Abstract
The aims of the current study were to examine, prospectively, 1) dynamic changes in affective state, self-efficacy, and urge in the hours before initial smoking and drinking lapses among individuals in concurrent alcohol and smoking treatment, and 2) the extent to which self-efficacy, urge to use, and/or the use of one substance predicted lapse to the other substance. Ninety-six men and women recruited for a clinical trial of concurrent alcohol and tobacco treatment were eligible for inclusion. Only data from those who experienced an initial lapse to drinking (n=29), or smoking (n=32) were included. Two outpatient substance abuse clinics provided concurrent alcohol and smoking treatment on a weekly basis for three months. Ecological Momentary Assessment (EMA) methods were employed over a 28-day monitoring period to assess antecedents to first drink and a 14-day monitoring period was examined for initial smoking lapses. Baseline and EMA measures of positive and negative affect, alcohol/smoking urge, alcohol/smoking abstinence self-efficacy, nicotine withdrawal, and quantity/frequency of alcohol and tobacco use were examined as lapse predictors. Analyses of EMA ratings controlled for the corresponding baseline measure. Smoking lapse among individuals in concurrent alcohol and tobacco treatment was foreshadowed by higher urges to smoke, lower positive mood, and lower confidence to resist smoking. Drinking lapse was preceded by lower confidence to resist smoking, but only among individuals who reported recent smoking. Concurrent alcohol and smoking treatment should focus on the enhancement of abstinence self-efficacy, positive mood, and the curbing of urges in order to offset lapse risk.
Keywords: alcoholism treatment, smoking cessation, ecological momentary assessment, smoking relapse, drinking relapse
Studies have shown that 56–65% of adults with alcohol abuse or dependence are current smokers (Daeppen et al., 2000; Lasser et al., 2000), which is nearly three times the rate (21%) in the general population (Pleis, Lucas, & Ward, 2009). Those with alcohol problems who smoke also are more dependent on nicotine, report lower smoking quit rates than those without alcohol problems (Hughes & Kalman, 2006), and die more often from diseases associated with cigarette smoking as opposed to alcohol-related causes (Hurt et al., 1996). Accordingly, the United States Department of Health and Human Services (Fiore et al., 2008) has recommended that smoking cessation be offered in the context of other substance abuse treatment. Although this is the recommended approach, concurrent treatment for alcohol and tobacco may pose unique challenges. For example, coping with withdrawal symptoms from both substances might impede treatment success (Joseph, Willenbring, Nugent, & Nelson, 2004). Alternatively, if one substance has become a conditioned stimulus for the use of the other, or if one substance elicits craving for the other substance (Istvan & Matarazzo, 1984; Littleton, Barron, Prendergast, & Jo Nixon, 2007), concurrent treatment may lead people to achieve abstinence from both substances more easily than would be expected from the treatment of one substance alone.
Outcome research has explored whether concurrent treatment leads to higher or lower rates of abstinence from tobacco and other substances compared to substance abuse or tobacco treatment alone (see Kodl, Fu, & Joseph, 2006 for a review). A meta-analysis of 11 randomized controlled trials of concurrent treatment for tobacco and other substances showed that those who received concurrent substance and tobacco treatment were 25% more likely to show long-term abstinence from alcohol and drugs than control participants who received substance treatment without tobacco treatment. However, there was no difference in long-term smoking abstinence rates (Prochaska, Delucchi, & Hall, 2004). In contrast, one well-designed study found that concurrent alcohol and tobacco treatment led to significantly lower alcohol abstinence rates following treatment compared to alcohol treatment with tobacco treatment deferred for six months (Joseph et al., 2004). While these outcome studies largely support the provision of concurrent treatment, there is little research on the process of lapse and relapse in this context (although see Cooney et al., 2007).
A careful examination of the lapse and relapse process among individuals aiming to quit smoking and drinking is important for several reasons: First, determining the extent to which dynamic changes in cognitive and affective variables are associated with initial lapses to tobacco and alcohol can advance theories of relapse, subsequently leading to the development of more effective interventions and relapse prevention strategies for individuals with comorbid alcohol and tobacco dependence. Traditionally, research on relapse has focused on traits, or stable characteristics of the individual that place them at risk for relapse (Shiffman, 2005). Since traits are, by definition, enduring characteristics, it may be more fruitful to examine factors that are more proximal and, therefore, potentially modifiable through intervention (Shiffman, 2005).
Second, an investigation of the factors leading up to early lapse among individuals in concurrent treatment affords researchers the unique opportunity to explore whether common factors are associated with lapse to both substances (e.g., do increases in negative affect predict both smoking and drinking lapse?), whether variables related to one substance are precursors of relapse to another substance (e.g., does cigarette urge increase the likelihood of a drinking lapse?), and the extent to which a lapse to one substance increases the risk of a lapse to another substance (e.g., does smoking lapse increase likelihood of a drinking lapse?).
The cognitive-behavioral model of lapse and relapse (Marlatt & Gordon, 1985; Witkiewitz & Marlatt, 2004) identifies several key intrapersonal factors, namely affective state, abstinence self-efficacy, and urge (or craving) as proximal determinants of alcohol and smoking lapse and relapse. Research on these factors often has been based on retrospective reports collected long after the lapse or relapse episode (e.g., Lowman, Allen, & Stout, 1996; Zhou et al., 2009). These reports are subject to recall errors and bias, and have been demonstrated to be inaccurate (Shiffman et al., 1997a). In contrast, ecological momentary assessment (EMA; Shiffman, Stone, & Hufford, 2008; Shiffman, 2009) methodology involves frequent sampling of participants’ cognitions and behaviors in their natural environments, in near real-time. Some EMA studies have asked participants to record details of a lapse right after it was over (Ferguson & Shiffman, 2010; Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996). This method may reduce retrospection bias, but it does not eliminate it. A small number of studies have employed a prospective analysis of EMA data, examining reports obtained during the day or hours just before the lapse episode. In this article we employed the latter approach and report on a prospective analysis of proximal measures of affective state, abstinence self-efficacy, and urge in the hours before first drinking and smoking lapses.
Affective state is a key component of one prominent theoretical model of relapse, which asserts that substance relapse is prompted largely by negative reinforcement, or as a means of coping with drug withdrawal (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004). According to this model, smoking or drinking lapses are most likely to occur in the context of rising negative mood states or falling positive mood states. In a prospective EMA study of smoking treatment (Shiffman & Waters, 2004) negative affect (NA) ratings collected across one day did not predict smoking lapse on the next day. Also, there was no significant trend in NA in the four days leading up to first smoking lapse. However, NA in the hours before was a marginal predictor of first smoking lapse. Moreover, for smoking lapses attributed to stress, NA in hours before the lapse was a strong predictor of first smoking lapse. In addition, a previous study of concurrent alcohol and tobacco treatment found that prospective EMA ratings of negative high arousal mood obtained hours earlier predicted smoking lapse (Cooney et al., 2007). However, prospective NA ratings did not predict drinking lapse. Taken together, EMA ratings of negative affect appear to be influential in smoking lapses; the role of NA in drinking lapses is less clear.
With respect to positive affect, research has shown that lower baseline levels of positive affect were associated with shorter time to smoking relapse following a smoking quit attempt (Leventhal, Ramsey, Brown, LaChance, & Kahler, 2008) and following smoking treatment (Doran et al., 2006). Moreover, among smokers with a history of depression, anhedonia was a significant predictor of smoking relapse, even after controlling for depressive symptoms prior to quitting (Cook, Spring, McChargue, & Doran, 2010). Interestingly, a recent review paper noted that both anhedonia and low levels of positive affect were more consistent predictors of smoking relapse when compared to negative affect and anxious arousal (Ameringer & Leventhal, 2010), suggesting that negative and positive affect should be regarded as unique predictors of smoking relapse. Findings from Borland (1990) further support this distinction, as positive and negative mood states served as lapse triggers with similar frequency (32% and 35%, respectively) among smokers making a quit attempt.
In contrast to the inverse relation between positive affect and relapse frequently reported in the smoking literature, some studies examining the relation between positive affect and drinking have shown a positive relation between these variables. For example, EMA measures of positive affect were positively associated with alcohol consumption later in the day among moderate to heavy college drinkers (Simons et al., 2005; Simons et al., 2010) and predicted excessive drinking in heavy drinking adults (Collins et al., 1998). Findings among people diagnosed with alcohol dependence, however, are mixed: positive mood was retrospectively reported as a trigger for some drinking lapses and relapses (Hodgins, el-Guebaly, & Armstrong, 1995), however, in another study, daily measures of positive affect were not associated with treatment outcomes (Oslin, Cary, Slaymaker, Colleran, & Blow, 2009). Additional research is needed to determine whether there is a relation between positive affect and drinking lapses in persons with alcohol use disorders.
Another potential determinant of lapse and relapse from the cognitive-behavioral model is urge to use, or craving. A prospective EMA smoking treatment study found that urge to smoke upon waking in the morning significantly predicted first smoking lapse later in the day (Shiffman et al., 1997b). Smoking urges assessed one day earlier also predicted first smoking lapse after controlling for baseline smoking urge intensity. In a previous concurrent alcohol tobacco treatment study (Cooney et al., 2007), prospective EMA ratings of higher smoking urge assessed hours earlier predicted first smoking lapse and drinking lapse, suggesting that urges might not need to be substance-specific to threaten abstinence. Interestingly, urge to drink was not a significant predictor of either type of lapse.
A low level of abstinence self-efficacy (ASE) is another key factor associated with smoking and drinking lapses. Self-efficacy has been examined as a prospective predictor of smoking lapse in four EMA studies. Shiffman et al. (2000) followed participants enrolled in a smoking cessation research clinic. Lower average daily smoking ASE predicted the first smoking lapse on the next day, but this prediction did not hold up after controlling for differences in baseline ASE, suggesting that between-person differences in ASE, not within-person variation in ASE, accounted for lapse risk. Gwaltney, Shiffman, Balabanis, and Paty (2005) followed a similar sample of participants and found that EMA reports of lower smoking ASE averaged across a day predicted first smoking lapse on the next day, even after controlling for differences in baseline ASE. Van Zundert, Ferguson, Shiffman, and Engels (2010) collected EMA reports from a sample of adolescent smokers who were trying to quit unaided. Lower daily smoking ASE predicted the first smoking lapse on next day, even after controlling for differences in baseline ASE. In a previous study of concurrent alcohol and tobacco treatment (Cooney et al., 2007) lower alcohol ASE assessed hours earlier predicted first drinking lapse, as was hypothesized. Smoking ASE was not assessed in that study.
To elucidate precursors to lapses and possible cross-substance interactions, the current study examined whether momentary measures of key intrapersonal factors (i.e., positive/negative affect, urge to smoke or drink, abstinence self-efficacy) predicted initial drinking and smoking lapses among individuals enrolled in a clinical trial of concurrent alcohol and tobacco treatment. Outcome data from this trial were reported in a previous report (Cooney et al., 2009). Specifically, it was hypothesized that momentary measures of negative affect and urge would be positively associated with smoking and drinking lapses, momentary measures of abstinence self-efficacy would be inversely associated with smoking and drinking lapses, and the momentary measure of positive affect would be inversely associated with smoking lapse. The current study also examined the extent to which ASE, urge, and/or the use of one substance predicted lapse to the other substance. Due to the paucity of research on cross-substance interactions in this context and the mixed findings regarding the role of positive affect in drinking lapses, no specific hypotheses regarding these relations were made.
Method
Participants and Procedure
Participants were 48 men and women who experienced a smoking (n=32) and/or drinking lapse (n=29) during an intensive one-month EMA monitoring period as part of a clinical trial of concurrent alcohol and tobacco treatment. Participants in the current study were a subset of the total number enrolled in the clinical trial (N=96). The methods for this trial are described in detail elsewhere (Cooney, et al., 2009). Briefly, two outpatient substance abuse treatment sites provided a platform of behavioral alcohol and smoking treatment delivered in three months of weekly one-hour sessions followed by three monthly booster sessions. Participants were randomly assigned to receive either nicotine patch and active nicotine gum or nicotine patch and placebo gum. Primary inclusion criteria were a DSM-IV diagnosis of alcohol abuse or dependence and a smoking rate of ≥15 cigarettes per day. Individuals with medical contraindications for nicotine replacement therapy, those taking medications known to influence alcohol or tobacco use (e.g., naltrexone, disulfiram, bupropion), and those with opiate or benzodiazepine abuse or dependence or intravenous drug abuse in the past year were excluded. When alcohol detoxification was necessary, it was completed prior to study enrollment. Table 1 summarizes the characteristics of the sample that provided data for the present study. Participants’ alcohol use was relatively heavy, with drinking reported on 72% of days, and the majority of days involving heavy drinking. Smoking rates also were fairly high, with participants smoking an average of 25 cigarettes per day, and a mean FTND score of 6.5, considered in the high dependence range (Fagerstrom, Heatherton, & Kozlowski, 1991).
Table 1.
Baseline participant characteristics (N=96)
| Characteristic | M (or %) | SD |
|---|---|---|
| Age (M, SD) | 44.96 | 10.10 |
| Sex (%) | ||
| Males | 70.80 | |
| Females | 26.40 | |
| Race (%) | ||
| Caucasian | 89.60 | |
| African American | 5.20 | |
| Hispanic | 3.10 | |
| Other (bi-racial) | 2.10 | |
| Education (%) | ||
| Grade/High school | 62.50 | |
| College degree | 37.50 | |
| Veteran (%) | 31.30 | |
| Baseline cigarettes/day (M, SD) | 25.48 | 9.73 |
| Carbon monoxide (M, SD) | 29.70 | 13.43 |
| Fagerstrom Test of Nicotine Dependence (FTND) | 6.46 | 2.56 |
| Baseline PDA (M, SD) | 0.28 | 0.27 |
| Baseline PDH (M, SD) | 0.57 | 0.31 |
| Other drug use (%) | ||
| Cocaine | 4.30 | |
| Cannabis | 16.00 | |
| Center for Epidemiological Studies Depression scale (CES-D) (M, SD) | 17.35 | 7.44 |
| CES-D (% who met cutoff) | 59.40 | |
| Profile of Mood States – Vigor Activity Scale (POMS) | 12.90 | 4.83 |
| Smoking Self-Efficacy Total Score (SSET) | 65.11 | 6.53 |
| Alcohol Abstinence Self-Efficacy (AASE) | 7.95 | 28.50 |
| Questionnaire on Smoking Urges Total (QSU) | 137.32 | 35.94 |
| Penn Alcohol Carving Scale Total (PACS) | 14.04 | 6.82 |
| Minnesota Withdrawal Scale | 13.82 | 7.58 |
Note. PDA=Proportion Days Abstinent from Alcohol in the previous 90 days; PDH= Proportion of days in the previous 90 in which heavy drinking was reported.
Baseline Measures
The following baseline measures were obtained immediately after informed consent and study enrollment, prior to the start of alcohol-tobacco treatment. At this point participants were abstinent from alcohol but were still smoking at baseline rates.
Affect
The 20-item Center for Epidemiologic Studies – Depression Scale was administered to assess negative affect (CES-D, Radloff, 1977). Although the entire CES-D scale was administered (α = .77 in this study), we used the seven-item Depressed Affect subscale (Shafer, 2006) as a baseline measure of negative affect, given that it likely captured this construct with a higher level of specificity than the total CES-D score. The internal consistency reliability of the Depressed Affect subscale in our study was good (α = .87). The Vigor-Activity scale from an abbreviated 30-item form of the Profile of Mood States (McNair, Lorr, & Droppelman, 1992) was used to assess baseline positive affect (PA). This scale consists of five items (lively, active, energetic, sluggish, weary) and is considered to be an indicator of global PA (Ameringer & Leventhal, 2010). Internal reliability of the scale in our sample was α = .93.
Craving
The five-item Penn Alcohol Craving Scale (PACS; Flannery, Volpicelli, & Pettinati, 1999) was administered to assess baseline alcohol craving, as well as the ability to resist alcohol if it were available (α = .89). Baseline cigarette craving was assessed with the 32-item Questionnaire of Smoking Urges (QSU; Tiffany & Drobes, 1991) (α = .94 in the current study).
Self-efficacy
Nine items from the Smoking Self-Efficacy/Temptation (SSE-T) scale were used to assess baseline smoking abstinence self-efficacy (α = .86). The SSE-T asks respondent to rate how tempted they would be to smoke across a variety of situations (see http://www.uri.edu/research/cprc/Measures/Smoking02.htm for 9-item scoring instructions; Velicer, DiClemente, Rossi, & Prochaska, 1990). The Alcohol Abstinence Self-Efficacy Scale (AASE; DiClemente, Carbonari, Montgomery, & Hughes, 1994) was used to assess baseline alcohol abstinence self-efficacy. The AASE inquires about temptation and confidence across 20 different drinking situations. Total abstinence self-efficacy was calculated as Confidence - Temptation (α = .92).
Nicotine withdrawal
The Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986) was administered at baseline to measures the severity of eight withdrawal symptoms on 5-point Likert scales (α = .88).
Ecological Momentary Assessment (EMA)
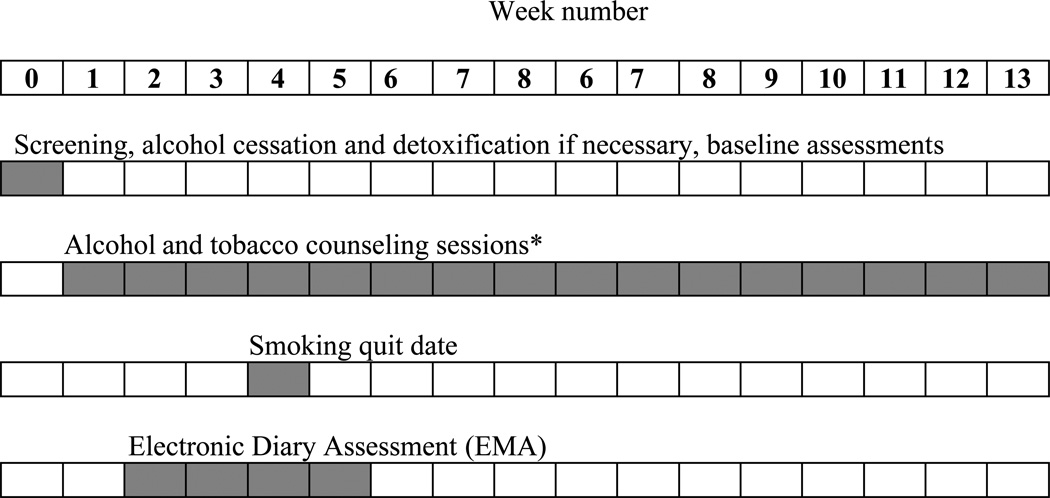
EMA was conducted for four weeks beginning soon after the start of alcohol treatment but two weeks before smoking quit date, continuing for two weeks after the smoking quit date. Figure 1 shows the timing of the EMA measures relative to the other study procedures. Participants engaged in real-time in vivo monitoring using cellular telephones and an Interactive Voice Response (IVR) system. The IVR system was programmed to call participants five times per day and administer a 23-item questionnaire. Cellular telephones were issued to participants upon study entry, and participants were trained in their use. Cellular telephones were programmed so that participants could not make outgoing calls.
Figure 1.
Weekly timeline for concurrent alcohol and tobacco treatment and assessments.
*Additional counseling sessions were provided at weeks 17, 21, and 25.
Participants used the telephone numeric keypad to respond to questions asked by recorded voice. System hardware and software were provided and supported by Telesage, Inc., of Chapel Hill, NC. Participants engaged in signal-contingent assessment only. Time-based sampling procedures (Shiffman et al., 2008) were used, whereby participants were prompted to respond on a quasi-random basis, five times per day, with one randomly scheduled prompt in each of five time periods from 8:00AM to 10:00PM. Participants had the option of delaying responding to the cellular telephone for 5, 10 or 15 minutes when answering would be inconvenient. If a person was unavailable when the phone rang, the system called them back at later intervals. Responses were time-and-date-stamped, and entry of out-of-range data was impossible. At the end of each assessment, the respondent was asked if all answers were correct and was able to correct any mistakes. Participants were unable to skip questions, making it impossible to complete the assessment with missing data. If data entry was abandoned in the midst of an assessment, the system called participant back to resume the assessment.
For every call participants were prompted to record their responses using a 5-point scale ranging from 1=none to 5=very much. The program provided recorded questions pertaining to the participant’s “Urge to drink?”, “Urge to smoke?”, mood state, self-efficacy to resist smoking and drinking urges ("Confident that you can keep from drinking?" and "Confident that you can keep from smoking?"), smoking and drinking behavior, and use of nicotine gum. Mood assessment was based on items derived from the circumplex model of mood experience (Larsen & Diener, 1992), in which mood states are classed along two major dimensions: pleasantness (pleasant - unpleasant) and activation (high - low). Four quadrants of moods are thus created: positive-high activation items (active; peppy: α = .76); positive-low activation (quiet; relaxed: α = .72); negative-high activation (anxious or nervous; angry, irritable or frustrated: α = .81); and negative-low activation (depressed or sad; tired: α = .74). Negative mood subscales were correlated at r=.60 and positive mood subscales were correlated at r= .35. Negative and positive subscales were correlated in the range of −.30. A momentary withdrawal symptoms scale was calculated by taking the mean of the following momentary assessments: Sadness, Sleep Problems, Angry, Trouble Concentrating, Nervous, Hungry, Impatient, and Urge to Smoke. Because the content of this scale overlapped others, it was always treated independently in analyses. The internal reliability of the momentary scale was α = .76.
Participants were paid $0.50 for each time they completed an IVR call. They also were paid an additional $5.00 for each day that they completed all five scheduled IVR assessments. The maximum total possible payment for IVR assessments was $210. The overall compliance rate with the EMA procedure over both phases was 65% of calls completed (range: 5% to 96%; 24 cases responding below 40%). There appeared to be no relation between occurrence of smoking or drinking lapses and loss of recording adherence. Of those who recorded lapses to either substance during the 28-day EMA period, the mean number of records completed was 96, versus 98 for those who did not lapse.
Data Analysis Plan
Analyses were conducted to determine the momentary affective and cognitive antecedents of first drink and first cigarette after beginning treatment. Since participants were required to stop drinking prior to beginning treatment, the entire 28-day monitoring period was evaluated for occurrence of the first drink. In order to detect a “true” drinking lapse (as opposed to a continuation of an intermittent drinking phase), drinking lapse was defined as the first drink recorded after a period of at least 7 days of abstinence. Smoking lapse was defined as the first cigarette recorded during the monitoring period after the smoking quit date (i.e., days 14–28), and after a period of least 24 hours of smoking abstinence. Single puffs of a cigarette or sips of a drink were not regarded as lapses. Of the 96 persons treated, 29 met criteria for having a drinking lapse, 32 for having a smoking lapse, and 13 for both. Earlier analyses indicated that treatment condition did not influence predictors of lapse to either substance, so treatment condition was not included as a factor in the analyses that follow.
Random effects logistic regressions were used in a within-subjects case-control design to evaluate predictors of occurrence of drinking lapse or smoking lapse. In these analyses, the IVR record in which either first drink or first cigarette was recorded was considered the “case” record. Records that preceded the “case” record served as “control” records (see, for example, Shiffman, 2009). Ratings from the participant’s IVR record immediately preceding the smoking or drinking lapse record were the predictors (i.e, lagged predictors). Thus, lagged predictors were used to determine the extent to which first cigarette or first drink was a function of affect, craving, or self-efficacy in the immediately preceding time period (up to 2.8 hours prior to the event). Lagged predictors had to be recorded on the same day as the event (i.e., affect or cognition scores from the evening before were not used to predict first cigarette or first drink the next morning). In analyses of determinants of first drink in the 28-day monitoring period, a total of 658 records from 29 participants (i.e., 29 events) were analyzed. In analyses of antecedents of first cigarette in the second 14-day monitoring phase, a total of 368 records from 32 participants were analyzed.
Analyses were conducted using generalized estimating equations (GEE) procedures (SAS Proc GENMOD; SAS, 1999). The GEE procedure was used because it is able to accommodate the multi-level, nested nature of the data. A logit link was used to characterize the outcome as having a binary distribution. Predictors included one of the relevant lagged affect or cognition variables (e.g., positive-high activation mood; confidence to resist drinking), day, time of day, and the interaction of day X time, to control for temporal variations in responding. The number of records completed by each participant, entered as a between-subjects variable, was used to control for IVR compliance levels. In addition, baseline values from questionnaires that provided an analog of each of the momentary predictor variables were entered to control for baseline between-subjects differences in responding, as per Kenny and Zautra (1995), who suggested that a person’s status on a given variable at a given time is a function of three sources of variance: a trait term that does not change over time; a state term that changes with circumstances, and a random error term. Neither the within-subjects temporal variables nor the between-subjects records variable emerged as significant predictors of occurrence of events in the succeeding time period, so these variables are not referred to in the results below.
Each of the planned lagged predictors was tested separately. Thus we conducted 10 separate analyses, evaluating Negative – Low Activation Mood, Negative – High Activation Mood, Positive – Low Activation Mood, Positive – High Activation Mood, Confidence to Resist Smoking, Confidence to Resist Drinking, Urge to Smoke, Urge to Drink, Withdrawal Symptoms, and occurrence of smoking (or drinking), as predictors of first drink (or first cigarette). All analyses of lagged predictors controlled for the corresponding baseline measure. Because of the exploratory nature of these analyses no corrections were made for multiple tests.
Results
Predictors of Alcohol Lapse
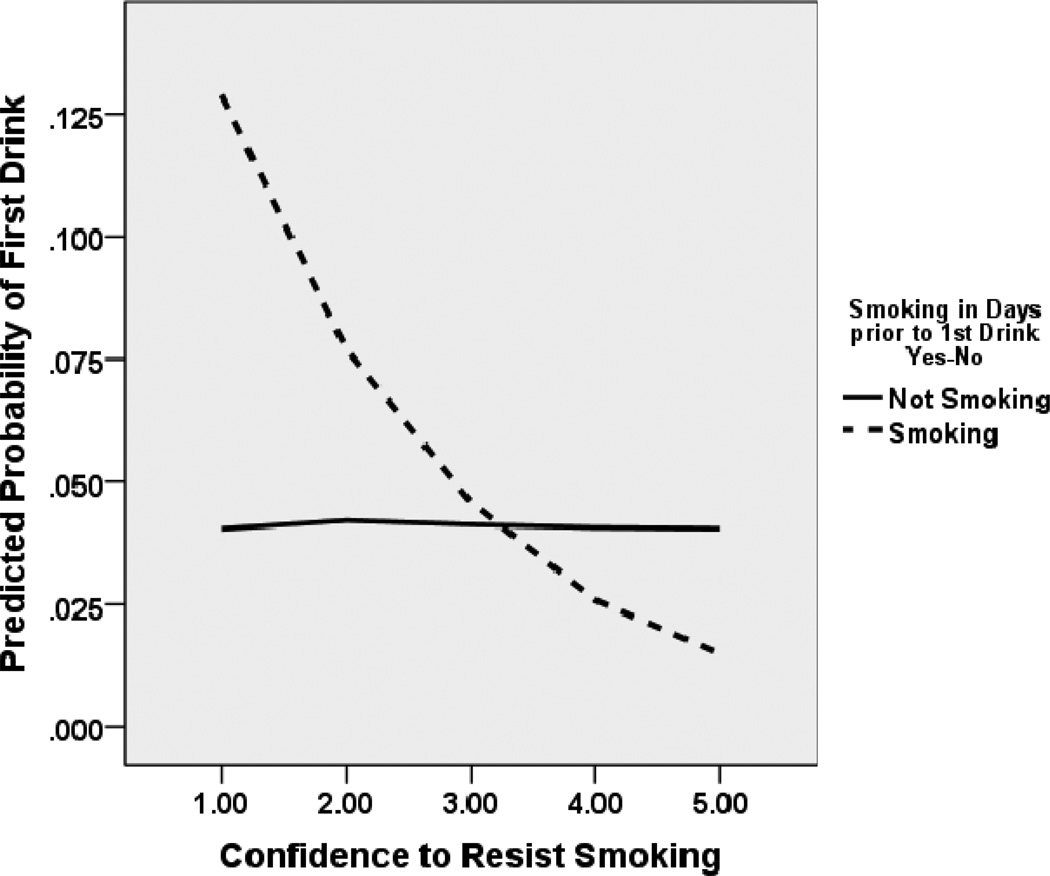
Table 2 shows the results of the random effects logistic regression analyses conducted using first drink in the 28-day monitoring period as the dependent variable. As seen in the table, confidence to resist smoking emerged as a momentary predictor of first drink in the succeeding hours such that those who had high confidence in their ability to stop smoking were significantly less likely to have their first drink in the next few hours. Given the observed cross-substance predictive relation, we conducted a follow-up analysis to determine whether smoking status prior to the drinking lapse interacted with confidence to resist smoking to predict drinking lapse. Indeed, smoking status at time of first drink predicted occurrence of first drink, such that those who reported smoking one or more cigarettes in any of the IVR records prior to the drinking lapse (n=19) were 6 times more likely to have a drinking lapse during the 28-day monitoring period than those not smoking (Wald χ2=15.03, df=1, p<.001, OR=6.01, 95% CIs 2.44, 15.09). Moreover, as depicted in Figure 2, smoking status interacted with momentary confidence to resist smoking to predict first drink, such that those who were still smoking, but had low self-efficacy regarding their ability to resist smoking on a momentary basis, were more likely to have a first drink compared to those with higher confidence and compared to those who were not smoking (Wald χ2=10.24, df=1, p=.001, OR=0.57, 95% CIs 0.40, 0.80).
Table 2.
Summary of Results of Random Effects Logistic Regression Analyses.
Dependent Variable = 1st Drink during 28-Day Monitoring Period. All analyses controlled for recording day, time of day, and day X time. (N=29 events; 658 records).
| Lagged Predictor | B | SE B | Wald | Odds | 95% CI for OR | |
|---|---|---|---|---|---|---|
| Chi-squarea | Ratio | Lower | Upper | |||
| Negative – Low Activation Moodb | .093 | .248 | 0.14 | 1.10 | 0.68 | 1.79 |
| Negative – High Activation Moodb | .292 | .206 | 2.00 | 1.34 | 0.89 | 2.01 |
| Positive – Low Activation Moodc | −.091 | .204 | 0.66 | 0.91 | 0.61 | 1.36 |
| Positive – High Activation Moodc | .247 | .280 | 0.78 | 1.28 | 0.74 | 2.22 |
| Confidence to Resist Smokingd | −.331 | .103 | 10.25*** | 0.72 | 0.59 | 0.88 |
| Confidence to Resist Drinkinge | −.117 | .122 | 0.92 | 0.89 | 0.70 | 1.13 |
| Urge to Smokef | −.177 | .129 | 1.88 | 0.84 | 0.65 | 1.08 |
| Urge to Drinkg | .225 | .134 | 2.83 | 1.25 | 0.96 | 1.63 |
| Withdrawal Symptomsh | .233 | .244 | 0.91 | 1.26 | 0.78 | 2.04 |
| Smoked (Yes/No) | −.409 | .310 | 1.74 | 0.66 | 0.36 | 1.22 |
Note.
p < .05;
p < .01;
p < .001
1 Degree-of-freedom tests
Analysis controlled for baseline CES-D depressed affect subscale score
Analysis controlled for baseline POMS vigor score
Analysis controlled for baseline SSET score
Analysis controlled for baseline AASE score
Analysis controlled for baseline QSU desire score
Analysis controlled for baseline PACS score
Analysis controlled for baseline Minnesota withdrawal scale score
Figure 2.
Relation between aggregated momentary confidence to resist smoking and probability of an initial drinking lapse at the next time point by smoking status. Probability estimates derived from GEE analyses. Among those who reported any smoking in the days prior to their first drinking lapse, lower momentary confidence to resist smoking was associated with a higher probability of initial drinking lapse occurring at the succeeding time point.
Predictors of Smoking Lapse
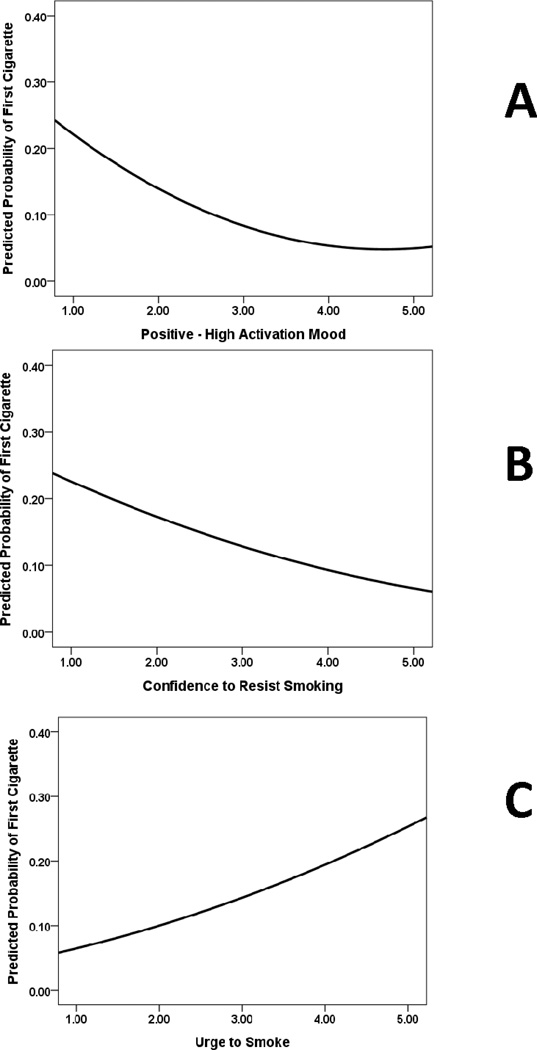
Table 3 shows the results of the random effects logistic regression analyses conducted using first cigarette in the 14-day post-quit monitoring period as the dependent variable. Positive, high activation mood, confidence to resist smoking, and urge to smoke emerged as significant momentary predictors of first cigarette in the succeeding hours. As shown in Figure 3, those who had low positive mood (panel A), low momentary confidence in their ability to stop smoking (panel B), and higher urge to smoke (panel C) were significantly more likely to have their first cigarette prior to the next recording period. Although neither low activation negative mood nor high activation negative mood predicted smoking lapse, baseline negative affect predicted smoking lapse in the analyses including low activation negative mood (Wald χ2=4.39, df=1, p<.05, OR=1.13, 95% CIs 1.01, 1.28) and high activation negative mood (Wald χ2=4.37, df=1, p<.05, OR=1.14, 95% CIs 1.01, 1.28).
Table 3.
Summary of Results of Random Effects Logistic Regression Analyses.
Dependent Variable = 1st Cigarette During 14-Day Post-Quit Monitoring Period. All analyses controlled for recording day, time of day, and day X time. (N=32 events; 368 records).
| Lagged Predictor | B | SE B | Wald | Odds | 95% CI for OR | |
|---|---|---|---|---|---|---|
| Chi-squarea | Ratio | Lower | Upper | |||
| Negative – Low Activation Moodb | −.284 | .288 | 0.98 | 0.75 | 0.43 | 1.32 |
| Negative – High Activation Moodb | −.056 | .282 | 0.04 | 0.95 | 0.54 | 1.65 |
| Positive – Low Activation Moodc | .012 | .296 | 0.00 | 1.01 | 0.57 | 1.81 |
| Positive – High Activation Moodc | −.667 | .232 | 8.30** | 0.51 | 0.33 | 0.81 |
| Confidence to Resist Smokingd | −.364 | .185 | 3.89* | 0.70 | 0.48 | 0.99 |
| Confidence to Resist Drinkinge | −.147 | .136 | 1.17 | 0.86 | 0.66 | 1.13 |
| Urge to Smokef | .392 | .173 | 5.11* | 1.48 | 1.05 | 2.08 |
| Urge to Drinkg | −.033 | .283 | 0.01 | 0.97 | 0.56 | 1.68 |
| Withdrawal Symptomsh | .207 | .380 | 0.30 | 1.23 | 0.58 | 2.59 |
| Drank (Yes/No) | .387 | .824 | 0.22 | 1.47 | 0.29 | 7.40 |
Note.
p < .05;
p < .01;
p < .001
1 Degree-of-freedom tests
Analysis controlled for baseline CES-D depressed affect subscale score
Analysis controlled for baseline POMS vigor score
Analysis controlled for baseline SSET score
Analysis controlled for baseline AASE score
Analysis controlled for baseline QSU desire score
Analysis controlled for baseline PACS score
Analysis controlled for baseline Minnesota withdrawal scale score
Figure 3.
Relation between aggregated high activation mood, confidence to resist smoking, and urge to smoke, and probability of an initial smoking lapse at the next time point. Probability estimates derived from GEE analyses. Higher momentary positive-high activation mood and higher momentary confidence to resist smoking were independently associated with lower probability of an initial smoking lapse occurring at the succeeding time point. Higher momentary urge to smoke was associated with a higher probability of an initial smoking lapse occurring at the succeeding time point.
Distribution Characteristics of Momentary Variables
We found it surprising that those variables related specifically to drinking failed to predict first drink in the monitoring period. One possibility for this is a floor or ceiling effect on the momentary predictor variables. To assess this possibility we looked at the distribution of the drinking-related variables. Table 4 shows the distribution characteristics of the variables aggregated over times and days for those records in the 28-day period that occurred before the first drink for the 29 patients who reported having a drink. Similarly, Table 5 shows the distribution characteristics of the momentary variables aggregated over times and days for those records in the post-quit 14-day period that occurred before the first cigarette for the 32 patients who reported a first cigarette. It is notable that in both tables the lowest mean score was for urge to drink and the highest mean score was for confidence to resist drinking.
Table 4.
Distribution Statistics of Aggregated Momentary Variables Recorded in the Records Prior to First Drink (n=29 events; 658 records)
| Momentary Measure | M | SD | Median | Minimum | Maximum | Skewness |
|---|---|---|---|---|---|---|
| Negative-Low Arousal Mood | 2.26 | .67 | 2.00 | 1.32 | 3.94 | .98 |
| Negative-High Arousal Mood | 2.11 | .66 | 2.05 | 1.09 | 3.23 | .07 |
| Positive-Low Arousal Mood | 2.66 | .52 | 2.62 | 1.75 | 3.88 | .79 |
| Positive-High Arousal Mood | 2.84 | .74 | 2.88 | 1.58 | 4.52 | .27 |
| Urge to Smoke | 3.21 | .95 | 3.18 | 1.62 | 4.89 | −.12 |
| Urge to Drink | 1.62 | .55 | 1.48 | 1.00 | 2.85 | .78 |
| Confidence Resist Smoking | 2.50 | .83 | 2.32 | 1.36 | 4.31 | .46 |
| Confidence Resist Drinking | 3.97 | .82 | 4.19 | 1.54 | 4.95 | −1.21 |
| Withdrawal Symptoms | 2.28 | .53 | 2.40 | 1.42 | 3.17 | −.14 |
Table 5.
Distribution Statistics of Aggregated Momentary Variables Recorded in the Records Prior to First Cigarette (n=32 events; 368 records)
| Momentary Measure | M | SD | Median | Minimum | Maximum | Skewness |
|---|---|---|---|---|---|---|
| Negative-Low Arousal Mood | 2.36 | .78 | 2.50 | 1.00 | 4.50 | .34 |
| Negative-High Arousal Mood | 2.26 | .74 | 2.00 | 1.00 | 4.33 | .76 |
| Positive-Low Arousal Mood | 2.62 | .76 | 2.59 | 1.00 | 4.75 | .51 |
| Positive-High Arousal Mood | 2.65 | .70 | 2.62 | 1.17 | 4.33 | .34 |
| Urge to Smoke | 2.25 | 1.27 | 2.00 | 1.00 | 5.00 | .66 |
| Urge to Drink | 1.51 | .99 | 1.00 | 1.00 | 5.00 | 2.65 |
| Confidence Resist Smoking | 3.32 | 1.31 | 3.00 | 1.00 | 5.00 | −.21 |
| Confidence Resist Drinking | 4.03 | 1.25 | 4.61 | 1.00 | 5.00 | −1.28 |
| Withdrawal Symptoms | 2.32 | .66 | 2.31 | 1.00 | 4.00 | .33 |
Discussion
Most previous research on concurrent alcohol and tobacco treatment has focused on outcomes, as opposed to the processes leading up to lapses and relapses. In addition, studies of smoking and drinking relapse largely have relied on retrospective or distant prospective measures of cognition or affect in predicting a lapse or relapse, or else have treated these variables as enduring, individual difference variables (Shiffman, 1989). The current study sought to address these shortcomings by using EMA methodology over a 28-day period during concurrent alcohol and tobacco treatment to elucidate intrapersonal proximal antecedents to initial smoking and drinking lapses, while simultaneously controlling for baseline measures of the study variables. Possible cross-substance predictions (e.g., smoking urge predicting drinking lapse, drinking predicting smoking lapse) also were tested, as these may be especially salient among individuals in concurrent treatment. Our prospective analysis suggested that smoking relapse was foreshadowed by higher urges to smoke, lower positive high-activation mood, and lower confidence to resist smoking. Drinking lapse also was preceded by lower confidence to resist smoking, but only among individuals who were smoking in the period prior to the drinking lapse.
Abstinence Self-Efficacy
Confidence in one’s ability to resist smoking was a significant predictor of both smoking and drinking lapses. For every 1-point decrease on the 5-point smoking ASE scale, there was a near 30% increase in lapse hazard rate over the base hazard of smoking or drinking lapse. These findings are consistent with the cognitive-behavioral model of relapse and prior research employing EMA methods to examine smoking ASE and smoking relapse. For example, among participants in smoking cessation research clinics, daily smoking ASE predicted first smoking lapse on the next day even after controlling for differences in baseline ASE (Gwaltney et al., 2005). Similarly, Van Zundert et al. (2010) noted that smoking ASE predicted first smoking lapse on the next day, also after controlling for differences in baseline ASE. Collectively, our research and previous research with both treatment-seeking and non-treatment-seeking individuals suggests that proximal declines in smoking ASE are an important and robust predictor of early smoking lapse and relapse.
An unexpected finding in the current study was that smoking ASE, and not alcohol ASE, predicted initial drinking lapses. Furthermore, this association was apparent only among those who were smoking prior to the drinking lapse. In an earlier investigation by our research group, lower alcohol ASE assessed hours earlier predicted initial drinking lapses (smoking ASE was not assessed in that study) (Cooney et al., 2007). Ceiling effects on the alcohol ASE measure, which have been observed in other studies (Cooney et al., 2007; Litt, Cooney, & Morse, 1998), might have precluded our ability to detect a relation between alcohol ASE and drinking lapse. Our finding that declining ASE for one substance predicted lapse to a different substance suggests the possibility that participants were experiencing a generalized decline in personal efficacy or self-control strength prior to their first alcohol lapse. Perhaps the smoking ASE measure, with its better distributional properties, was better at capturing this generalized decline than the drinking ASE measure. The fact that those who stopped smoking evidenced a lower alcohol lapse risk lends confidence in recommending smoking cessation concurrent with alcohol treatment.
Urge to Smoke or Drink
As hypothesized, higher urge to smoke hours earlier predicted smoking lapse. The effect size for smoking urge was sizeable: For every 1-point increase on the 5-point confidence scale, there was a 51% increase in the hazard rate of lapse over the base hazard of smoking lapse. These findings are in line with previous research showing that increases in smoking urge hours earlier predicted first smoking lapse in individuals in concurrent smoking and alcohol treatment (Cooney et al., 2007), and with a study that used EMA methods to collect retrospective information about smoking urge prior to a smoking lapse (Shiffman et al., 1996).
Contrary to our prediction, but in line with a previous study (Cooney et al., 2007), urge to drink was not a significant predictor of drinking lapse. An examination of the distribution of the drinking urge variable revealed possible floor effects, which might have precluded our ability to detect a relation between drinking urge and lapse to one or both substances. Other EMA studies with individuals in treatment also found that the frequency and intensity of alcohol craving was low (Cooney et al., 2007; Krahn, Henk, Grossman, & Gossnell, 2005; Litt, Cooney, & Morse, 2000; Litt et al., 1998; Lukasiewicz, Benyamina, Reynaud, & Falissard, 2005). There are several explanations that might account for low levels of reported drinking urge in this population. First, individuals in alcohol treatment settings may be less apt to experience urges because they are less likely to encounter cues that would trigger drinking urges (Rohsenow, 1999). Since patients in the current study were treated in an outpatient setting, however, it is likely that they would have readily encountered drinking cues outside the clinic. Other possibilities are that EMA methods do not sufficiently capture the experience of drinking urges, that recognizing an urge necessitates a higher level of self-awareness (Rohsenow, 1999) not yet developed among persons in treatment, that automatic processes, rather than conscious alcohol craving, determine initial alcohol relapse (Tiffany, 1990), or that urges (or the recognition of them) increased immediately prior to a lapse episode (Krahn et al., 2005) and thus were not detectable using lagged predictors obtained hours earlier.
Positive and Negative Affect
Low levels of positive high activation mood hours earlier predicted initial smoking lapses. Similar to the findings for smoking ASE and smoking urge, the effect size for positive affect was notable. For every 1-point decrease on the 5-point mood scale, there was a 49% increase in the hazard rate of smoking lapse over the base hazard rate. This finding was consistent with the affective processing model of negative reinforcement, which contends that the resumption of substance use serves to ameliorate low positive affect brought about by drug withdrawal (Baker et al., 2004). Low levels of positive affect may have been experienced as aversive and prompted a lapse, especially if study participants relied on tobacco or alcohol as a means of activating or energizing their mood or behavior. Our findings were not entirely consistent with the model, however, given that the model regards escape from negative affect as the primary motivation for resumption of use, and we did not find evidence of a relation between increasing levels of negative low/high activation mood and lapse. Instead, our findings corresponded more closely to recent research, which has found that low positive affect is associated with an increased risk of smoking relapse (Cook, Spring, McChargue, & Doran, 2010; Doran, 2006; Leventhal et al., 2008).
In the current study, high activation positive mood (i.e. happiness) was protective of smoking lapse, whereas low-activation positive mood, characterized by relaxation and quietness, was not. The literature generally corroborates our findings that high negative mood (Shiffman & Gwaltney, 2008; Oslin et al., 2009), or low positive mood (Strong et al., 2009) are predictive of relapses to substances of abuse. Generally these studies do not distinguish between low and high activation mood states. In the present study, the relative lack of predictive power for low activation positive mood may have been attributable to the lack of distinctiveness between low activation positive mood and negative mood. Whereas high-activation positive mood was strongly negatively correlated with negative mood (r = −.40), low activation positive mood was not (r =−.08). Thus, it may be the case that happiness is more protective than simple relaxation.
Given that the cognitive-behavioral and affective processing models of relapse regard negative affect as one of the key factors in the resumption of substance use, it is surprising that the current study did not find a relation between momentary measures of negative affect and smoking or drinking lapse. Research employing EMA methods to study smoking lapses has found that prospective measures of negative affect (Cooney et al., 2007) and retrospective measures (Shiffman et al., 1996) were associated with smoking lapses. This has not always been the case, however (Shiffman, 2009). The observed relation between baseline negative affect and smoking lapse in the current study was consistent with the findings of Berlin and Covey (2006), who found that pre-cessation depressive symptoms served as a risk factor for smoking relapse. Future research should continue to collect both proximal and distal measures of affect, and should examine changes in both negative and positive affect as possible predictors of lapse.
The cross-substance prediction observed has implications for the provision of concurrent treatment. In light of the observed associated between smoking ASE and drinking lapses in the current study, especially among those who are smoking, it would be advisable to work with persons in concurrent treatment to enhance their confidence to abstain from cigarettes, as well as from drinking. Given the small number of cross-substance interactions and the fact that alcohol use alone did not appear to trigger smoking lapses (and vice versa), concurrent treatment would still be advisable for individuals with alcohol and tobacco dependence.
The current study had numerous strengths, in that it elucidated the extent to which proximal cognitive-behavioral processes were associated with smoking and drinking lapses in a population of individuals rarely studied. Within-subjects analyses were employed, which permitted an examination of how unique changes in intrapersonal variables affected one’s risk of smoking and drinking lapse. Since both baseline measures and EMA lagged predictor variables were included in the analyses, the significant relations between the EMA measures and smoking/drinking lapses reflect unique within-subject variation in the hours prior to a lapse, as opposed to baseline differences between participants.
Several limitations to this study should be noted. First, the sample size was small due to the fact that EMA measures were obtained for only two weeks in the case of smoking lapse and four weeks in the case of drinking lapse and only a small percentage of participants lapsed to one or both substances during that time. However, according to Kreft and DeLeeuw (1998), the power for multilevel models like these is determined by both the number of level 1 (i.e., days) and level 2 (i.e., persons) units. Although the number of participants was relatively small, a large number of records (N=1026) was examined, providing for large power. Second, we used single item measures of urge and confidence to abstain, which might account for limited variability observed in the alcohol urge and alcohol abstinence confidence variables. More extensive EMA measures of confidence to abstain from drinking and urge to drink should be considered for future research. Third, compliance with the EMA protocol was somewhat lower than previous EMA studies of persons in tobacco treatment, but was comparable to studies with persons in alcohol treatment (Cooney et al., 2007).
Data from this subset of individuals may not generalize to all people in concurrent treatment, especially those who experience an initial lapse later in treatment or following the conclusion of treatment. Moreover, the brief EMA monitoring period only allows for an examination of the initial lapses, as opposed to an examination of processes that are involved in the progression from lapse to relapse. A longer monitoring period would permit a more comprehensive examination of the relapse process. Finally, the cognitive-behavioral model of relapse assigns a causal role to ASE, mood, and urges, but this study did not experimentally manipulate these processes, so it cannot be assumed that process changes caused smoking and drinking lapses.
Reactivity to the EMA measures could have affected participants’ reports of the variables under study. However, research largely has shown that while reactivity to EMA methods is possible (Rowan et al., 2007), it may be more common when the phenomenon of interest is a behavior (as opposed to a cognition), specifically one that an individual wishes to alter (Shiffman et al., 2008). Moreover, several EMA studies have detected little to no reactivity among substance users (Hufford, Shields, Shiffman, Paty, & Balabanis, 2002; Litt et al., 1998; Simpson, Kivlahan, Bush, & McFall, 2005). A review by Barta, Tennen and Litt (in press) concluded that when multiple behaviors and cognitions are monitored, and demand for change is minimized, reactivity to monitoring is reduced. Although our study design did not allow us to assess for reactivity, research would suggest that it did not considerably alter the findings.
This is the second study conducted by our research group that examined proximal antecedents to relapse in the context of concurrent alcohol and tobacco treatment. The first study (Cooney et al., 2007) also used randomly-timed signal contingent EMA assessments to examine lag predictors of smoking and drinking relapse. However, in the earlier study, EMA data collection began after the completion of a three-week intensive outpatient alcohol and tobacco treatment, with participants reporting a mean of 28 days alcohol abstinence at the outset of EMA collection, as compared to the present study with EMA data collection beginning at the very start of low intensity (weekly sessions) outpatient treatment. Also, the earlier study did not assess smoking abstinence self-efficacy, and the analysis did not control for baseline measures of the lagged predictor variables. In spite of these differences, both studies found that urge to smoke predicted smoking lapse and both found evidence for cross-substance predictive relations. The earlier study found that urge to smoke predicted both smoking and alcohol lapse while the present study found smoking abstinence self-efficacy predicted both smoking and alcohol lapse. Taken together, these two studies suggest that relapse theory needs to consider cross-substance processes in the context of concurrent treatment of multiple addictive behaviors. Advancing the research on the experiences of people in concurrent treatment may lead to more efficacious treatments and perhaps more widespread implementation of this treatment approach.
Acknowledgments
The project described was supported by award number R01 AA011197 from the National Institute on Alcohol Abuse and Alcoholism and by a Mental Illness Research, Education and Clinical Centers (MIRECC) award from the Department of Veterans Affairs. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institutes of Health, or the Department of Veterans Affairs. Ned Cooney has a family member who worked as a promotional speaker for Pfizer, Inc.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/adb.
Contributor Information
Laura J. Holt, Department of Psychology, Trinity College, Hartford, CT
Mark D. Litt, Division of Behavioral Sciences and Community Health, University of Connecticut Health Center, Farmington, CT
Ned L. Cooney, Yale University School of Medicine & VA Connecticut Healthcare System, Newington, CT
References
- Ameringer KJ, Leventhal AM. Applying the tripartite model of anxiety and depression to cigarette smoking: An integrative review. Nicotine & Tobacco Research. 2010;12:1183–1194. doi: 10.1093/ntr/ntq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Barta WD, Tennen H, Litt M. Measurement reactivity in diary research. In: Mehl MM, Conner TS, editors. Handbook of research methods for studying daily life. New York: Guilford Press; (in press) [Google Scholar]
- Berlin I, Covey LS. Pre-cessation depressive mood predicts failure to quit smoking: the role of coping and personality traits. Addiction. 2006;101:1814–1821. doi: 10.1111/j.1360-0443.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- Borland R. Slip-ups and relapses in attempts to quit smoking. Addictive Behaviors. 1990;15:235–245. doi: 10.1016/0306-4603(90)90066-7. [DOI] [PubMed] [Google Scholar]
- Cook J, Spring B, McChargue D, Doran N. Effects of anhedonia on days to relapse among smokers with a history of depression: A brief report. Nicotine & Tobacco Research. 2010;12:978–982. doi: 10.1093/ntr/ntq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Cooney JL, Perry BL, Carbone M, Cohen EH, Steinberg HR, et al. Smoking cessation during alcohol treatment: A randomized trial of combination nicotine patch plus nicotine gum. Addiction. 2009;104:1588–1596. doi: 10.1111/j.1360-0443.2009.02624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Cooney JL, Pilkey DT, Steinburg HR, Oncken CA. Alcohol and tobacco cessation in alcohol-dependent smokers: Analysis of real-time reports. Psychology of Addictive Behaviors. 2007;21:277–286. doi: 10.1037/0893-164X.21.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daeppen J, Smith TL, Danko GP, Gordon L, Landi NA, Nurnberger JI, et al. Clinical correlates of cigarette smoking and nicotine dependence in alcohol-dependent men and women. Alcohol and Alcoholism. 2000;35:171–175. doi: 10.1093/alcalc/35.2.171. [DOI] [PubMed] [Google Scholar]
- DiClemente CC, Carbonari JP, Montgomery RPG, Hughes SO. The alcohol abstinence self-efficacy scale. Journal of Studies on Alcohol. 1994;55:141–148. doi: 10.15288/jsa.1994.55.141. [DOI] [PubMed] [Google Scholar]
- Doran N, Spring B, Borrelli B, McChargue D, Hitsman B, Niaura R, Hedeker D. Elevated positive mood: A mixed blessing for abstinence. Psychology of Addictive Behaviors. 2006;20:36–43. doi: 10.1037/0893-164X.20.1.36. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear, Nose and Throat Journal. 1991;69:763–765. [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. Effect of high-dose nicotine patch on the characteristics of lapse episodes. Health Psychology. 2010;29:358–366. doi: 10.1037/a0019367. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ. Treating tobacco use and dependence: 2008 update. Public health service clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the penn alcohol craving scale. Alcoholism: Clinical and Experimental Research. 1999;23:1289–1295. [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman S, Balabanis MH, Paty JA. Dynamic self-efficacy and outcome expectancies: Prediction of smoking lapse and relapse. Journal of Abnormal Psychology. 2005;114:661–675. doi: 10.1037/0021-843X.114.4.661. [DOI] [PubMed] [Google Scholar]
- Hodgins DC, el-Guebaly N, Armstrong S. Prospective and retrospective reports of mood states before relapse to substance use. Journal of Consulting and Clinical Psychology. 1995;63:400–407. doi: 10.1037//0022-006x.63.3.400. [DOI] [PubMed] [Google Scholar]
- Hufford MR, Shields AL, Shiffman S, Paty J, Balabanis M. Reactivity to ecological momentary. assessment: An example using undergraduate problem drinkers. Psychology of Addictive Behaviors. 2002;16:205–211. [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Kalman D. Do smokers with alcohol problems have more difficulty quitting? Drug and Alcohol Dependence. 2006;82:91–102. doi: 10.1016/j.drugalcdep.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, et al. Mortality following inpatient addictions treatment: Role of tobacco use in a community-based cohort. JAMA: Journal of the American Medical Association. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Istvan J, Matarazzo JD. Tobacco, alcohol, and caffeine use: A review of their interrelationships. Psychological Bulletin. 1984;95:301–326. [PubMed] [Google Scholar]
- Joseph AM, Willenbring ML, Nugent SM, Nelson DB. A randomized trial of concurrent versus delayed smoking intervention for patients in alcohol dependence treatment. Journal of Studies on Alcohol. 2004;65:681–691. doi: 10.15288/jsa.2004.65.681. [DOI] [PubMed] [Google Scholar]
- Kenny DA, Zautra A. The trait-state-error model for multiwave data. Journal of Consulting and Clinical Psychology. 1995;63:52–59. doi: 10.1037//0022-006x.63.1.52. [DOI] [PubMed] [Google Scholar]
- Kodl M, Fu SS, Joseph AM. Tobacco cessation treatment for alcohol-dependent smokers: When is the best time? Alcohol Research & Health. 2006;29:203–207. [PMC free article] [PubMed] [Google Scholar]
- Krahn DD, Bohn MJ, Henk HJ, Grossman JL, Gosnell B. Patterns of urges during early abstinence in alcohol-dependent subjects. The American Journal on Addictions. 2005;14:248–255. doi: 10.1080/10550490590949424. [DOI] [PubMed] [Google Scholar]
- Kreft I, de Leeuw J. Introducing multilevel modeling. New York: Sage Publications; 1988. [Google Scholar]
- Larsen RJ, Diener E. Promises and problems with the circumplex model of emotion. In: Clark MS, editor. Emotion (Review of personality and social psychology. Vol. 13. Newbury Park, CA: Sage Publications; 1992. [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA: Journal of the American Medical Association. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. Nicotine & Tobacco Research. 2008;10:507–517. doi: 10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Cooney NL, Morse P. Ecological momentary assessment (EMA) with treated alcoholics: Methodological problems and potential solutions. Health Psychology. 1998;17:48–52. doi: 10.1037//0278-6133.17.1.48. [DOI] [PubMed] [Google Scholar]
- Litt MD, Cooney NL, Morse P. Reactivity to alcohol-related stimuli in the laboratory and in the field: Predictors of craving in treated alcoholics. Addiction. 2000;95:889–900. doi: 10.1046/j.1360-0443.2000.9568896.x. [DOI] [PubMed] [Google Scholar]
- Littleton J, Barron S, Prendergast M, Jo Nixon S. Smoking kills (alcoholics)! Shouldn't we do something about it? Alcohol and Alcoholism. 2007;42:167–173. doi: 10.1093/alcalc/agm019. [DOI] [PubMed] [Google Scholar]
- Lowman C, Allen J, Stout RL. Replication and extension of Marlatt's taxonomy of relapse precipitants: Overview of procedures and results. Addiction. 1996;91:S51–S71. [PubMed] [Google Scholar]
- Lukasiewicz M, Benyamina A, Reynaud M, Falissard B. An in vivo study of the relationship between craving and reaction time during alcohol detoxification using the ecological momentary assessment. Alcoholism: Clinical and Experimental Research. 2005;29:2135–2143. doi: 10.1097/01.alc.0000191760.42980.50. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR, editors. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. 1st ed. New York: Guilford Press; 1985. [Google Scholar]
- McNair DM, Lorr M, Droppleman LF, editors. Manual for the Profile of Mood States. San Diego, CA: EdITS/Educational and Industrial Testing Service; 1992. p. 23. [Google Scholar]
- Oslin DW, Cary M, Slaymaker V, Colleran C, Blow FC. Daily ratings measures of alcohol craving during an inpatient stay define subtypes of alcohol addiction that predict subsequent risk for resumption of drinking. Drug & Alcohol Dependence. 2009;103:131–136. doi: 10.1016/j.drugalcdep.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National health interview survey 2008. Vol. 10. Washington DC: National Center for Health Statistics; 2009. p. 242. [PubMed] [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. Journal of Consulting and Clinical Psychology. 2004;72:1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rohsenow DJ, Monti PM. Does urge to drink predict relapse after treatment? Alcohol Research & Health. 1999;23:225–232. [PMC free article] [PubMed] [Google Scholar]
- Rowan PJ, Cofta-Woerpel L, Mazas CA, Vidrine JI, Reitzel LR, Cinciripini PM, et al. Evaluating reactivity to ecological momentary assessment during smoking cessation. Experimental and Clinical Psychopharmacology. 2007;15:382–389. doi: 10.1037/1064-1297.15.4.382. [DOI] [PubMed] [Google Scholar]
- SAS. SAS/STAT software: Changes and enhancements through V7 and V8. Cary, NC: SAS Institute; 1999. [Google Scholar]
- Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. Journal of Clinical Psychology. 2006;62:123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- Shiffman SM. Conceptual issues in the study of relapse. In: Gossop M, editor. Relapse and addictive behaviour. New York: Tavistock/Routledge; 1989. pp. 149–179. [Google Scholar]
- Shiffman S. Dynamic influences on smoking relapse process. Journal of Personality. 2005;73:1715–1748. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment. 2009;21:486–497. doi: 10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Balabanis MH, Paty JA, Engberg J, Gwaltney CJ, Liu KS, et al. Dynamic effects of self-efficacy on smoking lapse and relapse. Health Psychology. 2000;19:315–323. doi: 10.1037//0278-6133.19.4.315. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hufford M, Hickcox M, Paty JA, Gnys M, Kassel JD. Remember that? A comparison of real-time versus retrospective recall of smoking lapses. Journal of Consulting and Clinical Psychology. 1997a;65:292–300. doi: 10.1037/0022-006x.65.2.292.a. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, et al. A day at a time: Predicting smoking lapse from daily urge. Journal of Abnormal Psychology. 1997b;106:104–116. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ. Does heightened affect make smoking cues more salient? Journal of Abnormal Psychology. 2008;117:618–624. doi: 10.1037/0021-843X.117.3.618. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: A prospective analysis. Journal of Consulting and Clinical Psychology. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Kivlahan DR, Bush KR, McFall ME. Telephone self-monitoring among alcohol use disorder patients in early recovery: A randomized study of feasibility and measurement reactivity. Drug and Alcohol Dependence. 2005;79:241–250. doi: 10.1016/j.drugalcdep.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Strong DR, Kahler CW, Leventhal AM, Abrantes AM, Lloyd-Richardson E, Niaura R, Brown RA. Impact of bupropion and cognitive-behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine & Tobacco Research. 2009;11:1142–1153. doi: 10.1093/ntr/ntp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Van Zundert, Rinka MP, Ferguson SG, Shiffman S, Engels RCME. Dynamic effects of self-efficacy on smoking lapses and relapse among adolescents. Health Psychology. 2010;29:246–254. doi: 10.1037/a0018812. [DOI] [PubMed] [Google Scholar]
- Velicer WF, DiClemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: An integrative model. Addictive Behaviors. 1990;15:271–283. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: That was zen this is tao. American Psychologist. 2004;59:224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- Zhou X, Nonnemaker J, Sherrill B, Gilsenan AW, Coste F, West R. Attempts to quit smoking and relapse: Factors associated with success or failure from the ATTEMPTS cohort study. Addictive Behaviors. 2009;34:365–373. doi: 10.1016/j.addbeh.2008.11.013. [DOI] [PubMed] [Google Scholar]