Abstract
Objective
To investigate the cross-sectional association between COPD severity and disturbed sleep and the longitudinal association between disturbed sleep and poor health outcomes.
Methods
98 adults with spirometrically-confirmed COPD were recruited through population-based, random-digit telephone dialing. Sleep disturbance was evaluated using a 4-item scale assessing insomnia symptoms as: difficulty falling asleep, nocturnal awakening, morning tiredness, and sleep duration adequacy. COPD severity was quantified by: FEV1 and COPD Severity Score, which incorporates COPD symptoms, requirement for COPD medications and oxygen, and hospital-based utilization. Subjects were assessed one year after baseline to determine longitudinal COPD exacerbations and emergency utilization and were followed for a median 2.4 years to assess all-cause mortality.
Results
Sleep disturbance was cross-sectionally associated with cough, dyspnea, and COPD Severity Score but not FEV1. In multivariable logistic regression, controlling for sociodemographics and body-mass index, sleep disturbance longitudinally predicted both incident COPD exacerbations (OR=4.7; p=0.018) and respiratory-related emergency utilization (OR=11.5; p=0.004). In Cox proportional hazards analysis, controlling for the same covariates, sleep disturbance predicted poorer survival (HR=5.0; p=0.013). For all outcomes, these relationships persisted after also controlling for baseline FEV1 and COPD Severity Score.
Conclusions
Disturbed sleep is cross-sectionally associated with worse COPD and is longitudinally predictive of COPD exacerbations, emergency health care utilization, and mortality.
Keywords: chronic obstructive pulmonary disease, cognitive performance, insomnia, mortality, outcomes
INTRODUCTION
Sleep quality is likely to be particularly important in the setting of a chronic, symptomatic, and progressive disease such as chronic obstructive pulmonary disease (COPD). COPD may lead to worse sleep quality and insomnia by virtue of respiratory symptoms, such as nocturnal cough and dyspnea. Moreover, poor sleep quality could contribute to poor COPD-related outcomes such as exacerbations or even mortality risk. Such adverse effects could operate through various pathways. Poor sleep quality could lead to impaired cognition, thus impairing COPD self-management behaviors [1–2]. Alternatively, poor sleep quality may impair immune function, contributing to the likelihood or severity of COPD exacerbations [3–4]. Poor sleep quality may act in ways that depend on the presence of underlying COPD, which underscores the need to study sleep disturbance specifically in COPD populations.
In a recent review of sleep abnormalities in COPD, Krachman and colleagues conclude that it is unknown whether there is a relationship between sleep quality and disease severity in COPD [5]. They furthermore note that it has yet to be determined whether poor sleep quality in patients with COPD has an effect on neurocognition [5]. This knowledge gap may reflect the cross-sectional nature of the limited studies that have demonstrated a link between respiratory symptoms and sleep complaints in COPD patients [6–7]. Indeed, there has been a dearth of longitudinal studies examining the consequences of disturbed sleep in COPD, especially with respect to the outcome of mortality.
In this analysis, we sought to address these gaps in the published literature. First, we wished to confirm the anticipated association between insomnia symptoms and COPD symptoms and severity, since such a cross-sectional relationship provides biological coherence to any sleep-related adverse effects we might observe longer-term. Next, we examined whether sleep disturbance predicted adverse outcomes, including: COPD exacerbations, emergency health services utilization, and overall survival. We also wished to investigate whether such an association with sleep disturbance, if present, might be explained solely by COPD severity and whether cognitive dysfunction, depression, or anxiety might mediate part of the relationship between disturbed sleep and poor outcomes. We conducted our study on an on-going population-based longitudinal COPD cohort, utilizing such data as COPD severity and lung function assessments, structured cognitive function evaluations, and longitudinal health care utilization information, with linkage to mortality data.
METHODS
Overview
In 98 subjects from a population-based study with spirometrically-confirmed COPD, we administered structured telephone interviews and, to obtain measurements of cognitive and lung function, we conducted subject home visits. Sleep disturbance was evaluated using four survey items which assessed insomnia symptoms. Disease severity was quantified with the COPD Severity Score based on interview responses. Lung function was measured spirometrically. Cognitive function was assessed using directly administered validated objective tests of memory and executive function during subject home visits. Depressive and anxiety symptoms were measured during structured interviews using validated scales. Subjects were re-assessed through structured interviews one year after baseline to ascertain COPD exacerbations and respiratory-related hospitalizations or emergency department (ED) visits. Subjects were followed for the outcome of all-cause mortality, which was determined by linkage to publically available databases. All study procedures were approved by the University of California San Francisco Committee on Human Research.
Study Population
This study was conducted on the UCSF COPD cohort, an ongoing population-based, longitudinal study of U.S. adults, ranging between ages 56 and 77 at the time of our baseline assessment [8]. Research subjects were initially identified from throughout the U.S. by random-digit telephone dialing. Subjects were included in longitudinal follow-up if they reported being diagnosed by a physician with an airway disease. In 2006 and 2007, participants who lived in Northern California, and were thus logistically accessible to investigators, were asked to participate in a single home visit closely following a structured telephone interview. In the home visit, research personnel conducted spirometry and cognitive function measures on research subjects. Of the 326 geographically eligible individuals from the larger cohort, 251 (77%) successfully participated in a home visit. From these 251 subjects, we identified 98 subjects who met criteria for COPD: FEV1/FVC ratio<0.7 and a self-reported physician’s diagnosis of COPD, emphysema, or chronic bronchitis.
Measurements
Sleep Disturbance
We measured sleep disturbance using four items consistent with major criteria for insomnia and drawn from the Medical Outcomes Study sleep battery: (1) difficult falling asleep, (2) nocturnal awakening, (3) morning tiredness, and (4) perceived sleep duration adequacy [9–12]. Items from the Medical Outcomes Study sleep battery suggestive of sleep-disordered breathing, such as morning headache and snoring, were excluded because the biologic pathways involved in sleep-disordered breathing may be distinct from those operating in insomnia [9–10]. For each of the four items, responses are elicited conforming to five response options, scored 0 to 4: none of the time, a little of the time, some of the time, most of the time, all of the time. Total sleep disturbance scores for the four items could therefore range from 0 to 16, with higher scores indicating greater insomnia, which we dichotomized at a cut-off: score <8 vs score ≥8. Our rationale for this cut-point was that is corresponds to a response of “some of the time” averaged over all four items (i.e. corresponds to an average score of 2 out of 4). This dichotomization is also consistent with that used in a large Italian study of sleep disturbance in obstructive lung disease, which utilized a comparable 16-point scale of insomnia symptoms [13]. Because the four items utilized represent a subset of the longer MOS scale, we wished to evaluate its performance characteristics as part of this analysis. As detailed below, we therefore evaluated its internal consistency, response distribution, and concurrent validity.
COPD Symptom and Severity
We conducted spirometry, according to American Thoracic Society guidelines,[14] using the EasyOne™ Frontline spirometer (ndd Medical Technologies, Chelmsford, MA) [15–16]. Based on spirometric results, COPD was staged by Global Obstructive Lung Disease criteria [17]. By inclusion criteria, all subjects had Global Obstructive Lung Disease (GOLD) stage ≥ 1. Oxygen saturation was assessed on room air during home visits with standard pulse oximetry, with the study participant sitting at rest.
We also assessed COPD severity using the previously-validated COPD Severity Score, which is based on responses to survey items that comprise 5 domains of severity: dyspnea, COPD-related requirement for systemic corticosteroids and antibiotics, regular COPD medication usage, prior COPD-related hospitalizations and intubations, and home oxygen use [18]. Scores can range from 0–35, with higher scores representing more severe COPD. The COPD Severity Score demonstrates both concurrent and predictive validity as a measure of COPD severity, including a prospective association with COPD exacerbations and hospitalizations that is independent of its association with exercise capacity and lung function [18–21].
COPD symptoms were assessed as both dyspnea and cough during baseline structured interviews. The dyspnea scale utilized is a 0–7 point scale which included both items from the Medical Research Council (MRC) Dyspnea Scale as well as the number of days or nights of dyspnea symptoms over the prior 2 weeks. Subjects were categorized as having cough symptoms if they verified either daily coughing attacks or coughing up phlegm from their “chest or lung for more than 3 months a year for each of the past 2 years,” the latter being consistent with MRC criteria for chronic bronchitis [22].
Psychological and Health Status Measurements
Depressive symptoms were obtained using the 15-item short-form Geriatric Depression Scale (GDS). The GDS has been validated both in non-geriatric populations generally as well as specifically in younger adults with obstructive lung disease [23–26]. Anxiety was assessed using the anxiety portion of the Hospital Anxiety and Depression (HAD) scale [27–28]. We utilized only the anxiety portion of this scale because the GDS is felt to be a better validated measure of depressive symptoms in COPD than the HAD.
Physical and mental health status were measured using the Short-Form (SF)-12 Physical Component Summary (PCS) and Mental Component Summary (MCS) scores [29]. The SF-12 PCS and MCS are derived from the Medical Outcomes Study SF-36 instrument, which has been extensively validated in the general population and among adults with COPD [30]. Higher scores reflect better health status.
Cognitive Status
During the home visit, examiners assessed subjects’ cognitive function using tests of both executive function and memory. Because of the complexity of executive function, we utilized three separate tests: the Stroop Color Word Interference test, the Delis Kaplan Executive Function System (DKEFS) Trail Making Set Shifting Condition test, and the Phonemic Verbal Fluency test, all of which are well-established direct measurements of executive function [32–36]. Learning and episodic memory was assessed using the revised Hopkins Verbal Learning Test (HVLT), which is essentially the number items from a 12-item word list recalled 20 minutes after immediate-recall learning trials [37]. The results of all cognitive function measures were standardized (i.e., converted to z-scores), where a z-score=0 is the population mean of healthy age-matched referents and a z-score=−1 is one standard deviation below this population mean [36–39].
Covariates
Age, gender, marital status, self-identified race (classified as non-Latino white vs other), and educational attainment were assessed as part of the telephone interview. Body mass index (BMI) was calculated from height and weight measured in the home visit and categorized as underweight (<18.5), normal (18.5 up to 25), overweight (25.0 up to 30), and obese (≥30) using standard classifications [40].
Health Outcomes: COPD Exacerbations and Emergency Utilization
Subjects were assessed one year after baseline to evaluate the longitudinal association of baseline sleep disturbance with both COPD exacerbations and respiratory-related emergency utilization (hospitalizations or ED visits). For this longitudinal analysis, 18 of the 98 subjects were unavailable for follow-up interview, due in part to subject deaths (see below). Thus, this portion of the analysis was restricted to the 80 subjects available for reassessment. To reduce possible bias from loss to follow-up, we incorporated probability-of-attrition weightings into our analyses for these outcomes, as described below in statistical analysis section.
Consistent with previously proposed guidelines, subjects were categorized as having had a COPD exacerbation if they reported the prescription and usage of new or additional systemic corticosteroids due to worsening respiratory symptoms [41]. Hospitalizations and ED visits were categorized as respiratory-related if subjects identified the primary cause of such utilization to be due to worsening respiratory status. For logistic regression analyses, COPD exacerbations and emergency utilization were dichotomized as ≥ 1 event vs none.
All-Cause Mortality
For the outcome of mortality, subjects were followed through 12/31/2008, a median follow-up period of 2.4 years (25th–75th interquartile range: 1.4 – 2.5 years). Minimum follow-up among surviving subjects was 1.2 years. Because we evaluated the longitudinal association between disturbed sleep and risk of all-cause mortality using proportional hazards analysis, this required not only confirmation of vital status but also date of death. We assessed vital status through 12/31/2008, not later, to leave adequate time for research subjects to be registered in public databases in the event of their death. Through contact with the research subjects’ household or family members, our research personnel were informed of the death of 12 of our 98 research subjects, and we were able to confirm the date of death using the Social Security Administration index for 11 out of those 12 subjects. We were unable to confirm vital status for the 12th subject and so omitted this subject from analysis; the results of our analysis were not substantively affected in sensitivity analyses in which we either assumed that the 12th subject had died, using an estimated death date based on contact with family members, or assumed that the 12th subject had not died (data not shown). We were able to confirm that the remaining 86 subjects were alive as of 12/31/2008 through direct contact with those subjects (n=73), contact with household or family members (n=9), or queries of the Accurint database of Lexis Nexis (n=4). These 86 subjects were also not listed as deceased in the Social Security Administration database.
Statistical Analysis
Internal Consistency and Concurrent Validity of Sleep Scale Items
We utilized Cronbach’s alpha analysis to examine the internal consistency of the 4-item sleep scale. Since psychological factors and overall physical and mental health status are theoretically relevant constructs to insomnia symptoms [42–44], we examined the cross-sectional correlation between the 4-item sleep disturbance scale and depressive symptoms, anxiety symptoms, and the SF-12 PCS and MCS using Pearson product-moment correlations.
Cross-Sectional Associations
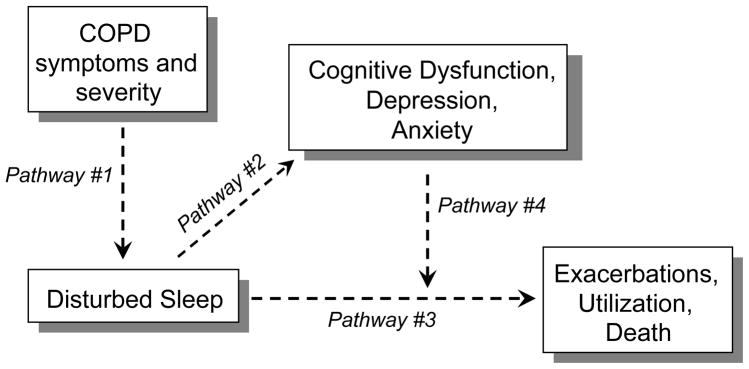
All multivariable analyses controlled for age, gender, race, marital status, educational attainment and BMI. We used multivariable logistic regression analysis to estimate the cross-sectional association between sleep disturbance and the various measures of COPD symptoms and severity (Figure 1: Pathway #1). In each of five distinct analyses, the presence or absence of sleep disturbance was the dependent (outcome) in a multivariable analysis, and a single measurement of COPD symptoms or severity was the independent (predictor) of interest: (1) cough symptoms, (2) dyspnea, (3) COPD Severity Score, (4) resting oxygen saturation, (5) FEV1.
Figure 1.
The study addressed questions of cross-sectional associations (Pathways #1 and #2), questions of longitudinal associations (Pathway #3), and questions of cognitive deficits or psychological factors as potential mediators in longitudinal associations (Pathway #4).
In our analysis of cognitive function, we utilized multivariable linear regression to determine the association between sleep disturbance and each measure of cognitive function, expressed as age-adjusted z-scores (Figure 1: Pathway #2). That is, each of four cognitive function variables was tested as a dependent variable in its own multivariable linear regression in which sleep disturbance was the independent variable of interest.
Longitudinal Analyses
Longitudinal analyses examined the predictive association between disturbed sleep and poor outcomes (Figure 1: Pathway #3). Multivariable logistic regression analyses were utilized to estimate the association between disturbed sleep at baseline and the likelihood, over the ensuing 12 months, of (1) COPD exacerbations or, in a separate model, (2) respiratory-related emergency utilization. Longitudinal analyses also examined the predictive association between disturbed sleep at baseline and subsequent all-cause mortality, occurring over the period from baseline assessment up to the end of the assessment period on 12/31/2008. For this analysis, we utilized multivariable Cox proportional hazards regression models (survival analysis) to determine the longitudinal association between baseline disturbed sleep and time until subsequent all-cause mortality; person time was censored for end of the study period without mortality. In all longitudinal analyses, we controlled for the same potentially confounding covariates (sociodemographics + BMI) as in our cross-sectional analyses. However, in addition to controlling for potentially confounding sociodemographic covariates and BMI (Model 1), we also tested longitudinal models in which we controlled for FEV1 (Model 2), and FEV1 + COPD Severity Score (Model 3). The purpose of Model 2 and Model 3 was to determine whether disturbed sleep was related to poor outcomes independent of the known association between FEV1/COPD severity and poor outcomes. That is, if the pathway is such that COPD severity causes disturbed sleep, does such disturbed sleep continue to predict poor outcomes after the role COPD severity is taken into account?
In longitudinal outcomes analyses, we also conducted a mediation analysis in which we examined whether poor cognitive function, depression, or anxiety might mediate the relationship between disturbed sleep and poor outcomes (Figure 1: Pathway #4) [45]. For example, if disturbed sleep is related to poor cognitive function, and disturbed sleep is also associated with poor outcomes, does the association between disturbed sleep and poor outcomes persist after controlling for such poor cognitive function? If not, this would suggest that poor cognitive function is mediating the relationship between disturbed sleep and poor outcomes. In these analyses, we controlled, in turn, for cognitive dysfunction, depressive symptoms, or anxiety symptoms, in addition to sociodemographic factors, BMI, FEV1, and the COPD Severity Score. Of the cognitive dysfunction measures, only memory impairment was associated with disturbed sleep and, therefore, memory impairment was the only cognitive dysfunction measure tested in this mediation analysis (along with depressive and anxiety symptoms).
To examine whether disturbed sleep at baseline might more strongly be associated with poor outcomes among subjects with more advanced COPD as compared to less advanced COPD, we conducted an exploratory secondary analysis in which we dichotomized our sample into subjects with more advanced COPD (GOLD stages 3 and 4; n=42) vs less advanced COPD (GOLD stages 1 and 2; n=56). In each of these two sub-groups, we then repeated logistic and Cox regression analyses. We conducted this analysis as a univariate analysis, including sleep disturbance as the only independent (predictor) variable, because the smaller sample size prohibited the inclusion of multiple independent covariates.
For all analyses of the outcomes of COPD exacerbations and respiratory-related emergency utilization, we applied probability-of-attrition weights to take losses to follow-up into account; probability-of-attrition weights have been shown to reduce bias from non-response in longitudinal cohort studies [46–48]. In our study, probability-of attrition weights were developed using the key sociodemographic factors of study interest as well as FEV1, COPD Severity Score, and sleep disturbance at baseline [46–48]. Thus, we developed a multivariate logistic regression model to determine the probability of follow-up and then weighted subjects based on their likelihood of follow-up such that those who are less likely to re-interview were weighted more heavily.
All analyses used Stata/SE version 9.2 (StataCorp; College Station, TX). For all multivariable logistic regression models, the Hosmer-Lemeshow test demonstrated adequate goodness-of-fit (p>0.20 for all models) [49]. For survival analyses, the proportional hazards assumption was verified by obtaining scaled Schoenfeld residuals (p >0.20 for all Cox models).
RESULTS
Subject Characteristics
Baseline subject characteristics are presented in Table 1. Among 98 study participants, the mean age was 67.6 years (SD=5.4), 56% of patients were women, and 85% where white, non-Latino. There was a broad range of BMI categorizations: 6% underweight, 32% normal weight, 34% overweight, and 29% obese or morbidly obese. The degree of COPD was moderately advanced, with a mean FEV1 % predicted of 54% (SD=22%). Staged by GOLD criteria, where higher stage represents more advanced COPD, 15% of subjects were Stage 1; 42% Stage 2; 30% Stage 3, and 13% Stage 4.
Table 1.
Baseline characteristics of 98 subjects with COPD
| N (%) or Mean ± SD | |
|---|---|
| Age, years | 67.6 ± 5.4 |
| Female gender | 55 (56%) |
| Married or Cohabitating | 51 (52%) |
| Education: | |
| High School or Less | 25 (26%) |
| White non-Latino | 83 (85%) |
| Body-Mass Index, kg/m2 | |
| BMI < 18.5 | 6 (6%) |
| BMI 18.5 – 24.9 | 31 (32%) |
| BMI 25.0 – 29.9 | 33 (34%) |
| BMI ≥ 30 | 28 (29%) |
| Cough Symptoms | 53 (54%) |
| Dyspnea Scale Score | 3.5 ± 2.5 |
| COPD Severity Score | 7.8 ± 6.1 |
| Oxygen Saturation ≤ 92% | 21 (21%) |
| FEV1 liters | 1.4 ± 0.7 |
| FEV1 % predicted | 54% ± 22% |
| GOLD Stage | |
| Stage 1 | 15 (15%) |
| Stage 2 | 41 (42%) |
| Stage 3 | 29 (30%) |
| Stage 4 | 13 (13%) |
Internal Consistency and Concurrent Validity of Sleep Scale Items
Overall, 24.5% of subjects (n=24) manifested disturbed sleep based on the sleep scale items. The Cronbach’s alpha for the four sleep scale items was 0.71, indicative of adequate internal consistency. At baseline, disturbed sleep on the four-item sleep scale was correlated cross-sectionally and in the anticipated directions with greater depressive symptoms (r=0.41; p<0.001) and greater anxiety symptoms (r=0.32; p=0.001). Furthermore, poorer sleep quality on the sleep scale correlated with worse overall physical health status (r=−0.31; p=0.002) and mental health status (r=−0.21; p=0.04).
Association of Disturbed Sleep with COPD Symptoms and Severity
As presented in Table 2, patients with cough symptoms (just over half of the group) had three-fold greater likelihood of disturbed sleep (odds ratio [OR]=3.3, 95% confidence interval [CI] = 1.1–9.7; p=0.034). The degree of dyspnea also was associated with a higher likelihood of disturbed sleep (OR=1.4 per 1 point increment in dyspnea scale; 95% CI 1.1 – 1.7; p = 0.004). Greater COPD severity, as measured by COPD Severity Score, was associated with greater likelihood of sleep disturbance (OR=1.9 per SD increment in score; 95% CI 1.1 – 3.2; p=0.015). The likelihood of sleep disturbance in patients with hypoxemia (oxygen saturation at rest ≤92%) was increased but not statistically significantly (OR=2.2; 95% CI 0.7 – 6.5; p=0.18). FEV1 was not related to sleep disturbance (OR=0.97; 95% CI 0.4 – 2.2; p=0.94).
Table 2.
Cross-sectional association of disturbed sleep with COPD symptoms and severity among 98 COPD subjects
| OR (95% CI) | p-value | |
|---|---|---|
| Cough Symptoms | 3.3 (1.1 – 9.7) | 0.034 |
| Dyspnea Scale* | 1.4 (1.1 – 1.7) | 0.004 |
| COPD Severity Score† | 1.9 (1.1 – 3.2) | 0.015 |
| Oxygen Saturation ≤ 92% | 2.2 (0.7 – 6.5) | 0.18 |
| FEV1‡ | 0.97 (0.4 – 2.2) | 0.94 |
All analyses control for age, gender, race, marital status, education, and BMI. Each COPD symptom or severity measurement listed above was used as an independent variable in its own multivariable logistic regression analysis in which sleep disturbance was the outcome (dependent variable).
Dyspnea Scale OR presented per 1 point change in scale.
OR per standard deviation change in COPD Severity Score (SD= 6.1).
Per liter increment in FEV1
Cognitive Function
Sleep disturbance was associated with incrementally worse HVLT memory test scores (difference in Z-score=−0.62; 95% CI −1.13 to −0.11; p=0.018), but was not significantly associated with incremental differences in any of the three tests of executive function (Table 3).
Table 3.
Association between sleep disturbance and cognitive dysfunction among 98 subjects with COPD*
| Difference in Z-score associated with presence of sleep disturbance (95% CI)† | P-value for difference | |
|---|---|---|
| Memory | ||
| Hopkins Verbal Learning Test | −0.62 (−1.13 to −0.11) | 0.018 |
| Executive Function | ||
| Stroop Color Word Test | −0.26 (−0.72 to 0.19) | 0.26 |
| Phonemic Fluency Test | −0.23 (−0.66 to 0.20) | 0.30 |
| DKEFS Trail Making Test | 0.05 (−0.45 to 0.55) | 0.86 |
Z-scores are standardized for age, based on a healthy referent population. A Z-score of 0 is the mean score for age while a Z-score of −1.0 is one standard deviation below the mean (i.e. one standard deviation lower cognitive function).
Each of the four cognitive function variables listed above was used as an outcome (dependent variable) in its own multivariable linear regression analysis in which sleep disturbance (present vs absent) was an independent variable. All analyses controlled for gender, race, marital status, educational attainment, and BMI. (Z-scores are already adjusted for age).
Mean difference in Z-score associated with the presence of sleep disturbance, relative to absence of sleep disturbance.
Longitudinal Analyses: Association between Sleep Disturbance and Health Outcomes
Sleep disturbance predicted both COPD exacerbations (OR=4.7; 95% CI 1.3 – 17; p=0.018) and respiratory-related emergency utilization (OR=11.5; 95% CI 2.1 – 62; p=0.004) over the ensuing year (Table 4). In multivariable Cox proportional hazards analysis over a median of 2.4 years, sleep disturbance was associated with five-fold increased hazard of poorer survival (hazard ratio [HR]=5.0; 95% CI 1.4 – 18; p=0.013). When controlling for FEV1 or FEV1 and COPD Severity Score, in addition to sociodemographic factors and BMI, sleep disturbance remained longitudinally associated with each of the three longitudinal outcomes examined.
Table 4.
Longitudinal Analyses: Multivariable analyses of the association between sleep disturbance at baseline and subsequent health outcomes
| COPD Exacerbations* | Respiratory-Related Emergency Utilization* | All-Cause Mortality† | |
|---|---|---|---|
| OR (95% CI) p-value | OR (95% CI) p-value | HR (95% CI) p-value | |
| Model 1: Controlling for Sociodemographics + BMI | 4.7 (1.3 – 17) p = 0.018 | 11.5 (2.1 – 62) p = 0.004 | 5.0 (1.4 – 18) p = 0.013 |
| Model 2: Model 1 + FEV1 | 6.3 (1.6 – 25) p = 0.008 | 15.2 (2.5 – 91) p = 0.003 | 9.5 (2.1 – 44) p = 0.004 |
| Model 3: Model 1 + FEV1 + COPD Severity Score | 4.0 (1.1 – 15) p = 0.042 | 9.7 (1.5 – 63) p = 0.017 | 8.8 (1.8 – 43) p = 0.007 |
All of the estimates (hazard ratio [HR] or odds ratio [OR]) presented above are for the association between sleep disturbance and a given health outcome (COPD exacerbations, or emergency utilization, all-cause mortality), with each of the 3 models controlling for an increasing number of covariates.
Analyses of COPD exacerbations and emergency utilization were multivariable logistic regressions in which the outcome was assessed one year after baseline. Emergency utilization is the combined end-point of either a hospitalization or ED visit.
Analyses of all-cause mortality were multivariable Cox regression analysis in which the outcome was assessed over a median 2.4 years of follow-up.
Model 1 controls for age, gender, race, marital status, educational attainment, and BMI
Model 2 controls for all variables in Model 1 + FEV1 (absolute)
Model 3 controls for all variables in Model 1 + FEV1 (absolute) + COPD Severity Score
Mediation Analyses
In these analyses, we controlled alternately for memory impairment, depressive symptoms, and anxiety symptoms. Adding the HVLT memory test to multivariable models, sleep disturbance maintained its predictive association with each of the three longitudinal outcomes and the point estimates did not decline (Table 5), thus failing to provide evidence that poor memory mediated the relationship between sleep disturbance and poor outcomes [45]. When controlling for depressive or anxiety symptoms, sleep disturbance was still predictive of death and COPD-related emergency utilization at the 95% confidence level, but confidence intervals included no association for the outcome of COPD exacerbations (Table 5). When adding depressive or anxiety symptoms to multivariable modeling, the point estimate for the association between sleep disturbance and poor outcomes also declined modestly for all longitudinal outcomes.
Table 5.
Mediation Analyses: Predictive association between sleep disturbance and poor outcomes after controlling for memory impairment, depressive symptoms, or anxiety symptoms
| COPD Exacerbations* | Respiratory-Related Emergency Utilization* | All-Cause Mortality† | |
|---|---|---|---|
| OR (95% CI) p-value | OR (95% CI) p-value | HR (95% CI) p-value | |
| Controlling for Memory Impairment | 10.4 (1.6 – 70) p=0.016 | 14.3 (1.7 – 118) p=0.013 | 14.8 (2.2 – 100) p = 0.006 |
| Controlling for Depressive Symptoms | 3.4 (0.7 – 15) p = 0.12 | 8.4 (1.2 – 57) p = 0.03 | 6.3 (1.2 – 32) p = 0.03 |
| Controlling for Anxiety Symptoms | 3.9 (0.99 – 15) p = 0.051 | 6.9 (1.1 – 45) p = 0.04 | 8.3 (1.7 – 41) p = 0.009 |
All models controlled for age, gender, race, marital status, educational attainment, BMI, FEV1 (absolute), and COPD Severity Score.
Analyses of COPD exacerbations and emergency utilization were multivariable logistic regressions in which the outcome was assessed one year after baseline. Emergency utilization is the combined end-point of either a hospitalization or ED visit.
Analyses of all-cause mortality were multivariable Cox regression analysis in which the outcome was assessed over a median 2.4 years of follow-up.
Secondary Analysis: Longitudinal Associations by GOLD Stage
As shown in Table 6, sleep disturbance at baseline predicted respiratory-related emergency utilization and mortality among subjects with more advanced COPD, but this relationship was not statistically significant among subjects with less advanced COPD. The presence of sleep disturbance at baseline perfectly predicted at least one subsequent exacerbation among subjects with COPD GOLD stages 3 to 4, and thus the OR for the longitudinal association between disturbed sleep and COPD exacerbations in this sub-group was undefined.
Table 6.
Secondary Analysis: Association between sleep disturbance at baseline and subsequent health outcomes, by COPD GOLD stage
| COPD Exacerbations | Respiratory-Related Emergency Utilization | All-Cause Mortality | |
|---|---|---|---|
| OR (95% CI) p-value | OR (95% CI) p-value | HR (95% CI) p-value | |
| GOLD 1 and 2 (n=56) | 1.9 (0.5 – 7.4) p = 0.38 | 3.9 (0.5 – 33) p = 0.21 | 3.2 (0.2 – 50) p = 0.42 |
| GOLD 3 and 4 (n=42) | N/A* | 15.1 (1.4 – 159) p = 0.02 | 4.5 (1.3 – 16) p = 0.02 |
OR could not be calculated because all GOLD stage 3 and 4 subjects who had disturbed sleep at baseline, and followed up in longitudinal reassessment, had suffered a COPD exacerbation (i.e. the presence of disturbed sleep at baseline perfectly predicted subsequent COPD exacerbation among subjects with more advanced COPD).
DISCUSSION
In this population-based, longitudinal study of persons with COPD, we found that disturbed sleep is predictive of exacerbations, respiratory-related emergency utilization, and all-cause mortality. This longitudinal relationship persisted even after controlling for both FEV1 and COPD Severity Score, suggesting that disturbed sleep is playing an independent role as a risk factor for poor outcomes in COPD, rather than simply being a marker of worse disease.
Although sleep disturbance was not related to FEV1, our findings demonstrated an association between sleep disturbance and both respiratory symptoms and COPD Severity Score, indicating that sleep disturbance is, on balance, associated with severity of disease in COPD. The lack of a relationship between sleep quality and FEV1 that we observed is consistent with the findings of Klink and colleagues [6]. Although perhaps surprising, and therefore important that the work of Klink and colleagues be replicated, we note that the correlation between dyspnea and FEV1 is often low [50]. Regardless, the COPD Severity Score, although modestly correlated with FEV1, is a strong predictor of poor outcomes in COPD, and higher COPD Severity Scores were associated with greater likelihood of disturbed sleep [18–21]. Thus, by virtue of an effect-response relationship, our findings suggest that COPD may in fact be contributing to disturbed sleep, which would potentially lower one’s threshold, in the clinic, for suspecting sleep disturbance in COPD patients.
Our findings, however, also support a bi-directionality to the relationship between disturbed sleep and disease severity in COPD. That is, COPD symptoms such as cough and dyspnea may be, in part, responsible for poor sleep quality, yet disturbed sleep may in turn contribute to COPD exacerbations and greater COPD severity. The fact that disturbed sleep was longitudinally predictive of poor COPD-related outcomes, even after rigorously controlling for COPD severity with both FEV1 and COPD Severity Score in multivariate models, supports this concept. This would be consistent with sleep disturbance being part of a vicious circle, whereby COPD contributes to poor sleep, which in turn contributes to worse COPD.
Hypothetically, if there were an interaction between COPD and disturbed sleep, we might expect that sleep disturbance would place subjects with more advanced COPD at higher risk of adverse outcomes. Our sample size limited a robust analysis of this question, but the higher estimates for mortality and respiratory-related emergency utilization associated with sleep disturbance at baseline, comparing subjects with GOLD stages 3–4 to subjects with GOLD stages 1–2, would be consistent with such an interaction. However, a substantially larger sample size adequate to statistically examine an interaction effect would be necessary to conclusively establish such a finding.
There are several theoretical mechanisms by which sleep disturbance could contribute to worsening COPD. It is thought that disturbed sleep, in general populations, may affect memory, learning, and abstract problem solving [51–53]. It is also known that verbal memory impairment in COPD patients is associated with poor adherence to medications [1, 51]. Alternatively, poor sleep quality is also known to affect immune function, and poor immunity may be particularly harmful for patients with COPD in whom infectious etiologies may precipitate COPD exacerbations [3–4]. Finally, although we attempted to measure insomnia symptoms rather than sleep-disordered breathing symptoms, it is possible that nocturnal hypoxemia or other nocturnal respiratory factors may have contributed to the observed findings.
In our modeling, we tested whether poor outcomes might have resulted from cognitive dysfunction, depression, or anxiety related to sleep disturbance. Although sleep disturbance was indeed cross-sectionally associated with poor memory, this did not appear to explain the risk for poorer outcomes. As expected, sleep disturbance was cross-sectionally associated with depressive and anxiety symptoms. This study cannot resolve whether such psychological factors were the result or the cause of insomnia symptoms; prior research, however, has shown that insomnia symptoms do strongly predict the subsequent development of depression [43]. Regardless, the central observation remains that sleep disturbance predicted death and COPD-related emergency utilization even after controlling for depressive or anxiety symptoms, suggesting that these psychological factors do not entirely explain the role of sleep disturbance.
This study has important limitations. The lack of objective sleep-related data, such as that obtained from polysomnography, does not permit us to more fully understand the etiology of the sleep disturbance we characterized; for example, whether it is related, at least in part, to sleep-disturbed breathing or hypoxemia. It is certainly possible that disturbed sleep, as assessed in our study, was a manifestation of nocturnal hypoxemia. Second, although the Medical Outcomes Study sleep scale has been psychometrically validated in a variety of populations, and we conducted our own analysis of performance characteristics, this sleep questionnaire has not been validated against objective measures of sleep quality such as polysomnography data. Nonetheless, these questions have strong face validity by virtue of their consistency with major criteria used for insomnia. Third, the wide confidence intervals in our longitudinal analyses, especially for the outcomes of COPD exacerbations and emergency utilization, limit our ability to accurately estimate the actual magnitude of the association between sleep disturbance and poor outcomes, although the fact that the confidence intervals excluded unity lends credence to the presence of an association between sleep disturbance and poor outcomes in the direction observed. Next, we measured lung function spirometrically but because of the population-based nature of recruitment with in-home assessments, additional measures of pulmonary function were not feasible. We were unable to determine, therefore, whether pulmonary impairments such as air-trapping or reduced diffusing capacity might be associated with sleep disturbance.
Loss to follow-up for the outcomes of COPD exacerbations and emergency utilization is an additional limitation. We attempted to compensate for potential biases by utilizing probability-of-attrition models, but less attrition would have provided greater confidence in these results. Nonetheless, it is reassuring that the mortality analysis reflected similar results, since this analysis did not require subject reassessment and therefore was not subject to follow-up loss. Also, the exacerbation and utilization outcomes were determined by self-report, albeit at subject follow-up. Objective confirmation might have provided more accurate measurement, but the approach of self-report has been shown to be valid and reasonably accurate, especially for emergency utilization services [54–56]. Although there is some evidence that older adults may tend to under-report utilization [55], under-reporting would tend to diminish any observed associations with sleep disturbance, and thus be a conservative bias (that is, biasing toward the null). Furthermore, it is again reassuring that the mortality outcome, which was confirmed through database queries, yielded results similar to the self-reported outcomes.
Our study underscores the potential importance of sleep quality in COPD. Sleep quality was assessed using items which elicited symptoms reflecting major criteria for insomnia, and disturbed sleep as such predicted poorer longitudinal outcomes in COPD, even after controlling for COPD severity. Interventional trials that specifically target insomnia symptoms, either by virtue of COPD control focused specifically on nocturnal symptoms or behavioral therapies targeted at improved sleep hygiene and sleep quality, would appear warranted. Although further investigations are needed to better delineate the causal pathways accounting for the potent adverse risks we observed, this study supports the conclusion that disturbed sleep, assessed in terms of insomnia symptoms, is a potential contributor to poor outcomes in COPD.
Acknowledgments
Funding: Dr. Omachi was supported by K23 HL102159-01 from the National Heart, Lung, and Blood Institute, National Institutes of Health. Dr. Katz, Dr. Blanc and recruitment of the UCSF COPD cohort were supported by R01 HL067438 from the National Heart, Lung, and Blood Institute, National Institutes of Health.
ABBREVIATIONS
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- DKEFS
Delis Kaplan Executive Function System
- ED
emergency department
- HR
hazard ratio
- HVLT
Hopkins Verbal Learning Test
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- GOLD
Global Obstructive Lung Disease
- MRC
Medical Research Council
- OR
odds ratio
- SD
standard deviation
Footnotes
No authors have any conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Theodore A. Omachi, Email: omachi@ucsf.edu.
Paul D. Blanc, Email: paul.blanc@ucsf.edu.
David M. Claman, Email: david.claman@ucsf.edu.
Hubert Chen, Email: hubert.chen@ucsf.edu.
Edward H. Yelin, Email: ed.yelin@ucsf.edu.
Laura Julian, Email: laura.julian@ucsf.edu.
Patricia P. Katz, Email: patti.katz@ucsf.edu.
References
- 1.Incalzi RA, Gemma A, Marra C, Capparella O, Fuso L, Carbonin P. Verbal memory impairment in COPD: its mechanisms and clinical relevance. Chest. 1997;112:1506–13. doi: 10.1378/chest.112.6.1506. [DOI] [PubMed] [Google Scholar]
- 2.Riegel B, Weaver TE. Poor sleep and impaired self-care: towards a comprehensive model linking sleep, cognition, and heart failure outcomes. Eur J Cardiovasc Nurs. 2009;8:337–44. doi: 10.1016/j.ejcnurse.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majde JA, Krueger JM. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005;116:1188–98. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Veeramachaneni SB, Sethi S. Pathogenesis of bacterial exacerbations of COPD. Copd. 2006;3:109–15. doi: 10.1080/15412550600651347. [DOI] [PubMed] [Google Scholar]
- 5.Krachman S, Minai OA, Scharf SM. Sleep abnormalities and treatment in emphysema. Proc Am Thorac Soc. 2008;5:536–42. doi: 10.1513/pats.200708-134ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klink ME, Dodge R, Quan SF. The relation of sleep complaints to respiratory symptoms in a general population. Chest. 1994;105:151–4. doi: 10.1378/chest.105.1.151. [DOI] [PubMed] [Google Scholar]
- 7.Dodge R, Cline MG, Quan SF. The natural history of insomnia and its relationship to respiratory symptoms. Arch Intern Med. 1995;155:1797–800. [PubMed] [Google Scholar]
- 8.Blanc P, Eisner M, Trupin L, Yelin E, Katz P, Balmes J. The association between occupational factors and adverse health outcomes in chronic obstructive pulmonary disease. Occup Environ Med. 2004;61:661–7. doi: 10.1136/oem.2003.010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hays RD, Martin SA, Sesti AM, Spritzer KL. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Med. 2005;6:41–4. doi: 10.1016/j.sleep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Manocchia M, Keller S, Ware JE. Sleep problems, health-related quality of life, work functioning and health care utilization among the chronically ill. Qual Life Res. 2001;10:331–45. doi: 10.1023/a:1012299519637. [DOI] [PubMed] [Google Scholar]
- 11.The International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 12.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22 (Suppl 2):S347–53. [PubMed] [Google Scholar]
- 13.Bellia V, Catalano F, Scichilone N, Incalzi RA, Spatafora M, Vergani C, et al. Sleep disorders in the elderly with and without chronic airflow obstruction: the SARA study. Sleep. 2003;26:318–23. doi: 10.1093/sleep/26.3.318. [DOI] [PubMed] [Google Scholar]
- 14.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Padilla R, Vazquez-Garcia JC, Marquez MN, Jardim JR, Pertuze J, Lisboa C, et al. The long-term stability of portable spirometers used in a multinational study of the prevalence of chronic obstructive pulmonary disease. Respir Care. 2006;51:1167–71. [PubMed] [Google Scholar]
- 16.Walters JA, Wood-Baker R, Walls J, Johns DP. Stability of the EasyOne ultrasonic spirometer for use in general practice. Respirology. 2006;11:306–10. doi: 10.1111/j.1440-1843.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- 17.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. American Journal of Respiratory and Critical Care Medicine. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 18.Eisner MD, Trupin L, Katz PP, Yelin EH, Earnest G, Balmes J, et al. Development and validation of a survey-based COPD severity score. Chest. 2005;127:1890–7. doi: 10.1378/chest.127.6.1890. [DOI] [PubMed] [Google Scholar]
- 19.Omachi TA, Yelin EH, Katz PP, Blanc PD, Eisner MD. The COPD severity score: a dynamic prediction tool for health-care utilization. Copd. 2008;5:339–46. doi: 10.1080/15412550802522700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisner MD, Omachi TA, Katz PP, Yelin EH, Iribarren C, Blanc PD. Measurement of COPD severity using a survey-based score: validation in a clinically and physiologically characterized cohort. Chest. 2010;137:846–51. doi: 10.1378/chest.09-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miravitlles M, Llor C, de Castellar R, Izquierdo I, Baro E, Donado E. Validation of the COPD severity score for use in primary care: the NEREA study. Eur Respir J. 2009;33:519–27. doi: 10.1183/09031936.00087208. [DOI] [PubMed] [Google Scholar]
- 22.Definition and classification of chronic bronchitis for clinical and epidemiological purposes. A report to the Medical Research Council by their Committee on the Aetiology of Chronic Bronchitis. Lancet. 1965;1:775–9. [PubMed] [Google Scholar]
- 23.van Ede L, Yzermans CJ, Brouwer HJ. Prevalence of depression in patients with chronic obstructive pulmonary disease: a systematic review. Thorax. 1999;54:688–92. doi: 10.1136/thx.54.8.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rule BG, Harvey HZ, Dobbs AR. Reliability of the Geriatric Depression Scale for younger adults. 1989;9:37–43. [Google Scholar]
- 25.Mancuso CA, Peterson MG, Charlson ME. Effects of depressive symptoms on health- related quality of life in asthma patients. J Gen Intern Med. 2000;15:301–10. doi: 10.1046/j.1525-1497.2000.07006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferraro FR, Chelminski I. Preliminary normative data on the Geriatric Depression Scale-Short Form (GDS-SF) in a young adult sample. J Clin Psychol. 1996;52:443–7. doi: 10.1002/(SICI)1097-4679(199607)52:4<443::AID-JCLP9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 27.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 28.Mykletun A, Stordal E, Dahl AA. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry. 2001;179:540–4. doi: 10.1192/bjp.179.6.540. [DOI] [PubMed] [Google Scholar]
- 29.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Benzo R, Flume PA, Turner D, Tempest M. Effect of pulmonary rehabilitation on quality of life in patients with COPD: the use of SF-36 summary scores as outcomes measures. J Cardiopulm Rehabil. 2000;20:231–4. doi: 10.1097/00008483-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Lezak MD. Neuropsychological Assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- 32.Golden CJ. Stroop Color and Word Test: A manual for clinical and experimental uses. Chicago, IL: Stoelting; 1978. [Google Scholar]
- 33.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 34.Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 35.Demakis GJ. Frontal lobe damage and tests of executive processing: a meta-analysis of the category test, stroop test, and trail-making test. J Clin Exp Neuropsychol. 2004;26:441–50. doi: 10.1080/13803390490510149. [DOI] [PubMed] [Google Scholar]
- 36.Delis DC, Kaplan E, Kramer JH, Ober BA. Delis-Kaplan Executive Function Scale (D-KEFS) San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- 37.Brandt J, Benedict RHB. Hopkins Verbal Learning Test - Revised. Lutz, FL: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- 38.Steinberg BA, Bieliauskas LA, Smith GE, Ivnik RJ. Mayo’s Older Americans Normative Studies: Age- and IQ-Adjusted Norms for the Trail-Making Test, the Stroop Test, and MAE Controlled Oral Word Association Test. Clin Neuropsychol. 2005;19:329–77. doi: 10.1080/13854040590945210. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg BA, Bieliauskas LA, Smith GE, Ivnik RJ, Malec JF. Mayo’s Older Americans Normative Studies: Age- and IQ-Adjusted Norms for the Auditory Verbal Learning Test and the Visual Spatial Learning Test. Clin Neuropsychol. 2005;19:464–523. doi: 10.1080/13854040590945193. [DOI] [PubMed] [Google Scholar]
- 40.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. [Accessed February 20, 2010];Obes Res. 1998 6(Suppl 2):51S–209S. Available at: www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.htm. [PubMed] [Google Scholar]
- 41.Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl. 2003;41:46s–53s. doi: 10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- 42.Stewart AL, Ware JE. Measuring functioning and well-being. Durham, NC: Duke University Press; 1992. [Google Scholar]
- 43.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 44.Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28:1457–64. doi: 10.1093/sleep/28.11.1457. [DOI] [PubMed] [Google Scholar]
- 45.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2. Hoboken, NJ: John Wiley & Sons; 2002. [Google Scholar]
- 47.Rao RS, Sigurdson AJ, Doody MM, Graubard BI. An application of a weighting method to adjust for nonresponse in standardized incidence ratio analysis of cohort studies. Ann Epidemiol. 2005;15:129–36. doi: 10.1016/j.annepidem.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–77. [PubMed] [Google Scholar]
- 49.Hosmer DW, Lemeshow S. Applied logistic regression. 2. Hoboken, NJ: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 50.Eltayara L, Becklake MR, Volta CA, Milic-Emili J. Relationship between chronic dyspnea and expiratory flow limitation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154:1726–34. doi: 10.1164/ajrccm.154.6.8970362. [DOI] [PubMed] [Google Scholar]
- 51.Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J. 2010;35:913–22. doi: 10.1183/09031936.00125109. [DOI] [PubMed] [Google Scholar]
- 52.Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64:180–7. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Killgore WD, Kahn-Greene ET, Lipizzi EL, Newman RA, Kamimori GH, Balkin TJ. Sleep deprivation reduces perceived emotional intelligence and constructive thinking skills. Sleep Med. 2008;9:517–26. doi: 10.1016/j.sleep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 54.Petrou S, Murray L, Cooper P, Davidson LL. The accuracy of self-reported healthcare resource utilization in health economic studies. Int J Technol Assess Health Care. 2002;18:705–10. doi: 10.1017/s026646230200051x. [DOI] [PubMed] [Google Scholar]
- 55.Richards SH, Coast J, Peters TJ. Patient-reported use of health service resources compared with information from health providers. Health Soc Care Community. 2003;11:510–8. doi: 10.1046/j.1365-2524.2003.00457.x. [DOI] [PubMed] [Google Scholar]
- 56.Roberts RO, Bergstralh EJ, Schmidt L, Jacobsen SJ. Comparison of self-reported and medical record health care utilization measures. J Clin Epidemiol. 1996;49:989–95. doi: 10.1016/0895-4356(96)00143-6. [DOI] [PubMed] [Google Scholar]