Abstract
Tissue factor (TF)-initiated coagulation plays a critical role in both hemostatsis and thrombosis. It is generally believed that most of the tissue factor expressed on cell surfaces is maintained in a cryptic, i.e., coagulantly inactive state and an activation step (decryption) is required for the expression of maximum TF procoagulant activity. However, what exactly constitutes cryptic or procoagulant TF, molecular differences between these two forms and mechanisms that are responsible for transformation from one to the other form are not entirely clear and remain highly controversial, thus are a matter of ongoing debate. This brief review discusses pertinent literature on TF encryption/decryption with specific emphasis on the role of membrane phospholipids and reduction/oxidation of the TF Cys186–Cys209 disulfide bond in regulating TF activity at cell surfaces.
Keywords: Decryption, Encryption, Factor VIIa, Phosphatidylserine, Protein disulfide isomerase, Tissue factor
Introduction
Tissue factor (TF) is a cell surface transmembrane glycoprotein that is responsible for initiating blood clotting following vascular injury. TF binds to both the single chain zymogen factor VII (FVII) and the two-chain enzyme factor VIIa (FVIIa), facilitate the conversion of FVII to FVIIa and allosterically activate FVIIa to trigger the activation of the coagulation cascade. TF is essential for hemostasis, but the aberrant activation of TF-mediated coagulation leads to intravascular thrombus formation, the precipitating event in acute myocardial infarction, unstable angina and ischemic stroke. The proper regulation of TF expression is critical for maintenance of the hemostatic balance. It is generally believed that most of the TF on surfaces of resting cells exist in an encrypted state with very little procoagulant activity, and it must undergo transformation to become fully active. However, the cellular microenvironment on the plasma membrane that controls TF activity and cellular mechanisms that are responsible for TF decryption are poorly defined. The present review aims to discuss the importance of phosphatidylserine in TF decryption, and recent conflicting data in the literature on the importance of protein disulfide isomerase (PDI)-mediated Cys186–Cys209 disulfide bond switching in regulating TF coagulant activity. This brief review represents the authors’ perspective and the reader is encouraged to refer recent reviews from us and others [1–4] for a broader perspective of this debate.
TF encryption
TF encryption was originally defined as the post-translational suppression of TF procoagulant activity on the cell surface where TF, despite expression on the cell surface and capacity to bind FVII or FVIIa, fails to activate factors IX or X (see rev [3]). Broadening the use of the term encryption to describe any TF form that exhibits low procoagulant activity, including TF forms that have severe impairment in FVIIa binding, in our view is misappropriation of the terminology. Such non-discriminate usage of the term TF encryption makes it difficult to compare and reconcile the published data on TF encryption/decryption and partly responsible for recent controversies in the field. One could avoid this problem by defining TF decryption as a process which expresses the latent proteolytic activity of the encrypted TF-FVIIa complex, rather than an increased procoagulant activity of encrypted TF. This requires relating TF procoagulant activity to TF-FVIIa complexes and not merely TF protein levels at the cell surface.
Expression of the latent proteolytic activity of the encrypted TF-FVIIa complex often requires a stimulus. At present it is unclear whether TF procoagulant activity at the cell surface is actively suppressed by a specific mechanism or if a component that may be essential for TF procoagulant activity is missing from the cell surface. It has been known for quite some time that TF procoagulant activity requires its association with phospholipids [5;6], and the presence of anionic phospholipids, such as phosphatidylserine (PS), in the phospholipid mixture greatly accelerates TF procoagulant activity [7–12]. The outer leaflet of the plasma membrane is enriched with phosphatidylcholine (PC) and sphingomyelin (SM) and contains only traces of PS, whereas the inner leaflet is abundant with PS, PE, phosphoinositide and phosphatidic acid. Thus, unavailability of anionic phospholipids at the cell surface is thought to be responsible for TF encryption. Recent studies have suggested that the disulfide bond status of Cys186–Cys209 in the extracellular domain of TF dictates whether TF at the cell surface is encrypted or procoagulantly active [13;14]. According to this hypothesis, TF with unpaired cysteine thiols at Cys 186 and Cys 209 is encrypted TF whereas TF with Cys186–Cys209 disulfide bond is the procoagulant TF, and PDI plays a critical role in transforming cryptic TF to active TF and vice versa by switching on/off the Cys186–Cys209 disulfide bond [13;14].
Tissue factor decryption: Role for phosphatidylserine
All stimuli that are known to increase TF procoagulant activity on cell surfaces without increasing TF protein levels - i.e., freezing and thawing, sonication, nonionic detergents, proteases, phospholipases, apoptosis, complement, calcium ionophores, oxidizing agents and sulfhydryl reactive compounds - also increase invariably the exposure of PS on the outer cell surface [8;13;15–23]. A number of studies with relipidated TF protein have established that the presence of anionic phospholipids, such as phosphatidylserine (PS) in the phospholipid mixture greatly accelerates TF procoagulant activity [7–12]. Thus, it is reasonable to conclude that exposure of PS or certain other phospholipids on the outer leaflet of the plasma membrane are responsible for TF decryption following various stimuli. However, the mechanism(s) through which the increased phosphatidylserine converts cryptic TF to procoagulant TF at the cell surface is not entirely clear.
There are at least two possible mechanisms by which increased anionic phospholipid could enhance TF-FVIIa proteolytic activation of FX (and FIX) at the cell surface. One of the mechanisms could be that the increased exposure of PS at the outer leaflet of the plasma membrane could increase the binding of FX to the membrane, which can effectively increase the interaction of the membrane-anchored TF-FVIIa complex with the membrane bound substrate. It is widely accepted that the ability of PS (and other anionic phospholipids) to bind vitamin-K dependent clotting proteins via their γ–carboxyglutamic acid-containing domain (Gla domain), which effectively increases the local concentrations of these proteins on the lipid surface, greatly accelerates the rate of catalysis of the clotting reactions. Although Forman and Nemerson [24] reported that TF-FVIIa activation of FX is independent of the concentration of phospholipid bound FX, others have found that the substrate-membrane interaction must precede catalysis for the efficient activation of FX by TF-FVIIa [9]. In this scenario, the major effect of PS in increased catalysis of FX comes from decreasing the apparent Km for FX [11;25]. However, the increased catalytic activity of TF-FVIIa toward FX activation following decryption of TF in fibroblasts treated with sulfhydryl reactive compound N-ethylmaleimide was found to come primarily from an increase in Vmax without an accompanying decrease in the apparent Km [20]. Although NEM treatment increased the exposure of PS at the outer leaflet of fibroblasts and the binding of FX to the cells, this increased binding of FX to the cell surface is not responsible for increased TF-FVIIa catalysis of FX [20]. The blockade of FX binding to the cell surface with a 10-fold molar excess of competing substrate prothrombin fragment 1 or annexin V failed to attenuate the increased TF-FVIIa activation of FX [20]. Thus, it is unlikely that enhanced binding of the substrate to the outer leaflet of the plasma membrane serves as the primary mechanism involved in TF-FVIIa decryption.
A second mechanism, which probably is a more likely mechanism but difficult to prove unequivocally at present, is the increased concentration of PS in the outer leaflet of the plasma membrane converts the inactive TF-FVIIa complexes to coagulant active TF-FVIIa complexes [3]. In this model, a direct interaction between PS and encrypted TF or TF-FVIIa would result in structural changes in TF that expose the macromolecular substrate binding sites for factors X and IX on TF. Consistent with such a possibility, recent molecular modeling has indicated potential direct interactions between the phospholipid membrane and TF [26]. At present, it is unclear what could be the PS-induced structural changes in TF at the cell surface. One of the proposed PS-induced changes in TF structure may involve the interactions between PS polar head groups and Lys165/Lys166 in the TF extracellular domain [3;18]. It has been suggested that possible electrostatic interactions between PS polar head groups and Lys165/Lys166 could change TF quaternary structure by altering the orientation of the extracellular domain relative to the membrane surface, which may facilitate the precise alignment of the TF-FVIIa active site to the scissile bonds of the membrane bound factors X and IX [3;18]. Such precise alignment may also be achieved by FVIIa (in TF-FVIIa complex) binding to the exposed PS on the cell surface via its Gla domain, which restricts the orientation of TF-FVIIa complex relative to the membrane surface. Here, it may be pertinent to note that a recent study indicates that any phospholipids with headgroup other than choline can strongly synergize with PS to enhance TF-FVIIa activation of FX in TF liposomes [27]. Thus it is possible that, in addition to PS, exposure of other phospholipids (anything but choline) along with PS could also play a role in TF decryption.
The role of PDI-mediated oxidation/reduction of TF Cys186/Cys209 in TF encryption/decryption
The extracellular domain of TF has two disulfide bonded loops (Cys49–Cys57 and Cys186–Cys209). Site-directed mutagenesis to selectively preclude the formation of disulfide loops in TF by pairwise substitution of cysteines with serine revealed that the Cys49–Cys57 bond is not essential for the proper folding of TF or for its procoagulant function, whereas the Cys186–Cys209 disulfide bond is required to maintain proper conformation, FVIIa binding and procoagulant activity [28]. Studies with purified TF showed no free sulfhydryl groups, indicating that the four cysteines in the extracellular domain of TF exist as disulfide linked cystines [29;30]. Nonetheless, it has been proposed recently that at least a fraction of TF on cell surfaces exists with unpaired cysteine thiols at Cys 186 and Cys 209 that constitutes cryptic TF and TF decryption involves the formation of the Cys186–Cys209 disulfide bond [13]. This hypothesis is firmly based on observations that ablation of the disulfide bond by mutating both cysteines to serine or alanine severely impaired TF procoagulant activity [14;28] and that treatment of cells with HgCl2, which oxidizes thiols to disulfides, increased TF activity at cell surfaces [13;31]. In a parallel publication, the same group of investigators also suggested that protein disulfide isomerase (PDI) plays a critical role in the disulfide isomerization of TF [14]. However, our studies failed to support this hypothesis [32]. Although we also found that exposure of cells to HgCl2 increased the cell surface TF coagulant activity as reported, the increase appeared to stem from the increased anionic phospholipids at the cell surface upon the exposure of cells to HgCl2 [32;33]. Further, no evidence was found for the presence of PDI at cell surfaces or association of PDI with TF [32]. Attenuation of PDI expression in cells or exogenous addition of PDI had no effect on TF procoagulant activity on cell surfaces [32]. Although recent studies of Furlan-Freguia et al. [34] suggest a regulatory role for PDI in P2X7 signaling-dependent TF decryption, there was no evidence in these studies for that TF decryption involves PDI-mediated thiol exchange reactions in TF.
It has been suggested that differences in cell-model systems might be responsible for opposing conclusions on the importance of disulfide isomerization in TF encryption/de-encryption [35]. However, this suggestion has been firmly repudiated [36]. In addition to us, others also questioned the validity of this model based on the lack of accessibility of the half-cystines for the proposed redox reactions [37] and the oxidative properties of HgCl2, which commonly oxidizes only single thiol groups [2]. More importantly, even after 5 years of original publications proposing the above hypothesis, still there is no direct quantitative evidence for the presence of free thiols in TF at the cell surface. Given that a majority of TF on many cell types exists as cryptic TF, if TF with free thiols is the cryptic TF, there should be no major technical limitation to prove this unequivocally.
Despite the lack of convincing evidence, the hypothesis that PDI plays a critical role in TF decryption gained much support from in vivo studies that showed PDI plays a critical role in thrombus formation [38;39]. Although these studies provide no direct evidence that TF actually exists in the reduced form in vivo, PDI decrypts TF by forming the Cys186–Cys209 disulfide bond, or that the decrypted TF is responsible for thrombus formation, they were viewed without critical evaluation as evidence that PDI-mediated TF decryption is responsible for thrombus formation.
In studies performed subsequent to the above in vivo findings, we convincingly showed that although the Cys186–Cys209 disulfide bond is critical for TF synthesis/processing and for FVIIa binding, it is not essential for TF decryption per se [33]. These studies showed that cells expressing the disulfide mutated TF exhibit low procoagulant activity because it binds FVIIa poorly, and use of high concentrations of FVIIa to allow binding of FVIIa to the mutant TF restored the procoagulant activity of this TF mutant [33]. Ruf and Versteeg [40] questioned the conclusion that the allosteric disulfide bond is not essential for TF’s procoagulant activity since the data reaching this conclusion were obtained at supraphysiological concentrations of FVIIa. However, it is important to note that since the physiological concentration of FVIIa was not sufficient to saturate the TF mutants at the cell surface, the use of higher concentrations of FVIIa in these studies is appropriate, necessary and well-justified [41].
Recently, van den Hengel et al. [42] reported that TFC209A mutant expressed in BHK cells exhibited no procoagulant activity even at supraphysiologic FVIIa concentrations. However, in the absence of specific data demonstrating that FVIIa actually bound to the TF mutant in this study, it is not feasible to conclude these data meaningfully. It is interesting to note that in the same study the authors have observed a substantial TF procoagulant activity, ~50% of the wild-type TF, in cells expressing TFC209S mutant [42].
Recently, Liang et al. [43] investigated the redox properties of the TF Cys186–Cys209 disulfide bond of recombinant wild-type sTF using oxidized and reduced dithiothreitol and found that the redox potential for the Cys186–Cys209 bond is in the range of the standard potentials of other RHStaple disulfides and a small fraction (~10%) of the recombinant wild-type sTF contained unpaired Cys186/Cys209 thiols. However, more recently Krudysz-Amblo et al. [30] provided very compelling evidence for non-existence of free thiols in both recombinant full-length TF and natural placental TF as all four cysteines are quantitatively involved in disulfide bridges. More importantly, TF with reduced or non-reduced cysteines, with or without alkylation, exhibited similar activity to that of the TF-FVIIa complex in membrane-dependent FX activation, indicating allosteric cysteine oxidation does not play a role in TF decryption.
Finally, PDI has also been shown to regulate TF activity by altering PS levels at the cell surface [44] or by chaperone function [45] on microvesicle- or soluble TF. The later observation was confounded by the fact that bovine PDI used in this study may be contaminated with PS [46;47] although it was reported recently that acetone extraction to remove any potential contaminant did not reduce the enhancing effect of PDI [48]. Merits and loopholes in these mechanisms have been discussed in a recent review [1].
Limitation in methodology in investigating TF encryption/decryption: Probable causes for controversies
The functional characteristics used to differentiate cryptic and procoagulant TF are largely arbitrary and non-quantifiable as there are no unique reagents at present that specifically recognize either cryptic or procoagulant TF. Although FVIIa is thought to have a lower affinity for cryptic TF relative to procoagulant TF, the difference between them may be trivial as the plasma concentration of FVII (10 nM) is sufficient to saturate all TF sites on cell surfaces. This could explain why 125I-FVIIa binding studies often fail to show that FVIIa binds to TF on cell surfaces with two different affinities [15;49–53]. Further, comparison of the affinities derived from functional activity assays (which often do not involve multiple washings) and radioligand binding assays (requires multiple washings) is error prone as it amplifies subtle differences that may exist in FVIIa affinity for procoagulant and cryptic TF [54].
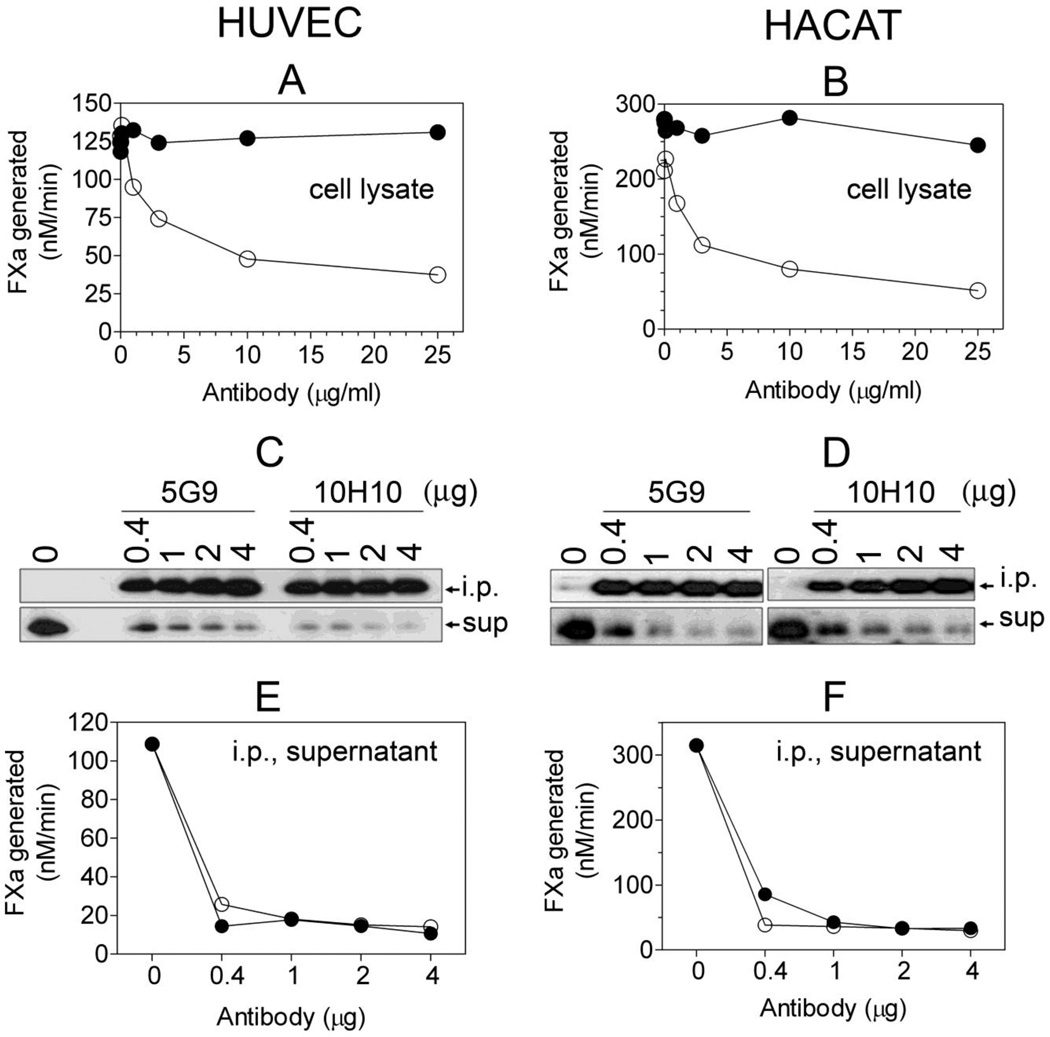
Interpretation of the data obtained with various TF mAb that are not fully characterized, which could exhibit differential sensitivities in recognizing TF antigen in different assays, requires cautious approach. For example, we have found that TF mAb 5G9, which effectively inhibits TF procoagulant activity, binds native TF at the cell surface and in cell lysates with good avidity but reacts poorly with TF on fixed cells or by Western blot analysis (unpublished data). Similarly, we found recently that all TF mAb tested failed to recognize effectively non-glycosylated TF mutants by Western blot analysis while they were fully capable of binding to the non-glycosylated TF at the cell surface [55]. Based on the observations that mAb 10H10 selectively blocks TF-FVIIa signaling but not TF procoagulant activity, it has been assumed that mAb 10H10 specifically recognizes cryptic TF and not the procoagulant TF [14]. A recent study, which showed that mAb 10H10 immunoprecipitated the majority of TF from the cell lysates of keratinocytes without depleting the TF procoagulant activity, further supported this notion [4]. However, we found no significant differences in the binding of mAb 10H10 and other TF mAb antibodies to cell surface TF regardless of whether TF is procoagulantly active or not [33;56]. More importantly, in contrast to the recently published study [4], we found that immunoprecipitation of cell lysates of keratinocytes as well as cytokine-stimulated endothelial cells with 10H10 mAb not only immunoprecipitated most of the TF antigen but also TF procoagulant activity (Fig. 1). It has been reported recently that mAb 5G9 recognized the wild-type TF and not the disulfide-mutated TF at the cell surface in immunofluorescence microscopy [42], implying that 5G9 poorly recognizes the cryptic TF. However, our radioligand binding studies clearly showed no significant differences between 5G9 and 10H10 binding to wild-type TF and various disulfide-mutated TF [33].
Fig 1.
TF mAb 10H10 does not inhibit TF procoagulant activity but efficiently depletes the procoagulant TF from cell lysates following immunoprecipitation. Human umbilical vein endothelial cells (HUVEC) and human keratinocytes (the HaCaT cell line) were cultured as monolayers in 100 mm culture dishes. HUVEC were stimulated with TNFα and IL-1β (20 ng/ml each) for 6 h. The cells were harvested in HEPES buffer containing 50 mM octylglucopyranoside, 1 ml/dish for HUVEC and 4 ml/dish for HaCaT). A and B: cell lysates were incubated with varying concentrations of TF mAb 5G9 or 10H10 for 1 h and the residual TF activity was analyzed in FX activation assay after adding FVIIa (10 nM) and FX (175 nM); C and D: cell lysates (200 µl) were incubated with the indicated amounts of TF mAb 5G9 or 10H10 for 2 h, the immunocomplexes were captured on protein G-agarose beads, and TF in immunoprecipitates (i.p.) or depleted supernatants (sup) was detected by Western blotting using a TF polyclonal antibody; E and F, residual TF activity in i.p. depleted supernatants was measured with FVIIa (10 nM) and FX (175 nM). Cell lysates and immunodepleted supernatants were diluted 10 to 100-fold for measuring TF activity. The symbols are: (○), 5G9; (●), 10H10.
Data obtained with TF Cys186/Cys209 disulfide mutants were used either to support or refute the hypothesis that reduction/oxidation of these cysteines plays an important role in TF encryption and decryption. The procoagulant activity of these mutants varied considerably in various studies, from no activity to similar activity as that of wild-type TF with significant differences among Cys186, Cys209 and Cys186/Cys209 mutants [14;28;33;40–42], even in studies from the same group [14;28;40;42]. Such varied results suggest that these mutants may have varied levels of structural deficiencies. Lack of procoagulant activity of these mutants in some reported studies may reflect unknown structural variance rather than them mimicking cryptic TF.
Annexin V, a protein that binds to PS in a calcium-dependent manner, is used commonly to investigate the role of PS in blood clotting reactions, including TF decryption. In a few earlier reports, based on the observation that annexin V treatment failed to completely block calcium ionophore-induced TF decryption, it had been suggested that TF could undergo decryption independent of PS [13;19]. The phospholipid binding property of annexin V is complex. Annexin V may not be very effective in blocking all PS on the cell surface and/or may require much higher concentrations to inhibit PS on cell surfaces than that are needed to inhibit PS in liposomes [57]. Annexin V binding to PS also depends on calcium concentration as well as the microenvironment in which PS is located [58]. Steric hindrance by membrane associated proteins and cofactor environment in the membrane may also influence annexin V binding to PS on cell surfaces [57;59]. Therefore, one may have to exercise caution in interpreting negative data obtained with annexin V in determining the role of phospholipids in TF decryption.
Conclusions
It is generally agreed that exposure of procoagulant PS is a major determinant in TF decryption. However, a role for PS does not automatically exclude the involvement of other mechanisms in TF decryption. It is possible that TF may undergo decryption independent of PS. Thus, recent studies that postulate that PDI and thiol pathways play an important role in TF encryption/decryption have gained much attention. However, despite many publications in the last few years on this subject, there is no convincing evidence to date that cryptic and procoagulant TF structurally differ with regard to the free thiols, disulfide bond status, or other post-translational modifications such as S-nitrosylaton, glutathionation or mixed disulfides. Further, there is no direct evidence for PDI-mediated modification of disulfide bond in TF. It is likely that PDI and thiol pathways, which influence various cellular functions or proteins, could regulate TF procoagulant activity or TF-dependent thrombosis in mechanisms not related to the disulfide bond. However, such mechanisms need to be defined more clearly and should be distinguished from PDI-mediated oxidation of cysteines in TF.
Acknowledgement
This work was supported by grants (HL58869 and HL65500) from National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None.
References
- 1.Rao LVM, Kothari H, Pendurthi UR. Tissue factor: Mechanisms of decryption. Front Biosci. 2012;E4:1513–1527. doi: 10.2741/477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butenas S, Orfeo T, Mann KG. Tissue factor in coagulation: Which? Where? When? Arterioscler Thromb Vasc Biol. 2009;29:1989–1996. doi: 10.1161/ATVBAHA.108.177402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach RR. Tissue factor encryption. Arterioscler Thromb Vasc Biol. 2006;26:456–461. doi: 10.1161/01.ATV.0000202656.53964.04. [DOI] [PubMed] [Google Scholar]
- 4.Versteeg HH, Ruf W. Thiol pathways in the regulation of tissue factor prothrombotic activity. Curr Opin Hematol. 2011;18:343–348. doi: 10.1097/MOH.0b013e32834981de. [DOI] [PubMed] [Google Scholar]
- 5.Chargaff E. Remarks on the role of lipids in blood coagulation. Arch Sci Physiol. 1948;2:269–271. [Google Scholar]
- 6.Nemerson Y. The phospholipid requirement of tissue factor in blood coagulation. J Clin Invest. 1968;47:72–80. doi: 10.1172/JCI105716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjorklid E, Storm E. Purification and Some Properties of the Protein Component of Tissue Thromboplastin from Human Brain. Biochem J. 1977;165:89–96. doi: 10.1042/bj1650089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bach R, Rifkin DB. Expression of tissue factor procoagulant activity: regulation by cytosolic calcium. Proc Natl Acad Sci U S A. 1990;87:6995–6999. doi: 10.1073/pnas.87.18.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnaswamy S, Field KA, Edgington TS, Morrissey JH, Mann KG. Role of the membrane surface in the activation of human coagulation factor X. J Biol Chem. 1992;267:26110–26120. [PubMed] [Google Scholar]
- 10.Rapaport SI, Rao LVM. The tissue factor pathway: How it has become a "prima ballerina". Thromb Haemost. 1995;74:7–17. [PubMed] [Google Scholar]
- 11.Neuenschwander PF, Bionco-Fisher E, Rezaie AR, Morrissey JH. Phosphatidylethanolamine augments factor VIIa-tissue factor activity: Enhancement of sensitivity to phosphatidylserine. Biochem. 1995;34:13988–13993. doi: 10.1021/bi00043a004. [DOI] [PubMed] [Google Scholar]
- 12.Shaw AW, Pureza VS, Sligar SG, Morrissey JH. The local phospholipid environment modulates the activation of blood clotting. J Biol Chem. 2007;282:6556–6563. doi: 10.1074/jbc.M607973200. [DOI] [PubMed] [Google Scholar]
- 13.Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochem. 2006;45:12020–12028. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- 14.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci U S A. 2006;103:13932–13937. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le DT, Rapaport SI, Rao LVM. Relations between factor VIIa binding and expression of factor VIIa/tissue factor catalytic activity on cell surfaces. J Biol Chem. 1992;267:15447–15454. [PubMed] [Google Scholar]
- 16.Maynard JR, Heckman CA, Pitlick FA, Nemerson Y. Association of tissue factor activity with the surface of cultured cells. J Clin Invest N Y. 1975;55:814–822. doi: 10.1172/JCI107992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carson SD, Archer PG. Tissue factor activity in Hela cells measured with a continuous chromogenic assay and elisa reader. Thromb Res. 1986;41:185–195. doi: 10.1016/0049-3848(86)90228-8. [DOI] [PubMed] [Google Scholar]
- 18.Bach RR, Moldow CF. Mechanism of tissue factor activation on HL-60 cells. Blood. 1997;89:3270–3276. [PubMed] [Google Scholar]
- 19.Wolberg AS, Monroe DM, Roberts HR, Hoffmann MR. Tissue factor de-encryption:ionophore treatment induces changes in tissue factor activity by phosphatidylserine-dependent and -independent mechanisms. Blood Coag Fibrinol. 1999;10:201–210. [PubMed] [Google Scholar]
- 20.Le DT, Rapaport SI, Rao LVM. Studies of the mechanism for enhanced cell surface factor VIIa/tissue factor activation of factor X in fibroblast monolayers after their exposure to N-ethylmalemide. Thromb Haemost. 1994;72:848–855. [PubMed] [Google Scholar]
- 21.Carson SD, Johnson DR. Consecutive Enzyme Cascades: Complement Activation at the Cell Surface Triggers Increased Tissue Factor Activity. Blood. 1990;76:361–367. [PubMed] [Google Scholar]
- 22.Carson SD. Manifestation of cryptic fibroblast tissue factor occurs at detergent concentrations which dissolve the plasma membrane. Blood Coag Fibrin. 1996;7:303–313. doi: 10.1097/00001721-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Otnaess AB, Prydz H, Bjorklid E, Berre A. Phospholipase C from Bacillus cereus and its use in studies of tissue thromboplastin. Eur J Biochem. 1972;27:238–243. doi: 10.1111/j.1432-1033.1972.tb01832.x. [DOI] [PubMed] [Google Scholar]
- 24.Forman SD, Nemerson Y. Membrane-dependent coagulation reaction is independent of the concentration of phospholipid-bound substrate: fluid phase factor X regulates the extrinsic system. Proc Natl Acad Sci U S A. 1986;83:4675–4679. doi: 10.1073/pnas.83.13.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruf W, Rehemtulla A, Morrissey JH, Edgington TS. Phospholipid-independent and -dependent interactions required for tissue factor receptor and cofactor function. J Biol Chem. 1991;266:2158–2166. [PubMed] [Google Scholar]
- 26.Ohkubo YZ, Morrissey JH, Tajkhorshid E. Dynamical view of membrane binding and complex formation of human factor VIIa and tissue factor. J Thromb Haemost. 2010;8:1044–1053. doi: 10.1111/j.1538-7836.2010.03826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavoosi N, Davis-Harrison RL, Pogorelov TV, Ohkubo YZ, Arcario MJ, Clay MC, Rienstra CM, Tajkhorshid E, Morrissey JH. Molecular determinants of phospholipid synergy in blood clotting. J Biol Chem. 2011;286:23247–23253. doi: 10.1074/jbc.M111.251769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rehemtulla A, Ruf W, Edgington TS. The integrity of the cysteine 186-cysteine 209 bond of the second disulfide loop of tissue factor is required for binding of factor VII. J Biol Chem. 1991;266:10294–10299. [PubMed] [Google Scholar]
- 29.Bach R, Konigsberg WH, Nemerson Y. Human tissue factor contains thioester-linked palmitate and sterate on the cytoplasmic half-cystine. Biochem. 1988;14:4227–4231. doi: 10.1021/bi00412a004. [DOI] [PubMed] [Google Scholar]
- 30.Krudysz-Amblo J, Jenny RJ, Knigy T, Matthews D, Mann KG, Butenas S. Allosteric cysteine oxidation does not play a role in tissue factor decryption. 2011 [Google Scholar]
- 31.Kaneko H, Kakkar VV, Scully MF. Mercury compounds induce a rapid increase in procoagulant activity of monocyte-like U937 cells. Br J Haematol. 1994;87:87–93. doi: 10.1111/j.1365-2141.1994.tb04875.x. [DOI] [PubMed] [Google Scholar]
- 32.Pendurthi UR, Ghosh S, Mandal SK, Rao LV. Tissue factor activation: is disulfide bond switching a regulatory mechanism? Blood. 2007;110:3900–3908. doi: 10.1182/blood-2007-07-101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kothari H, Nayak RC, Rao LV, Pendurthi UR. Cystine186-cystine 209 disulfide bond is not essential for the procoagulant activity of tissue factor or for its de-encryption. Blood. 2010;115:4273–4283. doi: 10.1182/blood-2009-09-241356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furlan-Freguia C, Marchese P, Gruber A, Ruggeri ZM, Ruf W. P2X7 receptor signaling contributes to tissue factor-dependent thrombosis in mice. J Clin Invest. 2011;121:2932–2944. doi: 10.1172/JCI46129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang HPH, Hogg PJ. Critical importance of the cell system when studying tissue factor de-encryption. Blood. 2008;112:912–913. doi: 10.1182/blood-2008-05-158766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pendurthi UR, Rao LVM. Tissue factor de-encyrption: the cell model sytem. Blood. 2008;112:913. [Google Scholar]
- 37.Bach RR, Monroe D. What is wrong with the allosteric disulfide bond hypothesis? Arterioscler Thromb Vasc Biol. 2009;29:1997–1998. doi: 10.1161/ATVBAHA.109.194985. [DOI] [PubMed] [Google Scholar]
- 38.Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–1122. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118:1123–1131. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruf W, Versteeg HH. Tissue factor mutated at the allosteric Cys186–Cys209 disulfide bond is severely impaired in decrypted procoagulant activity. Blood. 2010;116:500–501. doi: 10.1182/blood-2010-04-281287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kothari H, Rao LVM, Pendurthi UR. Cys186–Cys209 disulfide-mutated tissue factor does not equal cryptic tissue factor: no impairment in decryption of disulfide mutated tissue factor. Blood. 2010;116:502–503. [Google Scholar]
- 42.van den Hengel LG, Kocaturk B, Reitsma PH, Ruf W, Versteeg HH. Complete abolishment of coagulant activity in monomeric disulfide-deficient tissue factor. Blood. 2011;118:3446–3448. doi: 10.1182/blood-2011-06-364612. [DOI] [PubMed] [Google Scholar]
- 43.Liang HP, Brophy TM, Hogg PJ. Redox properties of the tissue factor Cys186–Cys209 disulfide bond. Biochem J. 2011;437:455–460. doi: 10.1042/BJ20110718. [DOI] [PubMed] [Google Scholar]
- 44.Popescu NI, Lupu C, Lupu F. Role of PDI in regulating tissue factor: FVIIa activity. Thromb Res. 2010;125(Suppl 1):S38–S41. doi: 10.1016/j.thromres.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Versteeg HH, Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J Biol Chem. 2007;282:25416–25424. doi: 10.1074/jbc.M702410200. [DOI] [PubMed] [Google Scholar]
- 46.Kothari H, Sen P, Pendurthi UR, Rao LV. Bovine protein disulfide isomerase-enhanced tissue factor coagulant function: is phospholipid contaminant in it the real culprit? Blood. 2008;111:3295–3296. doi: 10.1182/blood-2007-12-129825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Persson E. Protein disulfide isomerase has no stimulatory chaperone effect on factor X activation by factor VIIa-soluble tissue factor. Thromb Res. 2008;123:171–176. doi: 10.1016/j.thromres.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 48.Raturi A, Ruf W. Effect of protein disulfide isomerase chaperone activity inhibition on tissue factor activity. J Thromb Haemost. 2010;8:1863–1865. doi: 10.1111/j.1538-7836.2010.03918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drake TA, Ruf W, Morrissey JH, Edgington TS. Functional tissue factor is entirely cell surface expressed on lipopolysaccharide-stimulated human blood monocytes and a constitutively tissue factor-producing neoplastic cell line. J Cell Biol. 1989;109:389–395. doi: 10.1083/jcb.109.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ploplis VA, Edgington TS, Fair DS. Initiation of the extrinsic pathway of coagulation. Association of factor VIIa with a cell line expressing tissue factor. J Biol Chem. 1987;262:9503–9508. [PubMed] [Google Scholar]
- 51.Fair DS, MacDonald MJ. Cooperative interaction between factor VII and cell surface-expressed tissue factor. J Biol Chem. 1987;262:11692–11698. [PubMed] [Google Scholar]
- 52.Sakai T, Lund-Hansen T, Paborsky L, Pedersen AH, Kisiel W. Binding of human factors VII and VIIa to a human bladder carcinoma cell line (J82) J Biol Chem. 1989;264:9980–9988. [PubMed] [Google Scholar]
- 53.Broze GJ., Jr. Binding of human factor VII and VIIa to monocytes. J Clin Invest N Y. 1982;70:526–535. doi: 10.1172/JCI110644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sen P, Ghosh S, Ezban M, Pendurthi UR, Vijaya Mohan RL. Effect of glycoPEGylation on factor VIIa binding and internalization. Haemophilia. 2010;16:339–348. doi: 10.1111/j.1365-2516.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- 55.Kothari H, Rao LV, Pendurthi UR. Glycosylation of tissue factor is not essential for its transport or functions. J Thromb Haemost. 2011;9:1511–1520. doi: 10.1111/j.1538-7836.2011.04332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kothari H, Kaur G, Sahoo S, Idell S, Rao LV, Pendurthi U. Plasmin enhances cell surface tissue factor activity in mesothelial and endothelial cells. J Thromb Haemost. 2009;7:121–131. doi: 10.1111/j.1538-7836.2008.03218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravanat C, Archipoff G, Beretz A, Freund G, Cazenave JP, Freyssinet JM. Use of annexin-V to demonstrate the role of phosphatidylserine exposure in the maintenance of haemostatic balance by endothelial cells. Biochem J. 1992;282:7–13. doi: 10.1042/bj2820007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stuart MC, Reutelingsperger CP, Frederik PM. Binding of annexin V to bilayers with various phospholipid compositions using glass beads in a flow cytometer. Cytometry. 1998;33:414–419. [PubMed] [Google Scholar]
- 59.Rao LVM, Tait JF, Hoang AD. Binding of annexin V to a human ovarian carcinoma cell line (OC-2008). Contrasting effects on cell surface factor VIIa/tissue factor activity and prothrombinase activity. Thromb Res. 1992;67:517–531. doi: 10.1016/0049-3848(92)90013-z. [DOI] [PubMed] [Google Scholar]