This work used a chemical genetic screen to identify sulfamethazine, a small molecule that effectively suppresses gene silencing by impairing folate synthesis, thereby creating a methyl shortage; these findings provide a tool for probing epigenetic regulation and highlight the significance of primary metabolism to epigenetic regulation in planta.
Abstract
DNA methylation is a critical, dynamically regulated epigenetic mark. Small chemicals can be valuable tools in probing cellular processes, but the set of chemicals with broad effects on epigenetic regulation is very limited. Using the Arabidopsis thaliana repressor of silencing1 mutant, in which transgenes are transcriptionally silenced, we performed chemical genetic screens and found sulfamethazine (SMZ) as a chemical suppressor of epigenetic silencing. SMZ treatment released the silencing of transgenes as well as endogenous transposons and other repetitive elements. Plants treated with SMZ exhibit substantially reduced levels of DNA methylation and histone H3 Lys-9 dimethylation, but heterochromatic siRNA levels were not affected. SMZ is a structural analog and competitive antagonist to p-aminobenzoic acid (PABA), which is a precursor of folates. SMZ decreased the plant folate pool size and caused methyl deficiency, as demonstrated by reductions in S-adenosylmethionine levels and in global DNA methylation. Exogenous application of PABA or compounds downstream in the folate biosynthesis pathway restored transcriptional silencing in SMZ-treated plants. Together, our results revealed a novel type of chemical suppressor of epigenetic silencing, which may serve as a valuable tool for studying the roles and mechanisms of epigenetic regulation and underscores an important linkage between primary metabolism and epigenetic gene regulation.
INTRODUCTION
DNA methylation at the 5-position of cytosine is an important epigenetic feature that regulates gene and transposon silencing, imprinting, and genome stability. Unlike mammals, where DNA methylation occurs mainly in the symmetric CG context, in plants, cytosine methylation is commonly observed in all DNA sequence contexts, including CG, CHG, and CHH, with transposon-rich heterochromatic regions being the predominant targets (Henderson and Jacobsen, 2007). Cytosine methylation can trigger transcriptional silencing and can also influence protein–chromatin interactions (Law and Jacobsen, 2010; Zhang and Zhu, 2011). Closely linked with epigenetic histone modifications, genomic DNA methylation patterns are inherently more stable but are still under dynamic regulation by establishment, maintenance, and removal activities.
Establishment and maintenance of DNA methylation are mediated by cytosine methyltransferases, which use S-adenosyl-l-Met (SAM) as the methyl donor. DOMAIN REARRANGED METHYLTRANSFERASE2 (DRM2), a homolog of mammalian DNMT3, catalyzes de novo DNA methylation in plants (Cao and Jacobsen, 2002). The targeting of DRM2 for de novo methylation can be mediated by the RNA-directed DNA methylation (RdDM) pathway, in which genomic sequences for cytosine methylation are targeted by homologous small interfering RNAs (siRNAs). In current models, the biogenesis of siRNAs is proposed to be initiated by Pol IV, a plant-specific RNA polymerase, followed by RNA-DEPENDENT RNA POLYMERASE2–mediated synthesis of double-stranded RNAs. The double-stranded RNAs are then cleaved into 24-nucleotide siRNAs by DICER-LIKE3, and the siRNAs are loaded onto AGO4, an Argonaute protein that interacts with other downstream components in the RdDM pathway to guide DRM2 to the siRNA-generating genomic loci and other loci that are homologous to the siRNAs (Matzke et al., 2009; Law and Jacobsen, 2010; Zhang and Zhu, 2011; Haag and Pikaard, 2011). In plants, CG and CHG DNA methylation is maintained by DNA METHYLTRANSFERASE1 and CHROMOMETHYLASE3 (CMT3), respectively (Chan et al., 2005). CHH methylation cannot be maintained and thus has to occur de novo after DNA replication. In addition to DNA methyltransferases, DNA demethylases that actively convert 5-methylcytosine to cystosine have been characterized in plants. Arabidopsis thaliana contains four DNA demethylases, namely, REPRESSOR OF SILENCING1 (ROS1), DEMETER, DEMETER-LIKE2 (DML2), and DML3 (Gong et al., 2002; Gehring et al., 2006; Penterman et al., 2007; Ortega-Galisteo et al., 2008). These bifunctional DNA glycosylases remove the 5-methylcytosine base and then cleave the DNA backbone at the abasic site, resulting in a gap that is then filled with an unmethylated cytosine nucleotide by as yet unknown DNA polymerase and ligase enzymes (Gong et al., 2002; Agius et al., 2006; Gehring et al., 2006; Morales-Ruiz et al., 2006; Penterman et al., 2007; Zhu, 2009).
In plants and mammals, DNA hypermethylation coexisting with repressive histone marks is characteristic of heterochromatin that is transcriptionally inactive; by contrast, low DNA methylation levels are found to coexist with active histone marks in euchromatin (Vaillant and Paszkowski, 2007; Roudier et al., 2009). Epigenetic marks can be stably inherited, but transcription of the silenced targets can be naturally reactivated under certain circumstances, such as stress conditions (Madlung and Comai, 2004; Chinnusamy and Zhu, 2009; Pecinka et al., 2010; Tittel-Elmer et al., 2010; Ito et al., 2011), or during gametogenesis (Brennecke et al., 2008; Slotkin et al., 2009; Chen et al., 2010). Suppression of transcriptional gene silencing (TGS) is also frequently observed in eukaryotes when mutagenesis results in DNA hypomethylation and/or active histone modifications (Jeddeloh et al., 1999; Amedeo et al., 2000; Mathieu et al., 2007). Besides DNA glycosylase-mediated active demethylation, decreased DNA methylation can be caused by downregulated establishment and/or maintenance of cytosine methylation. In Arabidopsis, passive demethylation has been documented in mutants that are defective in DNA methyltransferases, the RdDM pathway, as well as in repressive histone modification enzymes (Saze et al., 2008; Law and Jacobsen, 2010; Furner and Matzke, 2011). Meanwhile, a shortage of methyl supply also leads to decreased DNA methylation levels, as in the example of the Arabidopsis homologous gene silencing1 (hog1), mutant, which is impaired in recycling the methyl donor SAM for transmethylation reactions (Rocha et al., 2005; Baubec et al., 2010).
In addition to genetic mutations, chemical reagents capable of causing chromatin alterations also provide valuable tools in epigenetics research and are important for therapeutic purposes. Small molecules, such as trichostatin A (TSA) and 5-aza-2'-deoxycytidine (5-adC), which inhibit histone deacetylases and DNA methyltransferases, respectively, are well-known examples of chemicals that are widely used to release epigenetic silencing (Monneret, 2005; Patra and Bettuzzi, 2009). Small molecules, such as adenosine, can also target SAM synthesis to reduce methyl supply and, as a consequence, DNA methylation levels (Kloor and Osswald, 2004). SAM is the universal methyl donor used by most methyltranferases to methylate DNA, RNA, and histones and other proteins (Loenen, 2006). The active methyl group donated by SAM originates from one-carbon (C1) metabolism mediated by folate, a term collectively representing derivatives from tetrahydrofolate (THF). Folate-dependent C1 metabolism is necessary for three biosynthetic pathways: de novo purine biosynthesis, de novo thymidylate biosynthesis, and methylation of homocysteine to Met (Stover, 2009).
Previous studies found TGS of two linked transgenes, ProRD29A:LUC (for LUCIFERASE under the promoter of RESPONSE TO DESSICATION 29A) and Pro35S:NPTII (for NEOMYCIN PHOSPHOTRANSFERASE II under the promoter of cauliflower mosaic virus 35S RNA), in the Arabidopsis ros1 mutant (Gong et al., 2002). Using ProRD29A:LUC as the reporter gene, genetic screening for suppressors of ros1 TGS identified several components of the RdDM pathway (He et al., 2009a, 2009b; Zheng et al., 2010). A similar strategy that focuses on Pro35S:NPTII TGS suppression revealed several DNA repair and replication proteins as well as a chloroplast phosphoenolpyruvate/phosphate translocator as important for RdDM-independent epigenetic silencing (Kapoor et al., 2005; Xia et al., 2006; Wang et al., 2007; Shen et al., 2009; Liu et al., 2010). These genetic studies suggested that the ProRD29A:LUC and Pro35S:NPTII reporter genes are silenced by RdDM-dependent and -independent mechanisms, respectively. In this study, we performed a chemical genetics screening and identified sulfamethazine (SMZ) as a novel chemical suppressor of TGS of both the ProRD29A:LUC and Pro35S:NPTII reporter genes in ros1. SMZ treatment also released the silencing of transposons. We provide evidence that SMZ is effective in reducing DNA methylation levels and a repressive histone mark, H3K9me2, through impairing folate-dependent C1 metabolism.
RESULTS
SMZ Suppresses TGS in ros1 Plants
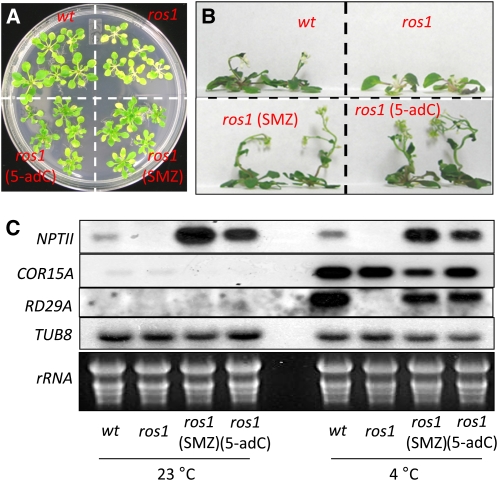
In searching for compounds that can influence epigenetic silencing, we performed a chemical genetic screen using 3580 biologically active small molecules from the Library of Active Compounds in Arabidopsis (http://cutlerlab.blogspot.com/2008/05/latca.html). To test the effect of these compounds on silencing, we used the Arabidopsis ros1 mutant that harbors two silenced reporter transgenes, ProRD29A:LUC and Pro35S:NPTII (hereafter referred to as ros1 in this study). During the screening, potential chemical suppression of TGS was evaluated based on visualization of luciferase activities in ros1 plants. SMZ was identified as a potential suppressor of TGS in the initial screening and confirmed in subsequent treatments with different doses of SMZ (data not shown). Treatment of ros1 plants with 50 μM SMZ in the growth medium clearly released TGS of ProRD29A:LUC, as evidenced by SMZ-induced bioluminescence in ros1 plants (Figures 1A and 1B). Similar results were observed in ros1 treated with an equal dose of 5-adC, which was used as a positive control since this DNA methyltransferase inhibitor is known to induce luminescence in ros1 (Gong et al., 2002). SMZ-induced luminescence in ros1 was weaker than the wild type (C24 containing ProRD29A:LUC and Pro35S:NPTII, referred to as C24 hereafter), suggesting a partial release of ProRD29A:LUC TGS. SMZ also had some cytotoxic effects, as indicated by the retarded growth in treated ros1 plants compared with untreated plants (Figure 1C). Untreated ros1 plants were sensitive to kanamycin; by contrast, SMZ-treated ros1 were resistant to kanamycin, produced green leaves, and could develop an inflorescence on kanamycin-containing medium (Figure 2A), suggesting that SMZ also released TGS of Pro35S:NPTII.
Figure 1.
SMZ Releases TGS of ProRD29A:LUC in ros1 Plants.
Seven-day-old ros1 seedlings were transferred from half-strength MS medium to fresh half-strength MS media with 50 μM SMZ or 50 μM 5-adC. Wild-type (wt; C24) and control ros1 plants were transferred to medium with the solvent DMSO but without SMZ or 5-adC.
(A) Plants were subject to luminescence imaging (upper panel) and regular photography (lower panel) at 6 days post transfer, with the last two days grown under 4°C to activate the cold-responsive RD29A promoter.
(B) SMZ induction of luciferase activity as quantified by plant population (n ≥ 80) distribution patterns based on luminescence intensity. AU, arbitrary units.
(C) Phenotypic comparison between SMZ-treated and untreated ros1. Bars = 0.5 cm.
Figure 2.
SMZ Releases TGS of Pro35S:NPTII in ros1 Plants.
(A) and (B) Six days after treatments as described in Figure 1, plants were transferred to MS medium supplemented with only 50 μg/mL kanamycin and allowed to grow for 2 (A) and 4 (B) weeks before imaging. wt, the wild type.
(C) Transcript accumulation of transgenic Pro35S:NPTII and endogenous RD29A in plants. Total RNA was extracted from plants with or without cold treatment and examined by RNA gel blot (4°C, 48 h). COR15A and TUB8 were used as cold treatment control and RNA loading control, respectively.
Consistent with the luminescence phenotype that requires activation of the RD29A promoter, cold stress–induced transcript accumulation of endogenous RD29A in SMZ-treated ros1 (Figure 2B), and RD29A expression in control ros1 was undetectable. In comparison, cold treatment triggered gene expression of COLD-REGULATED 15A (COR15A) in both SMZ-treated and control plants, suggesting that SMZ-induced RD29A expression is not due to an altered sensitivity to cold stress. Similarly, transcript from the Pro35S:NPTII transgene was not observed in untreated ros1, whereas plants treated with SMZ showed strong accumulation of NPTII mRNA (Figure 2B), which is in agreement with SMZ-induced resistance to kanamycin. It is noteworthy that SMZ-induced NPTII expression was much stronger than that induced by 5-adC (Figure 2C). Taken together, these results demonstrate the ability of SMZ to suppress epigenetic gene silencing.
Expression of Endogenous Silencing Targets Is Increased by SMZ
In addition to silencing ProRD29A:LUC and Pro35S:NPTII, the ros1 mutation also causes locus-specific DNA hypermethylation of endogenous targets, resulting in decreased transcript levels compared with wild-type plants (Penterman et al., 2007; Zhu et al., 2007). To assess the ability of SMZ to release known endogenous silencing targets in ros1, transcript levels of the following transposable elements were examined by quantitative real-time RT-PCR: transcriptionally silent information (TSI), the Mutator-like transposable element–related locus MULE-F19G14, the gypsy-class long-terminal repeat retroelement GP1, MULE DNA transposon MU1, and non-long-terminal repeat retroelements LINE1-4. SMZ treatment substantially increased the transcript levels of these endogenous loci, compared with untreated ros1 (Figures 3A and 3B). Transcript accumulation of these transposable elements was even greater than in wild-type plants (Figure 3A), indicating lower DNA methylation levels in SMZ-treated ros1 than in wild-type plants where transposons are normally hypermethylated. Similarly, treatment with 5-adC resulted in higher expression levels of these three transposons than either untreated ros1 or wild-type plants (Figure 3A). Similar to a previous report (Zhu et al., 2007), we also observed that GP1, MU1, and LINE1-4 accumulated more transcripts in wild-type plants than in ros1 mutants. In addition, an endogenous gene At4g18650 that is downregulated by ros1 mutation (Zhu et al., 2007) was examined. The result shows that the silencing of At4g18650 in ros1 was also released by SMZ (Figure 3A), further supporting that SMZ is an effective suppressor of ros1. Moreover, SMZ-induced release of transcriptional silencing is not specific to ros1 plants, since SMZ also activated the expression of endogenous silenced loci in wild-type plants (see Supplemental Figure 1 online).
Figure 3.
SMZ Activates the Transcription of Endogenous TGS Target Loci.
(A) SMZ elevates the transcript levels of TSI, MULE-F19G14, LINE1-4, and At4g18650. wt, the wild type.
(B) SMZ increases the expression of GP1 and MU1. Gene expression was determined by real-time RT-PCR. Transcript levels for each gene are relative to the values in untreated ros1 plants. 18S rRNA was used for RNA normalization during quantitative RT-PCR analysis. Mean ± sd for three technical repeats; similar results were observed for at least two biological replicates.
(C) SMZ reduces DNA methylation in GP1 and MU1. Genomic DNA was digested with the methylated DNA-specific restriction enzyme McrBC before amplification. Nondigested genomic DNA was used as a control.
SMZ Reduces DNA Methylation and Histone H3K9 Methylation but Not siRNA Accumulation
To test whether SMZ-induced transcriptional activation correlates with loss of DNA hypermethylation, we analyzed DNA methylation status at various loci. To examine DNA methylation levels at GP1 and MU1, the methylated DNA-digesting enzyme McrBC was applied to genomic DNA, followed by PCR. As shown in Figure 3C, SMZ reduced DNA methylation at GP1 and MU1, consistent with the elevated transcript levels of these loci. DNA methylation of the RD29A promoter was analyzed by bisulfite sequencing. For the endogenous RD29A promoter, heavy methylation in all cytosine contexts (CG, CHG, and CHH) was observed in untreated ros1, whereas SMZ treatment reduced methylation levels at CG, CHG, and CHH by 26, 63, and 72%, respectively, resulting in a 51% reduction of total cytosine methylation in this 361-bp region of the RD29A promoter (Figure 4A). DNA methylation of the transgenic RD29A promoter was reduced by SMZ to a similar extent as in the endogenous RD29A promoter (Figure 4A). Compared with the very low cytosine methylation levels in the wild type (Figure 4A), it is apparent that SMZ only partially decreased methylation at both the endogenous and transgene RD29A promoters. Such partial recovery was consistent with our observation that SMZ partially released TGS of ProRD29A:LUC (Figure 1) and endogenous RD29A (Figure 2B). Collectively, these results suggest that SMZ suppresses TGS at the RdDM target loci by reducing DNA methylation.
Figure 4.
SMZ Substantially Reduces DNA Methylation and the Histone H3K9me2 Mark but Not siRNA Accumulation.
(A) DNA cytosine methylation levels at the endogenous RD29A promoter (top), transgenic RD29A promoter (middle), and transgenic 35S promoter (bottom) as determined by bisulfite sequencing.
(B) ChIP analysis of H3K9me2 at the 35S promoter (35S), transgenic RD29A promoter (LUC), and endogenous RD29A promoter (RD29A) by quantitative RT-PCR. Actin was used as an internal control. Mean ± sd (n = 3).
(C) Detection of RD29A promoter siRNAs, At4g18650 siRNAs, siRNA1003, microRNA167, and microRNA171 in C24 (1), control ros1 (2), SMZ-treated ros1 (3), and 5-adC-treated ros1 (4). The ethidium bromide–stained gel corresponding to tRNAs and 5S rRNAs was used as a loading control. nt, nucleotides.
SMZ decreased total cytosine methylation levels in the 35S promoter region by 46%, with methylation at CG, CHG, and CHH contexts being reduced by 22, 57, and 69%, respectively (Figure 4A). Meanwhile, cytosine methylation contents at this promoter region in the wild type were similar as or slightly lower than in ros1 (Figure 4A). Therefore, consistent with previous findings that Pro35S:NPTII silencing can be released without decreasing DNA methylation (Kapoor et al., 2005; Xia et al., 2006; Wang et al., 2007; Shen et al., 2009; Liu et al., 2010), the release of Pro35S:NPTII silencing in SMZ-treated ros1 was not necessarily caused by a reduction in DNA methylation. However, this does not exclude the possibility that a reduction in DNA methylation levels could contribute to releasing TGS of Pro35S:NPTII. In fact, decreased DNA methylation at the 35S promoter (Figure 4A) correlates well with the substantially increased expression of Pro35S:NPTII in SMZ-treated ros1 relative to wild-type plants (Figure 2B). Since TGS of Pro35S:NPTII has been reported to correlate with levels of the repressive histone mark H3K9me2 at the 35S promoter, we then performed chromatin immunoprecipitation (ChIP) combined with quantitative PCR to examine histone modification in the 35S promoter and both the transgenic and endogenous RD29A promoters. H3K9 dimethylation (H3K9me2), a heterochromatic histone mark, at the 35S promoter in untreated ros1 was more than twofold that in wild-type plants; also, SMZ treatment drastically reduced H3K9me2 in ros1 to <5% of the level in untreated ros1 (Figure 4B), consistent with the effectiveness of SMZ in releasing TGS of Pro35S:NPTII. SMZ also reduced the H3K9me2 mark at transgenic and endogenous RD29A promoter by 53 and 68%, respectively (Figure 4B). Therefore, SMZ treatment resulted in not only decreased DNA methylation but also reduced H3K9me2, both of which would contribute to SMZ-triggered suppression of epigenetic silencing.
RD29A promoter siRNAs are required for the hypermethylation and silencing of both the transgenic and endogenous RD29A promoters in ros1 (Gong et al., 2002; He et al., 2009a, 2009b). To test whether SMZ may decrease DNA methylation by reducing siRNA levels, RD29A promoter siRNA accumulation was examined. In spite of a slight decrease in SMZ-treated ros1, accumulation of the 24-nucleotide RD29A siRNAs was obvious and comparable to that in 5-adC treated ros1, suggesting that SMZ-triggered loss of DNA methylation is not due to reduced siRNA triggers. siRNAs from the endogenous silencing locus At4g18650 showed a similar pattern as the RD29A siRNAs. In addition, the accumulation of siRNAs from 5S rDNA was not affected by SMZ (Figure 4C), further supporting that SMZ does not have a significant influence on heterochromatic siRNAs. Interestingly, the accumulation of two 21-nucleotide miRNAs, miR167 and miR171, was apparently reduced in SMZ-treated ros1 compared with 5-adC–treated ros1 (Figure 4C), suggesting that SMZ may somehow affect the biogenesis or stability of miRNAs.
SMZ Impairs Folate Biosynthesis and Downstream C1 Metabolism
SMZ belongs to the family of sulfonamides, which are known antibacterial compounds functioning through impairment of folate synthesis. As structural analogs and competitive antagonists to p-aminobenzoic acid (PABA), sulfonamides competitively inhibit hydropteroate synthase-catalyzed biosynthesis of dihydropteroic acid, the precursor of THF (Ortelli et al., 1999; Figure 5A). The observed reduction of DNA methylation triggered by SMZ is in agreement with the function of SMZ in decreasing methyl supply that relies on folate synthesis. Therefore, we examined whether sulfonamides can inhibit folate synthesis in planta to determine whether impairing folate biosynthesis affected plant epigenetic regulation. In SMZ-treated plants, gene expression of SAHH1 and ADK1 was induced (Figure 5B). Both SAHH1 and ADK1 are critical for removing S-adenosyl-l-homo-Cys (SAH) that inhibits SAM-dependent methyl transfer; SAHH1 is also required for SAM regeneration (Loenen, 2006). Therefore, the induction of SAHH1 and ADK1 genes indicates a demand for enhancing methyl donation from SAM in SMZ-treated plants. SMZ also downregulated gene expression of CHLM (Mg-protoporphyrin IX methyltransferase) (Figure 5B), which catalyzes methylation of Mg-protoporphyrin IX during chlorophyll synthesis (Van Wilder et al., 2009). Folate deficiency reduces chlorophyll levels, and chlorosis was observed in SMZ-treated plants (Figure 1C). Together, these gene expression patterns are consistent with the notion that SMZ treatment reduced folate-dependent methyl supply. To confirm that SMZ indeed impairs folate metabolism in planta, metabolite levels of folates, SAM, and SAH were measured. A significant reduction in the folate pool, including THF and its derivatives, was observed in SMZ-treated plants compared with untreated control (Figure 5C). Downstream of folate biosynthesis, SAM and SAH levels were decreased by up to 38 and 32%, respectively, within 24 h of SMZ treatment (Figure 6A). Consistent with the reduction in folate-dependent methyl supply, a global loss of DNA methylation was observed in SMZ-treated plants (Figure 6B). In addition, SMZ-induced hypomethylation was detected at the 180-bp centromeric repeats and the pericentromeric 5S rDNA repeats (see Supplemental Figure 2 online).
Figure 5.
SMZ-Triggered Differential Gene Regulation of Several C1 Metabolic Enzymes.
(A) Folate-dependent C1 metabolism. Active methyl group from 5-methyl THF is used for methylating homocysteine (Hcy) to Met, which can be converted to the methyl donor SAM through adenylation. SAM is the methyl donor for many methylation reactions, such as DNA cytosine methylation. SAH produced from SAM-dependent transmethylation inhibits these reactions and is cleaved by SAH hydrolase, yielding adenosine and homocysteine.
(B) SMZ triggers the upregulation of SAHH1 and ADK1 transcript levels and transcript downregulation of CHLM that is a methyltransferase necessary for chlorophyll synthesis. Expression of TSI was used as a treatment control. White and black bars represent untreated ros1 and SMZ-treated ros1, respectively. Mean ± sd for three technical repeats; similar results were observed for at least two biological replicates.
(C) SMZ reduces the pool size of folates in Arabidopsis (C24). Plants were harvested 6 d after treatments. Results represent mean ± sd for six biological replicates. FW, fresh weight.
Figure 6.
SMZ Induced Global Reduction of Methyl Supply in Arabidopsis (C24).
(A) SMZ decreased metabolite levels of SAM and SAH. Whole seedlings with or without treatment were used for analysis as described in Methods. Results represent mean ± sd for three biological replicates. FW, fresh weight.
(B) SMZ decreased global DNA methylation levels. Plants were harvested 6 d after treatments. The percentage of 5-mdC in total cytosine were presented as mean ± sd (n = 3 biological replicates).
Release of Epigenetic Silencing by SMZ Is Mediated through Folate-Dependent C1 Metabolism
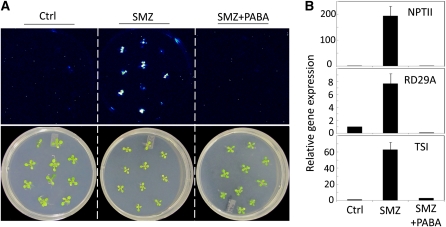
To confirm that SMZ releases TGS through competitive inhibition of PABA use, PABA was cosupplemented with SMZ in a 5:1 ratio into growth medium, and TGS was compared among different treatments. As shown in Figure 7, chemical treatment with SMZ alone released TGS in plants, but this effect was inhibited by exogenous PABA supplementation, indicating that SMZ releases TGS through impairing folate biosynthesis that requires PABA.
Figure 7.
PABA Treatment Reestablishes TGS in SMZ-Treated ros1 Plants.
(A) Seven-day-old seedlings were transferred from half-strength MS medium to fresh half-strength MS media with 50 μM SMZ, 50 μM SMZ + 250 μM PABA (SMZ+PABA), or the solvent DMSO alone as control (Ctrl). Plants were subject to luminescence imaging (top) and regular photography (bottom) at 6 d after transfer, with the last 2 d grown under 4°C.
(B) SMZ suppressed transcriptional silencing at transgenic ProRD29A:LUC and Pro35S:NPTII as well as endogenous TSI loci, while these effects were inhibited by PABA. Transcript levels for each gene are relative to the values in control plants. Actin2 was used for internal control during quantitative RT-PCR analysis. Mean ± sd (n = 3).
To further corroborate that suppression of TGS in SMZ-treated ros1 resulted from impairment of folate-dependent methyl supply, cosupplementation of THF or Met with SMZ was also examined. Luminescence from ProRD29A:LUC expression was observed in wild-type plants and in ros1 treated with SMZ alone, but not in untreated ros1 or ros1 that were treated with SMZ plus THF or Met (Figure 8A), suggesting that increasing methyl donor biogenesis by THF or Met was sufficient to restore methylation-dependent TGS at ProRD29A:LUC. In ros1 treated with SMZ + THF or SMZ + Met, TGS of the endogenous RD29A and Pro35S:NPTII was also restored (Figure 8B). Meanwhile, treatment with PABA, Met, or THF alone did not change the TGS status in ros1 plants (see Supplemental Figure 3 online). The effect of SMZ on TGS at endogenous transposons or repetitive sequences, as demonstrated by the expression patterns of TSI and MULE-F19G14 (Figure 8C), was also nullified by the THF or Met supplementation. These results suggest that a deficiency of methyl supply contributes to SMZ suppression of epigenetic silencing in plants.
Figure 8.
SMZ Suppression of Epigenetic Silencing Is Abolished by Repletion of THF or Met.
(A) Seven-day-old seedlings were transferred from half-strength MS medium to fresh half -strength MS media with 50 μM SMZ, 50 μM SMZ + 250 μM Met (SMZ+Met), or 50 μM SMZ + 250 μM THF. Wild type (C24) and control ros1 plants were transferred to medium with the solvent DMSO. Plants were subject to luminescence imaging (top) and regular photography (bottom) at 6 d after transfer, with the last 2 d grown under 4°C.
(B) SMZ-induced expression of endogenous RD29A, transgenic ProRD29A:LUC, transgenic Pro35S:NPTII, TSI, and MULE-F19G14 was inhibited by cosupplementation of THF or Met with SMZ. Quantitative PCR results are presented. Transcript levels for each gene are relative to the values in DMSO-treated ros1 plants. Mean ± sd for three technical replicates; similar results were observed for at least two biological replicates. Wt, wild type.
DISCUSSION
DNA and histone modifications play central roles in determining the status of chromatin. In many eukaryotes, DNA methylation at the 5-position of cytosine is a key epigenetic mark involved in diverse biological processes, including transcriptional silencing of genes and transposable elements (Lippman et al., 2003; Baulcombe, 2004), genomic imprinting (Scott and Spielman, 2004), and X chromosome inactivation (Heard, 2004). Therefore, it is of great interest to identify endogenous factors and exogenous stimuli that regulate DNA methylation and to understand the underlying mechanisms. In this study, we demonstrated that SMZ can release RdDM-dependent and -independent TGS of two transgenes and also release the silencing of endogenous loci. SMZ suppression of TGS was causally correlated with impairment of folate-dependent methyl supply and a concomitant reduction in DNA methylation as well as in the repressive histone mark H3K9me2. The suppression was abrogated by exogenous fortification of folate-dependent C1 metabolism. Compared with known molecules that directly inhibit DNA or histone modification enzymes (e.g., 5-adC or TSA), SMZ represents a novel class of small-molecule epigenetic regulators that emphasize the importance of primary metabolism on epigenetic gene regulation.
SMZ is a small molecule belonging to the sulfonamide family, which exert antibacterial effects through impairment of folate synthesis. One major function of folate is to provide 5-methylTHF for Met synthesis. In addition to being an essential amino acid, Met is also the precursor of SAM, which is required for polyamine synthesis and for various methylation processes, including DNA cytosine methylation and histone methylations. In mammals, which lack de novo folate synthesis, a Met-deficient diet causes locus-specific reduction of H3K9me2 levels (Dobosy et al., 2008), and genetic disruption of folate utilization for 5-methylTHF synthesis correlates with genomic DNA hypomethylation (Friso et al., 2002). The folate biosynthesis pathway is essentially the same in plants as in bacteria (Basset et al., 2005; Hanson and Gregory, 2011), both using dihydropterote synthase for dihydropteroic acid production. In fact, several sulfonamides, in addition to SMZ, such as sulfamerazine, were also identified as potential suppressors of TGS during our chemical genetic screen (data not shown). Release of TGS was also observed in plants treated with methotrexate (see Supplemental Figure 4 online), which is an antifolate compound that causes folate depletion in Arabidopsis (Loizeau et al., 2008). In addition to the observed changes in metabolites, SMZ-triggered folate deficiency and, as a consequence, shortages of C1 methyl supply were evident at phenotypic and molecular levels. Moreover, repletion of PABA, THF, or Met blocked SMZ-triggered transcriptional activation of transgenic and endogenous epigenetic silencing targets, supporting that SMZ suppression of TGS resulted from impairment of folate-dependent C1 metabolism.
Previous studies have shown that suppression of TGS at Pro35S:NPTII correlates well with decreased H3K9me2 levels (Kapoor et al., 2005; Xia et al., 2006; Wang et al., 2007; Shen et al., 2009; Liu et al., 2010). Supporting the observation that SMZ released TGS of Pro35S:NPTII, SMZ-treated ros1 exhibited a reduction in not only DNA methylation but also H3K9me2 levels at this transgenic promoter region. Given that histone methylation also relies on supply of methyl groups originated from folate metabolism, the observed reduction in H3K9me2 levels may be directly attributable to methyl deficiency due to SMZ-impaired folate synthesis. The decreases in DNA methylation, particularly non-CG methylation, could be largely a consequence of the low levels of H3K9me2. Alternatively, part of the observed decrease in H3K9me2 levels could be due to reduced DNA methylation. Genome-wide profiling of H3K9me2 and DNA methylation has revealed a strong correlation between these two epigenetic marks (Bernatavichute et al., 2008). As proposed in a reinforcing loop that controls the maintenance of DNA methylation at the CHG context, CHG methylation docks SUPPERESSOR OF VARIEGATION 3-9 HOMOLOG (SUVH4) that catalyzes histone H3K9 dimethylation, while H3K9me2 recruits the DNA methyltransferase CMT3 that is responsible for maintaining CHG methylation, resulting in enhancement of both epigenetic marks (Bernatavichute et al., 2008). In ros1 plants, cytosine methylation at CHG context was reduced by more than half when treated with SMZ (Figure 4A). Overall recruitment of SUVH4 by CHG methylation might then be impeded, which may contribute to a decreased H3K9me2 level as observed in SMZ-treated ros1. Such a scenario may be pertinent to explaining the reduction of H3K9me2 when plants were treated with 5-adC, which decreases DNA methylation levels via inhibiting DNA methyltransferase activity instead of reducing methyl supply.
In plants, the overwhelming majority of small RNAs are the 24-nucleotide siRNAs that function to guide the methylation of homologous genomic loci via the RdDM pathway (Zhang et al., 2007; Mosher et al., 2008). Defective RdDM in the ros1 background leads to reactivation of both transgenic and endogenous RD29A promoters, as well as transposable elements, such as GP1 and MU1 (He et al., 2009a, 2009b). Similarly, suppression of TGS at these loci was observed in SMZ-treated ros1. However, accumulation of siRNAs was at most only slightly reduced by SMZ treatment, thereby suggesting that SMZ-triggered reduction in DNA methylation is not caused by defects in the siRNAs. By contrast, accumulation of 21-nucleotide miRNAs was noticeably decreased in SMZ-treated ros1. Plant siRNAs and miRNAs are characterized by methylation of ribose at their 3′ end (Li et al., 2005; Yang et al., 2006). RNA methylation at the 3′ end protects miRNA and siRNA from degradation (Li et al., 2005; Yang et al., 2006;). SMZ-induced reduction in miRNA levels may result from decreased miRNA stability due to less 3′ end methylation, since SMZ impairs folate-dependent supply of the active methyl group. It is unknown why the steady state levels of siRNAs, compared with miRNA accumulation, appeared less sensitive to SMZ treatment. In any case, the slight reduction of siRNA levels may correspondingly decrease RdDM and thereby could provide a minor contribution to DNA hypomethylation in SMZ-treated plants.
Many enzymatic modifications of DNA or histones require cofactors, such as SAM, NAD, and acetyl-CoA. Therefore, metabolic regulation of gene expression can be mediated through epigenetic modifications. For instance, a study in mammals shows that histone acetylation is responsible for the regulation of metabolic genes by acetyl-CoA production from Glc metabolism (Wellen et al., 2009). Since folate-dependent C1 SAM metabolism is a central hub for various methylation reactions, disturbing folate biosynthesis and/or its downstream methyl supply can thus impact preexisting epigenetic marks, including DNA and histone methylations, as demonstrated in this study and previously in the Arabidopsis hog1 mutants (Rocha et al., 2005; Baubec et al., 2010). HOMOLOGUS GENE SILENCING1 (HOG1/SAHH1) breaks SAH down to homocysteine and adenosine, a process essential to maintain SAM-dependent transmethylation and to regenerate SAM (Figure 6A). In addition to its well-established function in regulating DNA methylation, dysfunction of HOG1 has also been recently shown to substantially reduce H3K9me2 levels (Baubec et al., 2010), showing a similar mode of epigenetic perturbation as observed in SMZ-treated plants.
In addition to genetic mutations, small molecules such as TSA, which inhibits histone deacetylation, and 5-adC or zebularine, which inhibit DNA methylation, have been important tools in investigating the mechanisms and roles of epigenetic regulation (Baubec et al., 2009). Our chemical genetic screen identified SMZ as a novel type of chemical epigenetic regulator, which exhibits stronger effects than 5-adC in releasing TGS at some loci. For example, whereas SMZ and 5-adC had similar effects in releasing TGS of the RD29A-LUC transgene (Figure 1A) and endogenous RD29A gene (Figure 2C), SMZ released TGS of 35S-NPTII much more strongly than 5-adC did (Figure 2C). The results suggest that H3K9me2 plays a much more important role in the TGS of 35S-NPTII than RD29A-LUC. Therefore, while DNA methylation is important for silencing at some genomic loci, heterochromatic histone methylation may be more important for silencing at some other loci.
METHODS
Plant Growth and Chemical Treatments
Wild-type C24 and the ros1 mutant plants that carry a homozygous stress-responsive RD29A-LUC transgene (Gong et al., 2002) are referred to simply as wild-type and ros1 plants, respectively, in this study. Seedlings were germinated on half-strength Murashige and Skoog (MS) medium containing 1.5% Suc and 0.7% agar. Seven-day-old seedlings were transferred to new plates supplemented with different chemicals as indicated in figure legends. For luminescence analysis (He et al., 2009a), plates were continuously incubated at 23°C for 4 d after transferring and then followed by 2-d incubation at 4°C to induced activation of RD29A promoter. For kanamycin resistance analysis, plants were grown at 23°C for 6 d after transferring and then transferred for a second time to half-strength MS plates supplemented only with 50 μg/mL kanamycin. SMZ, 5-adC, dl-Met, THF, and kanamycin were purchased from Sigma-Aldrich.
Transcription Analysis
Total RNA was extracted from whole plants using RNeasy plant kit (Qiagen). Twenty micrograms of total RNA per lane was used for RNA gel blot analysis as described by He et al. (2009a). Quantitative real-time PCR was performed as described by Zhang et al. (2009). cDNA was synthesized from 2 to 4 μg of total RNA using SuperScript III first-strand synthesis SuperMix (Invitrogen). Quantitative PCR was performed in technical triplicate and with a minimum of two biological replicates using a Bio-Rad C1000 thermal cycler with the Quantitech SYBR green kit (Bio-Rad). Results were analyzed using the CFX96 real-time system (Bio-Rad). The sequences of DNA oligo probes and primers are listed in Supplemental Table 1 online.
DNA Methylation Measurements
For ChIP-PCR, 500 ng of genomic DNA was incubated overnight with the methylated DNA-digesting enzyme McrBC (New England Biolabs). The digested DNA was used to amplify the RdDM targets GP1 and MU1 by RT-PCR. Nondigested genomic DNA was simultaneously amplified as controls. For bisulfite sequencing, 500 ng of genomic DNA was bisulfite converted following the protocol of the EpiTect bisulfite kit (Qiagen). The collected DNA was used for amplifying endogenous and transgenic RD29A promoter as well as transgenic 35S promoter. The PCR products were cloned into pGEM-T easy vector (Promega), and at least 10 individual clones were sequenced for each sample. Sequencing results were analyzed by the CyMATE program (http://www.gmi.oeaw.ac.at/en/cymate-index/cymate-v2/). Primers sequences are available in Supplemental Table 1 online. DNA gel blot hybridization was performed as described by He et al. (2009a) except that 15 μg of genomic DNA was used in this study.
ChIP
Plants were treated as described above for 4 d at 23°C and followed by 2 d at 4°C. Sample preparation and ChIP were performed as described by Saleh et al. (2008). The histone-chromatin complexes were precipitated with anti-dimethyl-histone H3 (Lys-9) antibody (Wako 302-32369). Recovered DNA fragments were subjected to quantitative real-time PCR using SYBR Green PCR Master Mix (Bio-Rad). Primers for amplifying the endogenous and transgenic RD29A promoters as well as the transgenic 35S promoter are listed in Supplemental Table 1 online.
Small RNA Analysis
Total RNA from whole plants was extracted using TRI reagent (Molecular Research Center). The low molecular weight RNA was isolated from total RNA by PEG precipitation of high molecular weight RNA, followed by isopropanol precipitation overnight at −20°C (He et al., 2009a). Small RNA gel blot analysis was performed as described by He et al. (2009a). Briefly, sixty micrograms of low molecular weight RNA was separated on a 15% polyacrylamide gel at 200 V for 3 h and electroblotted to Hybond-N+ membrane (Amersham), followed by cross-linking and hybridization overnight at 38°C with [γ-32P]ATP-labeled DNA oligonucleotides. The DNA oligo probes and primers for probe amplification are listed in Supplemental Table 1 online.
Metabolite Measurements
Metabolite measurement procedures were based on Gellekink et al. (2005). Whole plants were frozen and ground to fine powers with liquid nitrogen. Samples were extracted with 2 mL of extraction solvent (0.1 n HCl). Internal standards were added (50 μL of 2.5 μg/mL d3-AdoMet, dissolved in 0.1 n HCl), followed by sonication for 5 min. Most of the tissue debris was removed by centrifugation at 3200g for 5 min. The supernatant was transferred to a 2-mL microcentrifuge tube and centrifuged at 9.4g for 5 min for additional tissue removal. Samples were then filtered using a 2000 molecular weight cutoff spin filter (Vivaspin 2; 3200g for 45 min). The filtrate was submitted for analysis.
For liquid chromatography-tandem mass spectrometry analysis, 4 μL was submitted to an Agilent 1200 series HPLC coupled to an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies). Chromatography was performed using a Zorbax SB-CN column (4.6 × 150 mm × 5 μm). Compounds were analyzed using a solvent system composed of water with 0.1% formic acid (solvent A) and acetonitrile with 0.1% formic acid (solvent B). The elution gradient was as follows: 0 to 4 min 0% B; 4 to 5 min 80% B; 5 to 7 min 80% B; 7 to 8 min linear gradient to 0% B; and 8 to 18 min 0% B. Column flow was 0.75 mL/min. During analysis, the column effluent was directed to the tandem mass spectrometry only from 1.7 to 6.0 min to minimize instrument contamination. The Jetstream electrospray ionization source was operated in positive ion mode with the nozzle voltage set at 500 V and the capillary at 3000 V. Nebulizer pressure was set at 55 p.s.i., drying gas (nitrogen) was at 350°C with flow rate of 11 L/min, and the sheath gas was at 250°C with a flow rate of 7 L/min. The fragmentor voltage was 115 V for all analytes. Multiple reaction monitoring was used for selective detection. The mass transitions were 399.1 to 250.1 for AdoMet, 402.1 to 250.1 for d3-AdoMet, and 385.1 to 136.0 for AdoHcy, with a dwell time for each compound of 150 ms. Collision energies were 15 eV for AdoMet and d3-adoMet, and 16 eV for adoHcy.
Compound retention time was 2.0 min for adoMet and d3-AdoMet, and 4.0 min for adoHcy. Quantitation standards were prepared in the range of 15,000 ng/mL to 30 ng/mL in 0.1 n HCl. Responses for adoMet and AdoHcy were normalized against the internal standard response for d3-adoMet. Standard curves had a r2 > 0.99.
Analysis of Global 5-mdC Levels
HPLC–tandem mass spectrometry analysis was performed to determine the mol % of 5-mdC. Genomic DNA was digested by nuclease mix (nuclease P1 and DNase I), following the protocol as described by Rozhon et al. (2008). Ten microliters of digested sample was analyzed using an Agilent 1200 series HPLC coupled to an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies). Chromatography was performed using a Waters Atlantis T3 column (2.1 × 150 mm × 3 μm), maintained at room temperature. The solvent system was composed of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). Total run time per sample was 23 min. The solvent flow rate was 0.3 mL/min. The elution gradient was as follows: 0 to 2 min hold at 0% B; 2 to 8 min linear gradient to 10% B; 8 to 10 min linear gradient to 60% B; 10 to 12 min hold at 60% B; 12 to 13 min linear gradient to 0% B; and 13 to 23 min hold at 0% B. Elution time was 2.8 min for dC and 5.0 min for 5-mdC. Following separation, the column effluent was introduced by electrospray ionization into the mass spectrometer. The Jetstream electrospray ionization source was operated in negative ion mode with the nozzle voltage set at 500 V and a capillary voltage at 3000 V. Drying gas (nitrogen) was held at 325C, with flow rate of 9 L/min and a nebulizer pressure of 30 p.s.i. The sheath gas was at 250°C with a flow rate of 7 L/min. The fragmentor voltage was 115 V. Multiple reaction monitoring was used for selective detection. The transitions were 226 → 93 for dC and 240 → 107 for 5-mdC. The collision energy was set to 5 V. Calculations to determine mol % of 5-mdC were performed as described by Rozhon et al. (2008). The ratio of 5-mdC to total cytosine was presented as percentage of 5-mdC in Figure 6B.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: At2g01840 (LINE1-4), At4g03650 (GP1), At4g08680 (MU1), AT2G15810 (F19G14), AT4G13940 (SAHH1), AT3G09820 (ADK1), AT4G25080 (CHLM), AT5G52310 (RD29A), BD298459.1 (TSI), AT3G41768 (18S rRNA), AT2G42540 (COR15A), and AT3G18780 (Actin 2).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. SMZ Releases Transcriptional Silencing at Endogenous Loci in Wild-Type Plants.
Supplemental Figure 2. SMZ Reduces DNA Methylation at 180-bp Centromeric Repeats and 5S rDNA.
Supplemental Figure 3. TGS Was Not Released by PABA, Met, or THF.
Supplemental Figure 4. Methotrexate Releases Transcriptional Silencing in ros1 Plants.
Supplemental Table 1. Sequences of Primers and Probes.
Acknowledgments
The work in J.-K.Z.’s lab was supported by National Institutes of Health Grants R01GM070795 and R01GM059138. We thank Bruce Cooper (Bindley Bioscience Center, Purdue university) for performing metabolite and global 5-mdC measurements.
AUTHOR CONTRIBUTIONS
H.Z. performed research, analyzed the data, and wrote the article. X.D., D.M., H.L., Y.-J.H., and J.O. performed research. S.C. analyzed the data. J.-K.Z. designed the research, analyzed the data, and wrote the article.
References
- Agius F., Kapoor A., Zhu J.K. (2006). Role of the Arabidopsis DNA glycosylase/lyase ROS1 in active DNA demethylation. Proc. Natl. Acad. Sci. USA 103: 11796–11801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amedeo P., Habu Y., Afsar K., Mittelsten Scheid O., Paszkowski J. (2000). Disruption of the plant gene MOM releases transcriptional silencing of methylated genes. Nature 405: 203–206 [DOI] [PubMed] [Google Scholar]
- Basset G.J.C., Quinlivan E.P., Gregory J.F., Hanson A.D. (2005). Folate synthesis and metabolism in plants and prospects for biofortification. Crop Sci. 45: 449–453 [Google Scholar]
- Baubec T., Dinh H.Q., Pecinka A., Rakic B., Rozhon W., Wohlrab B., von Haeseler A., Mittelsten Scheid O. (2010). Cooperation of multiple chromatin modifications can generate unanticipated stability of epigenetic states in Arabidopsis. Plant Cell 22: 34–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baubec T., Pecinka A., Rozhon W., Mittelsten Scheid O. (2009). Effective, homogeneous and transient interference with cytosine methylation in plant genomic DNA by zebularine. Plant J. 57: 542–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe D. (2004). RNA silencing in plants. Nature 431: 356–363 [DOI] [PubMed] [Google Scholar]
- Bernatavichute Y.V., Zhang X., Cokus S., Pellegrini M., Jacobsen S.E. (2008). Genome-wide association of histone H3 lysine nine methylation with CHG DNA methylation in Arabidopsis thaliana. PLoS ONE 3: e3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J., Malone C.D., Aravin A.A., Sachidanandam R., Stark A., Hannon G.J. (2008). An epigenetic role for maternally inherited piRNAs in transposon silencing. Science 322: 1387–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Jacobsen S.E. (2002). Role of the Arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Curr. Biol. 12: 1138–1144 [DOI] [PubMed] [Google Scholar]
- Chan S.W., Henderson I.R., Jacobsen S.E. (2005). Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat. Rev. Genet. 6: 351–360 [DOI] [PubMed] [Google Scholar]
- Chen C., Farmer A.D., Langley R.J., Mudge J., Crow J.A., May G.D., Huntley J., Smith A.G., Retzel E.F. (2010). Meiosis-specific gene discovery in plants: RNA-Seq applied to isolated Arabidopsis male meiocytes. BMC Plant Biol. 10: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V., Zhu J.K. (2009). Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 12: 133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobosy J.R., Fu V.X., Desotelle J.A., Srinivasan R., Kenowski M.L., Almassi N., Weindruch R., Svaren J., Jarrard D.F. (2008). A methyl-deficient diet modifies histone methylation and alters Igf2 and H19 repression in the prostate. Prostate 68: 1187–1195 [DOI] [PubMed] [Google Scholar]
- Friso S., Choi S.W., Girelli D., Mason J.B., Dolnikowski G.G., Bagley P.J., Olivieri O., Jacques P.F., Rosenberg I.H., Corrocher R., Selhub J. (2002). A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc. Natl. Acad. Sci. USA 99: 5606–5611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furner I.J., Matzke M. (2011). Methylation and demethylation of the Arabidopsis genome. Curr. Opin. Plant Biol. 14: 137–141 [DOI] [PubMed] [Google Scholar]
- Gehring M., Huh J.H., Hsieh T.F., Penterman J., Choi Y., Harada J.J., Goldberg R.B., Fischer R.L. (2006). DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell 124: 495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellekink H., van Oppenraaij-Emmerzaal D., van Rooij A., Struys E.A., den Heijer M., Blom H.J. (2005). Stable-isotope dilution liquid chromatography-electrospray injection tandem mass spectrometry method for fast, selective measurement of S-adenosylmethionine and S-adenosylhomocysteine in plasma. Clin. Chem. 51: 1487–1492 [DOI] [PubMed] [Google Scholar]
- Gong Z., Morales-Ruiz T., Ariza R.R., Roldán-Arjona T., David L., Zhu J.K. (2002). ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell 111: 803–814 [DOI] [PubMed] [Google Scholar]
- Haag J.R., Pikaard C.S. (2011). Multisubunit RNA polymerases IV and V: Purveyors of non-coding RNA for plant gene silencing. Nat. Rev. Mol. Cell Biol. 12: 483–492 [DOI] [PubMed] [Google Scholar]
- Hanson A.D., Gregory J.F., III (2011). Folate biosynthesis, turnover, and transport in plants. Annu. Rev. Plant Biol. 62: 105–125 [DOI] [PubMed] [Google Scholar]
- He X.J., Hsu Y.F., Pontes O., Zhu J., Lu J., Bressan R.A., Pikaard C., Wang C.S., Zhu J.K. (2009a). NRPD4, a protein related to the RPB4 subunit of RNA polymerase II, is a component of RNA polymerases IV and V and is required for RNA-directed DNA methylation. Genes Dev. 23: 318–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X.J., Hsu Y.F., Zhu S., Wierzbicki A.T., Pontes O., Pikaard C.S., Liu H.L., Wang C.S., Jin H., Zhu J.K. (2009b). An effector of RNA-directed DNA methylation in Arabidopsis is an ARGONAUTE 4- and RNA-binding protein. Cell 137: 498–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard E. (2004). Recent advances in X-chromosome inactivation. Curr. Opin. Cell Biol. 16: 247–255 [DOI] [PubMed] [Google Scholar]
- Henderson I.R., Jacobsen S.E. (2007). Epigenetic inheritance in plants. Nature 447: 418–424 [DOI] [PubMed] [Google Scholar]
- Ito H., Gaubert H., Bucher E., Mirouze M., Vaillant I., Paszkowski J. (2011). An siRNA pathway prevents transgenerational retrotransposition in plants subjected to stress. Nature 472: 115–119 [DOI] [PubMed] [Google Scholar]
- Jeddeloh J.A., Stokes T.L., Richards E.J. (1999). Maintenance of genomic methylation requires a SWI2/SNF2-like protein. Nat. Genet. 22: 94–97 [DOI] [PubMed] [Google Scholar]
- Kapoor A., Agarwal M., Andreucci A., Zheng X., Gong Z., Hasegawa P.M., Bressan R.A., Zhu J.K. (2005). Mutations in a conserved replication protein suppress transcriptional gene silencing in a DNA-methylation-independent manner in Arabidopsis. Curr. Biol. 15: 1912–1918 [DOI] [PubMed] [Google Scholar]
- Kloor D., Osswald H. (2004). S-Adenosylhomocysteine hydrolase as a target for intracellular adenosine action. Trends Pharmacol. Sci. 25: 294–297 [DOI] [PubMed] [Google Scholar]
- Law J.A., Jacobsen S.E. (2010). Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 11: 204–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Yang Z., Yu B., Liu J., Chen X. (2005). Methylation protects miRNAs and siRNAs from a 3′-end uridylation activity in Arabidopsis. Curr. Biol. 15: 1501–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman Z., May B., Yordan C., Singer T., Martienssen R. (2003). Distinct mechanisms determine transposon inheritance and methylation via small interfering RNA and histone modification. PLoS Biol. 1: E67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Ren X., Yin H., Wang Y., Xia R., Wang Y., Gong Z. (2010). Mutation in the catalytic subunit of DNA polymerase alpha influences transcriptional gene silencing and homologous recombination in Arabidopsis. Plant J. 61: 36–45 [DOI] [PubMed] [Google Scholar]
- Loenen W.A. (2006). S-adenosylmethionine: Jack of all trades and master of everything? Biochem. Soc. Trans. 34: 330–333 [DOI] [PubMed] [Google Scholar]
- Loizeau K., De Brouwer V., Gambonnet B., Yu A., Renou J.P., Van Der Straeten D., Lambert W.E., Rébeillé F., Ravanel S. (2008). A genome-wide and metabolic analysis determined the adaptive response of Arabidopsis cells to folate depletion induced by methotrexate. Plant Physiol. 148: 2083–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlung A., Comai L. (2004). The effect of stress on genome regulation and structure. Ann. Bot. (Lond.) 94: 481–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu O., Reinders J., Caikovski M., Smathajitt C., Paszkowski J. (2007). Transgenerational stability of the Arabidopsis epigenome is coordinated by CG methylation. Cell 130: 851–862 [DOI] [PubMed] [Google Scholar]
- Matzke M., Kanno T., Daxinger L., Huettel B., Matzke A.J. (2009). RNA-mediated chromatin-based silencing in plants. Curr. Opin. Cell Biol. 21: 367–376 [DOI] [PubMed] [Google Scholar]
- Monneret C. (2005). Histone deacetylase inhibitors. Eur. J. Med. Chem. 40: 1–13 [DOI] [PubMed] [Google Scholar]
- Morales-Ruiz T., Ortega-Galisteo A.P., Ponferrada-Marín M.I., Martínez-Macías M.I., Ariza R.R., Roldán-Arjona T. (2006). DEMETER and REPRESSOR OF SILENCING 1 encode 5-methylcytosine DNA glycosylases. Proc. Natl. Acad. Sci. USA 103: 6853–6858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher R.A., Schwach F., Studholme D., Baulcombe D.C. (2008). PolIVb influences RNA-directed DNA methylation independently of its role in siRNA biogenesis. Proc. Natl. Acad. Sci. USA 105: 3145–3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Galisteo A.P., Morales-Ruiz T., Ariza R.R., Roldán-Arjona T. (2008). Arabidopsis DEMETER-LIKE proteins DML2 and DML3 are required for appropriate distribution of DNA methylation marks. Plant Mol. Biol. 67: 671–681 [DOI] [PubMed] [Google Scholar]
- Ortelli F., Maxwell C.A., Curtis J., Watkins W.M. (1999). Studies on anti-folate antimalarials in east Africa. Parassitologia 41: 313–314 [PubMed] [Google Scholar]
- Patra S.K., Bettuzzi S. (2009). Epigenetic DNA-(cytosine-5-carbon) modifications: 5-Aza-2′-deoxycytidine and DNA-demethylation. Biochemistry (Mosc.) 74: 613–619 [DOI] [PubMed] [Google Scholar]
- Pecinka A., Dinh H.Q., Baubec T., Rosa M., Lettner N., Mittelsten Scheid O. (2010). Epigenetic regulation of repetitive elements is attenuated by prolonged heat stress in Arabidopsis. Plant Cell 22: 3118–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penterman J., Zilberman D., Huh J.H., Ballinger T., Henikoff S., Fischer R.L. (2007). DNA demethylation in the Arabidopsis genome. Proc. Natl. Acad. Sci. USA 104: 6752–6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha P.S., Sheikh M., Melchiorre R., Fagard M., Boutet S., Loach R., Moffatt B., Wagner C., Vaucheret H., Furner I. (2005). The Arabidopsis HOMOLOGY-DEPENDENT GENE SILENCING1 gene codes for an S-adenosyl-L-homocysteine hydrolase required for DNA methylation-dependent gene silencing. Plant Cell 17: 404–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhon W., Baubec T., Mayerhofer J., Mittelsten Scheid O., Jonak C. (2008). Rapid quantification of global DNA methylation by isocratic cation exchange high-performance liquid chromatography. Anal. Biochem. 375: 354–360 [DOI] [PubMed] [Google Scholar]
- Roudier F., Teixeira F.K., Colot V. (2009). Chromatin indexing in Arabidopsis: An epigenomic tale of tails and more. Trends Genet. 25: 511–517 [DOI] [PubMed] [Google Scholar]
- Saleh A., Alvarez-Venegas R., Avramova Z. (2008). An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat. Protoc. 3: 1018–1025 [DOI] [PubMed] [Google Scholar]
- Saze H., Sasaki T., Kakutani T. (2008). Negative regulation of DNA methylation in plants. Epigenetics 3: 122–124 [DOI] [PubMed] [Google Scholar]
- Scott R.J., Spielman M. (2004). Epigenetics: Imprinting in plants and mammals—the same but different? Curr. Biol. 14: R201–R203 [DOI] [PubMed] [Google Scholar]
- Shen J., Ren X., Cao R., Liu J., Gong Z. (2009). Transcriptional gene silencing mediated by a plastid inner envelope phosphoenolpyruvate/phosphate translocator CUE1 in Arabidopsis. Plant Physiol. 150: 1990–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin R.K., Vaughn M., Borges F., Tanurdzić M., Becker J.D., Feijó J.A., Martienssen R.A. (2009). Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136: 461–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover P.J. (2009). One-carbon metabolism-genome interactions in folate-associated pathologies. J. Nutr. 139: 2402–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittel-Elmer M., Bucher E., Broger L., Mathieu O., Paszkowski J., Vaillant I. (2010). Stress-induced activation of heterochromatic transcription. PLoS Genet. 6: e1001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant I., Paszkowski J. (2007). Role of histone and DNA methylation in gene regulation. Curr. Opin. Plant Biol. 10: 528–533 [DOI] [PubMed] [Google Scholar]
- Van Wilder V., De Brouwer V., Loizeau K., Gambonnet B., Albrieux C., Van Der Straeten D., Lambert W.E., Douce R., Block M.A., Rebeille F., Ravanel S. (2009). C1 metabolism and chlorophyll synthesis: The Mg-protoporphyrin IX methyltransferase activity is dependent on the folate status. New Phytol. 182: 137–145 [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu J., Xia R., Wang J., Shen J., Cao R., Hong X., Zhu J.K., Gong Z. (2007). The protein kinase TOUSLED is required for maintenance of transcriptional gene silencing in Arabidopsis. EMBO Rep. 8: 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen K.E., Hatzivassiliou G., Sachdeva U.M., Bui T.V., Cross J.R., Thompson C.B. (2009). ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324: 1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia R., Wang J., Liu C., Wang Y., Wang Y., Zhai J., Liu J., Hong X., Cao X., Zhu J.K., Gong Z. (2006). ROR1/RPA2A, a putative replication protein A2, functions in epigenetic gene silencing and in regulation of meristem development in Arabidopsis. Plant Cell 18: 85–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Ebright Y.W., Yu B., Chen X. (2006). HEN1 recognizes 21-24 nt small RNA duplexes and deposits a methyl group onto the 2′ OH of the 3′ terminal nucleotide. Nucleic Acids Res. 34: 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Sun Y., Xie X., Kim M.S., Dowd S.E., Paré P.W. (2009). A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J. 58: 568–577 [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhu J.K. (2011). RNA-directed DNA methylation. Curr. Opin. Plant Biol. 14: 142–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Henderson I.R., Lu C., Green P.J., Jacobsen S.E. (2007). Role of RNA polymerase IV in plant small RNA metabolism. Proc. Natl. Acad. Sci. USA 104: 4536–4541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Xing Y., He X.J., Li W., Hu Y., Yadav S.K., Oh J., Zhu J.K. (2010). An SGS3-like protein functions in RNA-directed DNA methylation and transcriptional gene silencing in Arabidopsis. Plant J. 62: 92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Kapoor A., Sridhar V.V., Agius F., Zhu J.K. (2007). The DNA glycosylase/lyase ROS1 functions in pruning DNA methylation patterns in Arabidopsis. Curr. Biol. 17: 54–59 [DOI] [PubMed] [Google Scholar]
- Zhu J.K. (2009). Active DNA demethylation mediated by DNA glycosylases. Annu. Rev. Genet. 43: 143–166 [DOI] [PMC free article] [PubMed] [Google Scholar]