Abstract
Purpose
To measure accommodative performance as a function of refractive error in very young children.
Methods
This was a prospective study of accommodation lag in multiethnic typically developing children aged 5–24 months. Accommodation lag was measured by means of a modified bell retinoscopy technique. Refraction was measured by cycloplegic retinoscopy and right and left eye results were averaged. The study compared accommodative performance to cycloplegic spherical equivalent and astigmatic refractive error.
Results
Analysis of data from 189 of 203 subjects showed that larger lags and lower gain of the accommodative response were more common in younger children, although most children of all ages accommodated well, with 95% having lags <1.25 D. Larger accommodation lags were associated with higher spherical equivalent refractive error, although only with hyperopia ≥4.0 D were lags ≥1.25 D seen in a majority of children. Larger lags in the more hyperopic meridian were seen with increasing hyperopic with-the-rule astigmatism, but lag in the more hyperopic meridian varied little with the amount of myopic or mixed astigmatism.
Conclusions
Most 5- to 24-month-olds accommodate well over a range of moderate hyperopic refractive errors, but hyperopia ≥4.0 D is rarely associated with normal accommodative performance. Hyperopic and mixed or myopic astigmatic children show different patterns of accommodation, which may explain the patterns of visual acuity deficits seen in these children.
Accommodative performance is an important determinant of the developing brain’s early visual experience. Accurate accommodation develops early, with rapid maturation in the first months of life.1–6 Little is known, however, about how accommodative performance correlates with refractive error in very young children. Brookman5 reported no correlation between accommodation and refractive error in infants, except for one highly hyperopic infant. Recently, Mutti and colleagues7 reported larger discrepancies between accommodative responses and accommodative demands (larger accommodation lags) in highly hyperopic infants than across a range of moderate hyperopia, and Horwood and colleagues8 reported large accommodation lags in infants with ≥2.0 D hyperopia. Dobson and colleagues9 suggested that infants favor the less hyperopic meridian, unless they have myopic or mixed astigmatism. The purpose of this study was to characterize accommodative performance in a cross-section of infants and toddlers as a function of spherical equivalent (SE) and astigmatic refractive error.
Subjects and Methods
Participants were 5- to 24-month-old children in Early Head Start, Women Infants and Children centers, and community medical clinics in Los Angeles County, California. The research followed and conformed to the requirements of the United States Health Insurance Portability and Accountability Act, and was approved by the Institutional Review Board of Children’s Hospital Los Angeles. Written informed consent was obtained from subjects’ parents.
Accommodative lag was measured using a modified bell retinoscopy (MBR) technique, a dynamic retinoscopy technique that has been described in detail.10 Briefly, with the retinoscope held at a fixed distance from the child, an internally illuminated cube9 with high-contrast black- and-white cartoon images containing a range of spatial frequencies was presented at the same distance as the retinoscope. “Against-motion” of the retinoscopic reflex was noted as accommodative lead and not measured. If “with-motion” was seen, the target (attached to a retractable measuring tape) was advanced until the motion was neutralized or reversed, and then withdrawn again until “with-motion” was just seen, before final target–retinoscope distance was recorded (the withdrawal phase reduced overestimation of lag in initially inattentive children). Video 1 (available at jaapos.org) shows the examiner establishing a 50 cm retinoscopy distance, taking a measurement, and noting the final retinoscope–target distance.
“Nonneutralizable” lag was “with-motion” that was not neutralizable with any amount of target advancement. Measurements were attempted at 33 cm, 50 cm, and 67 cm retinoscope distances. Standardized estimates of lag for a 40 cm target and slopes of accommodative demand–response functions were derived using orthogonal regression. Two measurement sets were obtained by the same examiner and the results averaged. MBR was performed in, and lag expressed relative to, the horizontal meridian (vertical streak).
Following MBR, refraction was assessed by cycloplegic retinoscopy (30 minutes after drops containing cyclopentolate 0.5%, mydriacyl 0.5%, and phenylephrine 0.4%). Right and left eye results were averaged.
Lag over the 95th percentile was defined as “high.” Receiver operating characteristic (ROC) analysis was used to identify threshold levels of hyperopia for further analysis. Orthogonal regression analysis was performed using an open-source programming language and software for statistical analysis (the R programming environment).11 Excel was used for linear regression analyses. Excel and online software (http://www.graphpad.com/quickcalcs; http://stattrek.com/Tables/T.aspx) were used to calculate confidence intervals for regression slopes, and for the Fisher exact tests and t tests comparing proportions and means, respectively.
Results
MBR was attempted in 203 children aged 5–24 months. Nearly 60% of participants were of Hispanic ethnicity. The following subjects were excluded: 6 uncooperative children with no measurements; 3 children with strabismus (2 exotropic, 1 esotropic); and 5 children with >1.0 D SE or astigmatic anisometropia, in whom accommodation in the measured eye could differ from the eye determining accommodative performance, resulting in misclassification. Data from 189 participants were analyzed.
The mean age was 12.3 months (SD 5.2; range, 5–24; median, 12 months). Mean SE refractive error was 0.83 D (SD 1.26; range, −1.56 to 7.50; median, 0.50 D). Of 189 participants, 161 had with-the-rule (WTR) astigmatism, from 0.13 to 3.13 D (mean, 1.05; SD 0.71; median 0.88 D); 12 had against-the-rule (ATR) astigmatism, from 0.13 to 0.88 D (mean 0.45; SD 0.29; median 0.50 D); 2 had WTR in one eye and ATR in the other, ≤0.50 D; and 14 had no astigmatism. There was no correlation between SE refractive error and cylinder amount (Pearson’s r = 0.036; P = 0.62).
The averages of two standardized lag estimates and slopes were used in 166 children. Sixteen had only one standardized lag estimate and slope. Nonstandardized lag estimates were used in 2 children having only one neutralizable lag in each measurement set (lags of 1.6 and 1.3 D for target distances of 23 and 28 cm were averaged for one child, and lags of 2.0 and 1.3 D for 29 and 36 cm targets were averaged for the other). Three children having exclusively nonneutralizable lags, one with only one lag measurement of 2.5 D for a 25 cm target, and one with one lag measurement of 7.6 D for an 11 cm target were all assigned a magnitude of lag of 2.0 D for analysis.
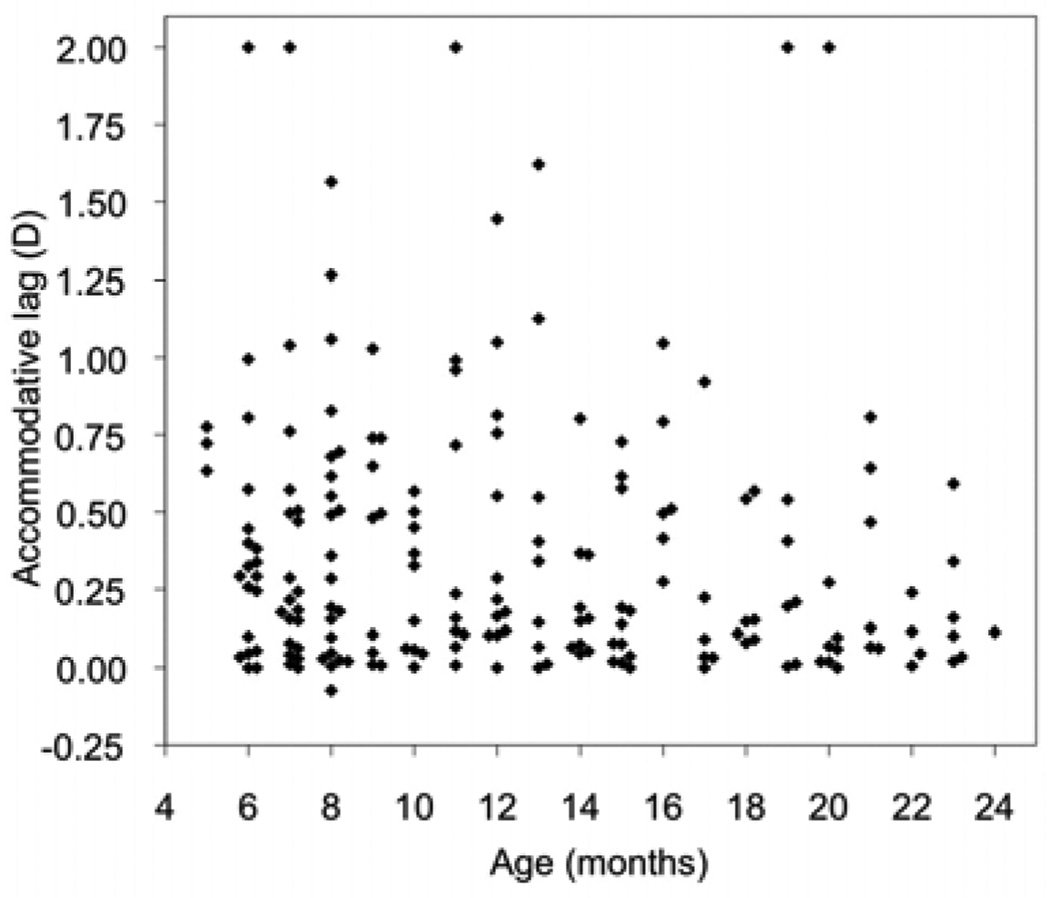
The mean lag was 0.37 D (SD 0.43; range, −0.07 to 2.00; median, 0.20 D). Higher lags were seen more frequently in younger children than in older children (Figure 1). The mean value did not differ between 5- to 11-month-olds (0.41 D; SD 0.44 D) and 12- to 24-month-olds (0.33 D; SD 0.41 D) (P = 0.20), but the median differed more (0.29 D vs 0.16 D); more younger children (53%) than older children (38%) showed lag >0.25 D (P = 0.04).
FIG 1.
Accommodative lag as a function of age. Superimposed data points are displaced along the x-axis for clarity.
The slope of the accommodative demand–response function, or gain of the accommodative response, is the ratio of increase in accommodative response to increase in accommodative demand. The mean gain was 0.97 (SD 0.23; range, 0.20–2.25; median, 1.01). Gain varied with age (e-Supplement 1, available at jaapos.org). Mean gain was lower in 5- to 11-month-olds (0.93; SD 0.24) than in 12–24 month-olds (1.02; SD 0.21) (P = 0.008). Younger children showed a gain of <0.75 more frequently than did older children (19% vs 7%; P = 0.02). No children >12 months had gain of <0.50, compared to 9% of younger children.
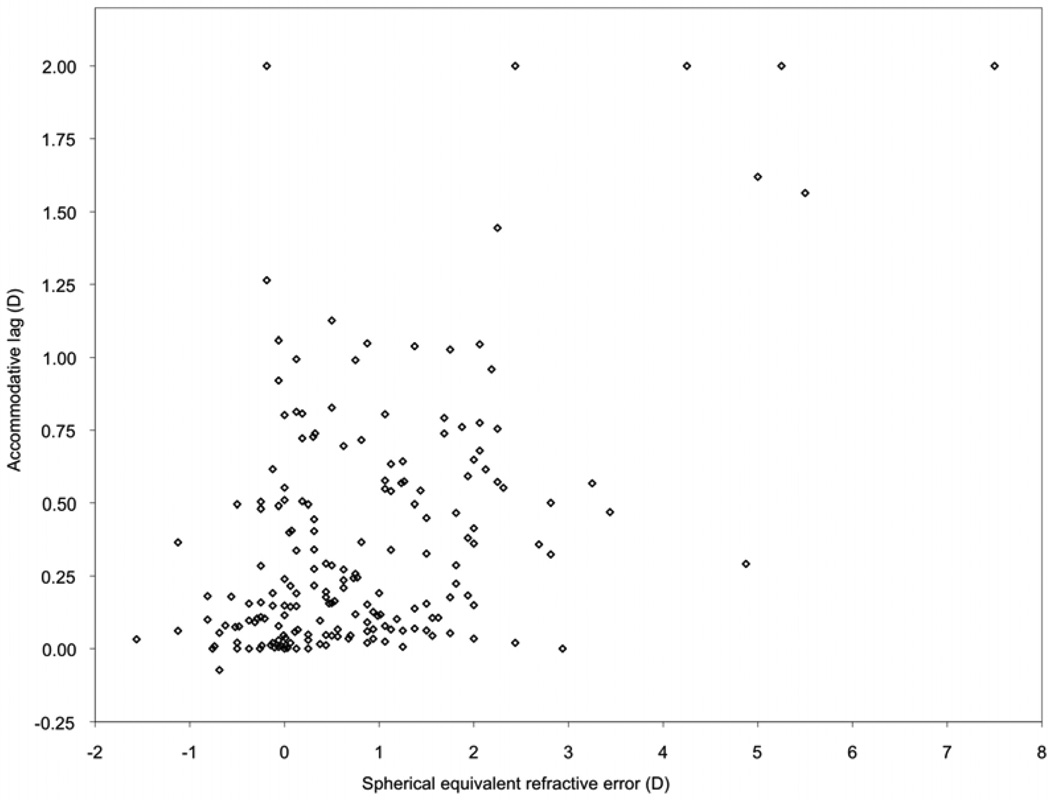
Accommodative lag varied with SE refractive error (Figure 2) (Pearson’s r = 0.52; P < 0.0001). In 5- to 11-month-olds, mean lag did not vary significantly across levels of hyperopia below 3.0 D but was increased in children with hyperopia ≥3.0 D, relative to those with 0 to <1.0 D (P < 0.0001) (e-Supplement 2A, available at jaapos.org). Among 12–24 month-olds, mean lag was increased even in children with 2.0 –3.0 D hyperopia (P = 0.0001) (e-Supplement 2B, available at jaapos.org).
FIG 2.
Accommodative lag as a function of spherical equivalent refractive error. Superimposed data points are displaced along the x-axis for clarity.
Ninety-five percent of children had lags <1.25 D; therefore high lag was defined as ≥1.25 D. ROC analysis evaluated sensitivity and specificity for high lag at different SE hyperopia thresholds. The area under the curve was 0.80 (e-Supplement 3, available at jaapos.org). A threshold ≥2.25 D SE hyperopia optimized both sensitivity (0.78) and specificity (0.94) for high lag. Children with hyperopia ≥2.25 D had lags ≥1.25 D more often (39%; 7/18) than children with <2.25 D (1%; 2/171) (P < 0.0001). The difference was seen both among 5–11 month-olds (P = 0.01), and 12- to 24-month-olds (P < 0.0001); 3 of 12 younger children, and 4 of 6 older children with hyperopia ≥2.25 D had high lags.
The hyperopia threshold yielding a specificity for high lag of at least 0.99 was ≥4.0 D (e-Supplement 3). Of 6 children with hyperopia ≥4.0 D, 5 showed lags ≥1.25 D (2 of 2 older children and 3 of 4 younger children), compared to 4 of 183 children with <4.0 D hyperopia (P < 0.0001).
In contrast to accommodative lag, the gain of the accommodative response did not vary with SE refractive error (Pearson’s r = 0.004; P = 0.96) (e-Supplement 4, available at jaapos.org).
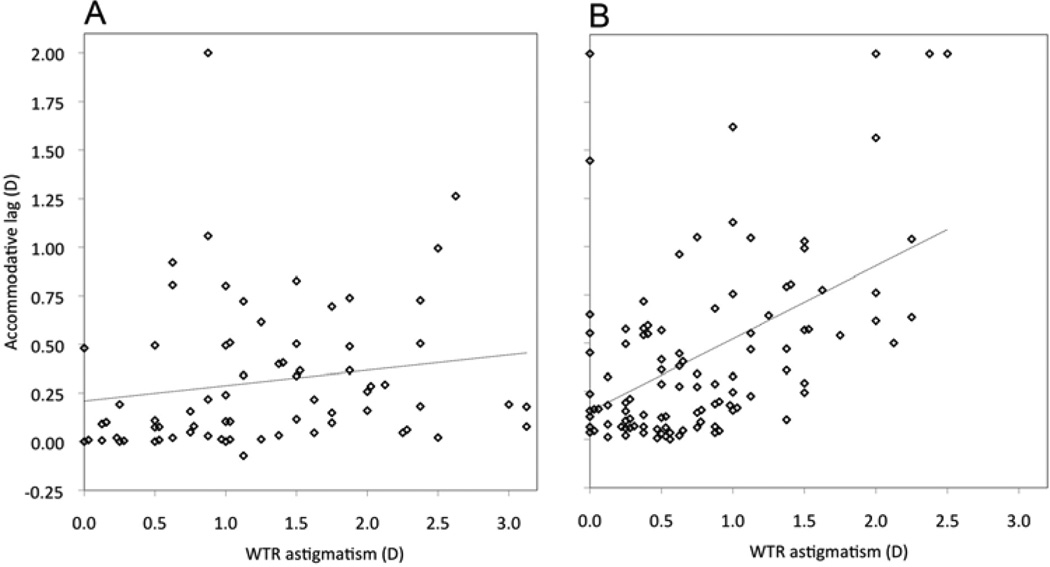
Accommodative lag in the horizontal meridian was also assessed as a function of the amount of WTR astigmatism. Analysis was stratified by type of WTR astigmatism: myopic or mixed (myopic in at least one meridian; Figure 3A) versus hyperopic (Figure 3B). ATR astigmatism was excluded from this analysis. For hyperopic astigmatism, lag varied according to the amount of astigmatism (Pearson’s r = 0.50; P < 0.0001); the slope of the regression line was 0.38 (95% CI, 0.25–0.51) (Figure 3B), that is, an increase in lag with regard to the more hyperopic meridian of nearly 0.40 D for each diopter increase in astigmatism.
FIG 3.
Accommodative lag as a function of with-the-rule astigmatic refractive error, stratified by type of astigmatism. A, Myopic or mixed astigmatism. B, Hyperopic astigmatism. Dotted lines show best-fitting linear regression functions. Superimposed data points are displaced along the x-axis for clarity. D, diopters; WTR, with-the-rule.
To address whether this could result from covariance of astigmatism with SE hyperopia among hyperopic astigmats (SE hyperopia itself being a predictor of lag), analysis was also performed excluding children with SE hyperopia ≥2.25 D. For hyperopia <2.25 D, lag remained correlated with hyperopic astigmatism (Pearson’s r = 0.51; P < 0.0001), with slope 0.29 (95% CI, 0.19–0.39). SE hyperopia was also correlated with lag in these children (Pearson’s r = 0.37; P = 0.0004), but the slope was only 0.18 D (95% CI, 0.08–0.28). Since a 1.0 D cylinder increase in hyperopic astigmatism implies a 0.50 D increase in SE hyperopia, hyperopia itself could not account for more than 0.09 D increase in lag per D increase in cylinder (95% CI, 0.04–0.14), less than the observed slope of 0.29.
Children with myopic or mixed astigmatism showed no significant correlation between lag and astigmatism (Pearson’s r = 0.17; P = 0.15) (Figure 3A); the slope of the regression line was 0.08 (95% CI, −0.03 to 0.19). Lag remained correlated with SE refractive error (Pearson’s r = 0.26; P = 0.03).
Discussion
This study employed a simple clinical technique to quantify accommodative lag in infants and toddlers aged 5–24 months with a range of refractive errors. Most children showed low accommodation lags and appropriate increases in accommodative response with increasing demand, though with greater variability of accommodative performance among younger children than older children. Ninety-five percent of children showed lag <1.25 D. Accommodative lag varied with refractive error: larger accommodation lags were associated with hyperopia, especially with levels ≥4.0 D. Lag in the more hyperopic meridian also increased with increasing amounts of astigmatism in children with hyperopic astigmatism, but did not vary with the amount of astigmatism in children with myopic or mixed astigmatism.
The term lag does not mean the accommodative response is deficient. Some discrepancy between accommodative demand and accommodative response is normal. Because there are few normative data for accommodative lag in young children, and none using MBR, the 95th percentile of observed values was used in this study to define the upper limit of normal, and lags ≥1.25 D were considered high.
The fact that most participants accommodated accurately to near targets is consistent with previous studies demonstrating mature accommodative performance emerging in the first months of life.1–6 Nevertheless, the present findings suggest maturation of accommodative performance over the age range studied in at least some individuals since the variability of lag measurements and the frequency of larger lags were higher in younger children. Also, the average gain of the accommodative response was lower in younger children, and more young children showed low gain, that is, a relatively flat accommodative response that did not vary appropriately in response to varying accommodative demand.
Accommodative lag increased with SE refractive error. High lags of accommodation (≥1.25 D) were more common in children with ≥2.25 D of SE hyperopia than in children with <2.25 D, and were especially frequent in children with hyperopia ≥4.0 D. Lyon and Candy similarly observed that children with ≤5.0 D of hyperopia showed lags no more than 1.0 D with Nott retinoscopy, while higher hyperopes exhibited larger lags (Lyon DW, Candy TR. Accommodative performance as a function of refractive error in infants and young children. IOVS 2006;47:ARVO E-Abstract 1182). For a given level of hyperopia, the lags observed in the present study were smaller than those reported by Mutti and colleagues7 at comparable ages. Lens-based retinoscopy, used by Mutti and colleagues, can yield higher lag estimates than Nott retinoscopy or MBR, which do not use lenses to neutralize the reflex.10,12 MBR may promote a child’s “best possible” accommodative performance, aided in part by “looming” cues to accommodation from an approaching target.13,14 Nonetheless, the overall pattern of the present findings is similar to Mutti and colleagues; children accommodated well over a range of moderate refractive errors, with consistently poor accommodative performance appearing only at higher levels of hyperopia.
Horwood and colleagues8 also reported hypoaccommodation in infants with SE hyperopia ≥2.0 D, using photorefraction, although their estimation of refractive error from noncycloplegic measurements creates a potential for bias from misclassification of hyperopes having good accommodation. In addition, Horwood and colleagues8 reported an association between hyperopia and the gain of the accommodative response. This study, on the contrary, found no such association, perhaps because it did not include target distances as large 1–2 m, where Horwood and colleagues8 observed the largest lags in hyperopic subjects, resulting in response functions with slopes steeper than those of emmetropic subjects.
Although hyperopia is a risk factor for the development of subsequent accommodative esotropia or bilateral ametropic amblyopia, not all hyperopic children develop these conditions,15 and it has been suggested that early accommodative performance might be predictive of later pathology.16 The range of accommodative performance seen in children with SE hyperopia in this study is consistent with this hypothesis, but additional, longitudinal studies of accommodative performance in hyperopic children and its relationship to subsequent alignment and vision outcomes are needed to clarify the prognostic significance, if any, of large lags at 5–24 months.
Accommodative performance in this study was also related to astigmatic refractive error. Children with 2.0 D of hyperopic WTR astigmatism showed, on average, close to 1.0 D of lag in the measured (more hyperopic) meridian. Increasing amounts of cylinder were associated with increasing amounts of lag, indicating that these children do not accommodate to the more hyperopic meridian; if they did, the slope of the regression function would be zero. A slope of 1.0, on the other hand, would indicate that the level of accommodation is driven by the less hyperopic axis. The observed intermediate slope suggests that these children accommodate somewhere between the two meridia (perhaps favoring the more hyperopic meridian somewhat, since the upper limit of the 95% confidence interval for the slope was near 0.5). Although cylinder amount in hyperopic astigmatism is correlated with SE hyperopia, itself a predictor of lag, SE hyperopia alone could not account for the observed relationship between lag and hyperopic cylinder amount. In contrast, children with myopic or mixed astigmatism showed little change in the amount of lag in the more hyperopic meridian with increasing amounts of cylinder, suggesting that they favor the more hyperopic (less myopic) meridian.
Dobson and colleagues9 similarly observed, in a photorefraction study of infants having mostly ATR astigmatism, that myopic astigmatic subjects favored the more hyperopic meridian, while hyperopic astigmatic subjects did not. The present findings support the hypothesis that children with different types of astigmatism may select different accommodative strategies to minimize defocus across a range of target distances,17 and can account for the patterns of visual acuity deficits observed in preschool children having predominantly WTR astigmatism: children with myopic or mixed astigmatism demonstrate meridional amblyopia, with reduced acuity for horizontal gratings, while those with hyperopic astigmatism have acuity deficits for grating of both orientations.18
The modified bell retinoscopy (MBR) technique employed in this study is simple and can be used to quantify lags of accommodation easily even in very young children.10 It represents a modification of the “bell retinoscopy” technique,19 and is a quantitative version of a technique proposed by Hunter20 as a way of qualitatively evaluating accommodative lag in children. MBR differs from Nott retinoscopy in that the retinoscopist is stationary while the position of the target is changed, whereas in Nott retinoscopy the target is fixed and the retinoscopist moves away until the retinoscopic reflex is neutralized. Measurements of lag by MBR correlate well with those by Nott retinoscopy and the monocular estimate method.10 Although the present study used multiple measurements of lag at different retinoscope distances and regression analysis to derive standardized lag estimates and slopes of accommodative demand–response functions, MBR can be employed in a clinical setting using a single retinoscopy distance; a simple published nomogram10 may be used to determine the accommodative lag on the basis of the distance of target advancement, or the target–retinoscope distance corresponding to a given lag. For example, with retinoscopy at 50 cm, neutrality is observed 50 cm from the eye, corresponding to an accommodative response of 2.0 D. A lag of 1.25 D would mean the demand was 3.25 D, corresponding to a target–eye distance of 31 cm, that is, a target-retinoscope distance of 19 cm. Thus, for retinoscopy at 50 cm, if the target must be advanced ≥19 cm to observe neutrality, the child has an accommodative lag in the observed meridian of ≥1.25 D. The threshold distance will differ at different retinoscopy distances.
Strengths of this study include the fact that the author did not assess the child’s refractive error until after measuring accommodative responses, avoiding bias in the assessment of accommodation; measurement of cycloplegic refraction in every child; the broad range of refractive errors studied; and recruitment from community settings, so that participants are representative of typically developing children in this age range. Several limitations must be acknowledged. Methodological weaknesses include the difficulty of quantifying leads or very large lags of accommodation using MBR and repeated measures by a single examiner. The relatively small number of children with large refractive errors limits the conclusions that may be drawn for these children. No attempt was made to exclude children with Down syndrome, static encephalopathy, or developmental delay, who might have deficits of accommodation; however, recruitment from community rather than ophthalmology clinic settings makes it unlikely that there were enough such children to confound the analysis. SE hyperopia and WTR hyperopic astigmatism coexisted, raising the question of whether these variables are independently associated with lag. Since SE hyperopia and cylinder amount were not correlated overall, it is unlikely that the association of lag with hyperopia is an artifact of measuring the more hyperopic meridian in astigmatic children; however, further study of larger samples of high pure spherical hyperopes would help confirm this. Among hyperopic astigmatic children, by definition, cylinder magnitude does correlate with hyperopia, but the observed association of lag with cylinder amount could not be accounted for on the basis of SE hyperopia alone. Finally, this study does not inform management of refractive error; a longitudinal study would be required to determine whether hyperopic children with high lag would experience improved retinal focus with spectacle correction.
In conclusion, this study, using a novel dynamic retinoscopy technique, confirms that mature accommodative behavior is seen at 5–24 months of age and that poor accommodation is associated with hyperopia, while providing new evidence for accommodative strategies in astigmatism that predict the patterns of visual acuity deficits resulting later in childhood.
Supplementary Material
Acknowledgments
This work was supported by NEI K23 EY016699. I am grateful to Velma Dobson† for valuable input with regard to data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haynes H, White BL, Held R. Visual Accommodation in Human Infants. Science. 1965;148:528–530. doi: 10.1126/science.148.3669.528. [DOI] [PubMed] [Google Scholar]
- 2.Braddick O, Atkinson J, French J, Howland HC. A photorefractive study of infant accommodation. Vision Res. 1979;19:1319–1330. doi: 10.1016/0042-6989(79)90204-9. [DOI] [PubMed] [Google Scholar]
- 3.Banks MS. The development of visual accommodation during early infancy. Child Dev. 1980;51:646–666. [PubMed] [Google Scholar]
- 4.Howland HC. Infant eyes: optics and accommodation. Curr Eye Res. 1982;2:217–224. doi: 10.3109/02713688208997697. [DOI] [PubMed] [Google Scholar]
- 5.Brookman KE. Ocular accommodation in human infants. Am J Optom Physiol Opt. 1983;60:91–99. doi: 10.1097/00006324-198302000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Horwood AM, Riddell PM. Gender differences in early accommodation and vergence development. Ophthalmic Physiol Opt. 2008;28:115–126. doi: 10.1111/j.1475-1313.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- 7.Mutti DO, Mitchell GL, Jones LA, et al. Accommodation, acuity, and their relationship to emmetropization in infants. Optom Vis Sci. 2009;86:666–676. doi: 10.1097/OPX.0b013e3181a6174f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwood AM, Riddell PM. Hypo-accommodation responses in hypermetropic infants and children. Br J Ophthalmol. 95:231–237. doi: 10.1136/bjo.2009.177378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobson V, Howland HC, Moss C, Banks MS. Photorefraction of normal and astigmatic infants during viewing of patterned stimuli. Vision Res. 1983;23:1043–1052. doi: 10.1016/0042-6989(83)90015-9. [DOI] [PubMed] [Google Scholar]
- 10.Tarczy-Hornoch K. Modified bell retinoscopy: Measuring accommodative lag in children. Optom Vis Sci. 2009;86:1337–1345. doi: 10.1097/OPX.0b013e3181be9d9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 12.del Pilar Cacho M, Garcia-Munoz A, Garcia-Bernabeu JR, Lopez A. Comparison between MEM and Nott dynamic retinoscopy. Optom Vis Sci. 1999;76:650–655. doi: 10.1097/00006324-199909000-00023. [DOI] [PubMed] [Google Scholar]
- 13.McLin LN, Jr, Schor CM, Kruger PB. Changing size (looming) as a stimulus to accommodation and vergence. Vision Res. 1988;28:883–898. doi: 10.1016/0042-6989(88)90098-3. [DOI] [PubMed] [Google Scholar]
- 14.Horwood AM, Riddell PM. Receding and disparity cues aid relaxation of accommodation. Optom Vis Sci. 2009;86:1276–1286. doi: 10.1097/OPX.0b013e3181bb41de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarczy-Hornoch K. The epidemiology of early childhood hyperopia. Optom Vis Sci. 2007;84:115–123. doi: 10.1097/OPX.0b013e318031b674. [DOI] [PubMed] [Google Scholar]
- 16.Ingram RM, Gill LE, Goldacre MJ. Emmetropisation and accommodation in hypermetropic children before they show signs of squint—a preliminary analysis. Bull Soc Belge Ophtalmol. 1994;253:41–56. [PubMed] [Google Scholar]
- 17.Harvey EM. Development and treatment of astigmatism-related amblyopia. Optom Vis Sci. 2009;86:634–639. doi: 10.1097/OPX.0b013e3181a6165f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobson V, Miller JM, Harvey EM, Mohan KM. Amblyopia in astigmatic preschool children. Vision Res. 2003;43:1081–1090. doi: 10.1016/s0042-6989(03)00014-2. [DOI] [PubMed] [Google Scholar]
- 19.Apell RJ. Clinical application of bell retinoscopy. J Am Optom Assoc. 1975;46:1023–1027. [PubMed] [Google Scholar]
- 20.Hunter DG. Dynamic retinoscopy: The missing data. Surv Ophthalmol. 2001;46:269–274. doi: 10.1016/s0039-6257(01)00260-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.