Abstract
Introduction
Tissue factor (TF) is a potent initiator of the extrinsic coagulation cascade. The role and source of TF in venous thrombotic disease is not clearly defined. Our study objective was to identify the contribution of myeloid cell TF to venous thrombogenesis in mice.
Materials and Methods
The mouse electrolytic inferior vena cava model was used to induce thrombosis. The following groups of mice were used (1) TFflox/floxLysMCre+ mice that have reduced TF expression in myeloid cells, (2) TFflox/floxLysMCre- littermate controls, (3) Wild type mice given a monoclonal anti-mouse TF antibody (1H1) to inhibit TF activity, and (4) Wild type mice given rat IgG. Evaluations at baseline, day 2, and day 6 post thrombosis included thrombus weight, vein wall inflammatory cell migration, vein wall TF mRNA, and plasma D-dimer levels.
Results
Inhibition of TF significantly decreased thrombus weight 2 days post venous thrombosis. In contrast, TFflox/floxLysMCre+ had no change in thrombus weight when compared to littermate controls. The absence of myeloid cell TF did not affect infiltration of neutrophils or monocytes into the vein wall. TF mRNA expression in the vein wall decreased at 2 days but then returned to baseline levels by 6 days post thrombosis. D-dimer levels peaked at 2 days post thrombosis in mice with or without myeloid cell TF.
Conclusions
TF is important in the formation of venous thrombi in the macrovasculature. However, TF expression by myeloid cells does not significantly contribute to venous thrombogenesis in this model.
Keywords: Venous thrombosis, Thrombogenesis, Tissue factor
Deep vein thrombosis (DVT) and pulmonary embolism, collectively known as venous thromboembolism, are major health problems in the United States. With over 900,000 cases and greater than 300,000 deaths each year, it is a significant cause of morbidity and mortality [1]. Furthermore, 30% of those patients initially treated for DVT develop a recurrence within 10 years [2, 3]. Understanding the underlying pathophysiology of venous thrombus formation, propagation, and recurrence is essential to developing earlier diagnostic assays, preventative therapies, and treatments.
Studies have shown that tissue factor (TF) plays a role in venous thrombosis [4-6]. TF is a transmembrane glycoprotein that initiates the coagulation cascade. After vessel injury, TF under the endothelium is exposed to the vascular lumen and serves as a cellular receptor for plasma factor VII/VIIa. Together, the factor VIIa:TF complex activates factor X and factor IX. Activated factor Xa in association with its cofactor Va, the prothrombinase complex, cleaves prothrombin to thrombin, which in turn cleaves fibrinogen to fibrin. Then, transglutaminase factor XIII cross-links fibrin to stabilize the thrombi [7]. TF is typically expressed by perivascular cells [8-10] and tissues that need additional hemostatic protection, like the heart (cardiac myocytes) [11], kidney (glomerular epithelial cells) [9], and brain (astrocytes) [9, 11-14]. Unlike all other components of the clotting system, little or no active TF is present in circulation in healthy individuals. The vessel wall [5, 6, 15] and hematopoietic cells [4, 5, 16] have been postulated as major and minor sources of TF, respectively that could participate in the initiation of venous thrombosis.
Endothelial disruption and exposure of underlying TF has been well documented in arterial thrombosis [6, 15]; however, venous thrombosis typically occurs in the absence of gross endothelial damage [7]. The cellular source of TF that contributes to venous thrombosis in the absence of severe endothelial injury is not known. This study examined the role of myeloid cell derived TF in a mouse model of venous thrombosis. Wild type mice that have had TF inhibited by a monoclonal, rat anti-TF antibody (1H1) [17] and transgenic mice that have reduced expression of TF in myeloid cells were utilized for this study. We used a mouse electrolytic inferior vena cava model (EIM) which consistently stimulates thrombosis in the presence of continuous blood flow that closely mimics clinical DVT [18, 19].
The objective of this study was to determine the contribution of myeloid cell TF to venous thrombogenesis. We hypothesized that a reduction of myeloid cell TF would decrease venous thrombogenesis in a mouse model of this disease.
Materials and Methods
Animals
Male C57BL/6 mice were obtained from Charles River Laboratories, Portage, Michigan (n=30, mean body weight=24.7 g). Male transgenic mice deficient in TF in myeloid cells (TFflox/floxLysMCre+ on a C57BL/6 background, n=51, mean body weight=25.9 g), and male Cre negative littermate controls (TFflox/floxLysMCre-, n=35, mean body weight=26.8 g) [20, 21] were generated at University of North Carolina at Chapel Hill (UNC-CH) and shipped to the University Michigan. Male mice were chosen for all groups because they produce a larger and more consistent IVC thrombus than their female counterparts [22]. This study was approved by the University of Michigan Committee on Use and Care of Animals and conducted in accordance with The Guide for the Care and Use of Laboratory Animals [23], and the National Institutes of Health guidelines [24]. All animals were healthy and free of specific pathogens. The University's animal program and facilities are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International. All mice were group housed and non-acute mice were provided enrichment upon arrival (paper bedding material or a plastic hut).
Animal model
We used the previously characterized electrolytic inferior vena cava model (EIM) to induce thrombogenesis [18, 19, 22]. In brief, mice were anesthetized with an inhalation mixture of 5% isoflurane gas and 0.5L oxygen per minute and a surgical plane of anesthesia was maintained with 2% isoflurane gas and 0.2L oxygen per minute. The inferior vena cava (IVC) was exposed and all side branches, inferior to the level of the left renal vein but superior to the iliac veins, were ligated using 7-0 Prolene (Ethicon, Inc, Somerville, NJ, USA). A 30 gauge silver coated copper wire (KY-1-GRN, Electrospec, Dover, NJ, USA) attached to a 25 gauge, 5mm stainless steel needle was placed into the caudal IVC and positioned against the anterior wall (anode). Another needle (cathode) was placed subcutaneously to complete the circuit. A current of 250 μAmps over 15 minutes was applied using a Grass S48 square wave stimulator and a constant current unit (Grass Technologies, An Astro-Med, Inc.,West Warwick, RI, USA). The EIM does not produce a thermal injury in order to initiate thrombogenesis, but instead the current results in the formation of free radicals that activates the IVC endothelial surface, thus promoting a thrombogenic environment and subsequent thrombogenesis [18]. After the required amount of time, needles were removed, the abdominal muscle and skin were closed, and the mouse recovered. Non-thrombosed true controls (TC), that were surgically naïve animals, were also utilized.
Animal groups
To determine the contribution of myeloid cell TF on early and later thrombosis in the macrovasculature, the following experimental groups were studied. TFflox/floxLysMCre+ EIM (n=40), TFflox/floxLysMCre+ true control (n=11), TFflox/floxLysMCre- EIM (n=26), TFflox/floxLysMCre-true controls (n=9). Mice expressing Cre recombinase under control of lysozyme (LysM) promoter were backcrossed 6 generations onto the C57BL/6 background [20, 21]. A sub-set of C57BL/6 mice (n=15 per group), received either 20 mg/kg of body weight rat anti-mouse TF monoclonal antibody 1H1 [17] or 20 mg/kg of body weight rat IgG control (Sigma-Aldrich, St. Louis, MO, USA) injected into the intraperitoneal (IP) space (100 μL total), 24 hours prior to EIM procedure. Mouse groups were evaluated at the following time points: day 2 and day 6 post EIM. Animals underwent the following analyses: thrombus and IVC weight, vein wall morphometrics, vein wall gene expression by quantitative Real-time PCR (qRT-PCR) for TF and β-actin, and plasma D-dimer levels [Table 1].
Table 1.
EIM=Electrolytic inferior vena cava model
TC=Non-thrombosed true controls
Day=Day of sample collection post EIM
TW=Total weight, weights of thrombus + IVC, thrombus alone, and IVC alone were obtained
n=number of samples collected for the specified analyses
| Strain | Model | Day | Tissue evaluation | Plasma evaluation | n |
|---|---|---|---|---|---|
| TFflox/flox ,LysMCre+ | TC | 0 | qRT-PCR & TW | 6 | |
| Vein Wall Morphometrics | D-dimer | 5 | |||
| EIM | 2 | qRT-PCR & TW | 10 | ||
| Vein Wall Morphometrics | D-dimer | 10 | |||
| EIM | 6 | qRT-PCR & TW | 10 | ||
| Vein Wall Morphometrics | D-dimer | 10 | |||
| TFf lox/f lox ,LysMCre- | TC | 0 | qRT-PCR & TW | 6 | |
| Vein Wall Morphometrics | D-dimer | 3 | |||
| EIM | 2 | qRT-PCR & TW | 10 | ||
| Vein Wall Morphometrics | D-dimer | 3 | |||
| EIM | 6 | qRT-PCR & TW | 10 | ||
| Vein Wall Morphometrics | D-dimer | 3 | |||
| C57BL/6 TF antibody 1H1 | EIM | 2 | qRT-PCR & TW | 10 | |
| EIM | 6 | qRT-PCR & TW | 5 | ||
| C57BL/6 IgG Controls | EIM | 2 | qRT-PCR & TW | 10 | |
| EIM | 6 | qRT-PCR & TW | 5 |
Total Thrombus Weight (TW)
In brief, groups of mice were analyzed for (wet) total thrombus weight (TW) at the time of euthanasia, in which the IVC thrombus and its associated vein wall were removed and weighed in grams (g) [25]. The thrombus was then dissected from the IVC and the IVC was used to assess vein wall expression of TF mRNA (see below).
Vein Wall Morphometrics
In selected samples the IVC, along with the aorta, were removed, paraffin-embedded, 4 μm sections onto slides, and stained with hematoxylin and eosin (H&E). Slides were examined under oil immersion light microscopy. Five representative high-power fields (5HPFs) around the vein wall were examined under high power (100×) and cell counts were averaged. Cells were identified as neutrophils or monocytes on the basis of standard histological criteria including nuclear size, cytoplasmic content, and total cell size in a blinded fashion by a board-certified veterinary pathologist. Results from all 5HPFs were added together and the mean ± standard error of the mean (SEM) was calculated for all vein segments per animal group and time point [25, 26].
Quantitative (Real-time) polymerase chain reaction (qRT-PCR)
Gene expression of TF and β-actin was determined as follows: Vein wall segments, separated from their respective thrombi, were placed in a 15 mL conical tube containing 1.5 mL of TRIzol (Invitrogen, Carlsbad, CA, USA) and then homogenized and RNA extracted according to the manufacturer's recommendations. The concentration of total RNA was determined using a Nanodrop spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE, USA). Five μg of vein total RNA was reverse transcribed using-MMLV as the reverse transcribing enzyme and oligo (dT) as the primer. The cDNA concentration was determined using a Nanodrop spectrophotometer. cDNA was diluted to 22 ng/μL and used as a template for Real-time PCR reaction. Real-time PCR was set up as a 25 μL reaction using 12.5 μL of 2× RT2 Real-time Sybr Green PCR Master Mix, 1.0 μL of 22 ng/μL cDNA, 1.0 μL of RT2 qPCR TF primer (band size 92bp, reference position 958; F3 PPM05163A) and β-actin (Actb, band size 154bp, reference position 182; PPM02945A) primers from SABiosciences (Frederick, MD, USA). The reaction was run at 95°C for 10 min via 40 cycles of (95°C, 15 sec 60°C, 60 sec) in a Corbett Rotocycler (Corbett Research Mortlake, New South Wales, Australia). Relative mRNA expression was calculated by the formula 2- (Ct target gene −Ct reference gene) and cycle lengths used within the exponential phase of the PCR.
D-dimer assay
Mice were anesthetized and a 500 μL blood sample was collected via cardiocentesis using a syringe primed with 50 μL of sodium citrate 0.1 M (10:1 respectively). The citrated blood was transferred into a 1.5 mL eppendorf and placed on a blood rocker until platelet poor plasma (PPP) preparation. PPP was obtained by centrifuging blood at 1,500 g for 15 minutes at 4°C. The plasma was then divided into 60 μL aliquots, immediately frozen in liquid nitrogen (-196°C) and stored at -80°C until analysis. Plasma D-dimer was quantitated according to manufacturer's recommendations by a sandwich-type ELISA from Asserachrom D-dimer (Diagnostica Stago Inc., Mount Olive, NJ, USA). The D-dimer activity was reported in ng/mL.
Statistical analysis
Statistical analysis included the mean ± SEM for each group. Statistical significance between animal groups and time points were calculated using Analysis of Variance (ANOVA) and unpaired t-test with Welch's Correction (GraphPad Software, Inc., La Jolla, CA, USA). Significance was defined as p≤0.05. The extreme studentized deviate test (Grubbs's test) was used to determine significant outliers to alpha=0.05 in groups of continuous data. Outliers identified were not included in statistical analysis.
Results
Tissue factor derived from myeloid cells does not contribute to venous thrombosis in mice
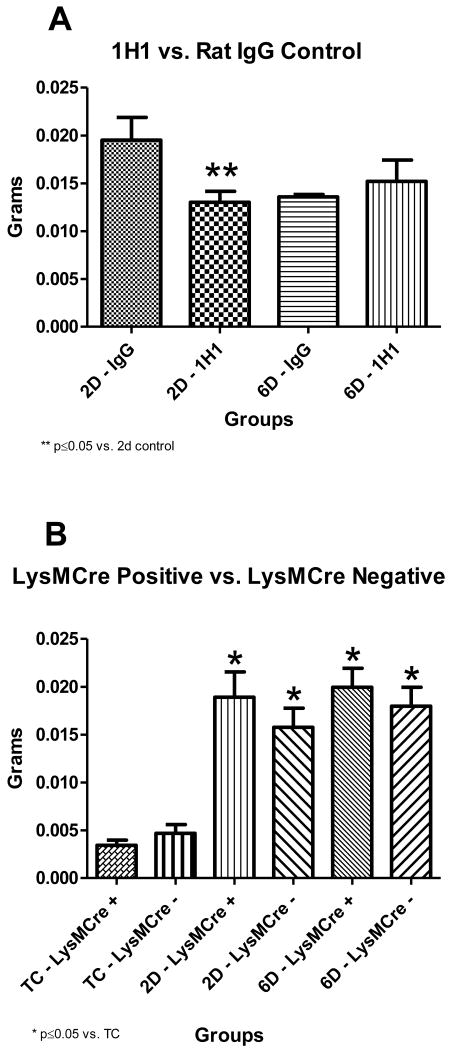
Mice given a TF inhibitory antibody (1H1) had significantly smaller thrombi at 2 days post thrombosis compared to mice given a rat IgG antibody control [Figure 1A]. No difference in thrombus size was seen between the two groups at 6 days post thrombosis [Figure 1A]. In contrast to the results with the anti-TF antibody, deletion of the TF gene in myeloid cells did not affect thrombosis at either 2 or 6 days post thrombosis (2 day: 189.1 ± 26.39 ×10-4 g vs. 157.7 ± 19.98 ×10-4 g, p=0.3569; 6 day: 199.4 ± 19.93 ×10-4 g vs. 179.6 ± 19.94 ×10-4 g, p=0.4919) [Figure 1B].
Figure 1A and Figure 1B. Thrombus Weight Composite.
* indicate significant differences, vs. non-thrombosed true controls, **indicate significant differences, vs. 2 days post thrombosis (1A: n=10 for 2D thrombosis and n=5 for 6D thrombosis; 1B: n=6 for TC, n=10 for 2D and 6D thrombosis). TC=Non-thrombosed true controls, D=days post thrombosis. Systemic inhibition of tissue factor significantly decreased thrombus size at 2 days post thrombosis. LysMCre + mice showed no decrease in thrombus size at any time point, compared to LysMCre - littermate controls. Tissue factor derived from myeloid cells did not contribute to thrombus size.
Vein Wall Morphometrics
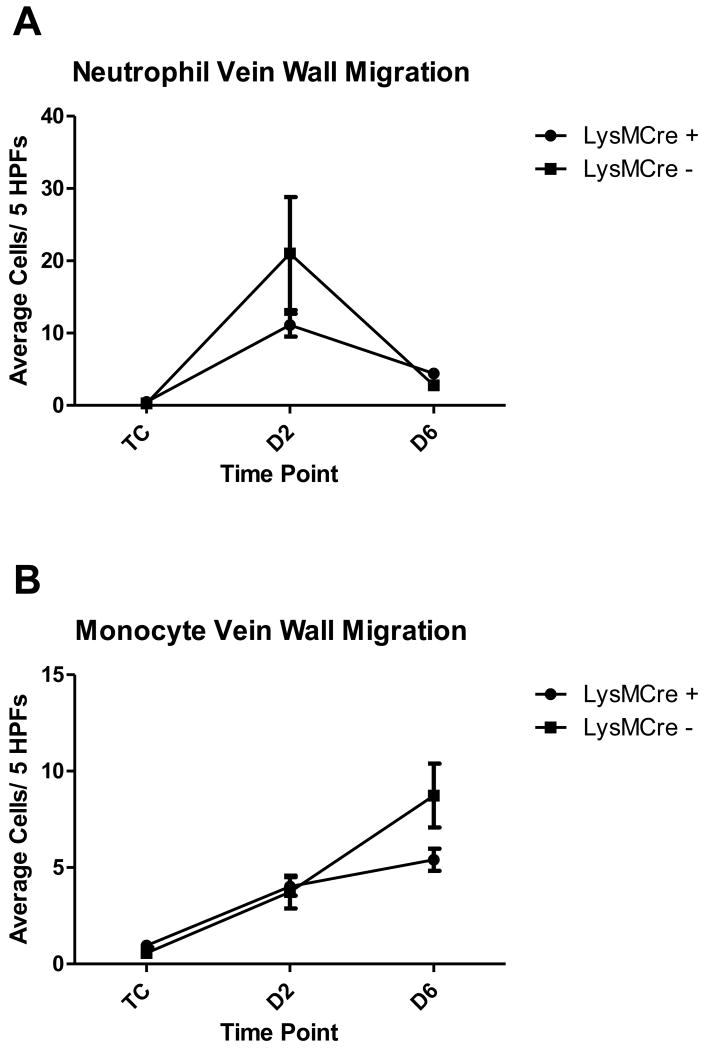
A significant increase was observed in neutrophil migration through the vein wall at 2 days post thrombosis in both LysMCre groups compared to their respective TC (LysMCre positive: 11.10 ± 1.571 cells/ 5 HPFs vs. 0.4800 ± 0.1306 cells/5HPFs, p < 0.0001; LysMCre negative: 21.00 ± 7.805 cells/ 5 HPFs vs. 0.2857 ± 0.1253 cells/5HPFs, p=0.0199). There was a significant decrease in neutrophil migration through the vein wall at 6 days in both groups compared to day 2 post thrombosis (LysMCre positive: 4.420 ± 0.6055 cells/ 5 HPFs vs. 11.10 ± 1.571 cells/5HPFs, p=0.0002; LysMCre negative: 2.800 ± 0.6340 cells/ 5 HPFs vs. 21.00 ± 7.805 cells/5HPFs, p=0.0370). There were no significant differences between LysMCre negative and LysMCre positive mice at any time points [Figure 2A].
There was a significant increase in monocyte migration through the vein wall at 2 days post thrombosis in both groups compared to their respective TC (LysMCre positive: 4.020 ± 0.4811Cells/ 5 HPFs vs. 0.9600 ± 0.2344Cells/5HPFs, p < 0.0001; LysMCre negative: 3.733 ± 0.8478Cells/ 5 HPFs vs. 0.5714 ± 0.2912Cells/5HPFs, p=0.0026). A significant increase in vein wall monocyte migration was found at 6 days post thrombosis in the LysMCre negative group, and a trending increase in the LysMCre positive group, compared to day 2 post thrombosis (LysMCre positive: 5.408 ± 0.5773Cells/ 5 HPFs vs. 4.020 ± 0.4811Cells/5HPFs, p=0.0679; LysMCre negative: 8.733 ± 1.655Cells/ 5 HPFs vs. 3.733 ± 0.8478Cells/5HPFs, p=0.0141). There were no significant differences between LysMCre negative and LysMCre positive mice at any time points [Figure 2B].
Figure 2A and Figure 2B. Vein Wall Morphometrics Composite.
average cell counts for each group (LysMCre +: n=5 for TC, n=10 for 2D and 6D thrombosis; LysMCre -: n=3 for all groups). TC=Non-thrombosed true controls, D=days post thrombosis, HPF=High power field. Both groups of mice (LysMCre + and LysMCre -) showed the same acute (neutrophil) to chronic (monocyte) inflammatory pattern. No differences were seen between the groups at any time-points.
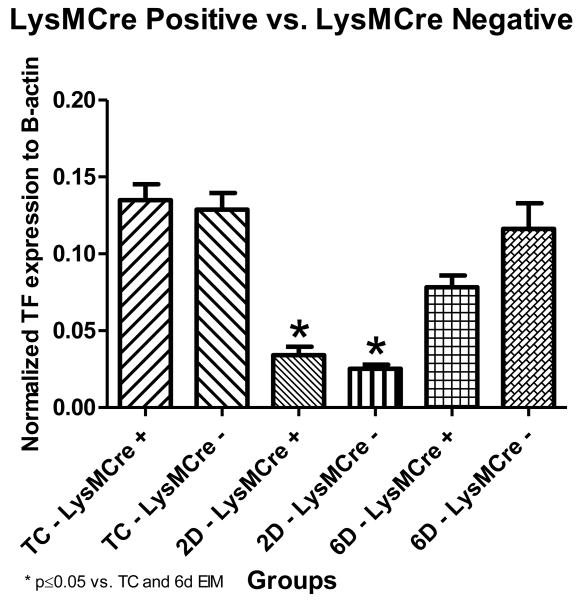
The absence of tissue factor in myeloid cells does not affect vein wall expression of tissue factor
No significant differences were observed in vein wall TF mRNA expression between LysMCre positive mice and their littermate controls (LysMCre negative mice) at any time point (Figure 3). Both groups showed a significant decrease in vein wall TF mRNA between the TC and 2 days post thrombosis (LysMCre positive: 0.1348 ± 0.01044 units normalized to β-actin vs. 0.0340 ± 0.005659 units normalized to β-actin, p < 0.0001; LysMCre negative: 0.1288 ± 0.01078 units normalized to β-actin vs. 0.0252 ± 0.002711 units normalized to β-actin, p=0.0026) before increasing at day 6. In addition, both the LysMCre negative and positive mice showed a significant increase in vein wall TF mRNA levels between 2 and 6 day post thrombosis (LysMCre positive: 0.0340 ± 0.005659 units normalized to β-actin vs. 0.0782 ± 0.007790, p=0.0004; LysMCre negative: 0.0252 ± 0.002711 units normalized to β-actin vs. 0.1163 ± 0.01658, p=0.0004) [Figure 3].
Figure 3. Tissue Factor Quantitative (Real-time) PCR.
*indicate significant differences, vs. non-thrombosed true controls and 6 days post thrombosis. (n=6 for TC, n=10 for 2D and 6D thrombosis). TC=Non-thrombosed true controls, D=days post thrombosis. There were no differences in vein wall TF mRNA levels between the LysMCre groups. LysMCre + mice showed no change in vein wall expression of tissue factor.
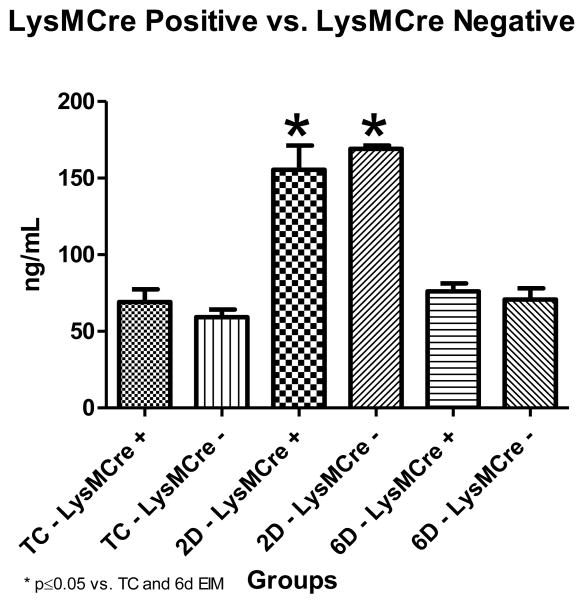
Plasma D-dimer Levels
Increases in circulating plasma D-dimer (fibrin degradation product) concentration can serve as an indication to active fibrinolysis post thrombosis. Evaluating LysMCre positive versus LysMCre negative animals showed no significant differences in D-dimer levels at any time point. Looking at temporal differences within the groups, both LysMCre positive and LysMCre negative mice had significant increases in plasma D-dimer levels between TC and 2 days post thrombosis. (LysMCre positive: 69.13 ± 8.247ng/mL vs. 155.4 ± 15.75ng/mL, p=0.0004; LysMCre negative: 59.25 ± 5.082ng/mL vs. 169.1 ± 2.173ng/mL, p=0.0025). In turn, there was a significant decrease in plasma D-dimer levels from 2 to 6 days post thrombosis (LysMCre positive: 155.4 ± 15.75ng/mL vs. 76.14 ± 5.097ng/mL, p=0.0007; LysMCre negative: 169.1 ± 2.173ng/mL vs. 70.70 ± 7.466ng/mL, p=0.0062) [Figure 4]. These findings demonstrate that peak fibrinolysis occurred 2 days post venous thrombosis in these animals.
Figure 4. Plasma D-dimer.
* indicate significant differences, vs. non-thrombosed true controls and 6 days post thrombosis. (LysMCre +: n=5 for TC, n=10 for 2D and 6D thrombosis; LysMCre -: n=3 for all groups). TC=Non-thrombosed true controls, D=days post thrombosis. LysMCre + and LysMCre - mice showed a peak in D-dimer levels at day 2 which returned to baseline levels by day 6 post thrombosis. The peak fibrinolysis occurred 2 days post venous thrombosis in both LysMCre groups.
Discussion
TF is important in venous thrombosis of the macrovasculature. Mice that had circulating and vascular wall TF inhibited by the use of a monoclonal, anti-mouse TF antibody (1H1) [17], had a significant decrease in total thrombus weight as documented at 2 day post thrombosis, compared to controls given rat IgG. This experiment showed that blocking TF, significantly decreases acute venous thrombus formation, and that TF is important to acute venous thrombogenesis in our animal model. There was no significant difference seen at day 6, suggesting that TF does not significantly contribute to propagation of thrombosis between day 2 and 6 post thrombosis. This finding is not unexpected since TF is thought to be involved in the initiation and acute stages of venous thrombosis [5, 6]. Other studies have also demonstrated the importance of TF in thrombus initiation using anti-TF antibody inhibition [4, 16, 27].
In the LyMCre positive mice that are TF deficient in myeloid cells, there was no effect on thrombus formation and total weight, versus their littermate controls (LysMCre negative mice). This led to the conclusion that in the thrombosis of DVT, myeloid cell derived TF has no contribution to venous thrombosis 2 and 6 days post-procedure. Thus TF from a non-myeloid source, most likely the vein wall, is the major contributor to venous thrombosis in this model. This finding is supported by other studies looking at the source of TF in venous thrombosis. Using a mouse IVC ligation model, Day et al. showed that mice with low levels of TF in hematopoietic cells developed a thrombus similar in size to the wild type controls. However, mice with low expression of TF in the vessel wall, regardless of hematopoietic cell expression, had significantly smaller thrombi [6]. Their conclusion was that TF is important in venous thrombosis and that hematopoetic cells were not a major source [6]. In our thrombosis model, the EIM needle is introduced into the lumen of the vein wall prior to electrolytic endothelial cell activation and injury. This process combined with electrolytic stimulation, could promote the exposure of vessel wall TF and other pro-inflammatory mediators like vein wall P-selectin, interleukin-1β, circulating P-selectin, in concert leading to the initiation of venous thrombogenesis as demonstrated by Alvarado et al. [22].
Thrombus formation initiates a local inflammatory response [28, 29]. During the acute stages of inflammation, neutrophils are the predominant cellular responder. Over time, monocytes increase at the site of thrombosis and are important in proteolytic digestion and resolution of the thrombus components [30-33]. Previous experience with venous thrombosis in our laboratory has shown that neutrophil migration through the vein wall increases early in thrombosis development (6 hour and 2 day), with a rapid decline over time. In contrast, monocytes have been shown to gradually increase with chronicity, surpassing neutrophil levels by day 6 [22, 25, 34, 35]. Using morphometric analysis of inflammatory cell migration through the vein wall during thrombosis, we demonstrated this acute to chronic inflammatory pattern in both the LysMCre positive and LysMCre negative mouse groups in this study. With regards to neutrophil migration, LysMCre positive and LysMCre negative mice both had a peak in neutrophil migration to the site of thrombosis at day 2 post thrombosis, followed by a significant decrease by day 6. In contrast, monocytes showed a gradual, but significant increase in migration at days 2 and day 6 post thrombosis, compared to non-thrombosed true controls. The peak in vein wall monocyte extravasation occurred at 6 days post thrombosis. Similar vein wall inflammatory cell extravasation patterns in wild type mice have been reported in both complete stasis induced and recent flow models of venous thrombosis [18, 19, 22, 25, 26]. Thus, the findings in this study suggest that myeloid cell TF is not required for promoting vein wall neutrophil and monocyte extravasations post venous thrombosis.
TF is constitutively expressed in the vein wall [36-38]. We quantified vein wall expression of TF mRNA at different stages of thrombosis. Both, LysMCre positive and LysMCre negative mice had a significant decrease in vein wall TF mRNA expression at day 2 post-EIM compared to TC before increasing at day 6 post-EIM [Figure 3]. We postulate that the TC value of TF mRNA in the vein wall represents the steady-state for TF expression in these cells. The decrease in vein wall TF mRNA at day 2 post-EIM may represent negative-feedback regulation of TF gene expression. Interestingly, a recent study [39] found that TF expression was down-regulated in cutaneous wounds.
D-dimer is a product of plasmin degradation of cross-linked fibrin [40]. D-dimer levels are elevated in initial thrombus formation [41] and serve as an indicator of fibrinolysis [42]. In this study D-dimer levels were used as a supporting indicator of thrombus development and resolution. In opposition to TF, which is important in thrombus initiation [4-6], D-dimer plasma levels increase at later time points in thrombosis, during fibrinolysis [42] and thrombus resolution. We showed that, both the LysMCre positive and LysMCre negative mice showed a peak in D-dimer levels at day 2 post thrombosis, which decreased to baseline levels by day 6, despite a large thrombus burden. This suggests that there is no active fibrinolysis occurring at day 6.
The study described focuses on the cellular contribution and role of tissue factor in and EIM of DVT formation in healthy mice. Although the EIM produces a DVT in the macrovascular without complete occlusion of the vessel, which is similar to the clinical picture in humans, there are some limitations to this model. In the mouse model, the DVT is created in the inferior vena cava with side branches ligated to maximize thrombus formation and size; however, in humans DVT is typically in a vein with different pathophysiologic features [18, 19]. These differences are examples of the inherent variables associated with use of animal models for human disease. The EIM is a well characterized venous thrombosis model which has the components of endothelial activation, blood stasis to some degree, and emphasizes the continuation of flow during thrombus formation. These elements are commonplace to clinical DVT. The findings in this study are important to understand the pathogenesis of DVT. Development of DVT in humans has been linked to numerous predisposing factors, such as malignant hematologic disorders, which may involve TF.
In conclusion, inhibition of all sources of TF with a mouse anti-TF antibody (1H1) showed that TF promotes early venous thrombus formation mouse thrombosis model. However, myeloid cell derived TF does not contribute to early venous thrombogenesis. This suggests that the vessel wall is the major source of TF that leads to venous thrombus.
Acknowledgments
Research supported by NIH grant 1K01HL080962-01A2 (DDM) and HL-006350 (NM). We also would like to thank the University of Michigan Medical School, Unit for Laboratory Animal Medicine for sponsoring the comparative medicine training program for Dr. Anna Hampton.
Abbreviations
- EIM
electrolytic inferior vena cava model
- IVC
inferior vena cava
- DVT
deep vein thrombosis
- TW
thrombus weight
- TF
tissue factor
Footnotes
Conflict of interest statement: The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heit JA, Cohen AT, Anderson FA Group VTEIA. Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (VTE) events in the US. Blood. 2005;106:267A–A. [Google Scholar]
- 2.Zhu T, Martinez I, Emmerich J. Venous thromboembolism: risk factors for recurrence. Arterioscler Thromb Vasc Biol. 2009;29:298–310. doi: 10.1161/ATVBAHA.108.182428. [DOI] [PubMed] [Google Scholar]
- 3.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–2. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Himber J, Wohlgensinger C, Roux S, Damico LA, Fallon JT, Kirchhofer D, et al. Inhibition of tissue factor limits the growth of venous thrombus in the rabbit. J Thromb Haemost. 2003;1:889–95. doi: 10.1046/j.1538-7836.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- 5.Nagai M, Yilmaz CE, Kirchhofer D, Esmon CT, Mackman N, Granger DN. Role of coagulation factors in cerebral venous sinus and cerebral microvascular thrombosis. Neurosurgery. 2010;66:560–5. doi: 10.1227/01.NEU.0000365745.49583.FD. discussion 5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day SM, Reeve JL, Pedersen B, Farris DM, Myers DD, Im M, et al. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105:192–8. doi: 10.1182/blood-2004-06-2225. [DOI] [PubMed] [Google Scholar]
- 7.Owens AP, 3rd, Mackman N. Tissue factor and thrombosis: The clot starts here. Thromb Haemost. 2010;104:432–9. doi: 10.1160/TH09-11-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greeno EW, Bach RR, Moldow CF. Apoptosis is associated with increased cell surface tissue factor procoagulant activity. Lab Invest. 1996;75:281–9. [PubMed] [Google Scholar]
- 9.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–97. [PMC free article] [PubMed] [Google Scholar]
- 10.Ranganathan G, Blatti SP, Subramaniam M, Fass DN, Maihle NJ, Getz MJ. Cloning of murine tissue factor and regulation of gene expression by transforming growth factor type beta 1. J Biol Chem. 1991;266:496–501. [PubMed] [Google Scholar]
- 11.Pawlinski R, Tencati M, Holscher T, Pedersen B, Voet T, Tilley RE, et al. Role of cardiac myocyte tissue factor in heart hemostasis. J Thromb Haemost. 2007;5:1693–700. doi: 10.1111/j.1538-7836.2007.02649.x. [DOI] [PubMed] [Google Scholar]
- 12.Edgington TS, Mackman N, Brand K, Ruf W. The structural biology of expression and function of tissue factor. Thromb Haemost. 1991;66:67–79. [PubMed] [Google Scholar]
- 13.Chou J, Mackman N, Merrill-Skoloff G, Pedersen B, Furie BC, Furie B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood. 2004;104:3190–7. doi: 10.1182/blood-2004-03-0935. [DOI] [PubMed] [Google Scholar]
- 14.Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis. 2006;36:104–7. doi: 10.1016/j.bcmd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Miller C, Swarthout RF, Rao M, Mackman N, Taubman MB. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood. 2009;113:705–13. doi: 10.1182/blood-2007-05-090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giesen PL, Rauch U, Bohrmann B, Kling D, Roque M, Fallon JT, et al. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci U S A. 1999;96:2311–5. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirchhofer D, Moran P, Bullens S, Peale F, Bunting S. A monoclonal antibody that inhibits mouse tissue factor function. J Thromb Haemost. 2005;3:1098–9. doi: 10.1111/j.1538-7836.2005.01253.x. [DOI] [PubMed] [Google Scholar]
- 18.Diaz JA, Hawley AE, Alvarado CM, Berguer AM, Baker NK, Wrobleski SK, et al. Thrombogenesis with continuous blood flow in the inferior vena cava. A novel mouse model. Thromb Haemost. 2010;104:366–75. doi: 10.1160/TH09-09-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz JA, Wrobleski SK, Hawley AE, Lucchesi BR, Wakefield TW, Myers DD., Jr Electrolytic Inferior Vena Cava Model (EIM) of Venous Thrombosis. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–77. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 21.Pawlinski R, Wang JG, Owens AP, 3rd, Williams J, Antoniak S, Tencati M, et al. Hematopoietic and non-hematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood. 2010 doi: 10.1182/blood-2009-12-259267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarado CM, Diaz JA, Hawley AE, Wrobleski SK, Sigler RE, Myers DD., Jr Male mice have increased thrombotic potential: Sex differences in a mouse model of venous thrombosis. Thromb Res. 2011;127:478–86. doi: 10.1016/j.thromres.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Institute of Laboratory Animal Resources (U.S.) Guide for the care and use of laboratory animals. 7th. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 24.Welfare NIoHUSOoLA. Publich Health Service policy on humane care and use of laboratory animals. Rev. August, 2002. Bethesda, MD: Office of Laboratory Animal Welfare, National Institutes of Health; 2002. [Google Scholar]
- 25.Myers D, Jr, Farris D, Hawley A, Wrobleski S, Chapman A, Stoolman L, et al. Selectins influence thrombosis in a mouse model of experimental deep venous thrombosis. J Surg Res. 2002;108:212–21. doi: 10.1006/jsre.2002.6552. [DOI] [PubMed] [Google Scholar]
- 26.Wakefield TW, Strieter RM, Wilke CA, Kadell AM, Wrobleski SK, Burdick MD, et al. Venous thrombosis-associated inflammation and attenuation with neutralizing antibodies to cytokines and adhesion molecules. Arterioscler Thromb Vasc Biol. 1995;15:258–68. doi: 10.1161/01.atv.15.2.258. [DOI] [PubMed] [Google Scholar]
- 27.Anthoni C, Russell J, Wood KC, Stokes KY, Vowinkel T, Kirchhofer D, et al. Tissue factor: a mediator of inflammatory cell recruitment, tissue injury, and thrombus formation in experimental colitis. J Exp Med. 2007;204:1595–601. doi: 10.1084/jem.20062354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saha P, Humphries J, Modarai B, Mattock K, Waltham M, Evans CE, et al. Leukocytes and the natural history of deep vein thrombosis: current concepts and future directions. Arterioscler Thromb Vasc Biol. 2011;31:506–12. doi: 10.1161/ATVBAHA.110.213405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakefield TW, Henke PK. The role of inflammation in early and late venous thrombosis: Are there clinical implications? Semin Vasc Surg. 2005;18:118–29. doi: 10.1053/j.semvascsurg.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Henke PK, Varga A, De S, Deatrick CB, Eliason J, Arenberg DA, et al. Deep vein thrombosis resolution is modulated by monocyte CXCR2-mediated activity in a mouse model. Arterioscler Thromb Vasc Biol. 2004;24:1130–7. doi: 10.1161/01.ATV.0000129537.72553.73. [DOI] [PubMed] [Google Scholar]
- 31.Humphries J, McGuinness CL, Smith A, Waltham M, Poston R, Burnand KG. Monocyte chemotactic protein-1 (MCP-1) accelerates the organization and resolution of venous thrombi. J Vasc Surg. 1999;30:894–9. doi: 10.1016/s0741-5214(99)70014-5. [DOI] [PubMed] [Google Scholar]
- 32.Ali T, Humphries J, Burnand K, Sawyer B, Bursill C, Channon K, et al. Monocyte recruitment in venous thrombus resolution. J Vasc Surg. 2006;43:601–8. doi: 10.1016/j.jvs.2005.10.073. [DOI] [PubMed] [Google Scholar]
- 33.Laouar A, Bauvois B. Characterization and modulation of cell surface proteases on human myeloblastic (HL-60) cells and comparison to normal myeloid cells. Immunol Lett. 1992;34:257–65. doi: 10.1016/0165-2478(92)90222-a. [DOI] [PubMed] [Google Scholar]
- 34.Myers DD, Jr, Rectenwald JE, Bedard PW, Kaila N, Shaw GD, Schaub RG, et al. Decreased venous thrombosis with an oral inhibitor of P selectin. J Vasc Surg. 2005;42:329–36. doi: 10.1016/j.jvs.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 35.Henke PK, Mitsuya M, Luke CE, Elfline MA, Baldwin JF, Deatrick KB, et al. Toll-like receptor 9 signaling is critical for early experimental deep vein thrombosis resolution. Arterioscler Thromb Vasc Biol. 2011;31:43–9. doi: 10.1161/ATVBAHA.110.216317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–97. [PMC free article] [PubMed] [Google Scholar]
- 37.Greeno EW, Bach RR, Moldow CF. Apoptosis is associated with increased cell surface tissue factor procoagulant activity. Lab Invest. 1996;75:281–9. [PubMed] [Google Scholar]
- 38.Ranganathan G, Blatti SP, Subramaniam M, Fass DN, Maihle NJ, Getz MJ. Cloning of murine tissue factor and regulation of gene expression by transforming growth factor type beta 1. J Biol Chem. 1991;266:496–501. [PubMed] [Google Scholar]
- 39.McDonald AG, Yang K, Roberts HR, Monroe DM, Hoffman M. Perivascular tissue factor is down-regulated following cutaneous wounding: implications for bleeding hemophilia. Blood. 2008;111:2046–8. doi: 10.1182/blood-2007-05-092916. [DOI] [PubMed] [Google Scholar]
- 40.Pizzo SV, Schwartz ML, Hill RL, McKee PA. The effect of plasmin on the subunit structure of human fibrin. J Biol Chem. 1973;248:4574–83. [PubMed] [Google Scholar]
- 41.Speiser W, Mallek R, Koppensteiner R, Stumpflen A, Kapiotis S, Minar E, et al. D-dimer and TAT measurement in patients with deep venous thrombosis: utility in diagnosis and judgement of anticoagulant treatment effectiveness. Thromb Haemost. 1990;64:196–201. [PubMed] [Google Scholar]
- 42.Janssen MC, Verbruggen H, Wollersheim H, Hoogkamer B, van Langen H, Novakova IR. D-dimer determination to assess regression of deep venous thrombosis. Thromb Haemost. 1997;78:799–802. [PubMed] [Google Scholar]