Abstract
Cardiotonic steroids signaling through the basolateral sodium pump (Na/KATPase) have been shown to alter renal salt handling in intact animals. As the relationship between renal salt handling and blood pressure is a key determinant of hypertension, and patients with insulin resistance are frequently hypertensive, we chose to examine whether there might be competition for resources necessary for receptor mediated endocytosis.
In LLC-PK1 cells, the Na/K-ATPase-α1 and carcinoembryonic antigen cell adhesion molecule (CEACAM1), a plasma membrane protein that promotes receptor-mediated endocytosis, co-localized in the plasma membranes and translocated to the intracellular region in response to ouabain. Either ouabain or insulin alone caused accumulation of CEACAM1 as well as IRβ, and EGFR in early endosomes, but no synergy was demonstrable. Like ouabain, insulin also caused c-Src activation. When caveolin or Na/K-ATPase-α1 expression was knocked down with siRNA, insulin but not ouabain induced CEACAM1, IRβ, and EGFR endocytosis.
To determine whether this might be relevant to salt handling in vivo, we examined salt loading in mice with null renal CEACAM2 expression (Cc2−/−). The Cc2−/− animals demonstrated greater increases in blood pressure with increases in dietary salt than control animals.
These data demonstrate that cardiotonic steroids and insulin compete for cellular endocytosis resources and suggest that under conditions where circulating insulin concentrations are high, cardiotonic steroid mediated natriuresis could be impaired.
Keywords: chronic renal insufficiency, renal proximal tubule cell, endocytosis
Introduction
Insulin binding induces phosphorylation of the insulin receptor (IR), carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), and its substrates to activate downstream signaling pathways. Phosphorylation of CEACAM1, a surface membrane glycoprotein, promotes insulin endocytosis via its receptor, followed by its degradation1. In agreement with receptor-mediated insulin uptake and degradation constituting the basic mechanism of its clearance in liver and kidney, null mutation of CEACAM1 impairs insulin clearance to cause hyperinsulinemia and insulin resistance2, 3.
We have previously shown that cardiotonic steroids induce endocytosis of the plasmalemmal Na/K-ATPase in renal proximal tubule cells to increase urinary sodium excretion4. Lingrel and colleagues have recently shown that although some cardiotonic steroids may promote hypertension, loss of their natriuretic effects may actually exacerbate salt-dependent hypertension5–7.
Similar to insulin receptor, the epidermal growth factor receptor (EGFR) also phosphorylates CEACAM1, an event that in turn mediates their complex formation and regulation of post-receptor signaling8. Given that EGFR is transactivated by Na/K-ATPase-initiated signaling, the current study tests whether CEACAM1 is also involved in this process.
Experimental Procedures
Chemicals and Antibodies
Chemicals of the highest purity available were obtained from Sigma (St. Louis, MO, USA).
Monoclonal and polyclonal antibodies against Na/K-ATPase α-1 subunit (clone C464.4), EGFR, and EEA-1 were obtained from Upstate Biotechnology (Lake Placid, NY, USA). Antibody against caveolin-1 (clone C060) was obtained from BD Transduction Laboratories (Lexington, KY, USA). Monoclonal antibody against clathrin heavy chain (CHC, clone ×22) was obtained from Affinity BioReagents (Golden, CO, USA). Polyclonal antibodies against insulin receptor β subunit, caveolin-1, c-Src, Rab7, as well as horseradish peroxidase-conjugated goat anti-mouse and goat anti-rabbit IgG were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and were used for Western blots. Monoclonal antibody against insulin receptor β subunit was obtained from AnaSpec (Fremont, CA). Monoclonal antibody against Na/K-ATPase α-1 subunit (clone α6F) was obtained from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA, USA). Normal mouse IgG and rabbit IgG were purchased from Sigma. Optitran nitrocellulose membrane was obtained from Schleicher&Schuell (Keene, NH, USA).
Cell Culture
The pig renal proximal tubule cell line, LLC-PK1, was obtained from the American Tissue Type Culture Collection (Manassas, VA, USA), and cultured to confluent condition as described before9.
In immunostaining, LLC-PK1 cells were grown to confluence on the 24-mm polycarbonate Transwell culture filter inserts (filter pore size 0.4 μm, Costar Co.; Cambridge, MA, USA) as reported previously9. LLC-PK1 cells expressing mock-vehicle (P-11, as control), Na/K-ATPase-α1 siRNA (PY-17, as α1-knock down cells), and caveolin-1 siRNA (C2–7, as caveolin-1 depleted cells) were cultured in the same manner as the parent LLC-PK1 cells.
Immunofluorescence
Cells grown to confluence on the 24-mm Transwell filters were fixed and permeabilized as described by Muth et al.10. The cells were then probed with primary antibody for 90 min. at room temperate or overnight at 4°C (monoclonal anti-α1 antibody, Upstate; polyclonal anti-CEACAM1 antibody8, 1:100 dilution in GSDB buffer). After 3 washes with permeabilization buffer, the cells were incubated with Alexa Fluor® 546-conjugated or Alexa Fluor® 488-conjugated secondary antibody for 1h at room temperature. After 3 additional washes, specimens were mounted using Prolong Anti-fade medium (Molecular Probes, Eugene, OR, USA).
All images were acquired via a Leica TCP SP5 broadband confocal microscope system (Leica, Mannheim, Germany) with a 63× oil-immersion objective, and analyzed with Leica software. The confocal microscope studies were performed using resources of the Advanced Microscopy and Imaging Center at the University of Toledo Health Science Campus.
Immunoprecipitation
Immunoprecipitation experiments were performed as described by Chibalin and others11 and published previously from our laboratory12, 13. LLC-PK1 cells were lysed in ice-cold RIPA lysis buffer. Insoluble material was removed by centrifugation (14,000 × 10 min at 4°C). Aliquots of supernatant (total of 1 mg protein) were immunoprecipitated overnight at 4°C with monoclonal anti-Na/K ATPase-α1 subunit antibody (Upstate). Immunoprecipitates were incubated with protein G-agarose beads, rotating for 2h at 4°C. Beads were washed five times in RIPA lysis buffer and immunoprecipitated proteins were eluted with Laemmli sample buffer and analyzed by Western Blot.
Preparations of Endosomes
Endosomes were fractionated on a floating gradient using the technique of Gorvel et al.14. The early endosomal fraction was collected at the 16%–10% sucrose interface. The identity of early endosomal fractions was determined with antibodies against early endosome protein marker EEA-1 as we have described previously12, 13.
Western Blot
Immunoblotting was performed as described previously12. Briefly, cell lysates (50 μg/lane), endosomal fractions (15 μg/lane), or immunoprecipitates (from 1 mg total protein per sample) were separated by 4–15% gradient sodium dodecyl sulfatepolyacrylamide gel electophoresis (SDS-PAGE; BioRad), and transferred to nitrocellulose membrane. After transfer, membranes were blocked with 5% milk in TBST (Tris-HCl 10 mmol/L, NaCl 150 mmol/L, Tween 20, 0.05%; pH 8.0) for one hour at room temperate, and immunoblotting was performed. Detection was performed with the enhanced chemiluminescence (ECL) Plus Western Blotting Detection System (Amersham, Buchinghamshire, UK). Multiple exposures were analyzed to assure that the signals were within the linear range of the film. Autoradiograms were scanned with a Bio-Rad GS-670 imaging densitometer (BioRad, Herculese, CA, USA) to quantify signals9.
Animals
The generation of Ceacam2-null (Cc2−/−) mice was described previously15. Male mice from 4–8 months of age were studied. Animals were kept in a 12-hour dark/light cycle and fed standard regular chow and tap water ad libitum. All procedures were approved by the Institutional Animal Care and Utilization Committee.
Statistical Analysis
Data are presented as the mean±standard error of mean (SEM). Data obtained were first tested for normality and then subjected to parametric analysis. For comparison of more than two groups, one-way ANOVA was employed using the Student t-test with Bonferroni's correction for multiple comparisons for post-hoc analysis16. Statistical analysis was performed with SPSS software.
Results
Na/K ATPase-α1 and CEACAM1 Co-Localize to the Plasmalemma of LLC-PK1 Cells
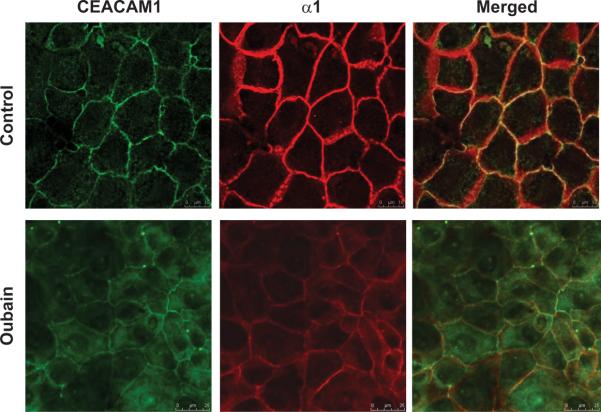
To determine whether CEACAM1 protein is expressed in porcine renal proximal tubule LLC-PK1 cells, an in vitro model extensively utilized in our laboratory, we initially carried out Western analysis of whole cell lystes. CEACAM1 protein was easily detected in LLC-PK1 cells, albeit to a much lower extent than rat hepatoma H4IIE cells (data not shown). Subsequently, immunofluorescence analysis demonstrated that CEACAM1 was mostly localized to the plasmalemma of LLC-PK1 monolayers grown on Transwell membranes (Fig. 1, top row, green fluorescence).
Fig. 1.
Representative images showing the cellular redistribution of CEACAM1 (green fluorescence) and Na/K-ATPase-α1 subunit (red fluorescence) following ouabain (100 nM, 30 min) treatment in renal proximal tubule LLC-PK1 cells. Merged image shows co-localization (yellow fluorescence). N=5 samples studied from each group.
This expression pattern of CEACAM1 was comparable to that of Na/K-ATPase-α1 subunit (Fig.1, top row, red fluorescence), as we and others have previously reported17. Furthermore, both of these proteins co-localized, as revealed by merging analysis (Fig. 1, top row, yellow fluorescence).
Ouabain- and Insulin-Induced Accumulation of CEACAM1 in Early Endosomal Fractions of LLC-PK1 Cells
Ouabain (100 nM, 30 min.) increased intracellular content of Na/K-ATPase–α1 subunit in LLC-PK1 cells (Fig. 1, bottom row, red fluorescence). Intracellular CEACAM1 content also increased in response to the same ouabain treatment (Fig. 1; bottom vs. top row; green fluorescence). These data suggested that the α1 subunit and CEACAM1 are co-expressed on the plasmalemma of LLC-PK1 cells, and both undergo internalization in response to ouabain.
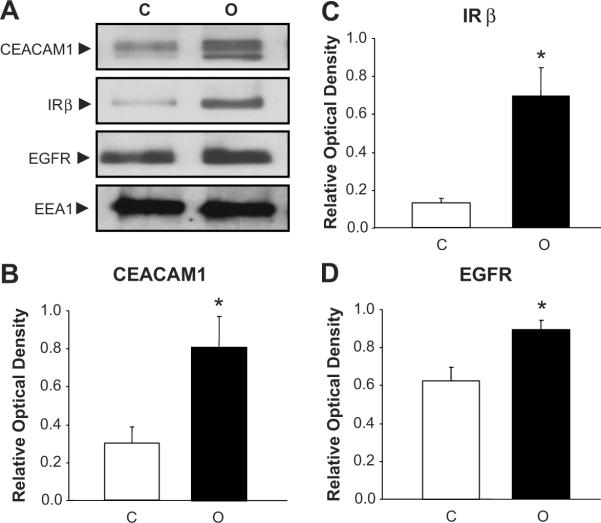
Western blot analysis revealed the presence of CEACAM1 in the early endosomal fractions of LLC-PK1 cells (Fig. 2A). In comparison to control cells, ouabain treatment (100 nM, 30 min) causes increased in early endosomal content of CEACAM1 by ~2.5-fold (Figs. 2A and B), Insulin receptor-β subunit (IRβ) by ~3-fold (Figs. 2A and C), and EGFR by ~1.5-fold (Figs.2A and D). Ouabain also increased the early endosomal content of the α1 subunit of the Na/K-ATPase as we have previously reported12 (data not shown).
Fig. 2.
Panel A shows autoradiographs of representative Western blot for CEACAM1, EGFR, and IRβ expression in early endosomal fractions of renal proximal tubule LLC-PK1 cells treated with ouabain (100 nM, 30 min). Panels B–D show corresponding quantitative data as the mean ± SEM of 5 experiments. Early Endosomal Antigen-1 (EEA1), an early endosomal marker, was used as loading control. *p<0.05 vs. Control. C, Control; O, Ouabain.
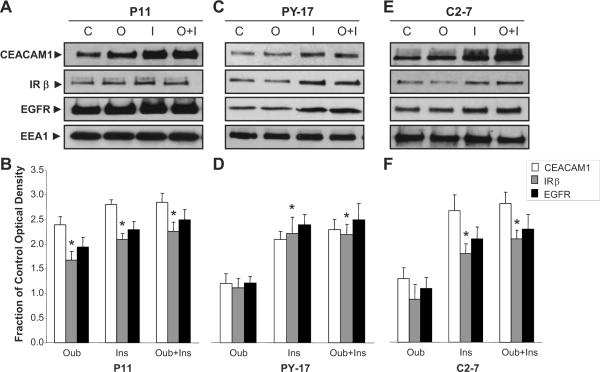
We next investigated whether insulin exerts a synergistic effect with ouabain on CEACAM1 internalization and whether CEACAM1 internalization depends on Na/K-ATPase signaling. To address this question, we utilized LLC-PK1 cells stably transfected with mock expressing vector alone (P11), or with siRNA expressing vectors of knockdown Na/K–ATPase α1-subunit expression (PY-17), or knockout caveolin-1 expression (C2–7)18. Like ouabain, insulin (100 nM, 30 min.) induced early endosomal accumulation of CEACAM1, IRβ, and EGFR proteins in P11 control cells (Fig. 3A and B). However, concurrent treatment with ouabain and insulin did not produce synergistic endocytic effects in P11 cells (Fig. 3A and B).
Fig. 3.
Representative Western blots and quantitative protein expression shown as the mean ± SEM of 5 samples for CEACAM1 (Panels A and B), EGFR (Panels C and D), and IRβ (Panels E and F) expression in early endosomal fractions of renal proximal tubule P11, PY-17, and C2-7 cells treated with exogenous ouabain (100 nM) and/or Insulin (100 nM) for 30 min. Early Endosomal Antigen-1 (EEA1) was used as loading control. *p<0.05 vs. Control. C, Control; I, Insulin; O, Ouabain; O+I, Ouabain+Insulin.
In the PY-17 and C2–7 cells where we have previously shown ouabain signaling through the Na/K-ATPase to be minimal19, insulin, but not ouabain, induced the endosomal accumulation of these receptors and CEACAM1 (Figs. 3C–F). Moreover, ouabain failed to influence the effect of insulin on the internalization of these proteins in both PY-17 and C2–7 cells.
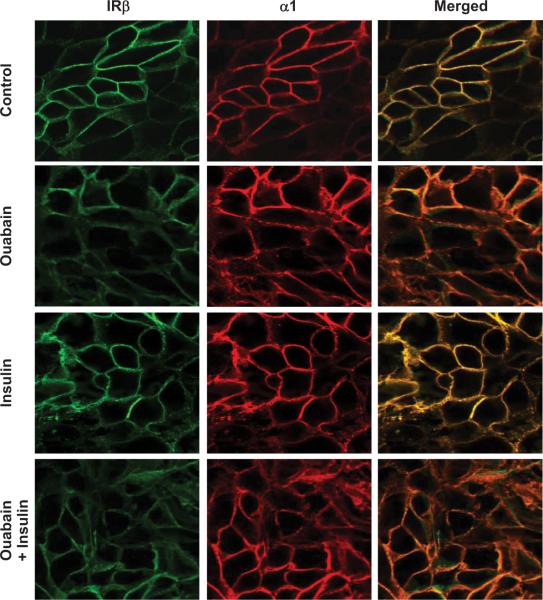
Na/K ATPase–α1 and Insulin Receptor β Co-Localize to the Plasmalemma of LLC-PK1 Cells
Immunofluorescence analysis demonstrated that sodium pump α1 and IRβ subunits were localized to the plasmalemma of monolayer LLC-PK1 cells grown on Transwell membranes. Ouabain increased internalization of α1 subunit as before, and insulin treatment, with or without ouabain, also caused internalization of both α1 and IRβ subunits (Fig 4).
Fig. 4.
Representative images showing the cellular distribution of Insulin Receptor β subunit (green fluorescence) and Na-K-ATPase-α1 subunit (red fluorescence) following ouabain (100 nM, 1 h) and/or insulin (100 nM, 1 h) treatment in renal proximal tubule LLC-PK1 cells. Merged image shows colocalization.
Ouabain- and Insulin-Induced Accumulation of Sodium pump-α1 subunits in Early Endosomal Fractions of LLC-PK1 Cells
Consistent with our previous reports, ouabain increased sodium pump-α1 subunit protein accumulation in early endosomal fraction of LLC-PK1 cells. Interestingly, insulin alone also caused early endosomal α1 subunit accumulation, but concurrent ouabain and insulin treatment was neither synergistic or additive (Fig 5A and B).
Fig. 5.
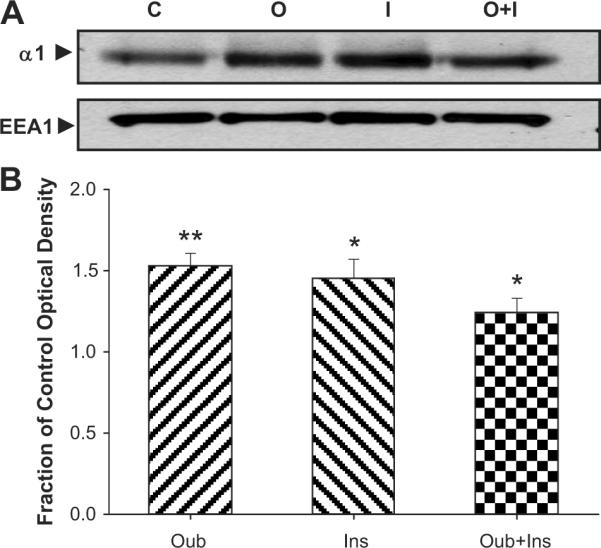
Representative Western blots (Panel A) and quantitative protein expression shown as the mean ± SEM (Panel B) of 8 samples of Na/K–ATPase-α1 subunit expression in early endosomal fraction of renal proximal tubule LLC-PK1 cells treated with ouabain (100 nM) and/or Insulin (100 nM) for 1 h. Early Endosomal Antigen-1 (EEA1) was used as loading control. *p<0.05 vs. Control. C, Control; I, Insulin; O, Ouabain; O+I, Ouabain+Insulin.
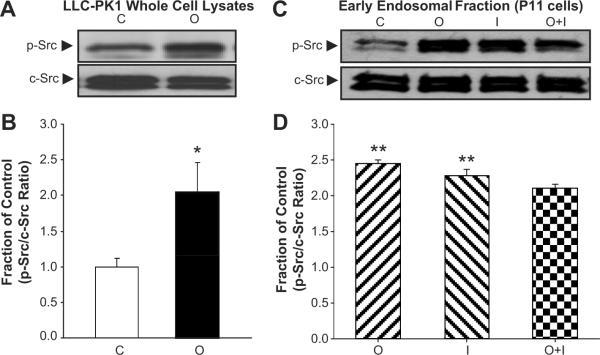
Ouabain- and Insulin-Induced c-Src Phosphorylation in LLC-PK1 Cells
As above, LLCPK1 cells were treated with ouabain and/or insulin for 30 min. and c-Src phosphorylation was determined. Relative to control, ouabain stimulated c-Src phosphorylation (p-Src) in whole cell lysates by ~2.5-fold (Figs. 6A and B) as well as in early endosomal fractionations (Figs. 6C and D). Interestingly, insulin induced a comparable effect to that of ouabain on c-Src phosphorylation in early endosomal fractions (Figs. 6C and D). Concomitant treatment with insulin and ouabain produced no synergistic induction on p-Src in either whole cell lysates (data not shown) or early endosomal fraction (Figs. 6C and D).
Fig. 6.
Panel A and C show representative Western blots for phosphorylated (p-Src) and total Src (c-Src) in LLC-PK1 whole cell lysates, and P11 cell early endosomal fractions, respectively. Panels B and D show corresponding quantitative relative optical densities of p-Src (relative to c-Src) in LLC-PK1 whole cell lysates and early endosomal fractions, respectively with exogenous ouabain (100 nM) and/or Insulin (100 nM) for 30 min. Quantitative data shown as the mean ± SEM of 5 experiments. *p<0.05 vs. Control. C, Control; I, Insulin; O, Ouabain; O+I, Ouabain+Insulin.
The Effect of Ouabain and Insulin on Interaction Amongst Na/K-ATPase- α1, CEACAM1, and Caveolin-1 in LLC-PK1 Cells
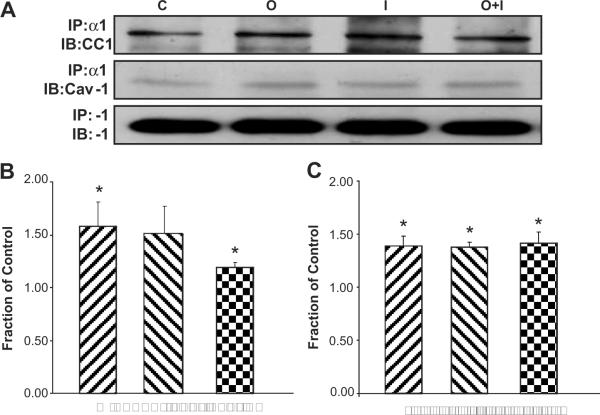
To elucidate if there is an interaction between α1 subunit and CEACAM1, LLC-PK1 cells were treated with ouabain and/or insulin for 1h and immunoprecipitated with anti-Na/K-ATPase-α1 subunit antibody (IP: α1). Immunoblotting with antibodies against CEACAM1 (IB: CC1) and α1 (IB: α1, as control) revealed that ouabain treatment enhanced the interaction between CEACAM1 and the α1 subunit (Fig. 7A and B). Insulin similarly augmented α1–CEACAM1 co-precipitation, though this did not reach statistical significance (Figs. 7A and B). Concurrent treatment with insulin and ouabain induced a mild statistically significant increase in α1–CEACAM1 co-precipitation (Fig. 7A and B).
Fig. 7.
Panel A shows representative Western blots for CEACAM1 and Caveolin-1 in renal proximal tubule LLC-PK1 cells treated with exogenous ouabain (100 nM, 1h) and/or insulin (100 nM, 1h) following immunoprecipitation with anti-Na/K-ATPase α1 subunit antibody. Panels B and C show corresponding quantitative data for CEACAM1 and Caveolin-1, respectively, as the mean ± SEM of 4 experiments. *p<0.05 vs. Control. C, Control; I, Insulin; O, Ouabain; O+I, Ouabain+Insulin.
Immunoblotting with an antibody against caveolin-1 (IB: Cav-1) revealed that ouabain and insulin increased the co-immunoprecipitation of Na/K-ATPase-α1 subunit and caveolin-1 and that this effect of insulin was not synergistic with ouabain (Figs. 7A and C).
Salt Increased Systolic Blood Pressure in Mice with Null Mutation of Ceacam2 (Cc2−/−)
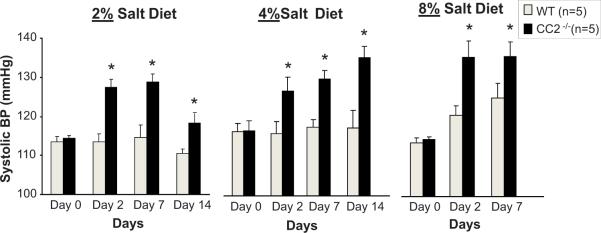
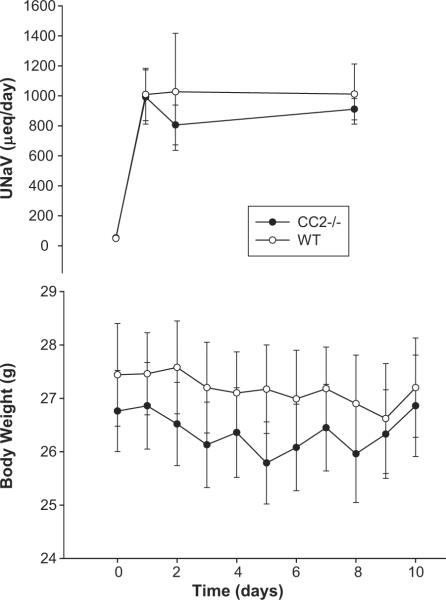
CEACAM2, a highly homologous related protein to CEACAM1, is the predominant protein in murine kidney20. With CEACAM proteins being expressed in kidney proximal tubules, we evaluated the role of CEACAM2 in regulating blood pressure following salt loading. Aged matched wild-type (WT) and Cc2−/− mice were switched from a control (low salt, 0.4% salt) diet to increased amounts of dietary salt (2%, 4%, or 8% NaCl). There was no difference in body weight, water consumed, and urine volume between WT and Cc2−/− mice regardless of their dietary salt content (Table 1). However, systolic blood pressures measured on Day 0, 2, 7, and 14 were relatively constant in WT mice when fed 2% and 4% high-salt diets, and trended to increase by Day 2 on 8% high-salt diet (Fig. 8). In comparison, Cc2−/− mice responded with increased blood pressure almost immediately (by Day 2) even on 2% salt diet, and underwent a progressive increase in response to higher salt concentration in diet (Fig. 8). On 8% salt-diet, systolic blood pressure reached a maximal blood pressure as early as Day 2, and remained maximally elevated during the remainder of the study in these Cc2−/− mice (Fig 8). Both WT and Cc2−/− mice responded with increased urinary sodium excretion with all of the high salt diet in a dose dependent manner. However, no differences in the change in body weight or urinary sodium excretion was evident between the wild type and Cc2−/− animals when placed on any experimental diet. The data for the 4% NaCl diet (which was the first experimental high salt diet the animals were exposed to) are shown in Fig 9.
Table 1.
Effect of high salt diet (2%, 4%, and 8%) vs. control (0.4%) on body weight, urine output, and water consumption in mice lacking Ceacam2− (Cc2−/−) vs. wild-type (WT). Data shown as the mean ± SEM of measurements performed on 5 animals in each group.
| Diet Collection Time | 0.4% Salt Diet Day 8 | 2% Salt Diet Day 8 | 4% Salt Diet Day 8 | 8% Salt Diet Day 8 | ||||
|---|---|---|---|---|---|---|---|---|
| WT | CC2−/− | WT | CC2−/− | WT | CC2−/− | WT | CC2−/− | |
| Body Weight (g) | 27.24±0.76 | 28.51±0.91 | 28.84±1.04 | 28.95±1.05 | 25.96±0.81 | 26.90±0.78 | 27.50±1.05 | 26.18±1.54 |
| Urine Output (mL/24hr) | 0.9±0.1 | 0.6±0.2 | 2.6±2.0 | 3.1±0.8 | 2.9±1.3 | 3.4±1.2 | 3.7±0.9 | 4.6±0.7 |
| UNaV (μeq/24 hrs) | 53±5 | 49±7 | 405±82 | 477±92 | 911±71 | 1011±200 | 1858±450 | 2025±124 |
Fig. 8.
Effect of high salt diet (2%, 4% or 8%) vs. control (0.4%) on systolic blood pressure (mmHg) in mice lacking Ceacam2 (Cc2−/−; solid bars) vs. wild-type (WT; open bars). Data shown as the mean ± SEM of measurements performed on 5 animals in each group. *p<0.05 vs. WT.
Fig. 9.
Effect of high salt diet (8%) on body weight (g) and urinary sodium excretion (UNa; μeq/day) in mice lacking Ceacam2 (Cc2−/−; solid circles) vs. wild-type (WT; open circles). Data shown as the mean ± SEM of measurements performed on 5 animals in each group.
Discussion
It is well established that ouabain binds to the Na/K-ATPase and induces its endocytosis via a signaling cascade, while binding of insulin to its receptor induces association of the receptor with CEACAM1 and internalization for ligand degradation and insulin receptor recycling 1, 21, 22. CEACAM1 is also present on the plasmalemma in various types of cells, where it can interact with the insulin receptor via Shc adaptor protein8, 23. Our data suggest that both the sodium pump and CEACAM1 are capable of responding to cardiotonic steroids in the renal proximal tubule, a crucial site of blood pressure regulation and insulin clearance. It has also been shown that the insulin receptor and CEACAM1 are expressed on both apical and basolateral aspects of polarized Madine-Darby canine kidney (MDCK) cells, though predominantly on the basolateral surface 24–26. Ouabain treatment of LLC-PK1 cells and a high salt diet result in the coordinated redistribution of the apical sodium proton antiporter NHE3 through mechanisms which are yet to be elucidated27, 28 as well as decreases in NHE3 expression on a transcriptional level29. It is the redistribution of the NHE3 which is felt to be rate limiting in terms of proximal tubular sodium handling and natriuresis, and our previous data suggest that this is a consequence of Na/K-ATPase signaling through Src27, 29. Although insulin has been shown to initiate phosphorylation of the sodium pump in skeletal muscle30, our current findings suggest that the sodium pump and insulin receptor compete for resources necessary for endocytosis. What is not clear from our data is whether insulin induces identical changes in proximal tubular sodium handling or whether redistribution of the NHE3 and other transport proteins are affected differently. Further studies will be necessary to explore this important area.
We also observed that the renal CEACAM molecule in mice (CEACAM-2) is involved in cardiotonic steroid induced Na/K-ATPase endocytosis and hence, contributes to blood pressure regulation. Interestingly, we have recently reported that in the Dahl salt sensitive strain of rat, impaired proximal tubular Na/K-ATPase endocytosis in response to salt loading in vivo or ouabain exposure in vitro can be demonstrated in comparison to the Dahl salt resistant strain. In other words, the lack of renal natriuretic response to circulating cardiotonic steroids corresponded to the increases in blood pressure seen in the Dahl salt sensitive strain with salt loading31. These data are consistent with the observed hypertension seen in the Cc2−/− mice subjected to a high salt diet. However, as mentioned above, additional studies are clearly required to further characterize abnormalities in insulin, or cardiotonic steroid-induced signaling through the Na/K-ATPase “signalosome”32–34 in the development of salt-sensitive hypertension in these Cc2−/− mice.
PERSPECTIVES.
The current studies demonstrate that ouabain-induced signaling through the sodium pump also interacts with insulin receptor-associated molecules, including CEACAM, in the kidney. Although further studies are required, the data identify a competition between ouabain and insulin mediated endocytosis and suggest a potential role for this competition in the regulation of salt excretion and, therefore, the pathogenesis and possibly treatment of hypertension seen in hyperinsulinemic states.
ACKNOWLEDGEMENTS
The authors would like to thank Ms. Carol Woods for her excellent secretarial assistance. We would also like to acknowledge Mats A. Fernstrom and Jennifer Kalisz for their technical assistance.
SOURCES OF FUNDING: This work was supported by grants from the National Institutes of Health (HL109015 to JI Shapiro and Z Xie, GM78565 to Z Xie and DK054254 and DK083850 to SM Najjar), an intramural contract with the National Institute on Aging (263-MA-707136-1 to D Malhotra and JI Shapiro) and an Ohio Valley Heart Association Beginning grant in aid (to J Liu).
Footnotes
AUTHOR DISCLOSURES: There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Najjar SM. Regulation of insulin action by ceacam1. Trends Endocrinol Metab. 2002;13:240–245. doi: 10.1016/s1043-2760(02)00608-2. [DOI] [PubMed] [Google Scholar]
- 2.Poy MN, Yang Y, Rezaei K, Fernstrom MA, Lee AD, Kido Y, Erickson SK, Najjar SM. Ceacam1 regulates insulin clearance in liver. Nat Genet. 2002;30:270–276. doi: 10.1038/ng840. [DOI] [PubMed] [Google Scholar]
- 3.DeAngelis AM, Heinrich G, Dai T, Bowman TA, Patel PR, Lee SJ, Hong EG, Jung DY, Assmann A, Kulkarni RN, Kim JK, Najjar SM. Carcinoembryonic antigen-related cell adhesion molecule 1: A link between insulin and lipid metabolism. Diabetes. 2008;57:2296–2303. doi: 10.2337/db08-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Periyasamy SM, Liu J, Tanta F, Kabak B, Wakefield B, Malhotra D, Kennedy DJ, Nadoor A, Fedorova OV, Gunning W, Xie Z, Bagrov AY, Shapiro JI. Salt loading induces redistribution of the plasmalemmal na/k-atpase in proximal tubule cells. Kidney Int. 2005;67:1868–1877. doi: 10.1111/j.1523-1755.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 5.Hou X, Theriault SF, Dostanic-Larson I, Moseley AE, Lingrel JB, Wu H, Dean S, Van Huysse JW. Enhanced pressor response to increased csf sodium concentration and to central ang i in heterozygous alpha2 na+ -k+ -atpase knockout mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1427–1438. doi: 10.1152/ajpregu.00809.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loreaux EL, Kaul B, Lorenz JN, Lingrel JB. Ouabain-sensitive alpha1 na,k-atpase enhances natriuretic response to saline load. J Am Soc Nephrol. 2008;19:1947–1954. doi: 10.1681/ASN.2008020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagrov AY, Shapiro JI. Endogenous digitalis: Pathophysiologic roles and therapeutic applications. Nat Clin Pract Nephrol. 2008;4:378–392. doi: 10.1038/ncpneph0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abou-Rjaily GA, Lee SJ, May D, Al-Share QY, Deangelis AM, Ruch RJ, Neumaier M, Kalthoff H, Lin SH, Najjar SM. Ceacam1 modulates epidermal growth factor receptor--mediated cell proliferation. J Clin Invest. 2004;114:944–952. doi: 10.1172/JCI21786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Periyasamy SM, Gunning W, Fedorova OV, Bagrov AY, Malhotra D, Xie Z, Shapiro JI. Effects of cardiac glycosides on sodium pump expression and function in llc-pk1 and mdck cells. Kidney Int. 2002;62:2118–2125. doi: 10.1046/j.1523-1755.2002.00672.x. [DOI] [PubMed] [Google Scholar]
- 10.Muth TR, Gottardi CJ, Roush DL, Caplan MJ. A basolateral sorting signal is encoded in the alpha-subunit of na-k-atpase. Am J Physiol. 1998;274:C688–696. doi: 10.1152/ajpcell.1998.274.3.C688. [DOI] [PubMed] [Google Scholar]
- 11.Chibalin AV, Kovalenko MV, Ryder JW, Feraille E, Wallberg-Henriksson H, Zierath JR. Insulin- and glucose-induced phosphorylation of the na(+),k(+)-adenosine triphosphatase alpha-subunits in rat skeletal muscle. Endocrinology. 2001;142:3474–3482. doi: 10.1210/endo.142.8.8294. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Kesiry R, Periyasamy SM, Malhotra D, Xie Z, Shapiro JI. Ouabain induces endocytosis of plasmalemmal na/k-atpase in llc-pk1 cells by a clathrin-dependent mechanism. Kidney Int. 2004;66:227–241. doi: 10.1111/j.1523-1755.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Liang M, Liu L, Malhotra D, Xie Z, Shapiro JI. Ouabain-induced endocytosis of the plasmalemmal na/k-atpase in llc-pk1 cells requires caveolin-1. Kidney Int. 2005;67:1844–1854. doi: 10.1111/j.1523-1755.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 14.Gorvel JP, Chavrier P, Zerial M, Gruenberg J. Rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- 15.Heinrich G, Ghosh S, Deangelis AM, Schroeder-Gloeckler JM, Patel PR, Castaneda TR, Jeffers S, Lee AD, Jung DY, Zhang Z, Opland DM, Myers MG, Jr., Kim JK, Najjar SM. Carcinoembryonic antigen-related cell adhesion molecule 2 controls energy balance and peripheral insulin action in mice. Gastroenterology. 2010;139:644–652. 652.e1. doi: 10.1053/j.gastro.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Najjar SM, Yang Y, Fernstrom MA, Lee SJ, Deangelis AM, Rjaily GA, Al-Share QY, Dai T, Miller TA, Ratnam S, Ruch RJ, Smith S, Lin SH, Beauchemin N, Oyarce AM. Insulin acutely decreases hepatic fatty acid synthase activity. Cell Metab. 2005;2:43–53. doi: 10.1016/j.cmet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J, Xie ZJ. Identification of a pool of non-pumping na/k-atpase. J Biol Chem. 2007;282:10585–10593. doi: 10.1074/jbc.M609181200. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Haas M, Liang M, Cai T, Tian J, Li S, Xie Z. Ouabain assembles signaling cascades through the caveolar na+/k+-atpase. J Biol Chem. 2004;279:17250–17259. doi: 10.1074/jbc.M313239200. [DOI] [PubMed] [Google Scholar]
- 20.Han E, Phan D, Lo P, Poy MN, Behringer R, Najjar SM, Lin SH. Differences in tissue-specific and embryonic expression of mouse ceacam1 and ceacam2 genes. Biochem J. 2001;355:417–423. doi: 10.1042/0264-6021:3550417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J. Ouabain-induced endocytosis and signal transduction of the na/k-atpase. Front Biosci. 2005;10:2056–2063. doi: 10.2741/1681. [DOI] [PubMed] [Google Scholar]
- 22.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: Physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61:9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poy MN, Ruch RJ, Fernstrom MA, Okabayashi Y, Najjar SM. Shc and ceacam1 interact to regulate the mitogenic action of insulin. J Biol Chem. 2002;277:1076–1084. doi: 10.1074/jbc.M108415200. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi F, Seta KA, Omura S, Roth RA. Insulin degradation by madin-darby canine kidney cells expressing the insulin receptor. Diabetes Res Clin Pract. 1997;37:81–90. doi: 10.1016/s0168-8227(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 25.Yeh TC, Roth RA. Insulin receptor signaling in madin-darby canine kidney cells overexpressing the human insulin receptor. Diabetes. 1994;43:1297–1303. doi: 10.2337/diab.43.11.1297. [DOI] [PubMed] [Google Scholar]
- 26.Sundberg U, Beauchemin N, Obrink B. The cytoplasmic domain of ceacam1-l controls its lateral localization and the organization of desmosomes in polarized epithelial cells. J Cell Sci. 2004;117:1091–1104. doi: 10.1242/jcs.00944. [DOI] [PubMed] [Google Scholar]
- 27.Cai H, Wu L, Qu W, Malhotra D, Xie Z, Shapiro JI, Liu J. Regulation of apical nhe3 trafficking by ouabain-induced activation of the basolateral na+-k+-atpase receptor complex. Am J Physiol Cell Physiol. 2008;294:C555–563. doi: 10.1152/ajpcell.00475.2007. [DOI] [PubMed] [Google Scholar]
- 28.McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2010;298:R851–861. doi: 10.1152/ajpregu.00002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oweis S, Wu L, Kiela PR, Zhao H, Malhotra D, Ghishan FK, Xie Z, Shapiro JI, Liu J. Cardiac glycoside downregulates nhe3 activity and expression in llc-pk1 cells. Am J Physiol Renal Physiol. 2006;290:F997–1008. doi: 10.1152/ajprenal.00322.2005. [DOI] [PubMed] [Google Scholar]
- 30.Al-Khalili L, Kotova O, Tsuchida H, Ehren I, Feraille E, Krook A, Chibalin AV. Erk1/2 mediates insulin stimulation of na(+),k(+)-atpase by phosphorylation of the alpha-subunit in human skeletal muscle cells. J Biol Chem. 2004;279:25211–25218. doi: 10.1074/jbc.M402152200. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Yan Y, Liu L, Xie Z, Malhotra D, Joe B, Shapiro JI. Impairment of na/k-atpase signaling in renal proximal tubule contributes to dahl salt-sensitive hypertension. J Biol Chem. 2011;286:22806–22813. doi: 10.1074/jbc.M111.246249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai T, Wang H, Chen Y, Liu L, Gunning WT, Quintas LE, Xie ZJ. Regulation of caveolin-1 membrane trafficking by the na/k-atpase. J Cell Biol. 2008;182:1153–1169. doi: 10.1083/jcb.200712022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Cai T, Yang C, Turner DA, Giovannucci DR, Xie Z. Regulation of inositol 1,4,5-trisphosphate receptor-mediated calcium release by the na/k-atpase in cultured renal epithelial cells. J Biol Chem. 2008;283:1128–1136. doi: 10.1074/jbc.M708025200. [DOI] [PubMed] [Google Scholar]
- 34.Liang M, Cai T, Tian J, Qu W, Xie ZJ. Functional characterization of src-interacting na/k-atpase using rna interference assay. J Biol Chem. 2006;281:19709–19719. doi: 10.1074/jbc.M512240200. [DOI] [PubMed] [Google Scholar]