Abstract
Autophagy allows cells to survive under conditions of nutrient deprivation. We have demonstrated that autophagy inhibitors are synthetically lethal with NFκB inhibitors in B-cell lymphomas because the NFκB pathway promotes survival by increasing glucose import. When NFκB is inhibited in B-cell lymphoma, glucose import decreases and cells become sensitive to perturbations in mitochondrial metabolism and autophagy. Thus, combined inhibition of autophagy and NFκB drives cells into metabolic crisis accelerating cell death.
Key words: Epstein-Barr virus, latent membrane protein-1, AKT, GLUT1, phosphoinositol-3-kinase, NFκB
IKKβ Controls Glucose Import
We determined that the NFκB activators Epstein-Barr virus (EBV) oncoprotein latent membrane protein 1 (LMP1), LPS, and CpG increase glucose uptake in Burkitt's lymphoma by promoting GLUT1 trafficking from intracellular vesicles to the plasma membrane (GLUT translocation). Chemical IKKβ inhibitors block the effects of all three stimuli on GLUT1 translocation and glucose import. Furthermore, IKKβ inhibitors cause GLUT1 retention in cells with constitutive GLUT1 membrane localization including EBV+ lymphoblastoid cells (LCLs), Kaposi's sarcoma herpes virus-infected peripheral effusion lymphomas (PEL) and diffuse large B cell lymphomas (DLBCL). Thus, IKKβ governs GLUT1 localization in multiple B-cell malignancies.
PtdIns3K, IKKβ and NFκB-Induced Transcription are all Necessary for GLUT1 Plasma Membrane Localization
GLUT1 trafficking in lymphocytes is regulated much like GLUT4 trafficking in adipocytes, where insulin triggers the PtdIns3K-AKT pathway to phosphorylate AKT substrate of 160 kDa (AS160). AS160 is a negative regulator of GLUT4 plasma membrane localization and is inactivated by phosphorylation. Using chemical inhibitors, we confirmed that PtdIns3K and AKT are both essential to sustain GLUT1 membrane localization in B-cell lymphomas. Likewise, constitutively active myristoylated AKT (myrAKT) renders AS160 phosphorylation, GLUT1 surface localization, and glucose import resistant to PtdIns3K inhibition.
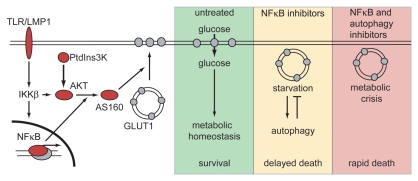
We established that the NFκB pathway controls GLUT1 trafficking by interacting with the PtdIns3K-AKT pathway at two distinct points. First LMP1, TLR4, and TLR9 require IKKβ and PtdIns3K activity for AKT activation. Second, NFκB-mediated transcription is necessary for AKT to phosphorylate AS160. myrAKT is unable to sustain AS160 phosphorylation after NFκB subunits are retained in the cytoplasm by the NFκB superrepressor, ΔNIκBα. Thus, PtdIns3K, IKKβ and NFκB-induced transcription are essential for TLR and LMP1 to promote AKT-mediated GLUT1 translocation (Fig. 1).
Figure 1.
The NFκB pathway induces glucose import to support survival of B-cell lymphomas; autophagy prolongs survival after NFκB inhibition. Stimulation of NFκB by TLRs or EBV-LMP1 promotes GLUT1 translocation to the plasma membrane at two distinct points. IKKβ and PtdIns3K cooperate to activate AKT, whereas NFκB-driven transcription is essential for AKT-mediated AS160 phosphorylation. In NFκB-high, untreated lymphomas, GLUT1-mediated glucose import supports proliferation and survival. After NFκB inhibition, lymphoma cells are deprived of glucose, causing starvation-induced autophagy that delays death. When NFκB and autophagy are inhibited simultaneously, lymphoma cells die rapidly of a metabolic crisis.
Although we had expected EBV-infected LCLs to die by apoptosis after NFκB inhibition, we have little evidence for it. We never observed cytochrome C release or Caspase 9 activation, suggesting that apoptosis is blocked at the mitochondria. Furthermore, caspase inhibitors cannot prevent LCL death after NFκB inhibition, indicating NFκB promotes survival independent of its function in apoptosis inhibition.
As increasing evidence indicates metabolism and cell survival are intertwined, we sought to determine the impact of NFκB-driven glucose import on NFκB-driven survival. The viability of LCLs after NFκB inhibition is increased from 40% to 60% by the addition of excess glutamine and α-ketoglutarate. These data indicate that an essential survival function of NFκB is linked to glucose import and, conversely, NFκB inhibitors cause cell death by restricting glucose availability.
Autophagy is a Prosurvival Pathway after NFκB Inhibition
Autophagy can be triggered by glucose restriction to prolong survival by providing energy through self-digestion. Consistent with a model in which NFκB inhibition causes starvation, we found that NFκB inhibition increases the number and size of autophagosomes (LC3 puncta and LC3B-II accumulation). Autophagy served as a prosurvival mechanism since the autophagy inhibitors, 3-methyladenine and chloroquine, kill LCLs only after NFκB inhibition. Importantly, glutamine and α-ketoglutarate suppress autophagosome formation and dependence on autophagy in NFκB-inhibited LCLs. Thus, autophagy is triggered by reduced glucose availability after NFκB inhibition and prolonged cell survival (Fig. 1).
Metabolic Sensors
The nutrient and energy sensing signaling pathway that regulates autophagy in the context of NFκB-inhibition is likely to involve either mTORC1 or AMPK; both directly target the autophagy machinery. Possibly, α-ketoglutarate and glutamine decreased autophagy in NFκB-inhibited, starved LCLs by altering intracellular amino acid pools and activating the autophagy-suppressor mTORC1. Alternatively, low intracellular glucose levels in NFκB-inhibited LCLs may increase autophagy in an AMPK-dependent manner despite the fact that we did not observe increased AMPK-Thr172 phosphorylation; the Meijer group has demonstrated that basal AMPK activity is sufficient to promote autophagy. It will be interesting in the future to assess the activity of AMPK, as well as of the mTORC1 pathway, after inhibition of NFκB to further elucidate how NFκB-signaling, metabolism and autophagy are intertwined.
NFκB Effects on GLUT1 may be Unique to Lymphocytes
We propose that the effect of NFκB on glucose import and autophagy may be specific to lymphocytes. Cells of the immune system are poised to proliferate in response to the same inflammatory signals that cause nonhematopoietic tissues to reduce their metabolic activity. Consistent with this idea, TNFα induced-IKKβ activity blocks PtdIns3K-induced GLUT4 translocation in adipocytes, whereas we and others have shown that TLR, CD40-R, BAFF-R and T-cell receptor signaling stimulates glucose import in lymphocytes. Furthermore, IKKβ promotes autophagy after heat shock, starvation, and rapamycin treatment in nonlymphoid cells, whereas we showed that IKKβ-induced glucose import suppresses autophagy.
Autophagy after antigen stimulation may play an important role in B cell maturation. In the primary B-cell response, NFκB activation will ensure ample glucose uptake for the proliferation of antigen specific B-cells. When antigen becomes limiting, NFκB activity and glucose import will decrease in the B cells with low affinity antigen receptors. Autophagy may be triggered to ensure B-cell survival despite low NFκB activity to provide the time required for affinity maturation and clonal selection.
Combinatorial Therapy for Lymphomas
As constitutive activation of NFκB is a common feature of many lymphomas, the NFκB pathway is actively pursued as a target for chemotherapy. Our findings suggest that combinatorial therapy of NFκB inhibitors and autophagy inhibitors, like choloroquine, may provide selective killing or allow a larger therapeutic window than either therapy alone. We found that NFκB regulates GLUT1 membrane localization and glucose import in the same germinal center type DLBCLs that are resistant to NFκB inhibitor monotherapy. Potentially, NFκB inhibitors will sensitize these cells to metabolic inhibitors. We wait with great interest to see the response of refractory multiple myeloma to chloroquine and bortezomib, a proteasome inhibitor that blocks NFκB transcription, now in clinical trial.
Abbreviations
- EBV
Epstein-Barr virus
- IKKβ
inhibitor of kappaB kinase beta
- LMP1
latent membrane protein 1
- TLR
toll-like receptor
- LPS
lipopolysaccharide
- LCL
lymphoblastoid cell line
- PEL
peripheral effusion lymphomas
- DLBCL
diffuse large B-cell lymphomas
- PtdIns3K
phosphatidylinositol 3-kinase
- AS160
AKT substrate of 160 kDa
- mTORC1
mTOR complex 1
- ΔNIκBα
deletion of amino acids 1–36 of IκBα
Punctum to: Sommermann TG, O'Neill K, Plas DR, Cahir-McFarland E. IKKβ and NFκB transcription govern lymphoma cell survival through AKT-induced plasma membrane trafficking of GLUT1. Cancer Res. 2011;71:7291–7300. doi: 10.1158/0008-5472.CAN-11-1715.