Abstract
The locus of attentional selection is known to vary with perceptual load (Lavie et al., 2004). Under voluntary attention, perceptual load modulates selective visual processing at an early cortical stage, as reflected in the posterior P1 and N1 components of the event-related potentials (ERPs). Adult aging also affects both behavioral and ERP signs of attentional selection. However, it is not known whether perceptual load modulates this relationship. Accordingly, in the present study ERPs were recorded in a voluntary attention task. Young and old participants were asked to discriminate the direction of a target line embedded within a display of four lines that appeared in the left or right visual field. Participants responded faster and more accurately to valid relative to invalid trials and to low-load relative to high-load condition. Older participants responded more slowly and with lower accuracy than young participants in all conditions. The amplitudes of the posterior contralateral P1 and N1 components in valid trials were larger than that in invalid trials in all conditions. N1 amplitude was larger under the high load condition than that in the low load condition. Moreover, in the high perceptual load condition, the old group had a larger N1 than the young group at contralateral sites. The findings suggest that under voluntary attention, perceptual load and aging modulates attentional selection at an early but not the earliest stage, during the N1 (120–200ms) time range. Increased N1 amplitude in older adults may reflect increased demands on target discrimination in high perceptual load.
Keywords: Event-related potentials, aging, perceptual load, voluntary attention
1. Introduction
Allocation of voluntary attention to a cued spatial location facilitates the processing of a target presented at that location, typically leading to faster and more accurate responses (Müller & Rabbitt, 1989; Posner, 1980). Such performance enhancement can occur even when attention is shifted covertly to the location, without eye movements (Fu et al., 2008; Mishra & Hillyard, 2009). Posner (1980) interpreted this effect as reflecting enhanced sensory processing of the attended target.
Event-related potential (ERP) studies have supported this interpretation by showing that stimulus-evoked neural activity is greater on validly cued trials compared to invalid or neutral cues (Mangun, 1995). ERP amplitude modulation occurs for early-latency components, typically for P1 (100–130ms) and N1 (120–200ms) and when attention is shifted to the cued location either voluntarily (Fu et al., 2008; Mishra & Hillyard, 2009) or involuntarily (Fu et al., 2001; Fu et al., 2005; for a review, see Eimer & Driver, 2001). Consistent with Posner’s (1980) view, P1 and N1 enhancement with spatial attention has been interpreted within a sensory gain control hypothesis (Hillyard et al., 1998). However, Doallo et al. (2004) only found the cue validity effect on P1 component under a peripheral cueing (involuntary attention) condition with short SOAs and not when informative-central cueing (voluntary attention) was provided. They suggested that central cueing may not affect sensory processing at the P1 level.
The P1 component, starting around 100 ms after stimulus onset, is generally thought to be the first ERP component influenced by attention (Heinze et al., 1994; Mangun et al., 1997), and it may reflect a mechanism that enhances perceptual processing of attended stimuli (Doallo et al., 2004). Neuroimaging studies (Heinze et al., 1994; Mangun et al., 1997) have provided evidence that the P1 component was generated in the ventral lateral occipital area within extrastriate cortex (the fusiform gyrus and surrounding areas). An earlier-latency component, C1, which is thought to be generated within primary visual or striate cortex, has been found in most studies to be unaffected by attention (Clark & Hillyard, 1996; Mangun & Buck, 1998; Mangun & Hillyard, 1991). In a spatially-cued task under high and low levels of attentional load, Fu et al., (2010) found that P1 was enhanced by increasing attentional load, whereas C1 was not. The term “attentional load” usually refers to the processing of the same stimulus (Rauss et al., 2009) and differs from “perceptual load” which refers to the processing of different (target and distracter) stimuli (Lavie, 1995). However, monkey single-unit (Motter, 1993; Schroeder et al., 1994) and fMRI studies (Gandhi et al., 1999; Hopf et al., 2004) have shown that neural activity in striate cortex can be attention sensitive. Some recent ERP studies have also provided evidence that C1 is attention sensitive, but only under certain conditions (Fu et al., 2009; Kelly et al., 2008; Rauss et al., 2009), although it is unclear whether this effect reflects a true C1 enhancement, given the temporal overlap between the C1 and P1 components (Fu et al., 2008). Thus, the current general consensus from ERP studies is that extrastriate cortex as indexed by the P1 component represents the earliest cortical stage at which visuospatial attention reliably and consistently influences brain activity. In visual spatial attentional tasks the posterior N1 component always appears around 160 ms after stimuli onset (Vogel & Luck, 2000). Although P1 and N1 are both sensitive to cue validity effects, they may reflect different mechanisms (Doallo et al., 2005; Luck et al., 1994). N1 could reflect enhanced processing of stimuli at the attended location (Luck et al., 1994; Luck and Hillyard, 1995) and also could reflect a limited-capacity process (Heinze et al., 1990). Most importantly, N1 may be related to a visual discriminative process at attended locations (Mangun and Hillyard, 1991; Vogel and Luck, 2000) associated with top-down modulation of discriminative processing.
The influence of visuospatial attention on behavior and neural activity is known to be affected by adult aging (Bennett et al., 2004; Greenwood & Parasuraman, 2003; 2004). However, whether the locus of attentional selection differs between young and older adults is not well understood. Lorist et al. (1995) used a selective search task to examine the effects of aging on ERPs to relevant and irrelevant stimuli. They found that the latency of a late negative ERP component (N1) was longer in an older group than in young adults, with an average delay of 25 ms. Kok (2000) provided an overview of age-related changes in ERPs in both voluntary and involuntary attention tasks. He concluded that old participants exhibited slower selection negativity than young participants, especially in tasks with complex stimulus features, and also were more strongly distracted by irrelevant peripheral stimuli than the young. These results indicate a decline of attentional orienting for older adults in complex attention tasks. The P1 and N1 components for both auditory and visual tasks were larger in the old group than in the young (Kok, 2000; Kok and Zeef, 1991; Pfefferbaum et al. 1980; Smith et al. 1980a,b) especially when the stimuli were more intense (Dustman and Snyder, 1981; Kramer et al., 1996), suggesting that there was an increase in mental effort in older adults when they have to perform complex tasks. Other ERP studies (Bashore, 1990; Strayer et al., 1987) have shown that the effects of aging mainly occurred at the stimulus encoding and response-related stages of information processing. In a visual search task, Lorenzo-López et al. (2008) found that the latency of N2pc component was smaller and approximately 68 ms later in old than in young group. As the N2pc amplitude was considered as index of attention focusing, they suggested a reduction in attentional processing resources for old adults. Moreover, the N2pc sources were located in occipito-temporal regions in a study using magnetoencephalography (MEG) (Lorenzo-López et al., 2011), in which greater activity was found in the young than in the old group, especially in the right hemisphere.
Age related slowing is also found in both visual search and Stroop tasks (Bélanger et al., 2010; Lorist, 1995), and is often interpreted as reflecting generalized slowing (Cerella, 1985). This hypothesis states that older adults have proportionately longer reaction times than the young, with the degree of slowing being linearly related to task complexity. However, other studies have not always supported this point of view (e.g., as in a study using a response competition paradigm, Hahn & Kramer, 1995).
These variable findings concerning ERP correlates of attention in young and older adults could reflect task differences that affect the locus of attentional selection. Lavie and colleagues (1994, 2004) have proposed that the temporal stage at which selection operates can vary, depending on perceptual load. They suggested that a low perceptual load favors late selection whereas early selection occurs only when the perceptual load is sufficiently high. The perceptual load in their studies not only indicated the difficulty of the tasks but also described conditions in which qualitatively different or additional processing of the stimuli was required. According to this view, when late selection occurs, perception is an unlimited process, so the limit will not be exceeded and perceptual analyses of relevant and irrelevant stimuli occur in parallel, so that distractor processing is achieved by sufficient attentional resources. When early selection is required, however, perception is a limited-capacity process, and distractor processing is reduced. Maylor & Lavie (1998) tested the effects of perceptual load on selective attention with different ages, and suggested that processing capacity decreased with age under low perceptual load conditions. In contrast, Madden & Langley (2003) provided a different view to the perceptual load theory. They conducted three experiments to test age differences in a selective attention task under different levels of perceptual load. In experiment 1 and 2, they found that there was no age difference under low perceptual load in a resource limited condition. Only under a data-limited condition (they changed the stimulus features, shortened the display during time, and added one singleton stimuli), there was age-related decline in selective attention. They suggested that this age difference may depend on the feature of the stimulus and could not be linked per se to attentional capacity limitations.
A study by Fu and colleagues (2008) specifically examined the interactive effects of perceptual load on ERP correlates of visuospatial attention in young adults. Perceptual load was manipulated by varying the demand for figure-ground segregation. They used a central cued-attention task with two different perceptual load conditions. In the high perceptual load condition, the target (left or right sloping diagonal line) was presented with a complex background of horizontal and vertical lines, making figure-ground segregation difficult; in the low perceptual load condition the non-diagonal lines were cut into small segments, which made the target more distinct from the background. They found that the interaction between perceptual load and attention was significant for the N1 (190 ms), compared with low perceptual load condition the attentional effect was more pronounced under high perceptual load condition. However, the interaction was not significant for the earlier P1 (110 ms) and C1 (84 ms) components, suggesting that perceptual load influenced the attentional effect at an early stage, but not before the discrimination stage as indexed by the N1 component (Vogel & Luck, 2000).
In the present study we used a perceptual load manipulation similar to the Fu et al. (2009) study to examine the effects of adult aging on ERP correlates of visuospatial attention. Based on previous research, we expected that attentional effects would be observed for the P1 and N1 components (Fu et al., 2008; Mangun, 1995; Mishra & Hillyard, 2009). We also hypothesized that the interaction of perceptual load and attention would be greater in older than in younger adults and would be primarily reflected in the N1 component, based on the results of Fu et al. (2008) and given that older adults show greater difficulty in target discrimination under high perceptual load. Given that the N1 component reflects the effort that individuals have to exert to discriminate targets from distractors (Vogel & Luck, 2000), we also predicted that N1 would be larger in older than in younger adults.
2. Methods
2.1. Participants
Two groups of healthy participants were tested, young and old. The young group consisted of 17 graduate and undergraduate students (aged 19–28 years) at George Mason University. The old group included 17 healthy older adults aged 65–73. They were recruited through advertisements in newspapers. The data from three young participants and three old participants were discarded because of low accuracy rate, eye movements, or excessive artifacts in the EEG, leaving 14 participants in each group. The fourteen young participants included 10 females and 4 males, aged between 19–28 years old (22.1±2.9 years). All were right handed, had normal or corrected to normal vision (SNELLEN eye chart: 20/19.5, ROSENBAUM eye chart: 20/20.4), with a mean Wide Range Achievement Test - 3rd Ed. (WRAT3) (Wilkinson, 1993) of 52±2.8, mean Wechsler Memory Scale (WMS) (Wechsler, 1987) scores 11.5±3.7 (immediate) and 11±3.7 (delayed). The fourteen older participants included 6 females and 8 males, aged between 65–73 years old (68.6 ± 4.7 years), all were right handed, with corrected vision (SNELLEN eye chart: 20/24.4, ROSENBAUM eye chart: 20/23.3). The old group had a mean Mini Mental score (Folstein et al., 1975) (available for 10 out of 14 participants) of 29.2±1 and a WRAT3 score of 52.8±4.09. The mean WMS scores were 11.3±2.4 for immediate recall and 9.4±2.8 for delayed recall.
2.2. Stimuli
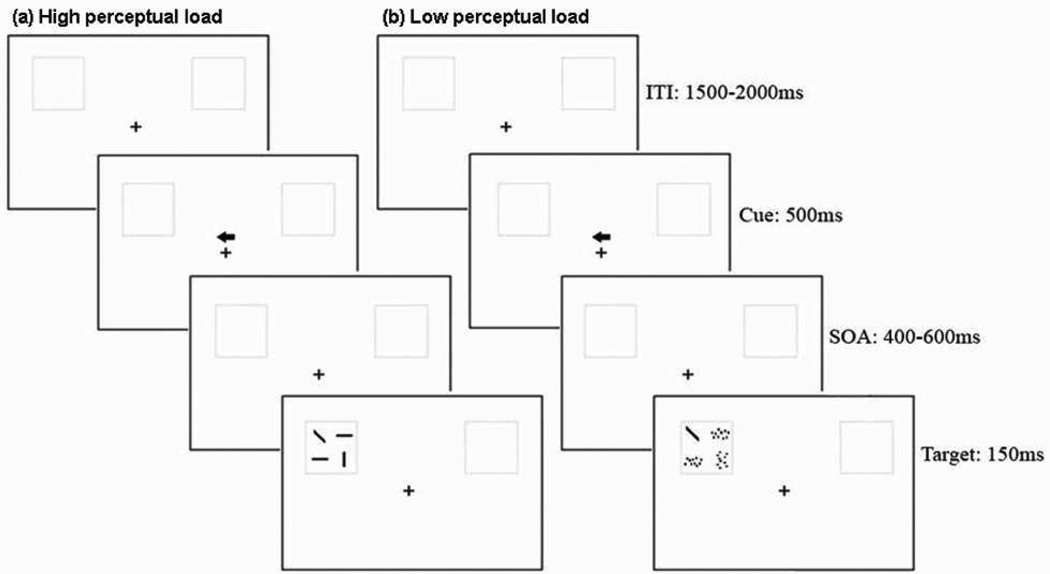
The stimuli for the visual voluntary attention task were presented using a Pentium 4 computer. The background of the display consisted of a central fixation cross (0.66°×0.66°) and two grey square boxes (3.44°×3.44°) on a white screen, with their center 6.06° lateral to and 2.62° above the fixation cross. There were two kinds of conditions: high perceptual load condition and low perceptual load condition. In the high perceptual load condition the visual search array (2.78°×2.78°) had four lines: two horizontal (“—”), one vertical (“|”), and one diagonal (it was the target line and it can be backward “\” or forward “/”). In the low load search condition, the non-target lines (horizontal and vertical lines) in the visual search array were cut into ten pieces, and these small pieces were scrambled in their respective quadrant. This made it easier for participants to find the target-diagonal line. The high-load and low-load search arrays were presented equally frequently in the experiment, and all search arrays appeared randomly in the left and right grey square boxes. The diagonal line (backward “\” and forward “/”with equal probability) could appear randomly at any one of the four quadrants of the grey square box. Each trial contained a predictive endogenous cue (75% validity). The cue was an arrow (1.1°×1.1°) which was presented 0.98° above the fixation cross (Fig.1). The luminance values of the screen background (24.2 cd/m2) and the stimuli (0.05 cd/m2) were the same between the low-load and high-load conditions.
Fig.1.
The procedure of the experimental paradigm, valid cue trials in high perceptual load condition (a), and low perceptual load condition (b).
2.3. Procedure
After signing consent form participants were tested for their vision using the Rosenbaum Pocket Vision Screener and the Snellen eye chart. The minimum visual acuity was at least 20/40. The WRAT3 and WMS tests were administered to all participants, while the Mini Mental test was given only to the old participants. Sufficient practice was provided to make sure that all participants’ accuracy rate could reach more than 80%.
The EEG test lasted approximately 1.5 hours. A total of 52 blocks were included in the task, each consisting of 32 trials. Participants could have a rest after each block, and the length of rest time was under their control. Each trial consisted of the following sequence (Fig.1). During the test, a fixation cross and two grey boxes were presented continuously on the computer screen. Each trial began with an arrow (representing an endogenous spatial cue) flashed above the fixation cross, randomly pointing to the left or right side. The arrow cue was displayed for 500ms, with 75% of the cues being valid and 25% invalid. After the cue, the visual search array (including the diagonal target line) was presented for 150 ms, with the stimulus onset asynchrony (SOA) between cue and target varied randomly in the range of 400–600ms. The inter-trial interval (ITI) was varied randomly between 1500–2000 ms.
After the EEG cap was fitted and prepared, participants were seated in a dimly lit, electrically isolated room, at about 70 cm in front of the monitor. During the test, they were required to sit in a relaxed position, fix their eyes on the cross in the center of the screen and limit eye blinks and body movements. When the search array appeared on the screen, they were required to respond to all the targets as quickly and accurately as possible. When the backward line “\” appeared, they need to use their right index finger to press the key “J” on the keyboard, and when the forward line “/” appeared, they need to use their left index finger to press the key “F”. Response time, accuracy, and EEG data were recorded.
2.4. ERP recording
The electroencephalogram (EEG) was recorded from 26 sites (M1, M2, F3, F4, F7, F8, C3, C4, Cz, CPz, Fz, P7, P8, P3, P4, Pz, PO7, PO8, PO5, PO6, POz, CB1, CB2, O1, O2, Oz) from 64 Ag/AgCl electrodes mounted in an elastic cap (Neuroscan Inc.). The physical reference electrode was approximately 2 cm posterior to CZ, and the EEG data were re-referenced to the average of left and right mastoid (M1 and M2). The vertical electrooculogram (VEOG) was recorded with electrodes placed above and below the left eye. The horizontal electro-oculogram (HEOG) was monitored by placing two electrodes 10 mm from the outer canthi of both eyes. All inter-electrode impedances were maintained below 5 kΩ. Signals were online digitized at 500 Hz, and band-pass filtered between 0.1 and 40 Hz. Reaction times were on-line recorded during the task.
2.5. Data analysis
Behavioral data were analyzed by means of repeated measures analysis of variance (ANOVA). Reaction times (RTs) were online recorded for all the participants. The hit rates and the RTs with correct responses were used for data analysis. The independent variables were cue validity (valid and invalid), perceptual load (low and high) and age group (young and old). Reaction times longer than 1500 ms were excluded from the data analysis.
The EEG data were online recorded and were digitally filtered with a 30 Hz low pass and ocular artifacts were removed from the EEG signal using a regression procedure implemented in the Neuroscan software (Semlitsch et al., 1986). The averaging epoch started 200ms before the target and ended 600ms after the stimulus. Only correct response trials were included in the ERP averages. Trials with response errors, body movements, or muscle activity were excluded from the analysis. The EEGs were averaged separately for all combinations of task conditions. The target ERPs were averaged separately from the time point of their onset. Peak amplitudes and peak latencies were used for statistical analyses. The time windows for C1, P1 and N1 components were 60–88 ms, 90–162 ms, and 150–204 ms, respectively. We selected the specific time windows for each ERP component by visual inspection of ERP grand averages. Repeated measures ANOVAs were applied to different components in the ERP data analysis. The factors were cue validity (valid and invalid), perceptual load (low and high), age group (young and old), visual field (left and right), hemisphere (left and right), and electrode site (8 sites for C1, P1 and N1: P3, P4, P7, P8, PO5, PO6, PO7, PO8). The Greenhouse–Geisser correction was used to compensate for sphericity violations. Post hoc analyses were conducted to explore interaction effects.
3. Results
3.1. Behavior
Young participants reacted more quickly and accurately than the old [F(1,13)=8.6, P<0.05; F(1,13)=10.58, P<0.01] (Table 1). The cue validity effect was significant, with all participants reacting more quickly and accurately with valid than with invalid cues [F(1,13)=72.9, P<0.001; F(1,13)=9.6, P<0.01]. Participants also had faster reaction times and were more accurate in the low perceptual load condition than in the high perceptual load condition [F(1,13)=216.94, P<0.001; F(1,13)=21.79, P<0.001].
Table.1.
Average and standard deviation of RT and accuracy rate for young and old groups in high perceptual load valid cue trial condition (HV), high perceptual load invalid cue trial condition (HI), low perceptual load valid cue trial condition (LV), and low perceptual load invalid cue trial condition (LI)
| HV | HI | LV | LI | |
|---|---|---|---|---|
| Reaction Time (ms) | ||||
| Young | 615.44±48.3 | 661.59±77.22 | 506.77±38.31 | 558.86±57.38 |
| Old | 759.67±147.46 | 780.29±154.58 | 592.39±98.67 | 638.99±122.94 |
| Accuracy Rate (%) | ||||
| Young | 98.27±1.6 | 94.96±8.2 | 98.73±1 | 98.25±1.7 |
| Old | 92.22±4.8 | 89.38±7.9 | 96.13±3.4 | 95.68±3.4 |
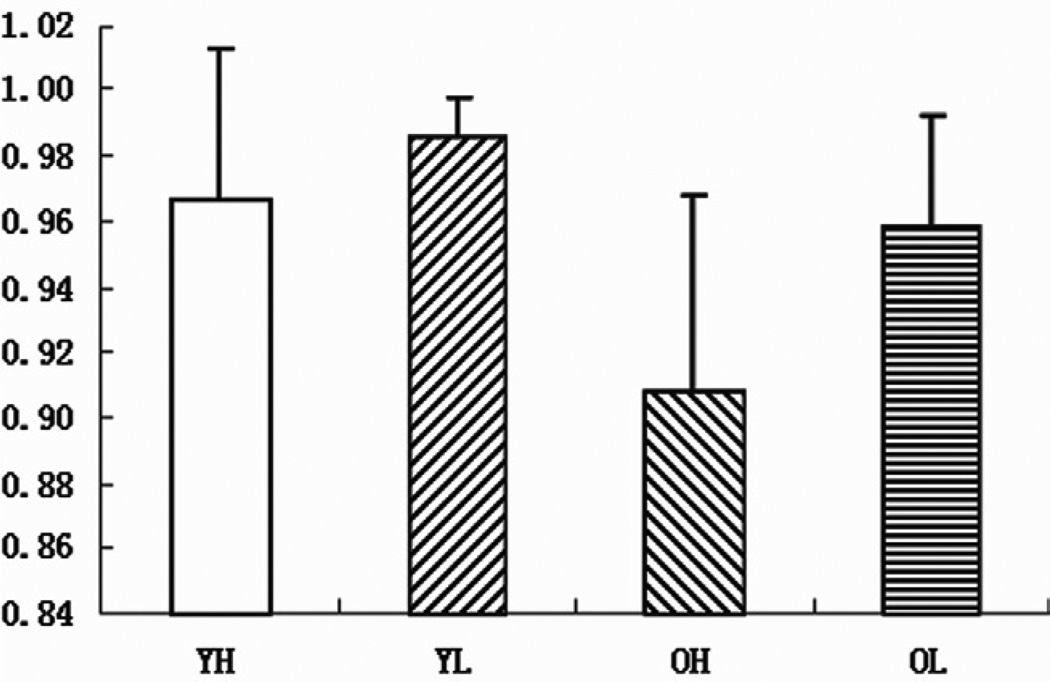
The interaction between perceptual load and validity was significant for both accuracy [F(1,13)=6.27, P<0.05] and reaction time [F(1,13)=6.23, P<0.05]. The highest accuracy rate and the shortest reaction time were found in the low perceptual load and valid cue trials condition [F(1,13)=6.42, P<0.05; F(1,13)=31.99, P<0.0001]. The interaction between age and perceptual load was also significant for reaction time [F(1,13)=8.05, P<0.05]; young participants reacted more quickly than old participants in both high and low perceptual load conditions [F(1,13)=9.4, P<0.01; F(1,13)=7.01, P<0.05]. The most accurate and quickest response was in the young group in the low perceptual load condition [F(1,13)=11.44, P<0.005; F(1,13)=7.01, P<0.05]. Perceptual load had a greater influence on accuracy rate in the old group than in young group [F(1,13)=19.42, P<0.001] (Fig.2).
Fig.2.
Mean accuracy rate and standard errors for young group in the high (YH) and low (YL) perceptual load conditions, and for old group in the high (OH) and low (OL) perceptual load conditions.
3.2. ERPs elicited by the target
3.2.1. C1
For C1 amplitude, the main effect of validity was significant [F(1,13)=6.19, P<0.05]. Invalid cues elicited a larger C1 component than valid cues. The validity × visual field × hemisphere interaction was significant [F(1,13)=11.44, P<0.005], indicating that the cue validity effect differed between contralateral and ipsilateral electrode sites. To analyze the interaction, a simple effect test was carried out on the combined visual field/hemisphere data (ipsilateral vs. contralateral). This analysis showed that for the ipsilateral condition, targets on invalid trials elicited more negative C1 than valid trials [F(1,13)=11.6, P<0.005] (−0.77±0.047µV, −1.19±0.043µV) (Table.2). No other effects were significant.
Table.2.
The mean amplitude and latency of C1 component (average ± SD)
| Contralateral | Ipsilateral | |||||
|---|---|---|---|---|---|---|
| Valid | Invalid | Valid | Invalid | |||
| C1 amplitude (µV) | Young | Low | −0.44±0.62 | −0.45±0.7 | −0.87±1.05 | −1.36±0.87 |
| High | −0.52±0.54 | −0.39±0.5 | −0.83±1.11 | −1.29±0.58 | ||
| Old | Low | −0.42±0.63 | −0.63±0.91 | −0.68±0.7 | −0.93±0.85 | |
| High | −0.32±0.91 | −0.48±0.58 | −0.71±0.84 | −1.16±0.96 | ||
| C1 latency (ms) | Young | Low | 68.39±5.02 | 68.18±3.3 | 78.39±5.31 | 80.27±5.44 |
| High | 64.57±3.92 | 68.98±7.76 | 77±3.87 | 76.6±4.92 | ||
| Old | Low | 73.46±4.99 | 74.21±4.8 | 79.77±5.7 | 80.25±4.65 | |
| High | 72.59±3.33 | 74.93±3.19 | 78.79±5.14 | 81.46±4.71 |
For the latency of C1, the main effects of validity and age were significant [F(1,13)=4.79, P<0.05; F(1,13)=9.61, P<0.01], with invalid trials and the old group eliciting longer C1 latency relative to valid trials and the young group. No other effects were significant.
3.2.2. P1
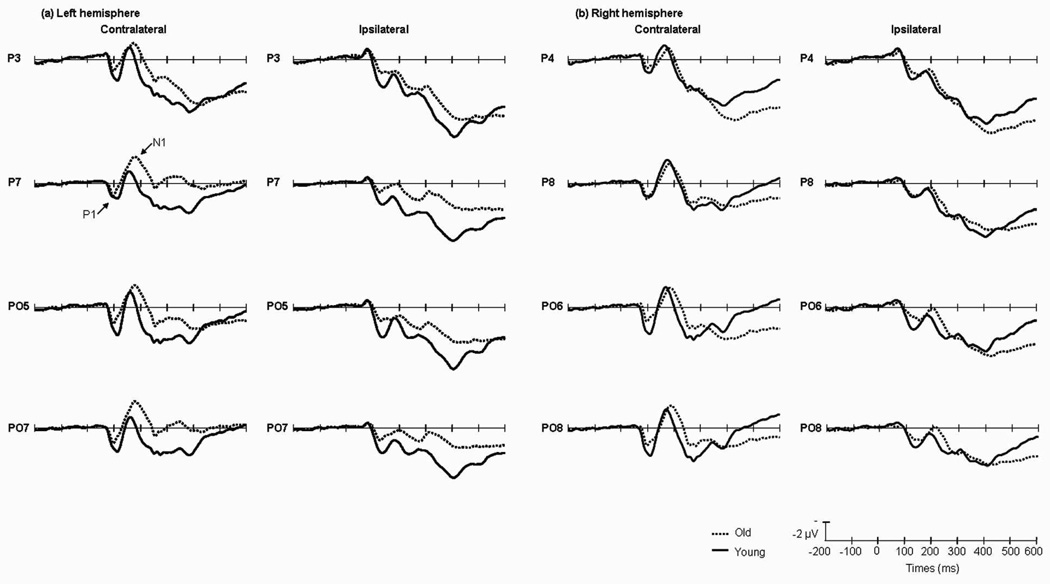
The main effect of age was significant [F(1,13)=9.38, P<0.01], indicating that P1 amplitude was greater in the young group than in old group (Fig.3). The main effect of validity was also significant [F(1,13)=9.96, P<0.01]; P1 was larger on valid than on invalid trials. No other main effects were significant. The validity × visual field × hemisphere interaction was significant [F(1,13)=16.27, P<0.001]. To analyze the interaction, a simple effect test was carried out on the combined visual field/ hemisphere data (ipsilateral vs. contralateral), valid trials elicited a larger P1 at contralateral temporal sites [F(1,13)=25.08, P<0.001]. The interaction between age × validity × visual field × hemisphere was significant [F(1,13)=17.81, P<0.001], for further analysis, in the young group the amplitude of P1 in valid cue trials were larger in the contralateral sites than the one elicited by invalid cue trials [F(1,13)=5.13, P<0.05] (Table.3) (Fig.4).
Fig.3.
Grand average of ERP elicited by old (dotted lines) and young (solid lines) groups at the posterior sites when the cues appeared in the left (a) and right (b) visual fields. Data were averaged across the low and high perceptual load conditions, and across valid and invalid cue trials.
Table.3.
The mean amplitude and latency of P1 component (average ± SD)
| Contralateral | Ipsilateral | |||||
|---|---|---|---|---|---|---|
| Valid | Invalid | Valid | Invalid | |||
| P1 amplitude (µV) | Young | Low | 3.66±0.62 | 1.97±0.7 | 3.49±1.05 | 3.65±0.87 |
| High | 4.03±0.54 | 2.6±0.5 | 3.32±1.1 | 3.75±0.58 | ||
| Old | Low | 2±0.62 | 1.5±0.91 | 1.77±0.7 | 1.43±0.85 | |
| High | 2.3±0.91 | 1.68±0.58 | 2.03±0.84 | 1.51±0.96 | ||
| P1 latency (ms) | Young | Low | 107.64±7.95 | 104.54±9.17 | 123.96±10.53 | 155.18±7.19 |
| High | 115.11±28.5 | 106.88±9.69 | 123.89±7.79 | 148.96±8.05 | ||
| Old | Low | 99.43±5.89 | 101.89±7.14 | 124.98±10.03 | 124.54±8.49 | |
| High | 104.23±11.27 | 99.36±7.61 | 126.73±7.89 | 124.91±8.15 |
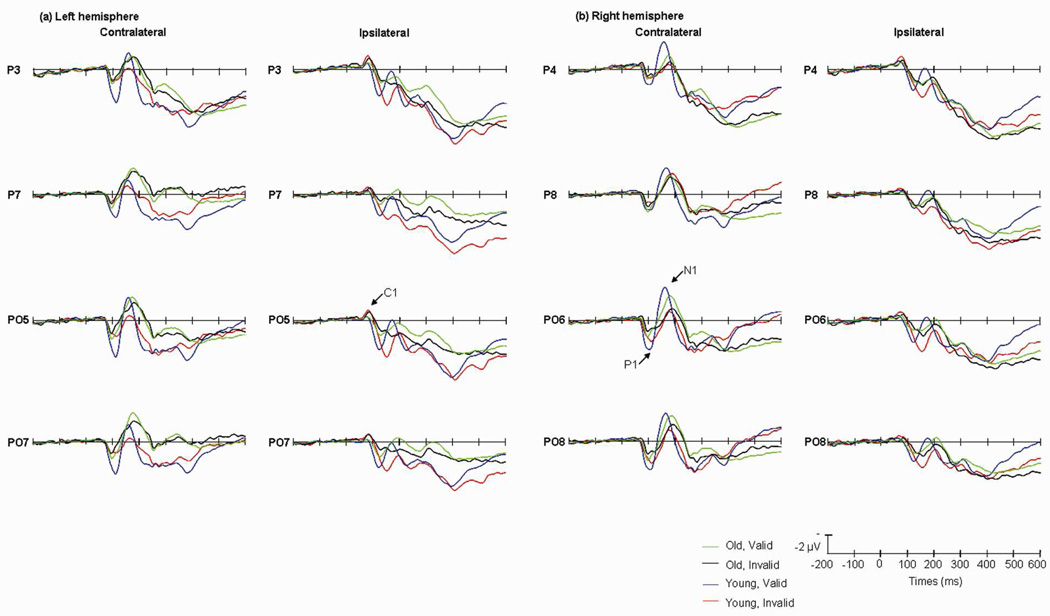
Fig.4.
Grand average of ERP elicited by old group in valid (green lines) and invalid (black lines) trials; and by young group in valid (blue lines) and invalid (red lines) trials at the posterior sites when the cues appeared in the left (a) and the right (b) visual fields. Data were averaged across the low and high perceptual load conditions.
For the latency of the P1 component, the main effects of validity [F(1,13)=9.69, P<0.01] visual field [F(1,13)=4.89, P<0.05], and age were significant [F(1,13)=15.19, P<0.01] (Table.3). No other main effects were significant. The interaction between validity × visual field × hemisphere was significant [F(1,13)=34.47, P<0.001], for further analysis, the validity effect on P1 latency was larger at the ipsilateral sites, and invalid trials elicited a longer P1 latency at ipsilateral temporal sites [F(1,13)=56.17, P<0.001]. The interaction between age × validity was significant [F(1,13)=16.41, P<0.001]. The interaction of age × validity × visual field × hemisphere was significant [F(1,13)=32.03, P<0.001]. For further analysis, in the ipsilateral sites, P1 latency of young group were longer in invalid trials than the one in valid trials [F(1,13)=82.52, P<0.001] (Table.3) (Fig.4).
3.2.3. N1
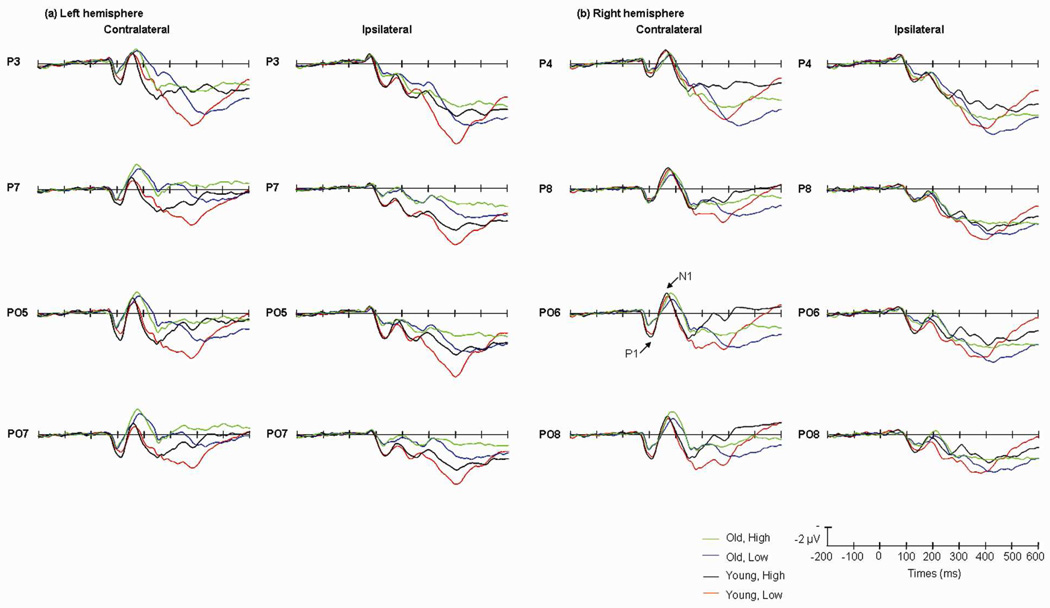
The main effect of perceptual load was significant [F(1,13)=15.16, P<0.005], with N1 in the high perceptual load condition being more negative than in the low perceptual load condition. The main effect of validity was significant [F(1,13)=7.34, P<0.05], N1 amplitude being greater on valid cue trials than on invalid trials. The main effect of age was not significant [F(1,13)=0.22, P<1]. The interaction between hemisphere and visual field was significant [F(1,13)=27.51, P<0.001], indicating that the effect was different in contralateral and ipsilateral sites. The interaction between perceptual load × validity × hemisphere × visual field was significant [F(1,13)=5.58, P<0.05]. To analyze the interaction, a simple effect test was carried out on the combined visual field/ hemisphere data (ipsilateral vs. contralateral), in the high perceptual load condition, compared to invalid trials, the amplitude of N1 elicited by the targets on valid cue trials was more negative at contralateral sites [F(1,13)=5.03, P<0.05], whereas in the low perceptual load condition, N1 amplitude was more negative at both contralateral [F(1,13)=6.57, P<0.05] and ipsilateral sites [F(1,13)=6.08, P<0.05] (Table.4). The age × perceptual load × hemisphere × visual field interaction was significant [F(1,13)=8.01, P<0.05] (Fig.5). To analyze the interaction, a simple effect test was carried out on the combined visual field/hemisphere data (ipsilateral vs. contralateral), in the old group, N1 amplitude was higher in the high perceptual load condition than in the low perceptual load condition at both contralateral [F(1,13)=23.95, P<0.001] and ipsilateral [F(1,13)=7.11, P<0.05] sites. In contrast, in the young group, the perceptual load effect was only significant at ipsilateral sites [F(1,13)=6.42, P<0.05]. In the high perceptual load condition, the old group had a larger N1 than the young group at contralateral sites [F(1,13)=5.37, P<0.05]. No other effects were significant.
Table.4.
The mean amplitude and latency of N1 component (average ± SD)
| Contralateral | Ipsilateral | |||||
|---|---|---|---|---|---|---|
| Valid | Invalid | Valid | Invalid | |||
| N1 amplitude (µV) | Young | Low | −2.71±0.62 | −1.18±0.7 | −0.39±1.05 | 1.19±0.87 |
| High | −2.83±0.54 | −1.42±0.5 | −1.23±1.1 | 0.64±0.58 | ||
| Old | Low | −2.67±0.63 | −2.13±0.91 | −0.36±0.7 | −0.11±0.85 | |
| High | −3.35±0.91 | −2.85±0.58 | −0.82±0.84 | −0.41±0.96 | ||
| N1 latency (ms) | Young | Low | 168.38±7.95 | 177.48±9.17 | 172±10.53 | 190.98±7.19 |
| High | 168.12±28.46 | 175.3±9.69 | 176.34±7.79 | 186.75±8.05 | ||
| Old | Low | 178.37±5.89 | 182±7.13 | 190.18±10.03 | 187.12±8.49 | |
| High | 177.08±11.27 | 180.48±7.61 | 189.25±7.89 | 189.14±8.15 |
Fig.5.
Grand average of ERP elicited by old group in high (green lines) and low (blue lines) perceptual load conditions; and by young group in high (black lines) and low (red lines) perceptual load conditions at the posterior sites when the cues appeared in the left (a) and right (b) visual fields. Data were averaged across the valid and invalid cue trials.
For the latency of N1, the main effect of age was significant [F(1,13)=5.35, P<0.05]. N1 was later in the old group than in the young group (Fig.3). The main effect of validity was significant [F(1,13)=16.28, P<0.001], indicating that N1 latency on invalid trials was longer than on valid trials. The main effect of visual field and hemisphere were significant [F(1,13)=5.52, P<0.05; F(1,13)=27.83, P<0.001]. No other main effects were significant. The interaction between age × validity was significant [F(1,13)=8.51, P<0.05]; targets in invalid trials elicited later N1 components in both the old [F(1,13)=4.06, P<0.05] and young group [F(1,13)=46.92, P<0.001]. The age × validity × hemisphere × visual field interaction was significant [F(1,13)=12.16, P<0.005]. To further analyze the interaction, a simple effect test was carried out on the combined visual field/ hemisphere data (ipsilateral vs. contralateral), in the young group, targets on invalid trials elicited longer latency than on valid trials at both ipsilateral [F(1,13)=19.31, P<0.01] and contralateral sites [F(1,13)=10.04, P<0.01] (Table.4). No other effects were significant.
4. Discussion
The present study investigated the relationship between aging, perceptual load and attention by using a selective attention task with endogenous cueing. Two kinds of search arrays were used to differentiate low and high perceptual load. The results confirmed several predictions we made.
4.1. Aging effect
Effect of age on reaction time is consistent with the processing speed theory of cognitive aging (Salthouse, 1996). The reaction time was longer in the old than in the young group, especially under the high perceptual load condition, suggesting that the speed of early perceptual processing is compromised with increasing age (Salthouse, 1996) and this effect is greater in the complex task. The reduction in speed may reflect the generalized cognitive slowing in the old group.
Aging effect was also reflected on the ERP result. There were significant effects of adult aging on the latency of P1 and N1 and on P1 amplitude. These effects point to differences in the magnitude of early visual processing between the young and old groups. The N1 latency was later in the old than in the young group, indicating that the older participants need more time to localized the target in the visual search array than young participants (Lorenzo-López et al., 2008). But the P1 latency was earlier in the old than in the young group. In Curran et al. (2001) study, they found that the old participants had slower latency of P1 components only at ipsilateral sites but not at contralateral sites, that maybe because of the age differences in interhemispheric transfer times. In our study, we also didn’t find that aging slowed latency of the contralateral P1. The ipsilateral P1 latency of young group was later than old group in the invalid trials (Table 3), that’s maybe why the P1 latency was later in the young group than in the old group. In a visual spatial attention task Greenwood & Parasuraman (2004) found that the young participants experienced greater cost-benefit than old participants in invalid trials. They suggested that old participants could not maintain their attention on the cue as good as the young participants, and they may have a broader attentional focus than the young participants. So when the cue was invalid the diffuse attentional focus in old group may reduce the costs of invalid cues, and in contrast the young group experienced greater costs of invalid cues. That may be why the later P1 latency in invalid trial was found in young group.
4.2. Spatial attention effect
The behavior data showed that the effects of endogenous cueing—the voluntary spatial attention effect—were significant in both young and old groups, as in previous studies (Curran et al., 2001; Lorist et al., 1995): participants responded more quickly and accurately to valid relative to invalid trials, suggesting that both young and old participants attention were attracted by the cue to the cued location.
For the ERP results, the amplitude of P1 was more positive and the amplitude of N1 component was more negative for targets on valid than on invalid trials, supporting the view that visuospatial attention selection occurs at an early stage of information processing (Hillyard et al., 1998; Mangun & Hillyard, 1991). These results are also consistent with findings of previous studies (Fu et al., 2005; Luck and Hillyard, 1994) and can be interpreted within a sensory gain control theory of visual attention (Hillyard et al., 1998), i.e, attention enhances perceptual processing of stimuli.
4.3. Attention effect on C1 component
Attentional effects on C1 amplitude and latency were significant. Some researchers (Clark, & Hillyard, 1996; Hopfinger, & West, 2006) have reported that C1 is immune to cue-driven attentional validity effects, but others (Fu et al., 2008; Kelly et al., 2008; Rauss et al., 2009) have found significant effects of attention on C1 amplitude. Rauss et al. (2009) suggested that the more attention is required by a task, the earlier are irrelevant stimuli filtered during perceptual processing. The task used in the present study may have similarly lead to participants having to process the distractors at a very early stage, and that may account for our observation of a cue validity effect on C1 amplitude. However, the cue validity effect on C1 component was reversed (i.e., invalid > valid) in the present study, possibly reflecting contamination of C1 by extrastriate neural activity indexed by P1 (Fu et al., 2008). The later latency of C1 for invalid relative to valid condition is consistent with such a possibility.
4.4. The relationship between attention, aging and perceptual load
The main focus of our study was the relationship between aging, perceptual load and attention. Although the interaction between these three factors was not significant, the interactions between each of two factors were significant, and separate ANOVAs indicated differences in the validity effect between the age groups under different perceptual load conditions, suggesting that perceptual load modulated the relationship of age and attention.
4.4.1. The relationship between aging and perceptual load
The interaction between age and perceptual load on reaction time was significant. The most accurate and fastest responses to targets were displayed by the young group under low perceptual load, while the old group was slowest under high perceptual load. Similar delays in reaction time and reduced accuracy has been reported in other cognitive aging studies (Greenwood & Parasuraman, 1997; Kok, 2000). The perceptual load effect in young group was not as larger as in old group, possibly because the high perceptual load in our task was not hard enough and resource demanding for the young group relative to the older group.
For the ERP results, the absolute magnitude of the perceptual load effect was larger for older than for young participants, as reflected in the N1 component. Also, N1 amplitude was larger in the old group than the young in the high perceptual load condition. Given that N1 amplitude reflects the difficulty of target discrimination (Vogel & Luck, 2000), the results are consistent with the view that the old group experienced more difficulty in target discrimination under high perceptual load, possibly due to a depletion of processing capacity. A similar age effect was also found in a previous PET (Positron Emission Computed Tomography) study (Grady et al., 1992) for both spatial and non-spatial visual tasks. Activation of occipito-temporal cortex and superior parietal cortex was stronger in the old group than in the young. The lack of age differences under low perceptual load is consistent with previous studies (Lavie et al., 2004; Maylor & Lavie, 1998). N1 latency was later in old group than in young group in our study. Čeponienė et al. (2008) also found that N1 latency increased with age, in a visual search task. The effect of age on N1 latency suggests that older adults showed not only a delay in processing but also a possible capacity limitation in target discrimination under high perceptual load.
4.4.2. The relationship between attention and perceptual load
Attention effect on behavior was modulated by perceptual load. Reaction time was shortest and accuracy was highest for targets for validly-cued trials in the low perceptual load condition; in contrast RT was high and accuracy was low under high perceptual load. This suggests that rejection of the distractors depended on the level of perceptual load--under the low perceptual load condition, resources were sufficient to process the distractors, but were limited under the high perceptual load condition, resulting in a delay in reaction time.
There was an interaction between perceptual load and cue validity for N1 amplitude, which is consistent with the view that perceptual load modulates attentional selection at an early processing stage, but following perceptual discrimination (Luck et al., 2000). Similar to our previous study using a sustained attention task (Fu et al., 2008), this interaction was only significant for the N1 component, and not for the C1 and P1 components. These findings suggest that modulation of voluntary attention effects (under either sustained attention or central cueing tasks) by perceptual load take place only at the stimulus discrimination stage but not at earlier cortical processing stages as indexed by the C1 and P1 components.
4.4.3. The relationship between attention and aging
The P1 latency in the invalid trial was later than in the valid trial in young group but there was no interaction between perceptual load and validity on P1 latency. In other words, the validity effect was independent of perceptual load in the young group. The interaction between age and validity effect was significant on N1 latency, invalid cue trials elicited later N1 components in both young and old groups. Therefore, the results may suggest that the attentional selection occur earlier in the young group (the process was reflected on P1 component) than in the old (the process was reflected on N1 component) even if a higher order interaction with perceptual load was not significant.
In summary, the present results indicate that perceptual load modulates the effects of attention on neural activity associated with target processing, and that this modulation varies with aging.
Highlights.
The spatial attention effect was significant in both young and old groups.
The speed of early perceptual processing is compromised with increasing age.
Perceptual load and aging effects take place at the stimulus discrimination stage.
Perceptual load modulates attention and this modulation varies with aging.
Cue validity effect was found on C1 component.
Acknowledgment
This research was supported by NIA grant AG19653 to R.P., SRF for ROCS, SEM (20111021085) and Initiative Scientific Research Program, Tsinghua University, China (20111081107) to S. F., NSFC (Y2JJ081004) and Project for Young Scientists Fund, Institute of Psychology, Chinese Academy of Sciences (Y0CX091S01) to Y.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bashore TR. Age-related changes in mental processing revealed by analyses of event-related brain potentials. In: Rohrbaugh JW, Parasuraman R, Johnson R, editors. Event-Related Brain Potentials. Basic Issues and Applications. New York: Oxford Univ. Press; 1990. pp. 242–275. [Google Scholar]
- Bélanger S, Belleville S, Gauthier S. Inhibition impairments in Alzheimer's disease, mild cognitive impairment and healthy aging: Effect of congruency proportion in a Stroop task. Neuropsychologia. 2010;48:581–590. doi: 10.1016/j.neuropsychologia.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Golob EJ, Starr A. Age-related differences in auditory event-related potentials during a cued attention task. Clinical Neurophysiology. 2004;115:2602–2615. doi: 10.1016/j.clinph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Čeponienė R, Westerfield M, Torki M, Townsend J. Modality-specificity of sensory aging in vision and audition: Evidence from event-related potentials. Brain research. 2008;1215:53–68. doi: 10.1016/j.brainres.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Cerella J. Information processing rates in the elderly. Psychol. Bull. 1985;98:67–83. [PubMed] [Google Scholar]
- Clark VP, Fan S, Hillyard SA. Identification of early visual evoked potential generators by retinotopic and topographic analyses. Human Brain Mapping. 1995;2:170–187. [Google Scholar]
- Clark VP, Hillyard SA. Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. J. Cogn. Neurosci. 1996;8:387–402. doi: 10.1162/jocn.1996.8.5.387. [DOI] [PubMed] [Google Scholar]
- Curran T, Hills A, Patterson MB, Strauss ME. Effects of aging on visuospatial attention: an ERP study. Neuropsychologia. 2001;39:288–301. doi: 10.1016/s0028-3932(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Hillyard SA. Source analysis of eventrelated cortical activity during visuo-spatial attention. Cereb. Cortex. 2003;13:486–499. doi: 10.1093/cercor/13.5.486. [DOI] [PubMed] [Google Scholar]
- Doallo S, Lorenzo-López L, Vizoso C, Rodrı´guez Holguı´n S, Amenedo E, Bara´ S, Cadaveira F. The time course of the effects of central and peripheral cues on visual processing: an event-related potentials study. Clinical Neurophysiology. 2004;115:199–210. doi: 10.1016/s1388-2457(03)00317-1. [DOI] [PubMed] [Google Scholar]
- Doallo S, Lorenzo-López L, Vizoso C, Rodrı´guez Holguı´n S, Amenedo E, Bara´ S, Cadaveira F. Modulations of the visual N1 component of event-related potentials by central and peripheral cueing. Clinical Neurophysiology. 2005;116:807–820. doi: 10.1016/j.clinph.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Snyder EW. Life-span changes in visually evoked potentials at central scalp. Neurobiol. Aging. 1981;2:303–308. doi: 10.1016/0197-4580(81)90039-7. [DOI] [PubMed] [Google Scholar]
- Eimer M, Driver J. Crossmodal links in endogenous and exogenous spatial attention: evidence from event-related brain potential studies. Neuroscience and Biobehavioral Reviews. 2001;25:497–511. doi: 10.1016/s0149-7634(01)00029-x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “MINI-MENTAL STATE” a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fu S, Caggiano DM, Greenwood PM, Parasuraman R. Event related potentials reveal dissociable mechanisms for orienting and focusing visuospatial attention. Cognitive Brain Research. 2005;23:341–353. doi: 10.1016/j.cogbrainres.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Fan S, Chen L, Zhuo Y. The attentional effects of peripheral cueing as revealed by two event-related potential studies. Clinical Neurophysiology. 2001;112:172–185. doi: 10.1016/s1388-2457(00)00500-9. [DOI] [PubMed] [Google Scholar]
- Fu S, Fedota JR, Greenwood PM, Parasuraman R. Dissociation of visual C1 and P1 components as a function of attentional load: An event-related potential study. Biological Psychology. 2010;85:171–178. doi: 10.1016/j.biopsycho.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Zinni M, Squire PN, Kumar R, Caggiano DM, Parasuraman R. When and where perceptual load interacts with voluntary visuospatial attention: An event-related potential and dipole modeling study. NeuroImage. 2008;39:1345–1355. doi: 10.1016/j.neuroimage.2007.09.068. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Heeger DJ, Boynton GM. Spatial attention affects brain activity in human primary visual cortex. Proc. Natl. Acad. Sci U S A. 1999;96:3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Haxby JV, Horwitz B, Schapiro MB, Rapoport SI, Ungerleider LG, Mishkin M, Carson RE, Herscowitch P. Disscociation of object and spatial vision in human extrastriate cortex: age-related changes in activation of regional cerebral blood-flow measures with (15O) water and positron emission tomography. J. Cogn. Neurosci. 1992;4:23–34. doi: 10.1162/jocn.1992.4.1.23. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R. Attention in aging and Alzheimers disease. In: Burack JA, Enns T, editors. Attention, Development, and Psychopathology. New York, NY: The Guilford Press; 1997. pp. 288–317. [Google Scholar]
- Greenwood PM, Parasuraman R. Normal genetic variation, cognition, and aging. Behavioral and Cognitive Neuroscience Reviews. 2003;2:278–306. doi: 10.1177/1534582303260641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R. The scaling of spatial attention in visual search and its modification in healthy aging. Perception and Psychophysics. 2004;66:3–22. doi: 10.3758/bf03194857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S, Kramer AF. Attentional flexibility and aging: You don’t need to be 20 years of age to split the beam. Psychology and Aging. 1995;10:597–609. doi: 10.1037//0882-7974.10.4.597. [DOI] [PubMed] [Google Scholar]
- Heinze HJ, Luck SJ, Mangun GR, Hillyard SA. Visual event-related potentials index focused attention within bilateral stimulus arrays. I. Evidence for early selection. Electroencephalogr Clin Neurophysiol. 1990;75:511–527. doi: 10.1016/0013-4694(90)90138-a. [DOI] [PubMed] [Google Scholar]
- Heinze HJ, Mangun GR, Burchert W, Hinrichs H, Scholz M, Muente TF, Goes A, Scherg M, Johannes S, Hundeshagen H, Gazzaniga MS, Hillyard SA. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature (London) 1994;372:543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplifi- cation) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philosophical Transactions of the Royal Society of London-Series B: Biological Sciences. 1998;353:1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf JM, Noesselt T, Tempelmann C, Braun J, Schoenfeld A, Heinze HJ. Popout modulates focal attention in the primary visual cortex. NeuroImage. 2004;22:574–582. doi: 10.1016/j.neuroimage.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, West VM. Interactions between endogenous and exogenous attention on cortical visual processing. Neuroimag. 2006;31:774–789. doi: 10.1016/j.neuroimage.2005.12.049. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Gomez-Ramirez M, Foxe JJ. Spatial Attention Modulates Initial Afferent Activity in Human Primary Visual Cortex. Cerebral Cortex. 2008;18:2629–2636. doi: 10.1093/cercor/bhn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok A. Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP) Biological Psychology. 2000;54:107–143. doi: 10.1016/s0301-0511(00)00054-5. [DOI] [PubMed] [Google Scholar]
- Kok A, Zeef EJ. Arousal and effort; a review and theoretical synthesis of studies of age-related changes in event-related potentials. In: Brunia CHM, Mulder G, Verbaten MN, editors. Event-Related Brain research (EEG Suppl. 42) Amsterdam: Elsevier Science Publishers B.V.; 1991. pp. 324–341. [PubMed] [Google Scholar]
- Kramer C, Hofman WF, Elton M, Kerkhof GA. The augmenting reducing paradigm studied with visually evoked potentials in normal aging. J. Psychophysiol. 1996;10:319–325. [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Lavie N. The role of perceptual load in visual awareness. Brain research. 2006;1080:91–100. doi: 10.1016/j.brainres.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, Fockert JW. Load Theory of Selective Attention and Cognitive Control. Journal of Experimental Psychology: General. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Lavie N, Tsal Y. Perceptual load as a major determinant of the locus of selection in visual attention. Perception & Psychophysics. 1994;56:183–197. doi: 10.3758/bf03213897. [DOI] [PubMed] [Google Scholar]
- Lorenzo-López L, Amenedo E, Cadaveira F. Feature processing during visual search in normal aging: Electrophysiological evidence. Neurobiology of Aging. 2008;29:1101–1110. doi: 10.1016/j.neurobiolaging.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Lorenzo-López L, Amenedo E, Cadaveira F. Age-related occipito-temporal hypoactivation during visual search: Relationships between mN2pc sources and performance. Neuropsychologia. 2011;49:858–865. doi: 10.1016/j.neuropsychologia.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Lorist MM, Snel J, Mulder G, Kok A. Aging, caffeine, and information processing: an event-related potential analysis. Electroencephalography and clinical Neurophysiology. 1995;96:453–467. doi: 10.1016/0168-5597(95)00069-5. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. The role of attention in feature detection and conjunction discrimination: an electrophysiological analysis. Int J Neurosci. 1995;80:281–297. doi: 10.3109/00207459508986105. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: psychophysical and electrophysiological evidence for early selection. J Exp Psychol Hum Percept Perform. 1994;20:887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Woodman GF, Vogel EK. Event-related potential studies of attention. Trends in Cognitive Sciences. 2000;4:432–440. doi: 10.1016/s1364-6613(00)01545-x. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Langley LK. Age-Related Changes in Selective Attention and Perceptual Load During Visual Search. Psychology and Aging. 2003;18:54–67. doi: 10.1037/0882-7974.18.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangun GR. Neural mechanism of visual selective attention. Psychophysiology. 1995;32:4–18. doi: 10.1111/j.1469-8986.1995.tb03400.x. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Buck LA. Sustained visual-spatial attention produces costs and benefits in response time and evoked neural activity. Neuropsychologia. 1998;36:189–200. doi: 10.1016/s0028-3932(97)00123-1. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual– spatial priming. Journal of Experimental Psychology: Human Perception and Performance. 1991;17:1057–1074. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hopfinger JB, Kussmaul CL, Fletcher E, Heinze HJ. Covariations in ERP and PET measures of spatial selective attention in human extrastriate visual cortex. Hum. Brain Mapp. 1997;5:273–279. doi: 10.1002/(SICI)1097-0193(1997)5:4<273::AID-HBM12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Lavie N. The influence of perceptual load on age differences in selective attention. Psychology and Aging. 1998;13:563–573. doi: 10.1037//0882-7974.13.4.563. [DOI] [PubMed] [Google Scholar]
- Mishra J, Hillyard SA. Endogenous attention selection during binocular rivalry at early stages of visual processing. Vision research. 2009;49:1073–1080. doi: 10.1016/j.visres.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2 and V4 in the presence of competing stimuli. Journal of Neurophysiology. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- Müller HJ, Rabbitt PMA. Reflexive and voluntary orienting of visual attention: Time course of activation and resistance to interruption. Journal of Experimental psychology: Human perception and performance. 1989;15:315–330. doi: 10.1037//0096-1523.15.2.315. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Roth WT, Kopell BS. Age-related changes in auditory even-related potentials. Electroencephalogr. Clin. Neurophysiol. 1980;49:266–276. doi: 10.1016/0013-4694(80)90221-7. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Rauss KS, Pourtois G, Vuilleumier P, Schwartz S. Attentional Load Modifies Early Activity in Human Primary Visual Cortex. Human Brain Mapping. 2009;30:1723–1733. doi: 10.1002/hbm.20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Constraints on theories of cognitive aging. Psychonomic Bulletin and Review. 1996;3:287–299. doi: 10.3758/BF03210753. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Mehta AD, Ulbert L. Intermodal selective attention I: Subcortical effects. Society for Neuroscience Abstracts. 1994;20:576. [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Smith DBD, Michalewsky HJ, Brent GA, Thompson LW. Auditory averaged evoked potentials and aging: factors of stimulus, task and topography. Biol. Psychol. 1980a;11:135–151. doi: 10.1016/0301-0511(80)90048-4. [DOI] [PubMed] [Google Scholar]
- Smith DBD, Thompson LW, Michalewsky HJ. Averaged evoked potential research in adult -aging: status and prospects. In: Poon LW, editor. Aging in the 1980s. Washington: American Psychological association; 1980b. pp. 135–151. [Google Scholar]
- Strayer DL, Wickens CD, Braune R. Adult age differences in speed and capacity of information processing. An electrophysiological approach. Psychol. Aging. 1987;2:99–110. doi: 10.1037//0882-7974.2.2.99. [DOI] [PubMed] [Google Scholar]
- Vogel E, Luck S. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37:190–203. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale- Revised Manual. New York: Psychological Corporation; 1987. [Google Scholar]
- Wilkinson GS. WRAT 3 administration manual. Delaware: Wide Range; 1993. [Google Scholar]