aschner et al., 2007; Crossgrove and Zheng, 2004; Mergler and Baldwin, 1997). However, the mechanism by which Mn induces neurotoxicity is still unclear. Our recent occupational study among Mn-exposed smelters reveals that blood copper (Cu) concentrations are significantly higher in smelters than in controls (Jiang et al., 2007). A separate study among welders also shows that the concentrations of Mn and Cu in saliva and serum of welders are significantly higher than those in controls (Wang et al., 2008a). Studies on non-human primates or rodents also demonstrate a significant increase in brain Cu concentrations following Mn exposure (Guilarte et al., 2006; Guilarte and Chen, 2007; Lai et al., 1999). More recently, we conducted an animal study, where rats were subchronically exposed to Mn for 4 weeks, and found a significant increase in Cu concentrations in striatum, hippocampus, motor cortex, and choroid plexus compared to saline controls (Zheng et al., 2009). These human and animal data clearly indicate an altered Cu homeostasis in the central nervous system following Mn exposure.
Cu is an essential trace element in all living organisms playing an important role as a catalytic cofactor for metalloenzymes (Horn and Barrientos, 2008); however, excessive intake of Cu can be pathogenic. Cumulative evidence has implied that an imbalanced Cu homeostasis in brain contributes to the pathogenesis of neurodegenerative disorders such as idiopathic Parkinson’s disease (IPD), Alzheimer’s disease (AD), and familial amyotrophic lateral sclerosis (ALS) (Deibel et al., 1996; Gaggelli et al., 2006; Rossi et al., 2004; Sparks and Schreurs, 2003; Strausak et al., 2001; Waggoner et al., 1999). It has been shown in some human studies that the Cu levels in the CSF are associated with IPD (Boll et al., 2008; Hozumi et al., 2011). Furthermore, the Cu-derived biochemical parameters in the CSF are correlated with the disease severity and the degree of disease progression (Jiménez-Jiménez et al., 1998; Pall et al., 1987). As a free metal ion, Cu can interact readily with oxygen to initiate a cascade of biochemical events leading to the production of the highly damaging hydroxyl free radicals. It is because of its essentiality to the cellular function and its cytotoxic nature in oxidative stress that Cu is strictly regulated in the body.
A stable Cu homeostasis in the brain is regulated by two barrier systems: the blood-brain barrier (BBB) and the blood-cerebrospinal fluid barrier (BCB) (Zheng and Monnot, 2011). The epithelial cells of the choroid plexus in brain ventricles provide the structural basis of the BCB (Zheng and Zhao, 2002), which restricts material transport between the blood and CSF, allowing selective essential nutrients, metal ions and drug molecules to enter the cerebrospinal fluid (CSF), and at the meantime removing metabolites or unwanted materials from brain extracellular fluid to the blood (Zheng, 2001; Zheng et al., 2003). At the BCB, the Cu transport is regulated by two major Cu transporters, i.e., copper transporter-1 (CTR1) and divalent metal transporter-1 (DMT1)
CTR1 is a plasma membrane protein with 3 transmembrane domains that form a homotrimeric pore essential for Cu uptake (Gaggelli et al. 2006). CTR1 has been shown to be present in the brain capillary endothelial cells of the BBB, choroid plexus of the BCB, and the brain parenchyma (Choi and Zheng, 2009; Kuo et al. 2006; Zheng and Monnot, 2011). DMT1, also known as divalent cation transporter 1 (DCT1) or natural resistance-associated macrophage protein 2 (Nramp2), is a proton driven transporter, transporting one proton and one atom of ferrous Fe(II) in the same direction. DMT1 nonselectively transports multiple divalent metals including Mn, Cu, cobalt (Co), zinc (Zn), cadmium (Cd) and lead (Pb) (Gruenheid et al., 1995; Gunshin et al 1997). DMT1 is expressed in most organs and cell types. While its presence in the BBB remains controversial, the data from our own studies and by others support the presence of DMT1 in the BCB (Gunshin et al., 1997; Wang et al., 2006, 2008b,c). Recent data from this laboratory (Zheng et al., 2009) have show that chronic exposure to Mn in rats results in a significant increase of Cu in the CSF as well as in the choroid plexus, the tissue that secretes and regulates the CSF. Since both CTR1 and DMT1 have been implicated in Cu transport by the BCB, it has become imperative to learn, from the basic biochemical conceptual point of view, what is the relative role of these two major Cu transporters in regulating Cu transmembrane transport in the brain barrier systems. From the toxicological point of view, it has also become necessary to know how Mn accumulation in the choroid plexus may affect the expression of CTR1, DMT1 or both, leading to an altered Cu transport by the BCB.
Thus, the purposes of this study were (1) to verify whether in vitro Mn exposure increased cellular Cu uptake in BCB cells, (2) to determine whether in vitro Mn exposure altered the expression of CTR1 and DMT1, and (3) to study the relative contribution of CTR1 and DMT1 in Cu uptake in choroidal cells by using the choroidal Z310 cell-based tetracycline-inducible cell lines that over-expressed CTR1 (i.e., iZCTR1 cell line) or DMT1 (i.e., iZDMT1 cell line). The results from this study should enable us to understand the relative role of CTR1 and DMT1 in regulating Cu transport by the BCB and the impact of Mn exposure on these processes.
Materials and methods
Materials and Cell culture
Manganese chloride (MnCl2) was purchased from Fisher scientific (Pittsburgh, PA); copper chloride, sodium pyruvate, calcium chloride and HEPES from Sigma Chemical Company (St. Louis, MO); Hank’s balanced salt solution (HBSS), fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), and antibiotic–antimycin solution from Gibco (Grand Island, NY); 14C-sucrose (specific activity: 495 mCi/mmol) from Moravek Biochemicals, Inc. (Brea, CA); Eco-lite-(+) scintillation cocktail from MP Biomedicals (Irvine, CA). 64CuCl2 (specific activity 15–30 mCi/μg) was obtained from Washington University at St. Louis, which was produced by cyclotron irradiation of an enriched 64Ni target by using methods reported (McCarthy et al., 1997). All reagents were analytical grade, HPLC grade, or the best available pharmaceutical grade.
The Z310 choroidal epithelial cell line was established from murine choroid plexus and used in the experiments. The detailed procedure for cell culture and maintenance can be found in the previous publications (Li et al., 2005; Wang et al., 2006; Zheng and Zhao, 2002). Briefly, the cells were grown in DMEM (Cellgro) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, 100 μg/ml streptomycin, and 10 μg/ml of gentamycin in a humidified incubator with 95% air-5% CO2 at 37°C. The cells were passaged (1:12–16) twice a week.
64Cu uptake assay
Z310 cells of each group were washed twice with PBS and incubated at 37°C for 60 min with serum-free medium containing 5 μCi/ml 64Cu and 5 μM CuCl2. After the incubation, cells were washed with PBS for 3 times and then were scraped into microcentrifuge tubes. The cellular 64Cu radioactivity was determined with a gamma-counter (PerkinElmer 1480 WIZARD 3”) and protein concentration determined by the Bradford assay.
RNA isolation and quantitative real-time PCR
Total RNA from cells was extracted using the TRIzol reagent (Invitrogen). The quality of RNA (A260/A280) was 1.8~2.0 for all RNA preparations. The amount (0.5 μg) of total RNA from each sample was reverse-transcribed in cDNA at 48°C for 1 h using Moloney murine leukemia virus reverse transcriptase (200 U; Applied Biosystems). For qPCR analysis, the levels of mRNAs were quantified by using FastStart Universal SYBR Green Master (Rox; Roche) in a M×3000p Real-Time PCR System. All amplification reactions were conducted in triplicate. Relative expression ratios were calculated by the ΔΔCt method where Ct is the threshold cycle value (Pfaffl, 2001). The GAPDH gene was used for normalization. The amplification efficiencies of both target genes and the endogenous reference were checked by examining the variation of the Ct with template dilutions.
The following primers were used in experiments: rat CTR1 (accession No. NM_133600.1), sense, 5′- TCG GCC TCA CAC TCC CAC GA -3′ (gene location 259–278), and antisense, 5′- CGA AGC AGA CCC TCT CGG GC -3′ (gene location 424–443); rat DMT1 (accession No. NM_013173.1), sense, 5′- TCG CAG GCG GCA TCT TGG TC -3′ (gene location 1537–1556), and antisense, 5′- TAC CGA GCG CCC ACA GTC CA -3′ (gene location 1717–1916); rat GAPDH (accession No. NM_017008.3), sense, 5′- TGC CGC CTG GAG AAA CCT GC -3′ (gene location 809–828), and antisense, 5′- AGC AAT GCC AGC CCC AGC AT -3′ (gene location 957–976).
Cell lysis and Western blotting
Monolayer cells were washed three times with ice-cold PBS followed by incubation in RIPA cell lysis buffer (Sigma) with PMSF and Protease Cocktail (Sigma) on ice for 10 min. Cells were scraped into microcentrifuge tubes, briefly sonicated, and then centrifuged for 15 min at 12,000×g to remove the cellular debris. Postnuclear supernatants were analyzed for protein concentration using the BCA method (Pierce).
For Western blotting, samples were boiled in Laemmli sample buffer (Sigma) and subjected to 12% tris-glycine SDS-PAGE and then transferred to polyvinylidene fluoride membranes (Millipore), which were blocked with 5% milk powder in TBST (0.1% Tween 20 in TBS) for 1 h and then incubated overnight at 4°C with the corresponding primary antibodies, which were diluted in blocking solution (rabbit anti-CTR1, 1: 300; Santa Cruz), rabbit anti-DMT1 (1: 500; Alpha Diagnostic International), rabbit anti-occludin, rabbit anti-ZO-1 (1: 300; Zymed), or mouse anti-β-actin (1:10000; Sigma). After incubation with the appropriate HRP-conjugated secondary antibody for 1 h at room temperature, the blots were developed using ECL Western Blotting Substrate (Pierce) and exposed to film (Kodak). The integrated densities of each band on the film were quantified using the Image Lab software.
siRNA knockdown studies
The siRNA transfection was performed following the manual of Silencer® siRNA Starter Kit were obtained from Ambion (Applied Biosystems). Briefly, the Z310 cells were cultured to ~50% confluent in 6-well plates at transfection. The cells were incubated with either stealth small interfering RNA (siRNA) duplexes against CTR1 or DMT1, or stealth siRNA Negative Control in Opti-MEM I medium (Invitrogen) for 8 h, and then an equal volume of normal DMEM medium was added to each well. The medium was replaced with fresh DMEM medium at 24 h after the transfection. Cells were harvested for the determination of mRNA and protein levels at 48 h following the siRNA transfection. Candidate sequences for rat CTR1 knockdown was self-designed by using the Ambion website. The sequences used were as follows. Forward target sequence: 5’-CCAUCCUUAUGGAGACACAtt-3’; Reverse target sequence: 5’-ttGGUAGGAAUACCUCUGUGU-3’. Candidate sequences for rat DMT1 knockdown was a pre-designed silencer select sequences DMT1 (ID: s130558) obtained from Ambion.
Construction of the Tet-regulated expression vector
Rat CTR1 and DMT1 gene inserts flanked by EcoR I and Xho I sites were amplified by RT-PCR with the cDNA template obtained from Z310 cells. The following primer sequences were used: CTR1 (accession No. NM_133600.1), sense, 5′- CCG GAA TTC GCC ACC ATG AGG ATG AAC CAC ATG GAG ATG-3′, and antisense, 5′- CCG CTC GAG ATG GCA GTG CTC TGT GAT GTC C-3′; DMT1 (accession No. NM_013173.1), sense, 5′- CCG GAA TTC ACC ATG GTG TTG GAT CCT GAA GAA-3′, and antisense, 5′- CCG CTC GAG CTT AGT ATT GCC ACC GCT GGT ATC-3′. Both the pcDNA4/TO vector (Invitrogen) and the inserts were double digested with EcoR I and Xho I; each insert was purified by the Zymoclean™ Gel DNA Recovery Kit (ZYMO RESEARCH) and then was ligated to open EcoR I.pcDNA™4/TO/myc-His.Xho I, respectively. The constructed expression vectors were transformed into One Shot® TOP10F’ Competent Cells (Invitrogen). Bacterial clones containing EcoR I.pcDNA™4/TO/myc-His.Xho I with CTR1 or DMT1 insert was selected using Luria-Bertani (LB)-agar plates containing 100 μg/mL ampicillin, followed by plasmid isolation by the QIAprep Spin Miniprep Kit (Qiagen).
Vector transfection and screening of stable integrants
The CTR1- and DMT1-inducible cell lines were created using a Tetracycline-Regulated Expression System for Mammalian Cells (T-REx™ System) (Invitrogen). The regulatory vector pcDNA6/TR was first transfected into Z310 cells using Lipofectamine™ 2000 reagent (Invitrogen), followed by selection of stable integrants with 5μg/mL blasticidin. Thirty stable cell clones were selected and tested for Tet-inducible gene expression by transiently transfecting with the positive control plasmid expressing β-galactosidase (pcDNA™4/TO/myc-His/lacZ), followed by β-gal assay. The clone that exhibited the lowest level of basal transcription and the highest levels of β-galactosidase expression after addition of Tet was selected for the transfection of the expression vector, EcoR I.pcDNA™4/TO/myc-His.Xho I with CTR1 or DMT1 insert, and then was dual-selected by 5μg/mL blasticidin plus 250 μg/mL Zeocin. Quantitative real-time PCR and Western blot were performed to test for Tet-regulated gene expression.
Statistical analysis
All data are reported as the mean ± SE from at least three independent biological samples. Statistical significance between multiple groups was performed using one-way ANOVA. If significant effects were found by ANOVA, the post hoc analyses were performed to compare individual groups using two-tailed paired Student’s t tests. A value of p<0.05 was considered statistically significant.
Results
Cu accumulation in Z310 cells following Mn exposure
When the choroidal Z310 cells were treated with Mn at 100 μM for 24 h and 48 h, the cellular 64Cu uptake was increased by 1.26 fold and 1.37 fold, respectively, as compared to the controls (p<0.05, Fig. 1). The data support our early in vivo observation that subchronic Mn exposure results in Cu accumulation in the choroid plexus, where the BCB is located (Zheng et al., 2009).
Fig. 1.
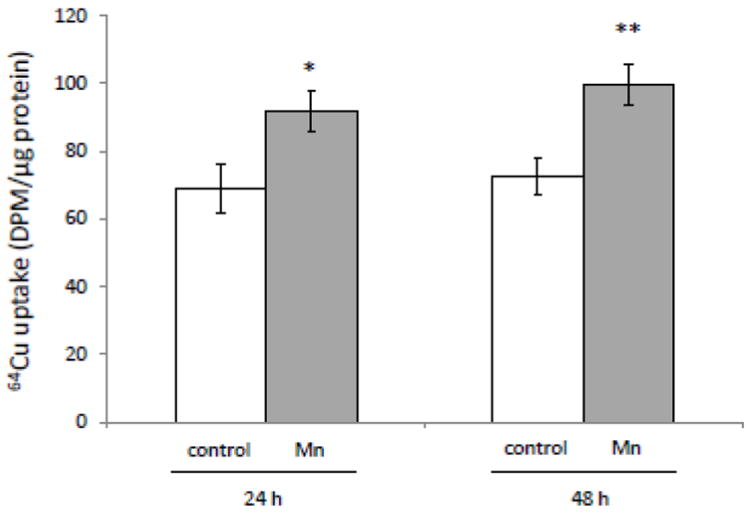
Increased Cu uptake in Z310 cells following Mn exposure in vitro. Z310 cells were treated with 100 μM Mn at time indicated, followed by the incubation with 5 mCi/ml 64Cu. The date represent Mean ± SE, n=8. *: p<0.05; **: p<0.01 as compared to controls.
Increased levels of both CTR1 and DMT1 following Mn exposure
Increased cellular update of Cu may be a direct result of Mn effect on Cu transporters, mainly CTR1 and DMT1. The quantitative real-time PCR (qRT-PCR) showed that in vitro Mn exposure (100 μM for 24 h and 48 h) caused a significant increase of mRNAs encoding CTR1 (1.38 fold and 1.47 fold, respectively, p<0.01, Fig. 2A) or DMT1 (1.41 and 1.63 fold, respectively, p<0.01, Fig. 2B) in Z310 cells. Further investigation of protein expression by the Western blot revealed that the protein levels of both CTR1 and DMT1 were also correspondingly increased in Mn-treated Z310 cells at different time points (Fig. 2C, D). Together, these data suggested that Mn exposure up-regulated the expression levels of both CTR1 and DMT1, which may contribute to the increased cellular uptake of 64Cu.
Fig. 2.
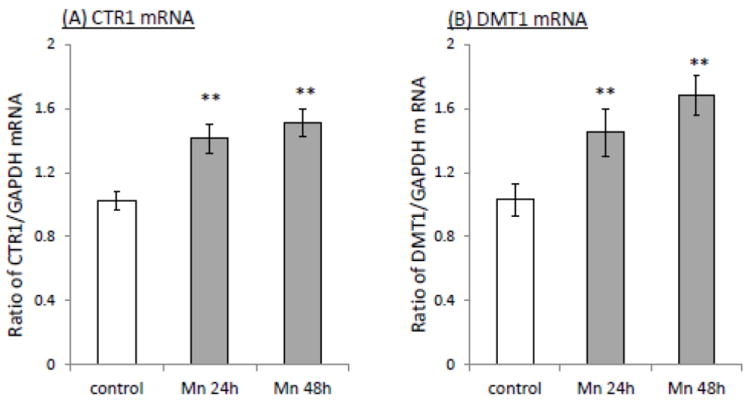
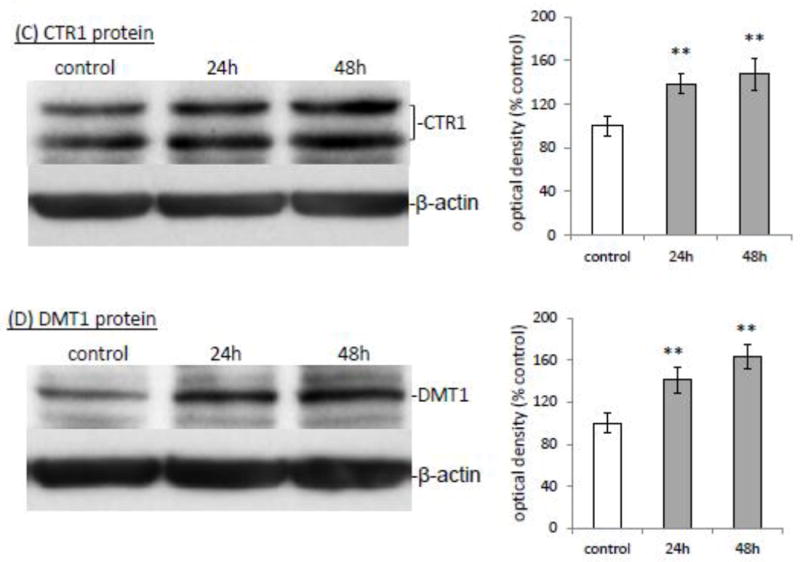
Increased expression of CTR1 and DMT1 following Mn exposure. Z310 cells were treated with 100 μM Mn for the time indicated. The levels of mRNA were quantified by real-time RT-PCR and expressed as the ratio of CTR1/GAPDH and the protein expressions were investigated by Western blot. (A). CTR1 mRNA. (B) DMT1 mRNA. (C) CTR1 protein. The bar graph represents the optical density of CTR1 bands normalized by the optical density of the corresponding β-actin band (n=3). (D) DMT1 protein. The bar graph represents the optical density of DMT1 bands normalized by the corresponding β-actin band (n=3). Data represent mean ± SE, n=6; *: p<0.05; **: p<0.01 as compared to the controls.
CTR1 and DMT1 siRNA knockdown and cellular 64Cu uptake
If CTR1 and DMT1 indeed regulated Cu uptake in the BCB, using siRNA to knock-down the expression of these two transporters would be expected to reduce the cellular 64Cu uptake. The data presented in Fig. 3 showed that after Z310 cells were transfected with either CTR1 siRNA or scramble siRNA (negative control), the siRNA duplexes against CTR1 caused ~63% reduction in the CTR1 mRNA level relative to the negative control, and Mn exposure for 24 h after CTR1 siRNA knockdown failed to up-regulate the mRNA level of CTR1 (Fig. 3A). Western blot confirmed the effectiveness of siRNA duplexes in knocking down CTR1, and Mn exposure for 24 h following the CTR1 siRNA knockdown did not significantly affect CTR1 protein expression (Fig. 3B).
Fig. 3.
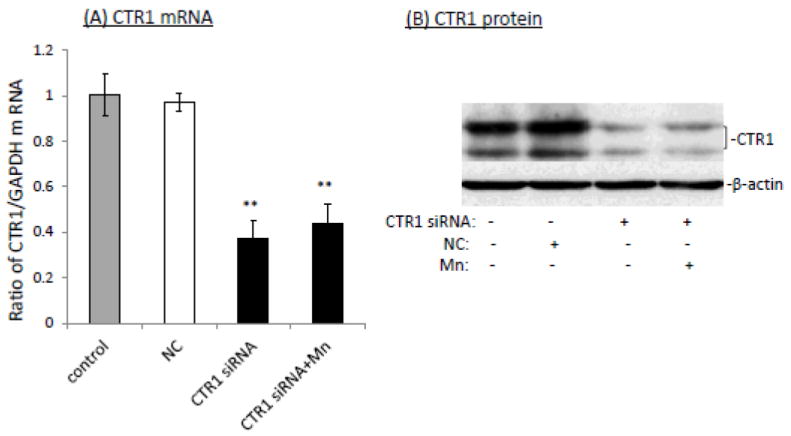
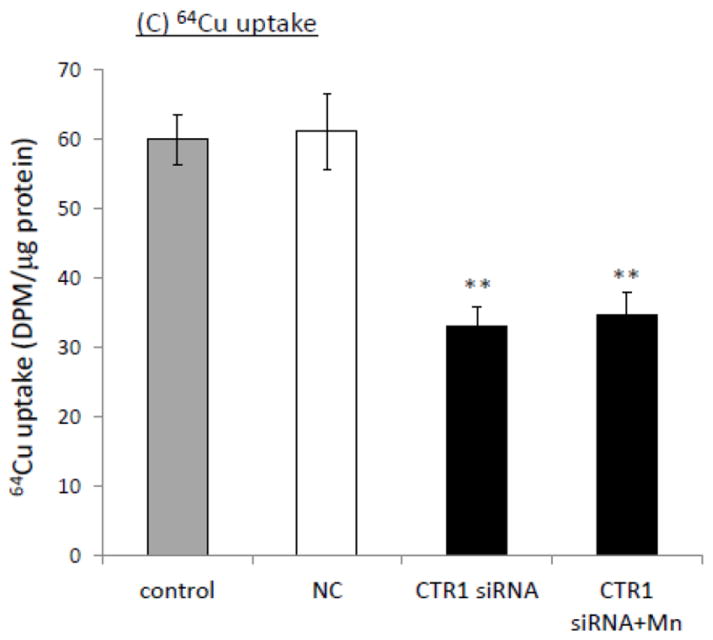
Impact of siRNA knockdown of CTR1 on the Mn-induced cellular Cu accumulation. (A) siRNA knockdown of CTR1decreased the CTR1 mRNA transcription. Data represent mean ± SE, n=6; **: p<0.01 as compared to NC (negative control or scramble siRNA). (B) CTR1 protein levels were decreased following the siRNA treatment. (C) CTR1siRNA knockdown decreased cellular 64Cu uptake. Data represent mean ± SE, n=6; **: p<0.01 as compared to NC.
To investigate the impact of CTR1 knockdown on cellular Cu uptake, the cells were exposed to 100 μM Mn for 24 h after the siRNA transfection, followed by incubation with 64Cu in serum-free medium for cellular 64Cu uptake assessment. The data showed that CTR1 knockdown resulted in a significant reduction in cellular Cu uptake, and Mn exposure following the CTR1 knockdown failed to cause the increase of Cu in the cells (Fig. 3C).
Similar approaches were used to investigate the impact of DMT1 siRNA knockdown on the cellular Cu uptake. In DMT1 siRNA transfected cells, there was about 56% reduction in the DMT1 mRNA level relative to the negative control; Mn exposure for 24 h after DMT1 siRNA knockdown did not result in the up-regulation of the DMT1 mRNA level (Fig. 4A). Western blot confirmed the down-regulation of DMT1 protein following siRNA knockdown (Fig. 4B). Unexpectedly, however, the 64Cu uptake assay revealed that the DMT1 siRNA knockdown did not reduce the cellular 64Cu uptake; instead Mn exposure after DMT1 knockdown led to a significant increase of 64Cu uptake by Z310 cells (Fig. 4C).
Fig. 4.
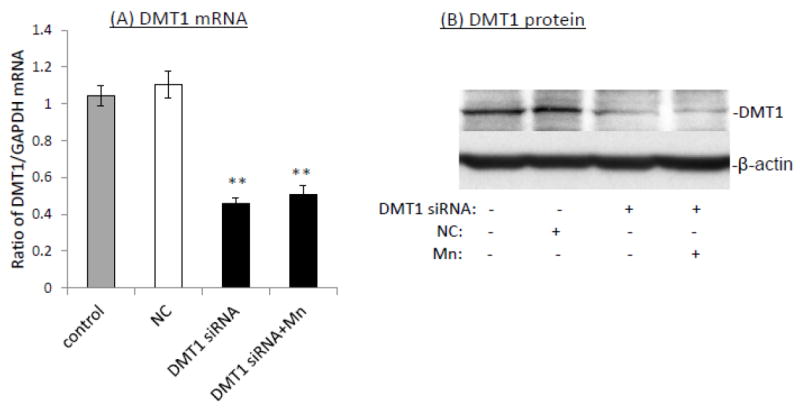
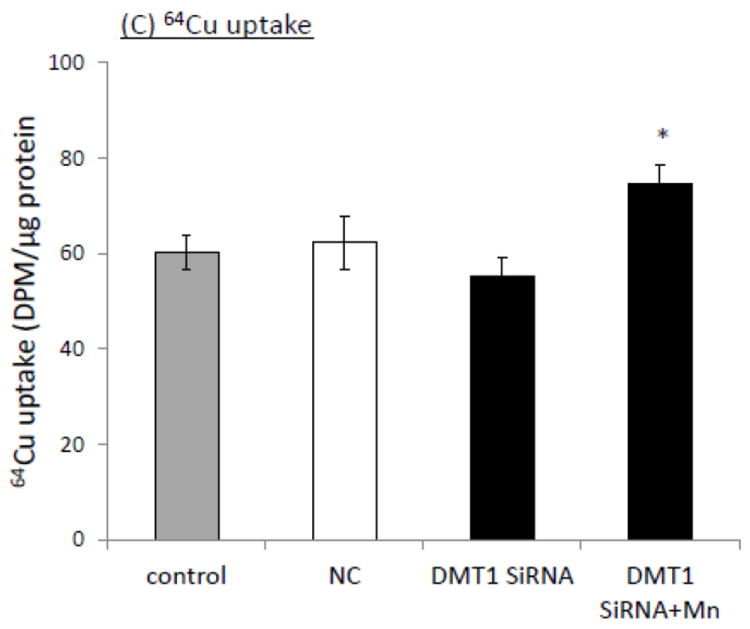
Impacts of siRNA knockdown of DMT1 on the Mn-induced cellular Cu accumulation. (A) siRNA knockdown of DMT1 decreased the DMT1 mRNA transcription. Data represent mean ± SE, n=6; **: p<0.01 as compared to NC. (B) DMT1 protein levels were decreased following the siRNA treatment. (C) DMT1 siRNA knockdown failed to decrease the 64Cu uptake, but instead increased the cellular Cu uptake, as compared to controls and NC. Data represent mean ± SE, n=6. *: p<0.05 compared to the NC.
These results appeared to suggest that CTR1 may play a more important role than DMT1 in cellular uptake of Cu by the choroidal epithelial cells. Since both transporters may be regulated by the intracellular Mn levels, it is possible that the down-regulation of one transporter may lead to the up-regulation of the other as a natural compensatory mechanism. To more precisely differentiate the effect of CTR1 from that of DMT1 in cellular uptake of Cu, it became necessary to adapt the strategy by using CTR1 or DMT-inducible cell line.
CTR1-inducible cell line (iZCTR1) and cellular 64Cu uptake
The expression of CTR1 in the CTR1-inducible cell line is normally suppressed; only under the selected condition, can the expression be turned on. Thus, at the steady state of Mn intoxication when DMT1 is in its full functionality, one can turn on CTR1 gene by the chemical other than Mn to confirm the CTR1’s contribution to cellular Cu uptake. By using this strategy, we can compare the relative role of both transporters in their magnitude and time response in handling Cu uptake.
To established the choroidal Z310 cell-based tetracycline (Tet)-inducible cell lines (i.e., iZCTR1 cell line), the rat CTR1 gene was inserted into the expression vector (pcDNA4/TO); the size and DNA sequence of the CTR1 inserts were confirmed by double-sites (EcoR I and Xho I) restriction enzyme digestion (supplemental Fig. 1A) and DNA sequencing (data not shown). Following transfection and selection by antibiotics, a stable cell clone that over-expressed CTR1 upon the addition of Tet was selected (supplemental Fig. 1B,C). The qRT-PCR assay confirmed that after addition of Tet into the culture medium, there was a time-dependent increase of CTR1 mRNA in the cells (Fig. 5A). Western blot further confirmed that the Tet-induced increase of CTR1 protein expression (Fig. 5B). The result from 64Cu uptake assay also showed that the cellular 64Cu uptake was drastically increased in a time-dependent manner upon the addition of Tet (Fig. 5C). Thus, the selected clone was named the iZCTR1 cell line.
Fig. 5.
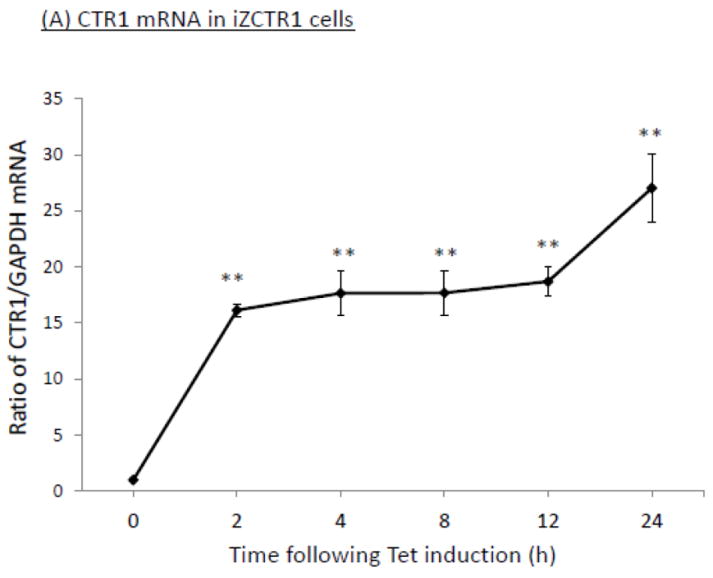
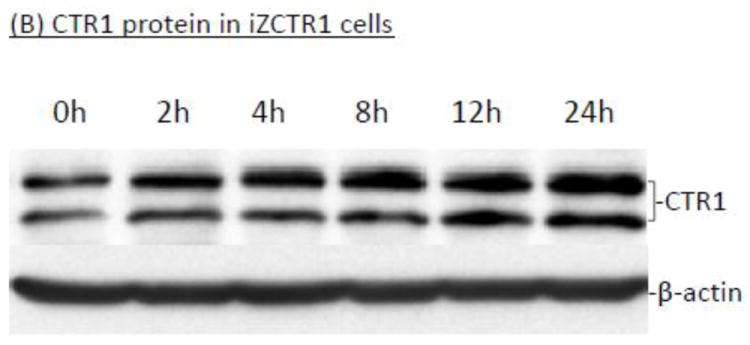
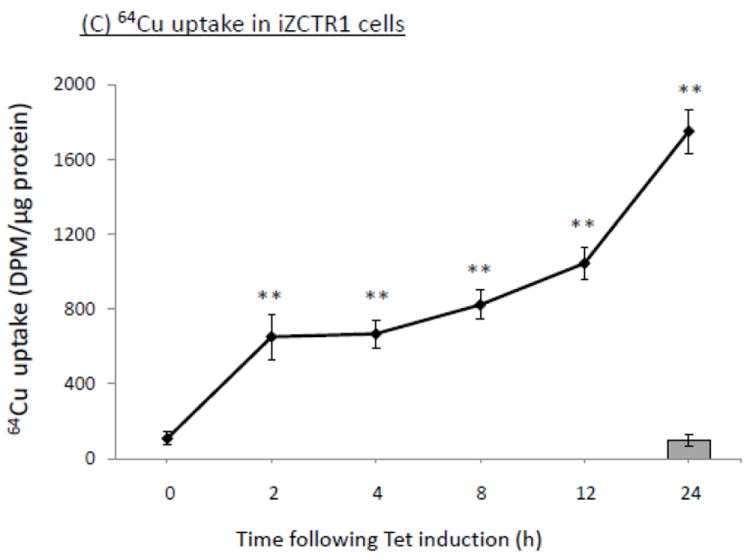
Tet-induced overexpression of CTR1 in iZCTR1 cells and the ensuing increase of cellular 64Cu uptake. The iZCTR1 cells were treated with Tet at time “0”. (A) Time course of CTR1 mRNA expression following Tet induction. Data represent mean ± SE, n=6; *: p<0.05 as compared to controls. (B) Time course of CTR1 protein level following Tet addition. (C) Time course of the cellular 64Cu uptake in iZCTR1 cells following Tet induction; the bar graph depicts the 64Cu uptake of the “normal” Z310 cells treated with Tet for 24 h (n=6). Data represent Mean ± SE, n=6. **: p<0.01 compared to controls and the Tet-treated Z310 cells.
To determine the contribution of CTR1 to Mn-induced cellular Cu accumulation, the iZCTR1 cells were exposed to 100 μM Mn for 24 h, followed by incubation with Tet for additional 4 h. The cells were then subjected to Cu uptake study as described above. The results showed that Tet alone led to more than 6 fold increase of Cu uptake as compared to controls (p<0.01, Fig. 6A); the incubation with Tet after Mn exposure further increased cellular Cu uptake by nearly 7 fold, which was statistically significantly different from the controls and cells treated only by Mn or Tet (Fig. 6A). Both qRT-PCR and Western blot confirmed the over-expression of CTR1 mRNA (16 fold, p<0.01, Fig. 6B) and protein level (10 fold, p<0.05, Fig. 6C) induced by Tet after Mn exposure. These data support an important role of CTR1 in Mn-induced intracellular Cu accumulation.
Fig. 6.
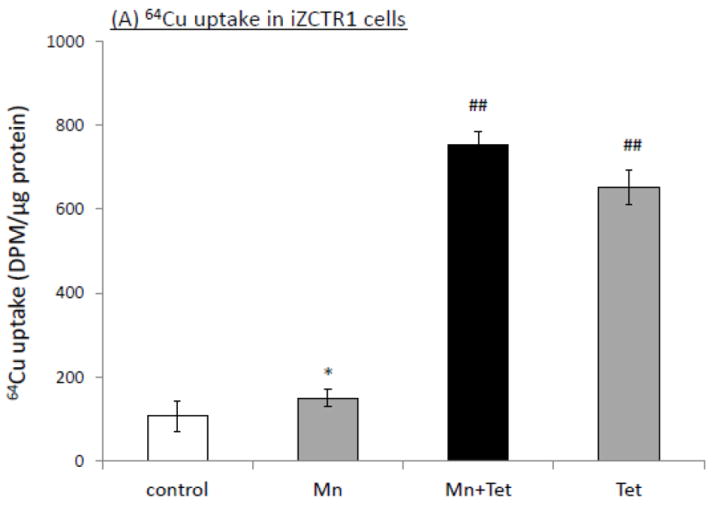
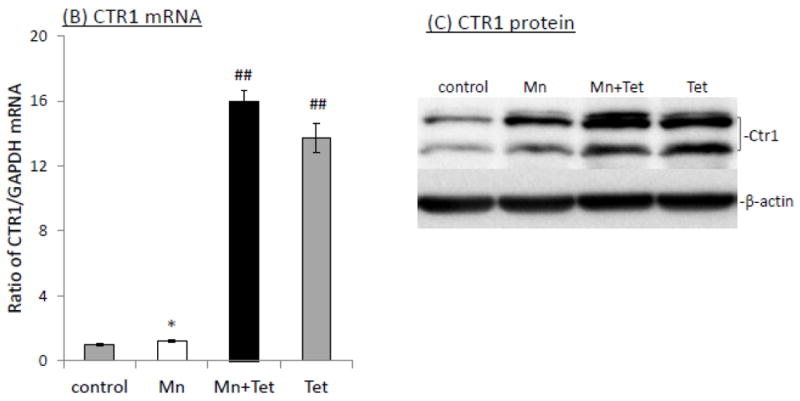
Induction of CTR1 in iZCTR1 cells at the steady state of Mn exposure and the cellular Cu accumulation. The iZCTR1 cells were exposed to 100 μM Mn for 24 h, followed by Tet treatment for 4 h. (A) Tet induced a drastic increase Cu uptake following Mn exposure in CTR1-inducible cells. (B) Tet induction increased CTR1 mRNA level in the Mn-treated iZCTR1 cells. Data represent mean ± SE, n=6; *: p<0.05 compared to controls; ##: p<0.01 compared to Mn-treated group. (C) Tet induction increased CTR1 protein levels in the Mn-treated iZCTR1 cells.
DMT1-inducible cell line (iZDMT1) and cellular Cu uptake
Using the similar approach, we selected a clone with a high expression of DMT1 mRNA and protein after Tet was added to the culture medium (supplemental Fig. 2A,B,C). We determined the time-course of the Tet-induced overexpression of DMT1 in iZDMT1 cells. Results by qRT-PCR and Western blot showed that after Tet treatment, there was a time-dependent increase of DMT1 expression in mRNA (Fig. 7A) and proteins (Fig. 7B), respectively. This transfected cell clone was then named iZDMT1 cell line. In 64Cu uptake assay, addition of Tet to the iZDMT1 cells indeed induced the cellular 64Cu uptake (Fig. 7C); however, the magnitude of Cu uptake was much less dramatic than that found in iZCTR1 cells (Fig. 5C).
Fig. 7.
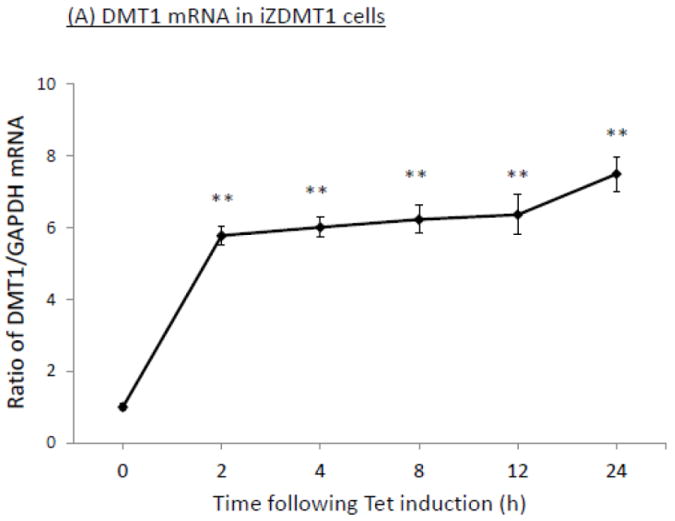
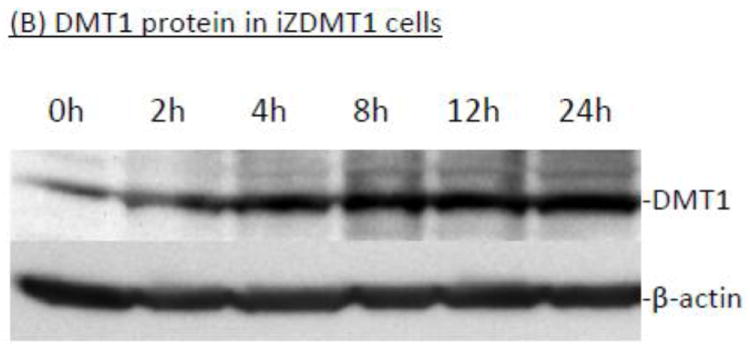
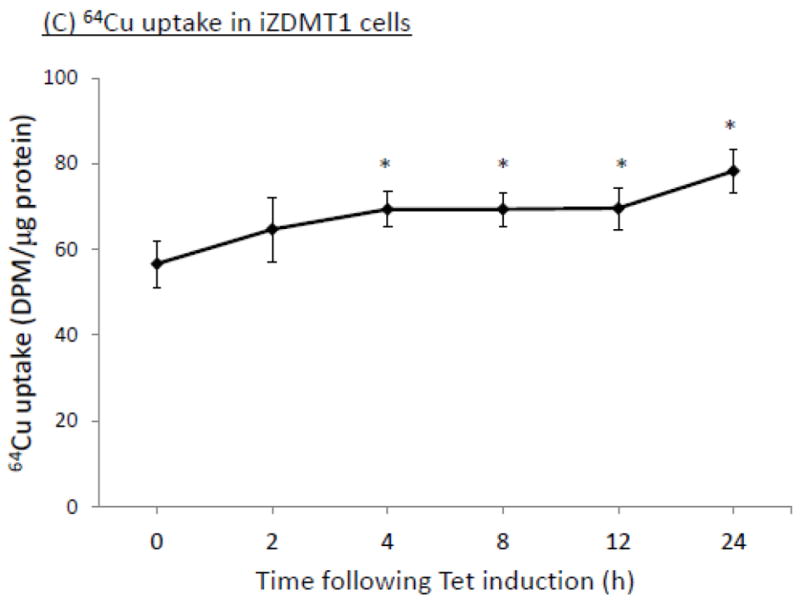
Tet-induced overexpression of DMT1 in iZDMT1 cells and the ensuing increase of cellular 64Cu uptake. The iZDMT1 cells were treated with Tet at time “0”. (A) Time course of DMT1 expression following Tet induction. Data represent mean ± SE, n=6;**: p<0.01 compared to controls. (B) Time course of DMT1 protein level following Tet addition. (C) Time course of the cellular 64Cu uptake in iZDMT1 cells following Tet induction. Data represent Mean ± SE, n=6. *: p<0.05 compared to controls.
When iZDMT1 cells were exposed to Mn, followed by Tet induction as did with the iZCTR1 cells, the incubation with Tet only increased cellular Cu uptake by 1.46 fold as compared to controls (Fig. 8A); Mn exposure plus Tet induction further increased cellular Cu uptake by 1.3 fold to a total of 1.78 fold in comparison to controls (Fig. 8A). Clearly, the extent to which the DMT1 induction was capable of increasing the cellular Cu uptake in iZDMT1 cells was much less than those observed in iZCTR1 cells; however, the induction with Tet in ZDMT1 cells after Mn exposure indeed resulted in a robust increase of the DMT1 mRNA (6.2 fold higher) and protein levels (4.6 fold higher) as compared to those in Mn-only group (Fig. 8B, C). Considering the substantial increase in DMT1 level in iZDMT1 cells but with only a moderate increase of intracellular Cu uptake, it seemed likely that DMT1 may play a marginal role in the Mn-induced intracellular Cu uptake.
Fig. 8.
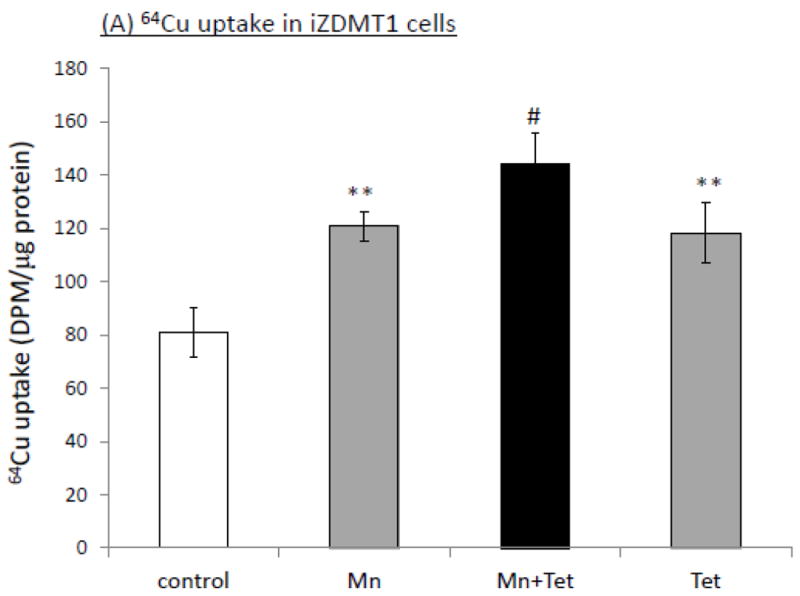
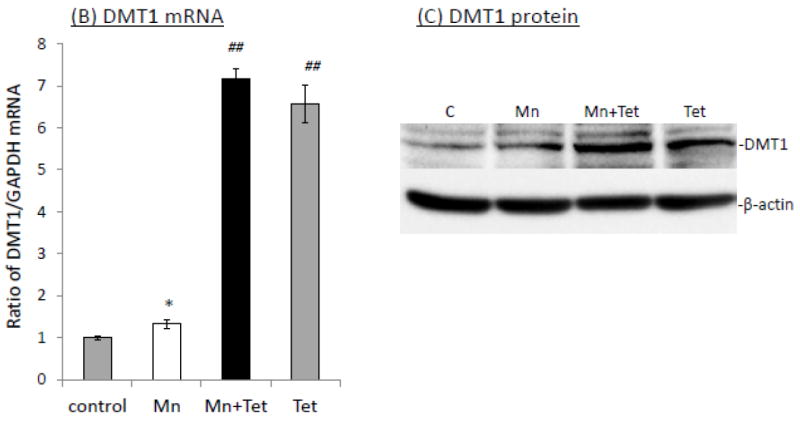
Induction of DMT1 in iZDMT1 cells at the steady state of Mn exposure and the cellular Cu accumulation. The iZDMT1 cells were exposed to 100 μM Mn for 24 h, followed by Tet treatment for 4 h. (A) Tet induced the increase of Cu uptake in Mn-treated iZDMT1 cells. Data represent mean ± SE, n=6; **: p<0.01 compared to controls; #: p<0.05 compared to Mn-treated group. (B) Tet induction increased DMT1 mRNA levels in Mn-treated iZDMT1 cells. Data represent mean ± SE, n=6; *: p<0.05 compared to controls; ##: p<0.01 compared to Mn-treated group. (C) Tet induction increased DMT1 protein levels in Mn-treated iZDMT1 cells.
Discussion
Previous studies from humans, nonhuman primates and rodent models clearly indicated that Mn exposure alters Cu homeostasis with a significant increase of Cu concentrations in brain and CSF (Guilarte et al., 2006; Guilarte and Chen, 2007; Jiang et al., 2007; Lai et al., 1999; Wang et al.2008a; Zheng et al., 2009). The results presented in this study support a significant cellular Cu uptake following Mn exposure. Furthermore, our observations suggest that a major Cu transporter CTR1, but less likely DMT1, plays a critical role in Mn-induced cellular overload of Cu in the BCB.
The data from this laboratory have shown an abundant expression of CTR1 in the choroid plexus (Choi and Zheng, 2009). Our past studies also provide the strong evidence of a bountiful expression of DMT1 in the choroid plexus (Wang et al., 2006, 2008b, 2008c). Interestingly, Mn exposure resulted in significant increases of both CTR1 and DMT1. This observation raised the question as to what is the relative contribution between these two transporters in Mn-induced increase of cellular Cu uptake. Two approaches were then used to compare the roles of CTR1 and DMT1 in Cu uptake.
First, we used the siRNA knockdown technique to determine the impact of gene silencing of the two transporters on Mn-induced cellular Cu accumulation. The knockdown of CTR1 clearly showed a significant decrease of cellular Cu uptake in control Z310 cells as well as the cells exposed with Mn. Unexpectedly, however, DMT1 knockdown resulted in only marginal reduction in cellular Cu uptake in the control cells. In the Mn-treated cells, knocking down DMT1, instead of reducing Cu transport, resulted in a significant increase of cellular Cu uptake. The data appeared to indicate a nonessential role of DMT1 in cellular Cu uptake by choroidal Z310 cells. However, the involvement of DMT1 in Cu uptake cannot be completely ruled out, because a down-regulation of one transporter may lead to the increased expression of other Cu influx transporters, such as Cu transporter 1-5 (CTR1-5) and presenilin (Bertinato et al., 2008; Pena MM et al., 2000; Beaudoin et al., 2011; Greenough et al., 2011), or a decreased expression of the Cu efflux transporters, such as ATP7A and ATP7B (Liu et al., 2010; Samimi et al., 2004), as parts of cell’s compensatory mechanism under the influence of Mn exposure.
To explore the mechanism, we established the choroidal Z310 cells-based CTR1-inducible cell line (iZCTR1) and DMT1-inducible cell line (iZDMT1), which allowed us to compare the relative contribution of either transporter in Cu uptake at the steady state of Mn exposure. These inducible cell lines carry Tet repressor (TetR). In the absence of Tet, the expression of CTR1 or DMT1 is repressed due to the binding of Tet operator (TetO2) in the promoter by the TetR. When Tet is added to the culture medium, binding of Tet to TetR frees the TetO2; consequently the transcription of the genes of interest becomes active. In our newly established iZCTR1 cell lines, addition of Tet caused a time-dependent increase of the mRNA and protein levels of CTR1, accompanied by a remarkable increase in cellular Cu uptake which followed the time-course of transporter expression. It is also noteworthy that a significant increase of expression levels of CTR1 was observed as early as within 2-4 h after addition of Tet, indicating that the increased Cu uptake was exclusively attributed to the up-regulation of CTR1, since the increase of CTR1 level was so sudden and robust within a few hours after Tet induction that the time would be too short for the cell’s compensatory mechanism to take effects. These results support a crucial role of CTR1 in mediating cellular Cu uptake in choroidal cells.
When we induced the DMT1expression with Tet in iZDMT1 cells, the levels of DMT1 mRNA and protein also increased in a time-dependent fashion. A significant increase of expression levels of DMT1were observed after adding Tet. Noticeably, however, the extent of the DMT1 increase was not as high as that of CTR1 in iZCTR1 cells. This could be due to the size of DMT1 molecule, which is much larger than CTR1 and thus it may need a longer time for transcription and translation. However, there was a sharp contrast between the cellular Cu uptake and the DMT1 induction following Tet addition. Tet treatment increased the DMT1 mRNA and protein expression by more than 6 and 4 fold, respectively, while at the same time, the cellular Cu uptake was increased merely by1.5 fold, These data strongly suggested a minor role of DMT1 in participating cellular Cu uptake.
The induction of cellular expression of CTR1 and DMT1 following Mn exposure was evident in the choroidal epithelial cells; but their contribution to cellular Cu overload was quite different. When the iZCTR1 or iZDMT1 cells were treated with Mn for 24 h, the changes of expression of the respective transporter would reach a stable steady status. Further induction of CTR1 by incubating the iZCTR1 cells with Tet resulted in an additional increase of Cu uptake by 5.6 fold. In contrast, when iZDMT1 cells were exposed to Mn followed by Tet treatment, the cellular Cu uptake was increased by 1.2 fold as compared to the cells treated only with Mn, whereas in the same cells, the expression of DMT1 was increased by 4 fold with Tet induction. These results suggest that the main portion of the increased Cu uptake may be mediated by the transporters other than DMT1, and presumably, by CTR1. Thus, it is likely that the contribution of DMT1 to the Mn-induced cellular Cu uptake is much smaller than that of CTR1.
Noticeably, overexposure to either Cu or Mn can lead to the cytotoxicity; the latter may contribute to the increased cellular Cu uptake. This hypothesis deserves further investigation.
In summary, our results provide the strong evidence to support a different role of CTR1 and DMT1 in mediating cellular Cu transport in the BCB. Under the normal physiological condition, CTR1 appears to play a much more significant role in transporting Cu into the cells than does DMT1. Mn exposure induces the expression of both transporters in BCB. However, it is the induction of CTR1, but much less likely the DMT1, that contributes to the Mn-induced cellular Cu uptake in BCB cells. Altered CTR1 expression in the BCB may lead to an increased uptake of Cu from the blood with ensuing release of it to the CSF; this may partially explain the elevated Cu concentrations in the CSF in chronic Mn-exposed animals in vivo.
Supplementary Material
Research Highlights.
This study compares the relative role of CTR1 and DMT1 in Cu transport by the BCB.
Two novel tetracycline-inducible CTR1 and DMT1 expression cell lines are created.
CTR1, but not DMT1, plays an essential role in transporting Cu by the BCB.
Mn-induced cellular Cu overload is due to its induction of CTR1 rather than DMT1.
Induction of CTR1 by Mn in the BCB contributes to an elevated Cu level in the CSF.
Acknowledgments
We are grateful to Dr. Andrew D. Monnot and Dr. Wendy Jiang for their technical assistance during experimentation. This study was supported by NIH/National Institute of Environmental Health Sciences Grant RO1-ES008146-14.
Abbreviations used
- BCB
blood-cerebrospinal fluid barrier
- CSF
cerebrospinal fluid
- CTR1
Cu transporter-1
- DMT1
divalent metal transporter-1
- Cu
copper
- Mn
manganese
Footnotes
Disclosure of Conflict of Interest
The authors declare that there is no any conflict of interest with regards to financial, personal, or their relationships with other people or organizations for the studies presented in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aschner M, Nass R, Guilarte TR, Schneider JS, Zheng W. Manganese: Recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin J, Thiele DJ, Labbé S, Puig S. Dissection of the relative contribution of the Schizosaccharomyces pombe Ctr4 and Ctr5 proteins to the copper transport and cell surface delivery functions. Microbiology. 2011;157:1021–1031. doi: 10.1099/mic.0.046854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertinato J, Swist E, Plouffe LJ, Brooks SP, L’abbé MR. Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem J. 2008;409:731–740. doi: 10.1042/BJ20071025. [DOI] [PubMed] [Google Scholar]
- Boll MC, Alcaraz-Zubeldia M, Montes S, Rios C. Free copper, ferroxidase and SOD1 activities, lipid peroxidation and NO(x) content in the CSF. A different marker profile in four neurodegenerative diseases. Neurochem Res. 2008;33:1717–1723. doi: 10.1007/s11064-008-9610-3. [DOI] [PubMed] [Google Scholar]
- Choi BS, Zheng W. Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res. 2002;1248:14–21. doi: 10.1016/j.brainres.2008.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossgrove JS, Zheng W. Manganese toxicity upon overexposure. NMR in Biomedicine. 2004;17:544–553. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibel MA, Ehmann WD, Markesbery WR. Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: possible relation to oxidative stress. J Neurol Sci. 1996;143:137–142. doi: 10.1016/s0022-510x(96)00203-1. [DOI] [PubMed] [Google Scholar]
- Gaggelli E, Kozlowski H, Valensin D, Valensin G. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis) Chem Rev. 2006;106:1995–2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- Greenough MA, Volitakis I, Li QX, Laughton K, Evin G, Ho M, Dalziel AH, Camakaris J, Bush AI. Presenilins promote the cellular uptake of copper and zinc and maintain Cu-chaperone of sod1-dependent Cu/Zn superoxide dismutase activity. J Biol Chem. 2011;286:9776–9786. doi: 10.1074/jbc.M110.163964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenheid S, Cellier M, Vidal S, Gros P. Identification and characterization of a second mouse Nramp gene. Genomics. 1995;25:514–525. doi: 10.1016/0888-7543(95)80053-o. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol. 2006;202:381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK. Manganese inhibits NMDA receptor channel function: Implications to psychiatric and cognitive effects. NeuroToxicology. 2007;28:1147–1152. doi: 10.1016/j.neuro.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Horn D, Barrientos A. Mitochondrial copper metabolism and delivery to cytochrome c oxidase. IUBMB Life. 2008;60:421–429. doi: 10.1002/iub.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozumi I, Hasegawa T, Honda A, Ozawa K, Hayashi Y, Hashimoto K, Yamada M, Koumura A, Sakurai T, Kimura A, Tanaka Y, Satoh M, Inuzuka T. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J Neurol Sci. 2011;303:95–99. doi: 10.1016/j.jns.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zheng W, Long L, Zhao W, Li X, Mo X, Lu J, Fu X, Li W, Liu S, Long Q, Huang J, Pira E. Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: search for biomarkers of manganese exposure. Neurotoxicology. 2007;28:126–135. doi: 10.1016/j.neuro.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Jiménez FJ, Molina JA, Aguilar MV, Meseguer I, Mateos-Vega CJ, González-Muñoz MJ, de Bustos F, Martínez-Salio A, Ortí-Pareja M, Zurdo M, Martínez-Para MC. Cerebrospinal fluid levels of transition metals in patients with Parkinson’s disease. J Neural Transm. 1998;105:497–505. doi: 10.1007/s007020050073. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Gybina AA, Pyatskowit JW, Gitschier J, Prohaska JR. Copper transport protein (Ctr1) levels in mice are tissue specific and dependent of copper status. J Nutr. 2006;136:21–26. doi: 10.1093/jn/136.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JC, Minski MJ, Chan AW, Leung TK, Lim L. Manganese mineral interactions in brain. Neurotoxicology. 1999;20:433–444. [PubMed] [Google Scholar]
- Li GJ, Zhao Q, Zheng W. Alteration at translational but not transcriptional level of transferrin receptor expression following manganese exposure at the blood–CSF barrier in vitro. Toxicol Appl Pharmacol. 2005;205:188–200. doi: 10.1016/j.taap.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Pilankatta R, Hatori Y, Lewis D, Inesi G. Comparative features of copper ATPases ATP7A and ATP7B heterologously expressed in COS-1 cells. Biochemistry. 2010;49:10006–10012. doi: 10.1021/bi101423j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DW, Shefer RE, Klinkowstein RE, Bass LA, Margeneau WH, Cutler CS, Anderson CJ, Welch MJ. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol. 1997;24:35–43. doi: 10.1016/s0969-8051(96)00157-6. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M. Early manifestations of manganese neurotoxicity in humans: an update. Environ Res. 1997;73:92–100. doi: 10.1006/enrs.1997.3710. [DOI] [PubMed] [Google Scholar]
- Pall HS, Williams AC, Blake DR, Lunec J, Gutteridge JM, Hall M, Taylor A. Raised cerebrospinal-fluid copper concentration in Parkinson’s disease. Lancet. 1987;2:238–241. doi: 10.1016/s0140-6736(87)90827-0. [DOI] [PubMed] [Google Scholar]
- Pena MM, Puig S, Thiele DJ. Characterization of the Saccharomyces cerevisiae high affinity copper transporter Ctr3. J Biol Chem. 2000;275:33244–33251. doi: 10.1074/jbc.M005392200. [DOI] [PubMed] [Google Scholar]
- Rossi L, Lombardo MF, Ciriolo MR, Rotilio G. Mitochondrial dysfunction in neurodegenerative diseases associated with copper imbalance. Neurochem Res. 2004;29:493–504. doi: 10.1023/b:nere.0000014820.99232.8a. Review. [DOI] [PubMed] [Google Scholar]
- Samimi G, Katano K, Holzer AK, Safaei R, Howell SB. Modulation of the cellular pharmacology of cisplatin and its analogs by the copper exporters ATP7A and ATP7B. Mol Pharmacol. 2004;66:25–32. doi: 10.1124/mol.66.1.25. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Schreurs BG. Trace amounts of copper in water induce beta-amyloid plaques and learning deficits in a rabbit model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2003;100:11065–11069. doi: 10.1073/pnas.1832769100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausak D, Mercer JF, Dieter HH, Stremmel W, Multhaup G. Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res Bull. 2001;55:175–185. doi: 10.1016/s0361-9230(01)00454-3. [DOI] [PubMed] [Google Scholar]
- Waggoner DJ, Bartnikas TB, Gitlin JD. The role of copper in neurodegenerative disease. Neurobiol Dis. 1999;6:221–230. doi: 10.1006/nbdi.1999.0250. Review. [DOI] [PubMed] [Google Scholar]
- Wang X, Li GJ, Zheng W. Upregulation of DMT1 expression in choroidal epithelia of the blood-CSF barrier following manganese exposure in vitro. Brain Res. 2006;1097:1–10. doi: 10.1016/j.brainres.2006.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Du X, Zheng W. Alteration of saliva and serum concentrations of manganese, copper, zinc, cadmium and lead among career welders. Toxicol Lett. 2008a;176:40–47. doi: 10.1016/j.toxlet.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li GJ, Zheng W. Efflux of iron from the cerebrospinal fluid to the blood at the blood-CSF barrier: effect of manganese exposure. Exp Biol Med (Maywood) 2008b;233:1561–1571. doi: 10.3181/0803-RM-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Miller DS, Zheng W. Intracellular localization and subsequent redistribution of metal transporters in a rat choroid plexus model following exposure to manganese or iron. Toxicol Appl Pharmacol. 2008c;230:167–174. doi: 10.1016/j.taap.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W. Toxicology of choroid plexus: special reference to metal-induced neurotoxicities. Microsc Res Tech. 2001;52:89–103. doi: 10.1002/1097-0029(20010101)52:1<89::AID-JEMT11>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Aschner M, Ghersi-Egea JF. Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol. 2003;192:1–11. doi: 10.1016/s0041-008x(03)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Jiang YM, Zhang Y, Jiang W, Wang X, Cowan DM. Chelation therapy of manganese intoxication with para-aminosalicylic acid (PAS) in Sprague-Dawley rats. Neurotoxicology. 2009;30:240–248. doi: 10.1016/j.neuro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhao Q. Establishment and characterization of an immortalized Z310 choroidal epithelial cell line from murine choroid plexus. Brain Res. 2002;958:371–380. doi: 10.1016/s0006-8993(02)03683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Monnot AD. Regulation of brain iron and copper homeostasis by brain barrier systems: Implication in neurodegenerative diseases. Pharmacol Ther. 2012;133:177–188. doi: 10.1016/j.pharmthera.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


