Abstract
Introduction
Serious mental illness (SMI) is associated with high rates of tobacco and other drug dependence, poor treatment compliance, obesity and low levels of physical activity, which have severe medical and psychosocial consequences. Interventions that effectively reduce these health risk behaviors among people with SMI are urgently needed.
Methods
Published reports from studies evaluating incentive-based treatments for promoting tobacco and other drug abstinence, treatment attendance, medication use and increased physical activity are reviewed.
Results
Results of this review indicate the efficacy of incentive-based treatments for reducing tobacco and other drug use among people with SMI. Few studies have examined whether incentive-based treatments improve treatment attendance, medication use and physical activity levels in people with SMI; however, initial evidence is positive and indicates that further research in these areas is warranted.
Conclusion
Given the medical and psychosocial costs of tobacco and other drug use, treatment non-compliance and physical inactivity, and the efficacy of incentive-based treatments for improving these behaviors, such interventions should be further developed and integrated into behavioral health treatment programs for people with SMI.
1. Health Status of Individuals with Serious Mental Illness
People with serious mental illness (SMI; e.g., schizophrenia, bipolar disorder, major depression) have a 20% (25-year) reduced lifespan compared to the general population, which is primarily due to high rates of cigarette smoking, physical inactivity, obesity, elevated blood cholesterol, hypertension and diabetes mellitus (Allison et al., 2009; Bresee et al., 2010; Brown et al., 2010; Goff et al., 2005; Grant et al., 2004; Hennekens et al., 2005; Lasser et al., 2000; Saha et al., 2007). In addition, SMI is associated with high rates of alcohol, cocaine and other substance use disorders (SUD), which can lead to reductions in medication compliance and increases in psychiatric symptoms, joblessness, homelessness, HIV and HCV infection (Drake et al., 2004; Regier et al., 1990; Rounsaville et al., 1982; Shaner et al., 1995). Given the severity of these medical and psychosocial consequences, and the high costs associated with their treatment, interventions that reduce these health risk behaviors among people with SMI are urgently needed (Allison et al., 2009; Banerjea et al., 2008; O’Brien et al., 2004; Watkins et al., 2011).
A vivid illustration of the relationship between drug use and hospitalization in people with SMI was provided by a study that tracked daily patterns of cocaine-positive toxicology screens and psychiatric admissions in men with schizophrenia who received SUD treatment at an urban medical center (Shaner et al., 1995). That study found a clear temporal relationship between cocaine use, psychiatric symptom severity and inpatient hospitalization. Furthermore, substance use and psychiatric symptom severity peaked in the early part of each month, indicating that receipt of disability payment may have triggered cocaine use and the subsequent exacerbation of psychiatric symptoms. This demonstration set the the stage for examining whether contingency management (CM) interventions, which reinforce drug abstinence or other therapeutic goals with vouchers, cash, clinic privileges or another tangible reinforcer (Higgins, Silverman & Heil, 2008), would reduce drug use in these patients. CM techniques have long been used to modify a variety of behaviors in people with SMI (e.g., Ayllon & Azrin 1965; Kale et al., 1968; King et al., 1960; Ullman & Krasner, 1965) – ironically, some of these study used cigarettes as reinforcers. However, using CM to reduce health risk behaviors in people with SMI is a fairly recent development. This review summarizes proof-of-concept and controlled treatment studies that have examined the effectiveness of CM interventions for improving the health of people with SMI and suggests directions for future research.
CM Interventions for Tobacco Dependence
People with SMI consume almost half of the cigarettes smoked in the United States and have lower smoking cessation rates than other smokers (Grant et al., 2004; Lasser et al., 2000; Ziedonis et al., 2008). An initial CM study for smoking in people with SMI used a within-subjects reversal (A-B-A) design to investigate whether cash reinforcement for reductions in exhaled breath carbon monoxide (CO) levels would reduce smoking in 11 outpatients with schizophrenia (Roll et al., 1998). During the 5-day baseline phases, CO samples were collected once per day and reinforced regardless of CO level (i.e., non-contingently), whereas during the 5-day intervention phase, CO samples were collected 3 times per day and CO levels of 11 parts per million (ppm) or lower were reinforced with cash payments that started at $3.00 per sample and increased in magnitude by $0.50 as the duration of abstinence increased. This study found that average CO values were reduced by 50% during the CM intervention compared to the initial baseline phase. These results were later replicated by a laboratory study that examined allocation of choices between cigarette puffs versus monetary reinforcement in smokers with schizophrenia (Tidey et al., 1999), and a study that examined the effects of contingent incentives for CO reductions, both alone and combined with 21 mg transdermal nicotine, in smokers with schizophrenia (Tidey et al., 2002).
A logical next step for this area of research was to test the effectiveness of a CM-smoking intervention for patients with SMI in a behavioral healthcare setting. Gallagher et al. (2007) randomized 180 smokers with psychotic-spectrum or affective disorders who were receiving case management services from a behavioral healthcare organization into one of three study conditions: (1) a CM intervention for smoking cessation, (2) the CM intervention combined with 21 mg transdermal nicotine, or (3) a minimal intervention, self-quit group. In the CM conditions, participants were seen weekly in Weeks 1–4, biweekly in Weeks 6–12, and monthly in Weeks 16–24, with a follow-up visit at week 36. During these visits, breath samples that met the CO criterion of 10 ppm or lower were reinforced with $20 in weeks 1–4, $40 in weeks 6–12 and $60 in weeks 16–24. In addition, saliva samples were collected in weeks 20 and 36 and analyzed for levels of the nicotine metabolite, cotinine, to validate the CO results. The CO results indicated that those randomized to the CM interventions were significantly more likely to meet the CO abstinence criterion at weeks 20 and 36 (40% abstinent; no difference between CM conditions) than those in the control condition (< 10% abstinent), demonstrating that reinforcing CO reductions effectively reduced recent smoking, although few met the cotinine abstinence criteria.
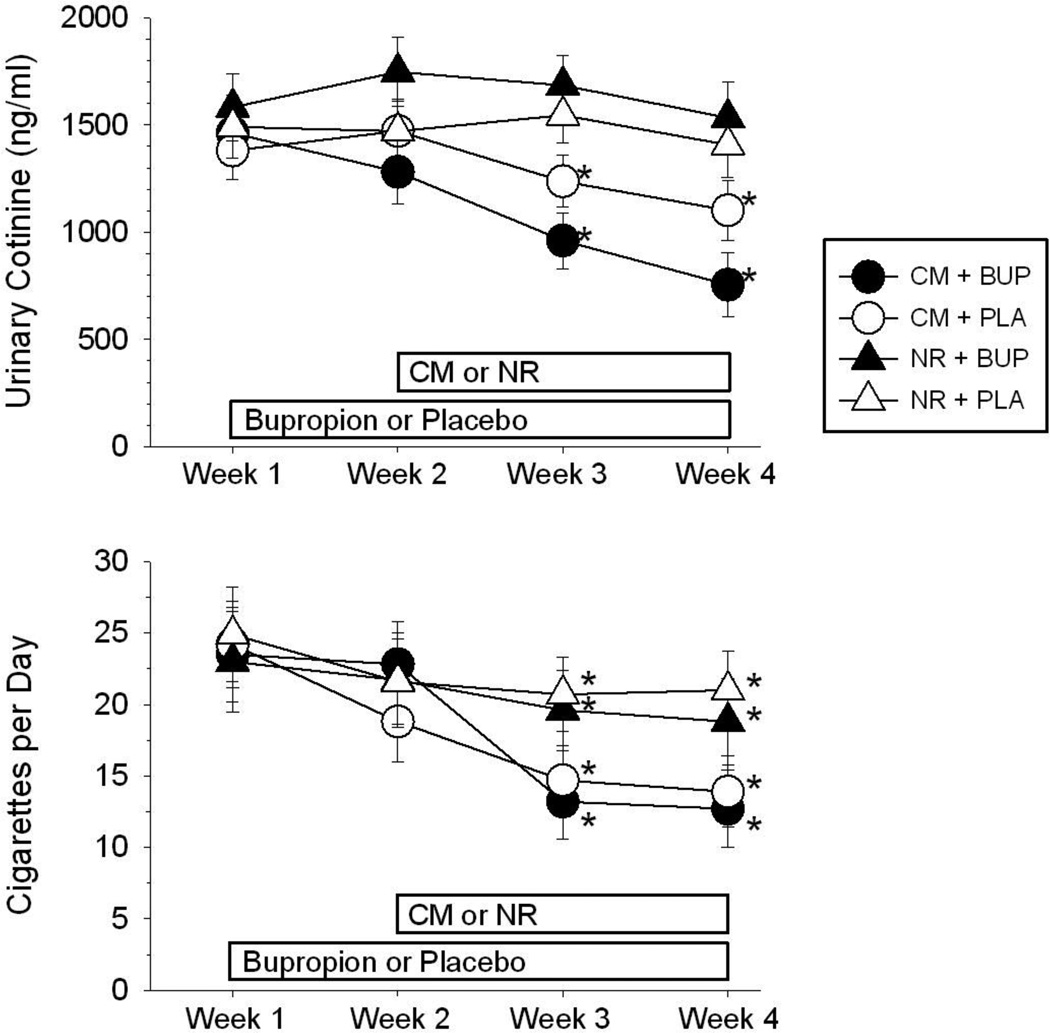
The results of the Gallagher et al. (2007) study illustrate that the choice of biomarker used to confirm smoking status is a significant consideration for CM-smoking studies. When breath CO levels are used as the biomarker, very frequent samples (i.e., twice-daily) are necessary to verify continuous smoking abstinence. An important development for CM-smoking interventions has been to instead reinforce reductions in the nicotine metabolite, cotinine, analyzed from urine or saliva samples (e.g., Higgins et al., 2004). Because cotinine levels from smoking are eliminated much more slowly than breath CO levels (SRNT, 2002), thrice-weekly cotinine analysis is sufficient to verify continuous smoking abstinence, which considerably increases the feasibility of implementing CM interventions for smoking. A recent study investigated the separate and combined effects of contingent reinforcement of reductions in urinary cotinine levels and 300 mg/day bupropion in heavy smokers with schizophrenia (Tidey et al., 2011). Using a placebo-controlled, between-groups design, 57 participants were randomized to receive bupropion or placebo. After one week on medication, 52 participants were randomized to a CM or a non-contingent reinforcement condition. In both conditions, participants received $25 for attending each session. In the CM condition, 25% or greater reductions in cotinine compared to the previous sample were reinforced with payments of $25 per sample, which increased by $5 for each consecutively reduced or abstinent sample. Over the next 2 weeks, participants visited the laboratory three times per week to provide urine samples for analysis of cotinine levels and to complete subjective measures of cigarette craving, nicotine withdrawal symptoms and psychiatric symptoms. As shown in Figure 1, cotinine levels decreased by 34% during the intervention in participants randomized to the CM conditions, compared to 4% reductions for those randomized to non-contingent reinforcement; bupropion did not reduce smoking by itself or increase the efficacy of CM (Tidey et al., 2011). Overall, these studies (Gallagher et al., 2007; Roll et al., 1998; Tidey et al., 1999; 2002; 2011) indicate that CM is an effective method of reducing smoking in people with schizophrenia and indicate the need for longer smoking CM interventions for smokers with SMI that target abstinence in addition to smoking reductions.
Figure 1.
Effects of contingency management (CM) versus non-contingent reinforcement (NR) in combination with 300 mg/day sustained-release bupropion (BUP) or placebo (PLA) on urinary cotinine levels (top) and self-reported number of cigarettes smoked per day (bottom) in 52 smokers with schizophrenia. Asterisks indicate significant differences from Week 1 (p < .05). Modified from Tidey et al., 2011. Copyright 2011 by Springer Berlin/Heidelberg. Reprinted by permission.
Major depression is also strongly associated with elevated current and lifetime rates of cigarette smoking (Lasser et al., 2000). Surprisingly, CM interventions for smoking have not been tested in people with major depression, although one study examined whether trait depression affected CM-smoking treatment response, hypothesizing that negative affect would predict rate of relapse (Gilbert et al., 1999). After a 3-week baseline period of biweekly CO monitoring, 56 male participants were randomized to either an immediate-quit group or a delayed-quit group. All participants received CO monitoring every 48 hours, but participants in the immediate-quit group could receive $300 plus their $50 deposit only if they maintained smoking abstinence (CO < 10 ppm) throughout the 31-day period, whereas those in the delayed-quit group only had to meet this abstinence contingency on days 32 and 33 of the study. This study found that 88% of the immediate-quit group, compared to 15% of the delayed-quit group, remained abstinent during the 31-day study period, indicating a strong effect of the contingency. Higher baseline scores on the Depression subscale of the Neuroticism scale of the NEO-Personality Inventory (Costa & McCrae, 1985) were associated with faster relapse to smoking, suggesting that, as hypothesized, the intervention was less effective in smokers with higher levels of negative affect (Gilbert et al., 1999).
CM INTERVENTIONS FOR OTHER SUD
Marijuana Use
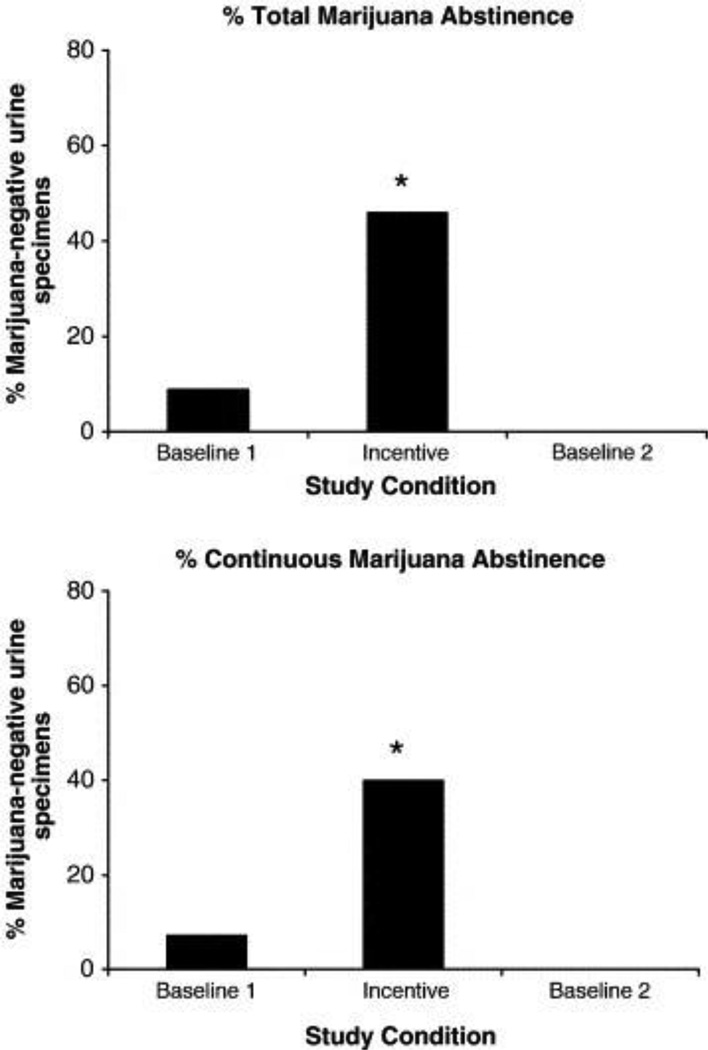
Two studies have examined the efficacy of CM interventions for marijuana use in people with schizophrenia. The first was a 25-week study with 18 participants (Sigmon et al., 2000). After a 5-week baseline period in which participants received monetary reinforcement for providing a sample twice per week, regardless of urinalysis results, participants were randomized to 5-week incentive conditions in which the amount of monetary reinforcement for negative samples was either $25, $50 or $100 per sample. After completing all three incentive conditions, participants underwent a second non-contingent baseline condition. This study found that marijuana use decreased during the CM period and that the $100 incentive condition produced the most abstinence. However, this study had high attrition, which is not surprising given its long (25-week) duration. Sigmon and Higgins (2006) later conducted an A-B-A study in 7 marijuana-dependent individuals, most of whom had schizophrenia. During the 4-week baseline periods, samples were non-contingently reinforced with $10 vouchers. During the 12-week CM intervention, marijuana-negative samples were reinforced with vouchers, the value of which started at $5 and increased by $2.50 with each subsequent negative sample, plus a $10 bonus for each set of two consecutive negative specimens. As shown in Figure 2, the CM intervention significantly increased marijuana abstinence, from 9% of samples in the first baseline period to 46% in the intervention phase, indicating that voucher-based CM interventions are an effective method of reducing marijuana use among people with SMI (Sigmon & Higgins, 2006).
Figure 2.
Effects of contingent reinforcement of marijuana-negative urine samples on percentage of total (top) and continuous (bottom) marijuana-abstinent samples among 7 weekly marijuana users with serious mental illness. Asterisks indicate significant difference from baseline conditions (p < .05). From Sigmon and Higgins, 2006. Copyright 2006 by Elsevier. Reprinted by permission.
Alcohol Use
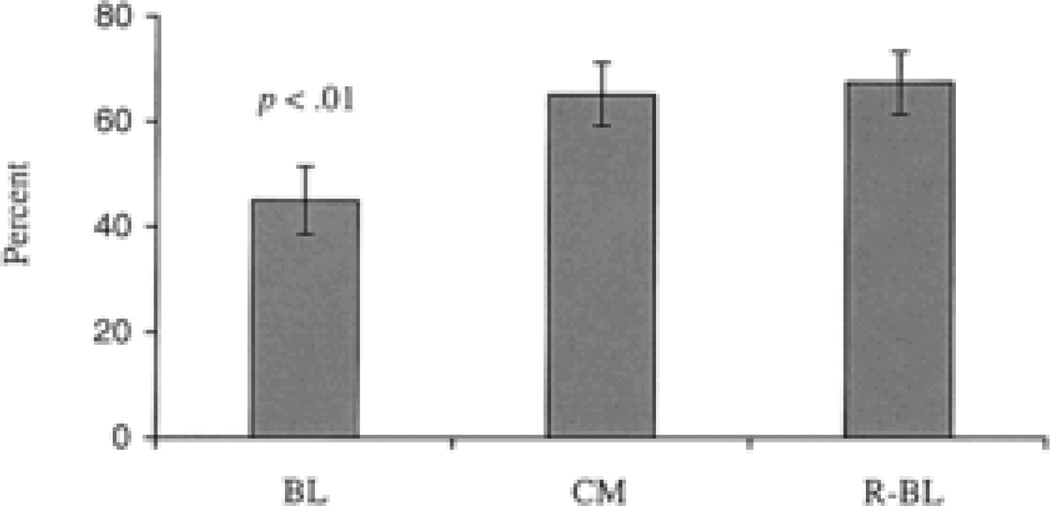
Two proof-of-concept studies have examined the efficacy of CM approaches for reducing alcohol use among people with SMI (Peniston, 1988; Helmus et al., 2003). In an early study (Peniston, 1988), 15 patients in an open psychiatric unit at a VA Medical Center received positive and response-cost contingencies aimed at improving social behaviors, grooming, treatment attendance and alcohol use. The interventions were successful for changing most target behaviors, but were less succcessful in those with excessive alcohol use. More recently, Helmus et al. (2003), examined the effectiveness of a 20-week CM intervention for improving treatment attendance and reducing alcohol use in 20 patients with major depressive disorder, bipolar I disorder, schizoaffective disorder or schizophrenia, within a community-based dual diagnosis treatment program. Using an A-B-A design, this study included a 4-week baseline phase in which patients attended twice-weekly group counseling sessions as part of the standard treatment regimen, a 12-week CM intervention period in which on-time treatment attendance and alcohol-negative breathalyzer readings were reinforced with low value ($2.50) gift certificates to a local retail store, and a 4-week return-to-baseline phase. Attendance rates improved significantly during the CM period, as shown in Figure 3, which is notable given that the average cost was only $31.50 per participant (Helmus et al., 2003). All breathalyzer results, regardless of phase, were negative during the study, which may indicate that simply monitoring alcohol levels was sufficient to reduce alcohol use. However, because of the very fast elimination rate of breath alcohol, confirming continuous alcohol abstinence with this biomarker requires frequent monitoring. CM studies have recently begun using transdermal alcohol monitors to collect and store information on alcohol use, which will greatly increase the feasibility of these interventions (Barnett et al., 2011).
Figure 3.
Effects of contingent reinforcement of on-time counseling attendance among 20 patients with serious mental illness and alcohol or other substance use disorders. From Helmus et al. (2003). Copyright 2003 by the Educational Publishing Foundation. Reprinted by permission.
Cocaine, Opiates and Polysubstance Abuse
Two small proof-of-concept studies used A-B-A designs to test the effectiveness of CM interventions for cocaine dependence in people with schizophrenia (Shaner et al., 1997; Roll et al., 2004). Participants in the Shaner et al. (1997) study were two homeless men who had been highly resistant to treatment. Each baseline and intervention phase lasted 2 months. During the intervention phase, urine samples were tested 5 times per week, and samples that were negative for the cocaine metabolite, benzoylecgonine, were reinforced with $25 cash. Although participants attended only about half of the scheduled sessions, their benzoylecgonine levels indicated that their cocaine use was significantly reduced during the CM intervention. Participants in the second study were 3 male veterans with schizophrenia and cocaine dependence (Roll et al., 2004). During each of the 2-week baseline phases, thrice-weekly urine samples were collected and reinforced with $3.00 in vouchers, regardless of immunoassay results. Vouchers were redeemable at the VA cafeteria or at a store located in the VA medical center. During the 4-week CM phase, cocaine-negative urine specimens were reinforced with vouchers that started at $3.00 and increased by $3.00 with each consecutive cocaine-negative sample. Participants could also earn a $10.00 bonus voucher for providing 3 consecutive negative samples. The voucher value was reset to $3.00 when patients provided a cocaine-positive sample. This CM intervention reduced cocaine use during the first 2 weeks of the intervention only; the reason why it apparently lost its effectiveness during the second 2 weeks of the intervention is unclear (Roll et al., 2004). A notable aspect of this study is its use of VA-redeemable vouchers as reinforcers, an innovation that is both cost-effective for the Veterans Health Administration (VHA) and convenient for patients. It was recently recognized that CM is an evidence-based practice for substance use disorders that is underutilized in the VHA (Watkins et al., 2011). Consequently, the VHA has developed an implementation plan that includes providing staff training in CM and funds for incentives, in order to increase the dissemination and utilization of CM within its intensive outpatient SUD treatment programs (Department of Veterans Affairs, 2011).
In a study conducted in a behavioral health center, Reis et al. (2004) tested the effectiveness of an innovative CM intervention for reducing alcohol and other drug use in 41 patients with SMI (schizophrenia, schizoaffective disorder, major depression or bipolar disorder) for whom the behavioral health center acted as the representative payee for disability payments. After a 3-month baseline period, patients were randomized to a contingent condition, in which alcohol and drug abstinence and attainment of other treatment goals were reinforced with greater control over the frequency and form (i.e., vouchers versus cash) of disability and study payments, or a non-contingent condition, in which the frequency and form of payments were not contingent upon attaining these goals. Results from the 27-week intervention period indicated that those randomized to the CM intervention had fewer weeks of alcohol use and better money management skills than those in the non-contingent group. Both groups reduced their drug use and sustained high attendance levels throughout the study. An advantage of this CM intervention is that the incentives could conceivably be sustained over long periods of time, although it does depend on having the behavioral health provider create a system for managing disability payments.
Co-morbid depression is prevalent among people with cocaine dependence (Rounsaville et al., 1982). Gonzalez et al. (2003) performed a secondary analysis to investigate whether major depression moderated treatment response to an intervention for cocaine and opiate use that crossed CM with desipramine (DMI; 150 mg/day). The parent study was a 12-week, double-blind, placebo-controlled study in which 160 patients were first stabilized on buprenorphine and then randomized to one of 4 groups: CM + DMI, CM + placebo, non-contingent reinforcement (NR) + DMI or NR + placebo (Kosten et al., 2003). All patients were seen weekly for group coping skills/relapse prevention therapy and individual therapy. Those randomized to CM received vouchers that escalated in value with consecutive drug-free urine samples, while those in NR received vouchers non-contingently. The parent study found that abstinence rates were highest in the CM + DMI condition (Kosten et al., 2003). The secondary analysis by Gonzalez et al. (2003) compared the treatment response of 53 patients who met DSM-IV criteria for a lifetime diagnoses of major depressive disorder with that of 96 patients who had no history of depression. This analysis indicated that the depressed patients had a stronger response to CM than the non-depressed patients (6.7- versus 4.0-fold increase in drug-free urines). However, only 31% of the depressed patients who were randomized to CM completed the study. Overall, these findings indicate that CM may reduce opiate and cocaine use in people with major depression if these patients can be retained in treatment.
Posttraumatic stress disorder (PTSD) is also strongly linked to SUD (e.g., Jacobsen et al., 2001; Najavits et al., 1998). Given its association with poorer SUD treatment outcome (e.g., Ouimette et al., 2003), several retrospective studies have examined whether PTSD diagnosis or symptom severity predicts treatment outcomes from CM interventions for SUD. Ford et al. (2007) examined relationships between PTSD symptoms and treatment outcome in 142 cocaine- or heroin-dependent patients randomized to a standard care condition or a CM intervention that reinforced drug abstinence and treatment-related activities with escalating cash and prize draws. PTSD symptom severity did not moderate response to standard treatment but moderated response to CM such that those with higher baseline PTSD symptoms were more likely to be abstinent at the 9-month follow-up assessment than those with lower baseline PTSD symptoms. However, more severe complex PTSD (Herman, 1992) predicted less in-treatment abstinence among those randomized to CM (no difference at follow-up; Ford et al. 2007). Mancino et al. (2010) examined whether PTSD moderated cocaine abstinence outcomes in 140 opiate-dependent LAAM-maintained individuals who were randomized to a CM or non-contingent reinforcement condition. Patients were randomized to one of 4 treatments: low-dose LAAM plus voucher-based CM for drug abstinence based on Higgins et al. (1994), low-dose LAAM without CM, high-dose LAAM plus CM, or high-dose LAAM without CM. Among the subsample that met diagnostic criteria for PTSD (23%), those randomized to CM had more cocaine abstinence than those in the non-contingent groups. Together, these studies indicate that CM for SUD is effective for people with PTSD, although complex PTSD symptoms may predict poor outcomes.
The Behavioral Treatment of Substance Abuse in SMI (BTSAS), a 6-month, small-group treatment that was developed for people with SMI, combines motivational interviewing, contingency management, social skills, drug refusal skills, education and relapse prevention training (Bellack & Gearon, 1998). Bellack et al. (2006) compared the efficacy of BTSAS versus a manualized standard group treatment for reducing drug use among 126 outpatients with major affective disorders, schizophrenia, schizoaffective disorder or another Axis-I disorder. At baseline, patients primarily abused cocaine (69%), followed by opiates (25%) and cannabis (7%). The CM piece of this treatment consisted of modest cash incentives for abstinence that started at $1.50 and increased by $0.50 per consecutive clean urine test up to a maximum of $3.50 per sample. Urine test results were announced and discussed in the context of the group treatment (Bellack et al., 2006). The results of this study showed that those randomized to BTSAS were retained longer in treatment, attended more sessions (29 vs. 19), and had higher in-treatment abstinence rates (59% vs. 25%) than those randomized to the standard treatment. The mean CM payment per subject was $60.27 (Bellack et al., 2006). Although the effects of the CM component cannot be determined independently of the other treatment elements, this study illustrates how CM can be effectively integrated with other elements of group treatment for SUD in people with SMI. A secondary analysis that examined predictors of CM response in these participants found that schizophrenia diagnosis and severity of drug use at baseline and comorbid alcohol dependence predicted lower CM earnings (Strong Kinnaman et al., 2007), suggesting that these participants may need either higher contingent incentive values to compete with the reinforcing effects of drugs, or a period of time in which reduction of drug use is an intermediate goal prior to abstinence.
Morbidity and mortality stemming from health risk behaviors are particularly prevalent among homeless individuals (e.g., Galea & Vlahov, 2002). One comprehensive approach incorporates abstinence-contingent housing and work therapy into behavioral day treatment for homeless individuals with SUD (primarily cocaine) and SMI (Milby et al., 1996; 2000). In the abstinence-contingent housing intervention, participants receive housing in furnished apartments or group homes contingent upon weekly drug-free urine drug tests. In the abstinence-contingent work therapy intervention, participants have access to construction, food service or lawn care jobs, or computer training. On-time attendance and the attainment of other vocational goals are reinforced with stipends that are then used to pay the modest rent for the abstinence-contingent housing. In 131 participants, the majority of whom had mental illness (non-psychotic), those randomized to the CM-enhanced treatment achieved better long-term cocaine abstinence outcomes than those randomized to a usual care condition (50% versus 40% abstinent at 12-month follow-up; Milby et al., 1996). A subsequent, more rigorous study found abstinence and housing results that favored the CM-enhanced intervention at the 6-month but not the 12-month follow-up (Milby et al., 2000). McNamara et al. (2001) examined whether the presence of major depression, PTSD or other psychiatric disorders predicted treatment response in the Milby et al. (2000) study and found that it did not: those with SMI/SUD stayed in treatment as long and had comparable improvements in alcohol, drug, and functional outcomes as the SUD-only clients. In fact, considering that the dually-diagnosed clients had more severe problems at baseline, their gains from baseline to end-of-treatment were stronger than those of the SUD-only clients in several areas of functioning (McNamara et al., 2001).
Another study in homeless individuals with SMI/SUD used a more typical CM approach to treating SUD, but tailored the intervention to this population by conducting it in a homeless shelter. Tracy et al. (2007) randomized 30 individuals with SMI (unspecified) and co-occurring cocaine or alcohol use disorders to a CM or an assessment-only condition. All participants had access to shelter services during the study and received assessments at baseline, weekly during the 4-week treatment period, and at a post-treatment follow-up visit. In addition, using the “fishbowl” CM procedure developed by Petry et al. (2000), the CM group earned drawings from a prize bowl for attending twice-weekly sessions and submitting negative breathalyzer and cocaine-free urine specimens. The number of drawings earned increased with successive negative samples and was reset to the initial number of drawings when participants missed a sample or submitted a drug-positive sample. A notable aspect of this study is its remarkably-high completion rate (100% for the CM condition, 87% in the assessment-only condition). Cocaine and alcohol abstinence rates were high overall, but were significantly higher in the CM condition than in the assessment-only condition (96% versus 75% cocaine-abstinent days).
Future Directions
The studies reviewed support the efficacy of contingent incentives for reducing tobacco and other drug use among people with SMI. As recently reviewed elsewhere (Tidey and Ries, 2008), the efficacy of these interventions for reducing health risk behaviors in people with SMI warrants their integration into behavioral health treatment programs for these patients. One example of how this can be accomplished is the incentive program described by Ries et al. (2004), reviewed above, which reinforced treatment attendance and drug abstinence by giving patients more control over the distribution of their own disability payments. Another example was reported by Drebing et al. (2007), who randomized 100 veterans with SMI and SUD in a VHA vocational rehabilitation (VR) program to VR-only or a VR + CM group in which cash incentives were used to reinforce drug abstinence and job-search activities. Those in the VR + CM condition had higher abstinence rates and significantly longer periods of sustained abstinence at the 16-week follow-up than those in the VR-only condition (11.8 ± 4.7 weeks versus 9.4 ± 5.3 weeks), although the groups were not significantly different at the 9-month follow-up. Furthermore, those in the VR + CM group completed 39% more job-search activities and achieved competitive employment more quickly than those in the VR-only group (Drebing et al., 2007). Both studies provide examples of how incentive systems can use the infrastructure and resources of existing programs to provide cost-effective and sustainable interventions for reducing health risk behaviors among people with SMI.
Incentive-based treatments for people with SMI could also be more widely applied to reducing other health risk behaviors such as low medication adherence, poor treatment attendance and low physical activity levels. Low medication adherence and treatment attendance are chronic problems for people with SMI, due to numerous factors including complicated medication regimens, limited illness insight and poor psychosocial functioning (Goff et al., 2010; Herbeck et al., 2005). Several of the studies reviewed above included incentives for treatment attendance as well as drug abstinence, with positive results (e.g., Helmus et al., 2003; Ries et al., 2004). In addition, Carey and Carey (1990) found positive effects of contingent incentives on attendance at a day treatment for SUD in 53 people with SMI, and Post et al. (2006) found that incentives ($10 per appointment) increased therapy attendance and reduced appointment rescheduling among 50 people with depression during a 12-week active intervention period. Priebe et al. (2009) recently described a randomized controlled trial aimed at investigating whether financial incentives contingent upon depot antipsychotic medication adherence, compared to treatment as usual, improve clinical and psychosocial functioning among non-adherent patients with psychotic disorders. Given the positive findings reviewed above, and the high psychosocial and financial costs of poor adherence, it is surprising that that adherence-contingent incentives are not routinely used, at least with chronically non-compliant individuals. Negative attitudes by treatment providers may be a major reason for the poor uptake of these incentives; ironically, they are regarded by many treatment providers as being too effective, thus potentially undermining patient autonomy (Claassen 2007; Claassen et al. 2007; Priebe et al., 2009; Burton et al., 2010).
People with SMI have high rates of obesity, hypertension, high blood cholesterol and diabetes mellitus (Dickerson et al., 2006; Goff et al., 2005; Allison et al., 2009), stemming from physical inactivity, poor dietary choices and the propensity of some atypical antipsychotic medications to increase weight and other metabolic risk factors (Brown et al., 1999; Daumit et al., 2005; Lean & Pajonk, 2003; Lindamer et al., 2008; Roick et al., 2007). Motivational and cognitive-behavioral interventions that focus on physical activity and diet have been shown to improve activity levels, weight and other health outcomes among people with SMI (Beebe et al., 2011; Faulkner et al., 2007; Gorczynski and Faulkner 2011; Loh et al., 2006; Methapatra and Srisurapanont, 2011). Only a few small-scale studies have examined whether contingent incentives can promote exercise and weight loss in people with SMI. Several early case studies in institutional settings found that token economy systems or other material reinforcers were successfully used to reinforce exercise or weight loss in people with schizophrenia (Haffey et al., 1972; Thyer et al., 1984; Upper and Newton, 1971; reviewed in Bradshaw et al., 2005). Likewise, Winkler (1970) reported that a token economy system in an inpatient setting increased exercise attendance and completion, among other behaviors, among individuals with a variety of chronic diagnoses. Such incentives have not been used more broadly in people with SMI, at least in part because the behaviors involved have been difficult to objectively monitor outside of institutional settings. However, with the recent development of pedometers, glucose meters, heart rate monitors and scales that can transmit readings to health care providers, these incentives could be incorporated into routine health care for people with SMI.
In summary, the studies reviewed support the efficacy of incentive-based treatments for reducing tobacco and other drug use among people with SMI and indicate several areas for future research. First, given the high rates of smoking-related morbidity and mortality among people with SMI, and the promising findings of the proof-of-concept studies, longer incentive-based smoking cessation interventions for people with SMI should be developed. Technological advances, including the use of cotinine as the biomarker of recent smoking and the development of web-based interventions for smoking (Dallery et al., 2007) should increase the feasibility of retaining participants with SMI in longer incentive-based smoking cessation treatments. A second important area for development is the integration of incentive-based treatments for substance use and other health risk behaviors into behavioral health treatment programs for people with SMI. Evidence from a recent randomized controlled trial of a non-incentive-based smoking cessation treatment indicates that patients are more likely to access and benefit from integrated treatments than non-integrated treatments (McFall et al., 2010). Furthermore, integrated treatments can build upon the infrastructure of the behavioral health treatment (Ries et al., 2004, Drebing et al., 2007). Finally, given the high medical and psychosocial costs associated with low treatment adherence and physical activity levels, and preliminary evidence that contingent incentives can increase these behaviors, larger -scale studies of incentive-based treatments for these health risk behaviors are warranted and needed.
Highlights.
>People with serious mental illness have high rates of tobacco and other drug use. >These patients also have low medication compliance and physical activity levels. >Contingent incentives effectively reduce health risk behaviors in these patients. >Incentives should be integrated into behavioral health treatment for these patients.
Acknowledgments
Preparation of this paper was supported in part by research grants DA017566 and DA026829 from the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. I appreciate the assistance of Ms Emily Xavier in the preparation of this paper.
Conflict of Interest Statement
The author declares that she has no conflict of interest with this work.
References
- Allison DB, Newcomer JW, Dunn AL, Blumenthal JA, Fabricatore AN, Daumit G, et al. Obesity among those with mental disorders: A National Institute of Mental Health meeting report. Am J Prev Med. 2009;36:341–350. doi: 10.1016/j.amepre.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Ayllon T, Azrin NH. The measurement and reinforcement of behavior of psychotics. J Exp Anal Behav. 1965;8:357–383. doi: 10.1901/jeab.1965.8-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjea R, Sambamoorthi U, Smelson D, Pogach L. Expenditures in mental illness and substance use disorders among veteran clinic users with diabetes. J Behav Health Serv Res. 2008;35:290–303. doi: 10.1007/s11414-008-9120-8. [DOI] [PubMed] [Google Scholar]
- Barnett NP, Tidey J, Murphy JG, Swift R, Colby SM. Contingency management for alcohol use reduction: A pilot study using a transdermal alcohol sensor. Drug Alcohol Depend. 2011;118:391–399. doi: 10.1016/j.drugalcdep.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe LH, Smith K, Burk R, McIntyre K, Dessieux O, Tavakoli A, Tennison C, Velligan D. Effect of a motivational intervention on exercise behavior in persons with schizophrenia spectrum disorders. Community Ment Health J. 2011;47:628–636. doi: 10.1007/s10597-010-9363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellack AS, Bennett ME, Gearon JS, Brown CH, Yang Y. A randomized clinical trial of a new behavioral treatment for drug abuse in people with severe and persistent mental illness. Arch Gen Psychiatry. 2006;63:426–432. doi: 10.1001/archpsyc.63.4.426. [DOI] [PubMed] [Google Scholar]
- Bellack AS, Gearon JS. Substance abuse treatment for people with schizophrenia. Addict Behav. 1998;23:749–766. doi: 10.1016/s0306-4603(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Bradshaw T, Lovell K, Harris N. Healthy living interventions and schizophrenia: a systematic review. J Adv Nurs. 2005;49:634–654. doi: 10.1111/j.1365-2648.2004.03338.x. [DOI] [PubMed] [Google Scholar]
- Bresee LC, Majumdar SR, Patten SB, Johnson JA. Prevalence of cardiovascular risk factors and disease in people with schizophrenia: A population-based study. Schizophr Res. 2010;117:75–82. doi: 10.1016/j.schres.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Brown S, Birtwistle L, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychol Med. 1999;29:687–701. doi: 10.1017/s0033291798008186. [DOI] [PubMed] [Google Scholar]
- Brown S, Kim M, Mitchell C, Inskip H. Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry. 2010;196:116–121. doi: 10.1192/bjp.bp.109.067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton A, Marougka S, Priebe S. Do financial incentives increase treatment adherence in people with severe mental illness? A systematic review. Epidemiol Psichiatr. 2010;19:233–242. [PubMed] [Google Scholar]
- Carey KB, Carey MP. Enhancing the treatment attendance of mentally ill chemical abusers. J Behav Ther Exp Psychiatry. 1990;21:205–209. doi: 10.1016/0005-7916(90)90008-9. [DOI] [PubMed] [Google Scholar]
- Claasen D. Financial incentives for antipsychotic depot medication: ethical issues. J Med Ethics. 2007;33:189–193. doi: 10.1136/jme.2006.016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen D, Fakhoury WK, Ford R, Priebe S. Money for medication: financial incentives to improve medication adherence in assertive outreach. Psychiatr Bull. 2007;31:4–7. [Google Scholar]
- Costa PT, McCrae RR. Psychological Assessment Resources. Odessa, FL: 1985. Manual of the NEO Personality Inventory. [Google Scholar]
- Dallery J, Glenn IM, Raiff BR. An Internet-based abstinence reinforcement treatment for cigarette smoking. Drug Alcohol Depend. 2007;68:230–238. doi: 10.1016/j.drugalcdep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Daumit GL, Goldberg RW, Anthony C, Dickerson F, Brown CH, et al. Physical activity patterns in adults with severe mental illness. J Nerv Mental Dis. 2005;193:641–646. doi: 10.1097/01.nmd.0000180737.85895.60. [DOI] [PubMed] [Google Scholar]
- Department of Veterans Affairs (VA) Washington, DC: Office of Management; 2011. Performance and Accountability Report. http://www.va.gov/budget/report. [Google Scholar]
- Dickerson FB, Brown CH, Daumit GL, LiJuan F, Goldberg RW, Wohlheiter K, Dixon LB. Health status of individuals with severe mental illness. Schizophr Bull. 2006;32:584–589. doi: 10.1093/schbul/sbj048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RE, Mueser KT, Brunette MF, McHugo GJ. A review of treatments for people with severe mental illnesses and co-occurring substance use disorders. Psychiatr Rehabil J. 2004;27(4):360–374. doi: 10.2975/27.2004.360.374. [DOI] [PubMed] [Google Scholar]
- Drebing CE, Van Ormer EA, Mueller L, Herbert M, Penk WE, et al. Adding contingency management intervention to vocational rehabilitation: Outcomes for dually diagnosed veterans. Journal of Rehabilitation Research and Development. 2007;44:851–866. doi: 10.1682/jrrd.2006.09.0123. [DOI] [PubMed] [Google Scholar]
- Faulkner G, Cohn T, Remington G. Interventions to reduce weight gain in schizophrenia. Cochrane Database Syst Rev. 2007 May 12; doi: 10.1002/14651858.CD005148.pub2. 2010: CD004412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JD, Hawke J, Alessi S, Ledgerwood D, Petry N. Psychological trauma and PTSD symptoms as predictors of substance dependence treatment outcomes. Behav Res Ther. 2007;45:2417–2431. doi: 10.1016/j.brat.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Galea S, Vlahov D. Social determinants and the health of drug users: Socioeconomic status, homelessness, and incarceration. Public Health Rep. 2002;117:135–145. [PMC free article] [PubMed] [Google Scholar]
- Gallagher SM, Penn PE, Schindler E, Layne W. A comparison of smoking cessation treatments for persons with schizophrenia and other serious mental illnesses. J Psychoactive Drugs. 2007;39:487–497. doi: 10.1080/02791072.2007.10399888. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Crauthers DM, Mooney DK, McClernon FJ, Jensen RA. Effects of monetary contingencies on smoking relapse: Influences of trait depression, personality, and habitual nicotine intake. Exp Clin Psychopharmacol. 1999;7:174–181. doi: 10.1037//1064-1297.7.2.174. [DOI] [PubMed] [Google Scholar]
- Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, Lamberti S, D’Agostino RB, Stroup TS, Davis S, Lieberman JA. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80:45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Goff DC, Hill M, Freudenreich O. Strategies for improving treatment adherence in schizophrenia and schizoaffective disorder. J Clin Psychiatry 2010. 2010;71:20–26. doi: 10.4088/JCP.9096su1cc.04. [DOI] [PubMed] [Google Scholar]
- Gonzalez G, Feingold A, Oliveto A, Gonsai K, Kosten TR. Comorbid major depressive disorder as a prognostic factor in cocaine-abusing buprenorphine-maintained patients treated with desipramine and contingency management. Am J Drug and Alcohol Abuse. 2003;29:497–514. doi: 10.1081/ada-120023455. [DOI] [PubMed] [Google Scholar]
- Gorczynski P, Faulkner G. Exercise therapy for schizophrenia. Cochrane Database Syst Rev. 2010 Jan 24; doi: 10.1002/14651858.CD004412.pub2. 2007: CD005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Haffey VA, Soroko ML, McCormack JH. Use of modeling and of operant reinforcement procedures in a group weight reduction programme. Newsletter Research in Psychology. 1972;14:17–22. [Google Scholar]
- Helmus TC, Saules KK, Schoener EP, Roll JM. Reinforcement of counseling attendance and alcohol abstinence in a community-based dual-diagnosis treatment program: A feasibility study. Psychol Addict Behav. 2003;17:249–251. doi: 10.1037/0893-164X.17.3.249. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150:1115–1121. doi: 10.1016/j.ahj.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Herbeck DM, Fitek DJ, Svikis DS, Montoya ID, Marcus SC, West JC. Treatment compliance in patients with comorbid psychiatric and substance use disorders. Am J Addict. 2005;14:195–207. doi: 10.1080/10550490590949488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J. Complex PTSD. J Trauma Stress. 1992;5:377–391. [Google Scholar]
- Higgins S, Budney A, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Plebani Lussier J, Lynch ME, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res. 2004;6:1015–1020. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Silverman K, Heil SH, editors. Contingency Management in Substance Abuse Treatment. New York, NY: The Guilford Press; 2008. [Google Scholar]
- Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. Am J Psychiatry. 2001;158:1184–1190. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- Kale RJ, Kaye JH, Whelan PA, Hopkins BL. The effects of reinforcement on the modification, maintenance, and generalization of social responses of mental patients. J Appl Behav Analysis. 1968;4:307–314. doi: 10.1901/jaba.1968.1-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GF, Armitage SG, Tilton JR. A therapeutic approach to schizophrenics of extreme pathology: an operant-interpersonal method. J Abnorm Soc Psychol. 1960;61:276–286. doi: 10.1037/h0041947. [DOI] [PubMed] [Google Scholar]
- Kosten T, Oliveto A, Feingold A, Poling J, Sevarino K, McCance-Katz E, Stine S, Gonzalez G, Gonsai K. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70:315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. J Am Med Assoc. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Lean ME, Pajonk FG. Patients on atypical antipsychotic drugs: another high-risk group for type 2 diabetes. Diabetes Care. 2003;26:1597–1605. doi: 10.2337/diacare.26.5.1597. [DOI] [PubMed] [Google Scholar]
- Lindamer LA, McKibbin C, Morman GJ, Jordan L, Harrison K, et al. Assessment of physical activity in middle-aged and older adults with schizophrenia. Schizophr Res. 2008;104:294–301. doi: 10.1016/j.schres.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh C, Meyer JM, Leckband SG. A comprehensive review of behavioral interventions for weight management in schizophrenia. Ann Clin Psychiatry. 2006;18:23–31. doi: 10.1080/10401230500464646. [DOI] [PubMed] [Google Scholar]
- Mancino MJ, McGaugh J, Feldman Z, Poling J, Oliveto A. Effect of PTSD diagnosis and contingency management procedures on cocaine use in dually-diagnosed cocaine- and opioid-dependent individuals maintained on LAAM: A retrospective analysis. Am J Addict. 2010;19:169–177. doi: 10.1111/j.1521-0391.2009.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall M, Saxon AJ, Malte CA, Chow B, Bailey S, Baker DG, Beckham JC, Boardman KD, Carmody TP, Joseph AM, Smith MW, Shih MC, Lu Y, Holodniy M, Lavori PW CSP 519 Study Team. Integrating tobacco cessation into mental health care for Posttraumatic stress disorder: a randomized controlled trial. JAMA. 2010;304:2485–2493. doi: 10.1001/jama.2010.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara C, Schumacher JE, Milby JB, Wallace D, Usdan S. Prevalence of nonpsychotic mental disorders does not affect treatment outcome in a homeless cocaine-dependent sample. Am J Drug Alcohol Abuse. 2001;27:91–106. doi: 10.1081/ada-100103120. [DOI] [PubMed] [Google Scholar]
- Methapatara W, Srisurapanont M. Pedometer walking plus motivational interviewing program for Thai schizophrenic patients with obesity or overweight: A 12-week, randomized, controlled trail. Psychiatry Clin Neurosci. 2011;65:374–380. doi: 10.1111/j.1440-1819.2011.02225.x. [DOI] [PubMed] [Google Scholar]
- Milby JB, Schumacher JE, Raczynski JM, Caldwell E, Engle M, Michael M, Carr J. Sufficient conditions for effective treatment of substance abusing homeless persons. Drug Alcohol Depend. 1996;43:39–47. doi: 10.1016/s0376-8716(96)01286-0. [DOI] [PubMed] [Google Scholar]
- Milby JB, Schumacher JE, McNamara CL, Wallace D, Usdan S, Michael M. Initiating abstinence in cocaine abusing dually diagnosed homeless persons. Journal of Drug and Alcohol Dependence. 2000;60:55–67. doi: 10.1016/s0376-8716(99)00139-8. [DOI] [PubMed] [Google Scholar]
- Najavits LM, Gastfriend DR, Barber JP, Reif S, Muenz LR. Cocaine dependence with and without PTSD among subjects in the National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Am J Psychiatry. 1998;155:214–219. doi: 10.1176/ajp.155.2.214. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Charney DS, Lowis L, Cornish JW, Post RM, Woody GE, et al. Priority actions to improve the care of persons with co-occurring substance abuse and other mental disorders: A call to action. Biol Psychiatry 2004. 2004;56:703–713. doi: 10.1016/j.biopsych.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Ouimette PC, Moos RH, Finney JW. PTSD treatment and 5-year remission among patients with substance use and posttraumatic stress disorders. J Consult Clin Psychol. 2003;71:410–414. doi: 10.1037/0022-006x.71.2.410. [DOI] [PubMed] [Google Scholar]
- Peniston EG. Evaluation of long-term therapeutic efficacy of behavior modification program with chronic male psychiatric patients. J Behav Ther Exp Psychiatry. 1988;19:95–101. doi: 10.1016/0005-7916(88)90022-5. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Post EP, Cruz M, Harman J. Incentive payments for attendance at appointments for depression among low-income African Americans. Psychiatr Serv. 2006;57:414–416. doi: 10.1176/appi.ps.57.3.414. [DOI] [PubMed] [Google Scholar]
- Priebe S, Burton A, Ashby D, Ashcroft R, Burns T, David A, et al. Financial incentives to improve adherence to anti-psychotic maintenance medication in non-adherent patients – a cluster randomized controlled trial (FIAT) BMC Psychiatry. 2009;9:61–69. doi: 10.1186/1471-244X-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Myers JK, Kramer M, Robins LN, George LK, Karno M, Locke BZ. Comorbidity of mental disorders with alcohol and other drug abuse: Results from the Epidemiologic Catchment Area (ECA) study. J Am Med Assoc. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Ries RK, Dyck DG, Short R, Srebnik D, Fisher A, Comtois KA. Outcomes of managing disability benefits among patients with substance dependence and severe mental illness. Psychiatr Serv. 2004;55:445–447. doi: 10.1176/appi.ps.55.4.445. [DOI] [PubMed] [Google Scholar]
- Roick C, Fritz-Wieacker A, Matschinger H, Heider D, Schindler J, Riedel-Heller S, Angermeyer MC. Health habits of patients with schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2007;42:268–276. doi: 10.1007/s00127-007-0164-5. [DOI] [PubMed] [Google Scholar]
- Roll JM, Chermack ST, Chudzynski JE. Investigating the use of contingency management in the treatment of cocaine abuse among individuals with schizophrenia: a feasibility study. Psychiatry Research. 2004;125:61–64. doi: 10.1016/j.psychres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Steingard S, McGinley M. Use of monetary reinforcement to reduce the cigarette smoking of persons with schizophrenia: A feasibility study. Exp Clin Psychopharmacol. 1998;6:157–161. doi: 10.1037//1064-1297.6.2.157. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Weissman MM, Kleber H, Wilber C. Heterogeneity of psychiatric diagnosis in treated opiate addicts. Arch Gen Psychiatry. 1982;39:161–166. doi: 10.1001/archpsyc.1982.04290020027006. [DOI] [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: Is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- Shaner A, Eckman TA, Roberts LJ, Wilkins JN, Tucker DE, Tsuang JW, Mintz J. Disability income, cocaine use, and repeated hospitalization among schizophrenic cocaine abusers: A government-sponsored revolving door? New England Journal of Medicine. 1995;333:777–783. doi: 10.1056/NEJM199509213331207. [DOI] [PubMed] [Google Scholar]
- Shaner A, Roberts LJ, Eckman TA, Tucker DE, Tsuang JW, Wilkins JN, Mintz J. Monetary reinforcement of abstinence from cocaine among mentally ill patients with cocaine dependence. Psychiatr Serv. 1997;48:807–810. doi: 10.1176/ps.48.6.807. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Higgins ST. Voucher-based contingent reinforcement of marijuana abstinence among individuals with serious mental illness. J of Substance Abuse Treat 2006. 2006;30:291–295. doi: 10.1016/j.jsat.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Steingard S, Badger GJ, Anthony SL, Higgins ST. Contingent reinforcement of marijuana abstinence among individuals with serious mental illness: a feasibility study. Exp Clin Psychopharmacol. 2000;8:509–517. doi: 10.1037//1064-1297.8.4.509. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Strong Kinnaman JE, Slade E, Bennett ME, Bellack AS. Examination of contingency payments to dually-diagnosed patients in a multi-faceted behavioral treatment. Addict Behav. 2007;32:1480–1485. doi: 10.1016/j.addbeh.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyer BA, Irvine S. Contingency management of exercise by chronic schizophrenic patients. Percept Mot Skills. 1984;58:419–425. doi: 10.2466/pms.1984.58.2.419. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Higgins ST, Bickel WK, Steingard S. Effects of response requirement and the presence of an alternative reinforcer on cigarette smoking by schizophrenics. Psychopharmacol. 1999;145:52–60. doi: 10.1007/s002130051031. [DOI] [PubMed] [Google Scholar]
- Tidey JW, O’Neill SC, Higgins ST. Contingent monetary reinforcement of smoking reductions, with and without transdermal nicotine, in outpatients with schizophrenia. Exp Clin Psychopharmacol. 2002;10:241–247. doi: 10.1037//1064-1297.10.3.241. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Ries R. Contingency management of substance abuse in people with major mental illness. In: Higgins S, Silverman K, Heil S, editors. Contingency Management in Substance Abuse Treatment. New York: Guildford Press; 2008. pp. 202–221. [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Reid N. Effects of contingency management and bupropion on cigarette smoking in smokers with schizophrenia. Psychopharmacol. 2011;217:279–287. doi: 10.1007/s00213-011-2282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy K, Babuscio T, Nich C, Kiluk B, Carroll KM, Petry NM, Rounsaville BJ. Contingency management to reduce substance use in individuals who are homeless with co-occurring psychiatric disorders. Am J Drug Alcohol Abuse 2007. 2007;33:253–258. doi: 10.1080/00952990601174931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman LP, Krasner L. Case Studies in Behavior Modification. New York, Holt: Rinehart and Winston, Inc; 1965. [Google Scholar]
- Upper D, Newton JG. A weight reduction program for schizophrenic patients on a token economy unit: two case studies. J Behav Ther Exp Psychiatry. 1971;2:113–115. [Google Scholar]
- Watkins KE, Pincus HA, Smith B, Paddock SM, Mannle TE, Woodroffe A, Solomon J, Sorbero ME, Farmer CM, Hepner KA, Adamson DM, Forrest L, Call C. Santa Monica, CA: RAND Corporation; 2011. Veterans Health Administration Mental Health Program Evaluation: Capstone Report. http://www.rand.org/pubs/technical_reports/TR956. [Google Scholar]
- Winkler RS. Management of chronic psychiatric patients by a token reinforcement system. J Appl Behav Anal. 1970;3:47–55. doi: 10.1901/jaba.1970.3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008;10:1691–1715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]