Abstract
Background and purpose
Perfusion MRI can be used to identify patients with acute ischemic stroke that may benefit from reperfusion therapies. The risk of nephrogenic systemic fibrosis, however, limits the use of contrast agents. Our objective was to evaluate the ability of arterial spin labeling (ASL), an alternative non-invasive perfusion technique, to detect perfusion deficits compared with dynamic susceptibility contrast (DSC) perfusion imaging.
Methods
Consecutive patients referred for emergency assessment of suspected acute stroke within a seven-month period were imaged with both ASL and DSC perfusion MRI. Images were interpreted in a random order by two experts blinded to clinical information for image quality, presence of perfusion deficits and diffusion-perfusion mismatches.
Results
156 patients were scanned with a median time of 5.6 (3.0–17.7) hours from last seen normal. Stroke diagnosis was clinically confirmed in 78 patients. ASL and DSC imaging were available in 64 of these patients. A perfusion deficit was detected with DSC in 39 of these patients; ASL detected 32 of these index perfusion deficits, missing 7 lesions. The median volume of the perfusion deficits as determined with DSC was smaller in patients which were evaluated as normal with ASL than in those with a deficit (median, interquartile range; 56 (10–116) vs. 114 (41–225) ml, p=0.01).
Conclusions
ASL can depict large perfusion deficits and perfusion/diffusion mismatches in correspondence with DSC. Our findings show that a fast 2½ minute ASL perfusion scan may be adequate for screening acute stroke patients with contraindications to gadolinium-based contrast agents.
Keywords: acute stroke, stroke management, cerebral hemodynamics, imaging, MRI
INTRODUCTION
In patients presenting with stroke-like symptoms MRI can be used to identify ischemic brain tissue and evaluate the amount of tissue at risk for infarction.1 Perfusion imaging identifies brain tissue that has reduced blood flow, the potential target for reperfusion therapies. In patients who present to the emergency room beyond the standard time window for intravenous tissue plasminogen activator (IV-tPA) MRI has been postulated as a tool to identify individuals with salvageable brain tissue by detecting whether or not hypoperfused tissue has developed irreversible ischemic injury.2–4
Perfusion is assessed in routine clinical practice with dynamic susceptibility contrast (DSC) imaging. In DSC-MRI a gadolinium contrast agent is injected and a time series of fast T2*-weighted images is acquired. The use of gadolinium-based contrast agents is however limited because of the risk of inducing nephrogenic systemic fibrosis (NSF) in patients with poor renal function. Gadolinium is therefore contraindicated in patients with an estimated glomerular filtration rate (GFR) < 30 mL/min and in those on hemodialysis.5–7 Arterial spin labeling (ASL) is an alternative non-invasive MR technique for visualizing perfusion and quantifying cerebral blood flow.
ASL perfusion imaging uses blood as an endogenous contrast agent by magnetically labeling it with radiofrequency pulses and does not require gadolinium-based contrast agents. The perfusion contrast is given by the difference in magnetization induced by the exchange of these labeled spins at brain tissue level and a non-labeled control image.8–10 Limited by its low intrinsic signal-to-noise ratio, ASL perfusion measurements generally take several minutes for an accurate perfusion measurement. An improved pseudo-continuous labeling scheme, however, has increased the SNR as it has a higher labeling efficiency and enables the combined use of the body transmit coil with the multi-detector coils.11,12 By combining it with background suppression the SNR is increased further, by almost a twofold, allowing for shorter imaging time and increased spatial resolution.13
Previous studies have shown that ASL can detect perfusion deficits in patients with acute and subacute stroke.14–16 The aims of our study were to test the feasibility of using ASL in the clinical setting for evaluating hyperacute stroke patients and to evaluate the ability of ASL to detect perfusion deficits and perfusion-diffusion mismatch of varying volumes compared with DSC perfusion imaging.
MATERIALS AND METHODS
Patients
This prospective study was conducted in compliance with human subjects protection requirements. All patients who had an acute MRI for the assessment of suspected ischemic stroke at the Washington Hospital Center in Washington D.C. over a seven-month period were considered. At this hospital MRI is the initial imaging modality for the evaluation of acute stroke. Patients with a suspected stroke who were 18 years of age or older were included and patients who had a contraindication to MRI or were pregnant were excluded. A vascular neurologist or stroke fellow evaluated all patients and scored the National Institutes of Health Stroke Scale (NIHSS). Patients who met standard eligibility criteria and were within 4.5 hours of symptom onset were treated with IV-tPA.17
Imaging protocol
Imaging was performed on a clinical 3 Tesla MRI scanner (Achieva, Philips Medical Systems, Best, The Netherlands) equipped with an eight-channel coil and locally developed software to enable ASL perfusion imaging. The imaging protocol was the standard imaging protocol used to screen all stroke patients was part of a quality improvement study. IT included diffusion-weighted imaging (DWI), T2-weighted fluid attenuation inversion recovery (FLAIR) imaging, DSC and ASL perfusion-weighted imaging. DSC images were not acquired in patients with a GFR less than 30 mL/min in accordance with published guidelines due to the increased risk of developing nephrogenic systemic fibrosis.18
The DSC perfusion-weighted images were acquired with the vendor's standard commercially available gradient echo sequence. A single dose of 0.1 mmol/kg of gadolinium (gadolinium-DTPA, Magnevist, Bayer Schering Pharma, Germany) was administered at 5 ml/sec. The scan parameters were: repetition time (TR), 1000 ms; echo time (TE), 25 ms; field-of-view (FOV), 256 × 256 mm; 20 slices of 7 mm; scan time 1.43 minutes. ASL perfusion-weighted images were acquired using a pseudo-continuous labeling technique according to a previously published protocol.19 In short, arterial spin labeling was performed by employing a train of 18 degrees, 0.5 ms, Hanning shaped RF pulses at an interval of 1 ms, for a duration of 1650 ms, with a balanced gradient scheme.12,13 The control images were acquired by adding 180° to the phase of all even RF pulses. After a 1525 ms delay, twenty slices were acquired in ascending fashion with an in-plane resolution of 3 × 3 mm2 with single shot gradient echo imaging in combination with background suppression and parallel imaging (SENSE factor 2.5). Background suppression consisting of a saturation pulse immediately before labeling and inversion pulses at 1680 and 2830 ms after the saturation pulse.20 The other ASL MRI parameters were: TR, 4000 ms; TE, 14 ms; pairs of control/label, 12; 20 slices with a 3 × 3 × 7 mm resolution; scan time 2.5 minutes.
FLAIR and DWI images were acquired with the vendor's standard commercially available sequences. The FLAIR images were balanced (roughly) across field strength for conspicuity of chronic ischemic parenchyma, with the following parameters: TR/TE = 9000/120 m and; TI, 2600 ms; 40 slices with 1 × 1 × 4 mm resolution (SENSE factor 1.75); scan time 2.27 minutes. For DWI the parameters were: TR/TE = 4500/62.1 ms; 40 slices with 1 × 1 × 3.5 mm resolution (SENSE factor 1.75); scan time 3.05 minutes.
Perfusion-weighted image analyses
DSC perfusion-weighted images were calculated from the acquired from series of T2*-weighted images with the vendor's standard available perfusion software (Advanced Brain Perfusion, Philips Healthcare, Cleveland, OH) on the MRI console. The time-to-peak (TTP) images were used for perfusion deficit analyses. ASL perfusion-weighted images were generated according to a previously published model that corrects for T1 decay, T2* decay and the different delay times of the imaging slices.21 In patients with motion artefacts, in-plane motion was first corrected for by coregistering all dynamic pairs with SPM5 (Wellcome Trust Centre for Neuroimaging, Oxford, United Kingdom) using the normalized mutual information and a rigid body transformation.
Quantitative perfusion deficit and mismatch
The acute perfusion and diffusion ischemic volumes were measured from DWI and the DSC TTP series using a semi-automated quantitative method in Cheshire™ (Perceptive Informatics, Waltham, Massachusetts) by a core lab rater who has extensive experience and established rater reliability statistics.22 Lesion areas were segmented on a slice-by-slice basis with user selected seed points followed by user-driven editing. DWI lesions were identified on affected hyperintense areas visible from the b=1000 mm/s2 trace or isotropic images. The rater was careful not to include bilateral artefacts, chronic lesions and if necessary reviewed apparent diffusion coefficient maps to isolate acute lesions. PWI lesions on the TTP maps were identified as hyperintense areas, excluding susceptibility artefacts adjacent to the paranasal sinuses. The volumes were automatically calculated by multiplying the total lesion area by the slice thickness.
Qualitative image evaluation
Unaware of the patient's identity, clinical information and diagnosis, two experienced readers (SW, JGM) reviewed the DSC and ASL perfusion-weighted images. The images were viewed independently in a random order. The readers also had access to the DWI and FLAIR images. They rated image quality, presence / absence of perfusion deficits, DWI lesions, perfusion-diffusion mismatch and significance of the mismatch. Image quality was scored as excellent, good, fair, poor and uninterpretable. A perfusion deficit was defined as an area with visually significant increased time-to-peak on DSC and with decreased perfusion signal on ASL when compared to the surrounding brain tissue and contralateral hemisphere.
When there was a discrepancy in the evaluation of a perfusion deficit between both readers, they looked at the images together and reached a consensus. The readers used commercially available software to view the images (MIPAV, NIH, Bethesda, Maryland, version 4.4.1) and were able to adjust for contrast, color scheme and size of the images.
Statistical analyses
To compare acute DWI ischemic lesion volume, acute perfusion lesion volume on TTP and diffusion-perfusion mismatch, logarithmic transformation was applied to correct for normality and comparison was performed with an independent t-test. Values are expressed as mean ± standard deviation (SD) or median (1st interquartile – 3rd interquartile) unless otherwise specified. A p-value< 0.05 was considered statistically significant. Inter-rater reliability analyses was performed using the Kappa statistic to determine consistency among raters.23 Statistical analysis was performed using SPSS (SPSS Inc., Chicago, Illinois, version 15.0.1) for Windows.
RESULTS
One hundred fifty-six consecutive patients (83 women, 73 men, 62±17 years) had an MRI as part of the initial evaluation of stroke at Washington Hospital Center between June 2009 and January 2010. The median time from symptom onset to imaging was 5.6 (3.0 – 17.7) hours. Of the156 patients, 30 patients were excluded because they could not receive contrast due GFR less than 30 mL/min and 21 patients were excluded because of incomplete imaging data. A total of 105 patients underwent both ASL and DSC perfusion imaging. Of the 31 patients in whom DSC was contraindicated, 14 had a stroke.
The quality of the DSC and ASL images was variable. 96% of all DSC images were interpretable (100 of 105 patients): in 75 patients the DSC images were judged as good to excellent (71%), in 15 they were fair (14%), in 10 they were poor (10%), and in 5 they were uninterpretable (5%). 97% of all ASL images were interpretable (100 of 105 patients): in 76 patients the ASL images were judged as good to excellent (76%), in 17 they were fair (16%), in 9 they were poor (9%), and in 3 they were uninterpretable (3%).
Of the 105 patients with both ASL and DSC perfusion imaging, 64 (61%) had a clinically confirmed stroke. The median baseline NIHSS score was 6 (range, 0 – 33). The median time between symptom onset and imaging was 6.5 (3.0 – 23.2). Twenty-nine (45%) of the 64 patients with confirmed stroke received IV-tPA. In these patients the median time to imaging was 3.0 (1.7 – 4.0) hours.
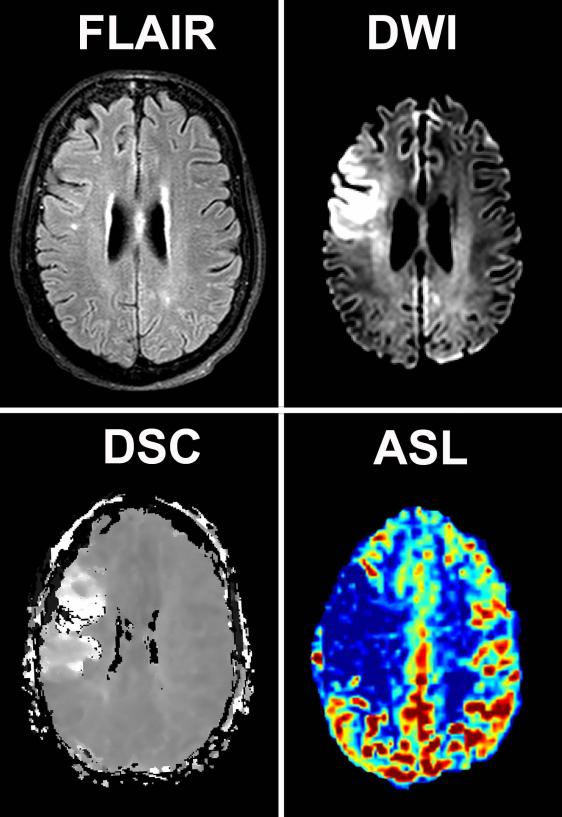
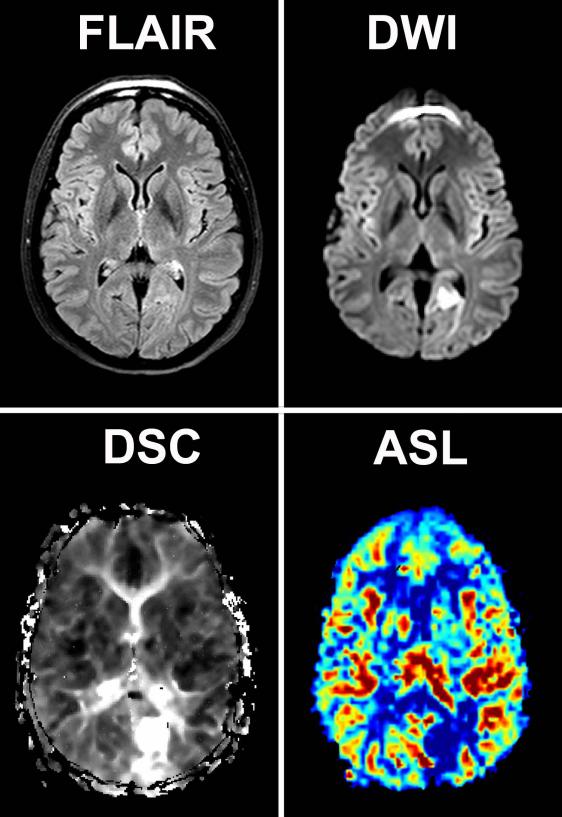
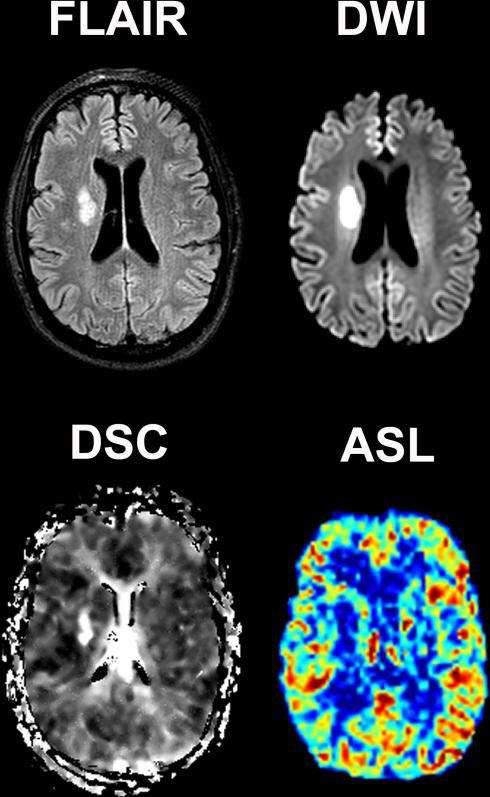
Figure 1 and 2 illustrates two examples of ASL and DSC perfusion imaging in patients with acute stroke. A perfusion deficit was detected with DSC in 39 (61%) of the 64 patients with confirmed stroke (table 1). ASL identified a perfusion deficit in 32 (82%) of the 39 patients with a deficit on DSC. In the seven stroke patients with a deficit depicted on DSC but not on ASL; five of these patients had a cortical gray matter deficit and two a deficit in the basal ganglia (figure 3). The ASL image quality was scored as poor to uninterpretable in four of the seven patients, all with a cortical perfusion deficit. Four of the 25 stroke patients who were classified as having normal perfusion by DSC were classified as having a perfusion deficit with ASL. The quality of the DSC images in 3 of these 4 patients was poor. A perfusion deficit was depicted in one of these four patients in the basal ganglia with ASL but not with DSC. The inter-rater agreement for detecting perfusion deficits with DSC and ASL perfusion imaging was, respectively, 0.64 and 0.6.
Figure 1.
Perfusion, diffusion and FLAIR images of a 66-year old woman presenting within 1 hour after symptom onset. Restricted diffusion and increased time-to-peak times can be appreciated in the flow territory of the right middle cerebral artery on the DWI and DSC images. The corresponding ASL image shows a corresponding decrease in perfusion.
Figure 2.
Perfusion, diffusion and FLAIR images of a 48-year old woman presenting within 6 hours after symptom onset. Restricted diffusion and increased time-to-peak times can be appreciated in the flow territory of left posterior circulation on the DWI and DSC images. The corresponding ASL image shows a corresponding decrease in perfusion.
Tabla 1.
Agreement between the perfusion deficits depicted with ASL and DSC perfusion imaging.
| DSC (n = 64) | ||
|---|---|---|
| ASL | Yes | No |
| Yes | 32 | 4 |
| No | 7 | 21 |
Figure 3.
Perfusion, diffusion and FLAIR images of a 53-year old male presenting within 10 hours after symptom onset. Restricted diffusion and increased time-to-peak times can be appreciated in the right basal ganglia on the DWI and DSC images. The perfusion deficit was however not depicted with ASL.
Table 2 lists how many patients were identified as having a significant perfusion / diffusion mismatch on ASL and DSC perfusion imaging. Twenty (31%) out of the 64 patients with a confirmed stroke had a significant mismatch on DSC, and out of these 18 (90%) were also determined to have a mismatch on ASL. The two patients that had a mismatch on DSC but not on ASL had cortical lesions; one had an uninterpretable ASL. In six (33%) out of the 18 patients the mismatch was categorized as significant with ASL, but not with DSC. The inter-rater agreement for detecting perfusion/diffusion mismatch with DSC and ASL perfusion imaging was 0.71 and 0.51, respectively.
Tabla 2.
Agreement between the significant perfusion/diffusion mismatched depicted with ASL and DSC perfusion imaging.
| DSC (n = 64) | ||
|---|---|---|
| ASL | Yes | No |
| Yes | 18 | 6 |
| No | 2 | 38 |
It was not possible to measure the perfusion deficit volume in 7 (11%) of 64 patients with clinically confirmed stroke because the DSC images were too poor of quality. The median volume of the perfusion deficit on DSC for all stroke patients was 110 (35 – 215) ml. The median volume of the perfusion deficit (on DSC) in patients that did not have a defect present on ASL was 56 (10 – 116) ml. This was significantly smaller than the median volume of 114 (41 – 225) ml in patients that did have a defect on ASL, p=0.01 t-test performed on log-transformation of volumes.
DISCUSSION
This study demonstrates that fast evaluation of hyperacute stroke patients in a clinical setting is feasible with ASL perfusion imaging. Detection of large perfusion deficits and the presence of a perfusion/diffusion mismatch with ASL is comparable to that of DSC perfusion imaging. In the patients in whom a perfusion deficit was detected with DSC but evaluated as normal with ASL, the perfusion deficit volume was smaller.
Our findings that perfusion deficits are detectable with ASL correspond with previous studies in small groups of pediatric and adult patients with both acute and subacute stroke.14–16 Using a prototype single slice pulsed ASL sequence, Siewert et al showed that ASL could detect perfusion abnormalities in a group of 18 subacute stroke patients in comparison to gadolinium-enhanced DSC imaging.24 With a more recent pulsed ASL scan that uses a FAIR alternating labeling scheme combined with a QUIPS2 bolus cut-off, Viallon et al showed similar results in a group of 41 acute stroke patients within two weeks of symptom onset.25 Their results showed that ASL can identify territorial hypoperfusion in correspondence with DSC, however for lacunar infarctions, the spatial resolution of ASL was not sufficient to predict local perfusion deficits. This is in line with our findings that in those patients where ASL did not detect a perfusion deficit, the lesion volume as defined with TTP DSC was smaller, indicating that ASL is relatively insensitive for small perfusion deficits.
There are important differences between both perfusion imaging techniques used in this study. The ASL perfusion-weighted images are based on cerebral blood flow and ischemic tissue is reflected by loss of signal. This is substantially different to DSC, where the measured mean transit times are predominantly used for lesion detection in stroke. This hemodynamic parameter derived through deconvolution reflects the transit time of the administered contrast bolus through the brain parenchyma. Ischemia will lead to increased transit times and a lesion is reflected by increased signal or hypointensity. When comparing both techniques this is an important difference, as the contrast-to-noise of the ASL perfusion-weighted maps is lower and ischemic lesions are less clearly delineated. Although our study shows correspondence, the ischemic lesions that were not detected with ASL were of smaller volume. By using an ASL technique with image acquisition at multiple delay times after the initial labeling, it is also possible to measure the arterial arrival times with ASL.26 In a recent study of 15 patients with acute minor stroke and TIA, MacIntosh et al demonstrated that a whole-brain 3D-GRASE PASL sequence with prolonged arrival times values can be measured within the affected hemisphere.16 With further research, this potentially may be a valuable additive to the currently acquired perfusion-weighted images, as small inconspicuous lesions, for instance in the basal ganglia, may be easier to detect. Recent acute ischemic stroke imaging guidelines recommend MR imaging for detection of ischemic changes and to exclude potential intracerebral hemorrhage.1 Currently, there is increasing evidence supporting that perfusion imaging may play an important role on the selection of patients beyond the strict three hour window that could benefit from thrombolysis treatment. In our study however, DSC imaging was not performed in 20% of the patients presenting with stroke-like symptoms due to increased risk of developing NSF. This significant amount of patients illustrates the importance of having an alternative noninvasive method for perfusion imaging. Since ASL uses radiofrequency pulses and does not require injection of gadolinium-based contrast agents, it may potentially be a viable alternative for those patients with a poor GFR or on hemodialysis. A relatively long 2 ½ minute ASL sequence was used in this study, Fernández-Seara et al showed however, that perfusion maps can be acquired in less than 1 minute by combining pseudo-continuous ASL with background suppression and a single-shot 3D GRASE readout.27
A potential limitation of our study may be that an ASL perfusion sequence was used that acquires the images after a fixed time point following the labeling. In patient with delayed inflow, for instance caused by collateralization, this may lead to an underestimation of cerebral perfusion. The perfusion deficit in ASL imaging is as a result different to that reflected by prolonged transit times in DSC as the perfusion signal drop may be explained by both decreased cerebral blood flow and delayed arrival of the blood bolus. However, with the sequence the effective delay time from begin of labeling to the readout is 3 seconds and should allow appropriate inflow time. An additional limitation is that ASL is predominately sensitive to gray-matter perfusion. Due to the limited signal-to-noise ratio, it is therefore difficult to detect small CBF changes in white matter with ASL. For the evaluation of perfusion deficit presence on DSC no threshold other than visual assessment was used. Areas with benign oligemia may have therefore been erroneously evaluated to be at risk for infarction and potentially have lead to discrepancies when compared to ASL.
CONCLUSIONS
ASL can depict large perfusion deficits and perfusion/diffusion mismatches in correspondence with DSC. Our findings show that a fast 2½ minute ASL perfusion scan can be used a fast non-invasive method to image for screening patients suspected of ischemic stroke in a clinical setting.
ACKOWLEDGEMENTS
The authors thank Dr. Marie Luby and Dr. Li An for their help with this study.
SOURCES OF FUNDING This work was supported by the Intramural Division of the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland.
Dr. M.J.P. van Osch receives support from the Technology Foundation STW, applied science division of The Netherlands Organisation for Scientific Research and the technology program of the Dutch Ministry of Economic Affairs.
Dr. J. Hendrikse receives support from the Netherlands Organization for Scientific Research (grant 916-76-035).
Footnotes
CONFLICT(S) OF INTEREST / DISCLOSURES The authors have no disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Latchaw RE, Alberts MJ, Lev MH, Connors JJ, Harbaugh RE, Higashida RT, et al. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009;40:3646–3678. doi: 10.1161/STROKEAHA.108.192616. [DOI] [PubMed] [Google Scholar]
- 2.Warach S, Dashe JF, Edelman RR. Clinical outcome in ischemic stroke predicted by early diffusion-weighted and perfusion magnetic resonance imaging: a preliminary analysis. J Cereb Blood Flow Metab. 1996;16:53–59. doi: 10.1097/00004647-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Heiss WD, Thiel A, Grond M, Graf R. Which targets are relevant for therapy of acute ischemic stroke? Stroke. 1999;30:1486–1489. doi: 10.1161/01.str.30.7.1486. [DOI] [PubMed] [Google Scholar]
- 4.Kidwell CS, Alger JR, Saver JL. Evolving paradigms in neuroimaging of the ischemic penumbra. Stroke. 2004;35:2662–2665. doi: 10.1161/01.STR.0000143222.13069.70. [DOI] [PubMed] [Google Scholar]
- 5.Penfield JG, Reilly RF., Jr. What nephrologists need to know about gadolinium. Nat Clin Pract Nephrol. 2007;3:654–668. doi: 10.1038/ncpneph0660. [DOI] [PubMed] [Google Scholar]
- 6.Kuo PH, Kanal E, bu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242:647–649. doi: 10.1148/radiol.2423061640. [DOI] [PubMed] [Google Scholar]
- 7.Kanal E, Barkovich AJ, Bell C, Borgstede JP, Bradley WG, Jr., Froelich JW, et al. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol. 2007;188:1447–1474. doi: 10.2214/AJR.06.1616. [DOI] [PubMed] [Google Scholar]
- 8.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water [published erratum appears in Proc Natl Acad Sci U S A 1992 May 1;89(9):4220] Proc Natl Acad Sci U S A. 1992;89:212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 10.Edelman RR, Siewert B, Darby DG, Thangaraj V, Nobre AC, Mesulam MM, Warach S. Qualitative mapping of cerebral blood flow and functional localization with echo-planar MR imaging and signal targeting with alternating radio frequency. Radiology. 1994;192:513–520. doi: 10.1148/radiology.192.2.8029425. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Seara MA, Edlow BL, Hoang A, Wang J, Feinberg DA, Detre JA. Minimizing acquisition time of arterial spin labeling at 3T. Magn Reson Med. 2008;59:1467–1471. doi: 10.1002/mrm.21633. [DOI] [PubMed] [Google Scholar]
- 12.Wu WC, Fernandez-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med. 2007;58:1020–1027. doi: 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]
- 13.Garcia DM, Bazelaire CM, Alsop DC. Pseudo-continuous Flow Driven Adiabatic Inversion for Arterial Spin Labeling. ISMRM. 2005:37. [Google Scholar]
- 14.Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31:680–687. doi: 10.1161/01.str.31.3.680. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Licht DJ, Smith SE, Agner SC, Mason S, Wang S, et al. Arterial spin labeling perfusion MRI in pediatric arterial ischemic stroke: initial experiences. J Magn Reson Imaging. 2009;29:282–290. doi: 10.1002/jmri.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacIntosh BJ, Lindsay AC, Kylintireas I, Kuker W, Gunther M, Robson MD, et al. Multiple inflow pulsed arterial spin-labeling reveals delays in the arterial arrival time in minor stroke and transient ischemic attack. AJNR Am J Neuroradiol. 2010;31:1892–1894. doi: 10.3174/ajnr.A2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 18.Perazella MA, Rodby RA. Gadolinium-induced nephrogenic systemic fibrosis in patients with kidney disease. Am J Med. 2007;120:561–562. doi: 10.1016/j.amjmed.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 19.Bokkers RP, van Osch MJ, van der Worp HB, de Borst GJ, Mali WP, Hendrikse J. Symptomatic carotid artery stenosis: impairment of cerebral autoregulation measured at the brain tissue level with arterial spin-labeling MR imaging. Radiology. 2010;256:201–208. doi: 10.1148/radiol.10091262. [DOI] [PubMed] [Google Scholar]
- 20.Ye FQ, Frank JA, Weinberger DR, McLaughlin AC. Noise reduction in 3D perfusion imaging by attenuating the static signal in arterial spin tagging (ASSIST) Magn Reson Med. 2000;44:92–100. doi: 10.1002/1522-2594(200007)44:1<92::aid-mrm14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab. 1996;16:1236–1249. doi: 10.1097/00004647-199611000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Luby M, Bykowski JL, Schellinger PD, Merino JG, Warach S. Intra- and interrater reliability of ischemic lesion volume measurements on diffusion-weighted, mean transit time and fluid-attenuated inversion recovery MRI. Stroke. 2006;37:2951–2956. doi: 10.1161/01.STR.0000249416.77132.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 24.Siewert B, Schlaug G, Edelman RR, Warach S. Comparison of EPISTAR and T2*-weighted gadolinium-enhanced perfusion imaging in patients with acute cerebral ischemia. Neurology. 1997;48:673–679. doi: 10.1212/wnl.48.3.673. [DOI] [PubMed] [Google Scholar]
- 25.Viallon M, Altrichter S, Pereira VM, Nguyen D, Sekoranja L, Federspiel A, et al. Combined use of pulsed arterial spin-labeling and susceptibility-weighted imaging in stroke at 3T. Eur Neurol. 2010;64:286–296. doi: 10.1159/000321162. [DOI] [PubMed] [Google Scholar]
- 26.Golay X, Hendrikse J, Lim TC. Perfusion imaging using arterial spin labeling. Top Magn Reson Imaging. 2004;15:10–27. doi: 10.1097/00002142-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Seara MA, Edlow BL, Hoang A, Wang J, Feinberg DA, Detre JA. Minimizing acquisition time of arterial spin labeling at 3T. Magn Reson Med. 2008;59:1467–1471. doi: 10.1002/mrm.21633. [DOI] [PubMed] [Google Scholar]