This study shows that the 14-3-3 λ protein, which interacts with PHOT2, is required for normal PHOT2-mediated blue light–induced stomatal opening. Furthermore, the PHOT2 residue responsible for the interaction with 14-3-3 λ was identified.
Abstract
The 14-3-3 λ isoform is required for normal stomatal opening mediated by PHOT2 in Arabidopsis thaliana. Arabidopsis PHOTOTROPIN2 (PHOT2) interacts with the λ-isoform 14-3-3 protein both in yeast two-hybrid screening and in an in vitro pull-down assay. Further yeast two-hybrid analysis also showed that the PHOT2 C-terminal kinase domain was required for the interaction. Site-directed mutagenesis indicated that PHOT2 Ser-747 is essential for the yeast interaction. Phenotypic characterization of a loss-of-function 14-3-3 λ mutant in a phot1 mutant background showed that the 14-3-3 λ protein was necessary for normal PHOT2-mediated blue light–induced stomatal opening. PHOT2 Ser-747 was necessary for complementation of the blue light–activated stomatal response in a phot1 phot2 double mutant. The 14-3-3 λ mutant in the phot1 mutant background allowed normal phototropism and normal chloroplast accumulation and avoidance responses. It also showed normal stomatal opening mediated by PHOT1 in a phot2 mutant background. The 14-3-3 κ mutant had no effect on stomatal opening in response to blue light. Although the 14-3-3 λ mutant had no chloroplast movement phenotype, the 14-3-3 κ mutation caused a weaker avoidance response at an intermediate blue light intensity by altering the balance between the avoidance and accumulation responses. The results highlight the strict specificity of phototropin-mediated signal transduction pathways.
INTRODUCTION
Phototropins are plant blue light receptors that regulate a series of light responses that function to optimize photosynthesis and plant growth under a wide range of environmental conditions (Christie, 2007; Goh et al., 2009). Blue light receptors with chromophore domains similar to those of the Arabidopsis thaliana phototropins are widespread among green plants, algae, fungi, and bacteria, though the protein domains adjacent to the chromophore domains vary widely (Swartz et al., 2007; Takahashi et al., 2007; Krauss et al., 2009; Djouani-Tahri et al., 2011).
In Arabidopsis, two phototropins, PHOTOTROPIN1 (PHOT1) and PHOT2, function redundantly or individually to regulate several blue light responses. Both PHOT1 and PHOT2 mediate the phototropic response toward strong blue light, while PHOT1 mediates the phototropic response toward weak blue light. Both PHOT1 and PHOT2 can independently activate blue light–induced stomatal opening (Kinoshita et al., 2001), leaf flattening (Sakamoto and Briggs 2002), and cotyledon positioning (Inoue et al., 2008). PHOT1 is responsible for rapid inhibition of hypocotyl elongation during deetiolation (Folta and Spalding 2001) and induces chloroplast accumulation (Kagawa et al., 2001). PHOT2 induces the chloroplast accumulation response under low light and the chloroplast avoidance response under high light intensities (Sakai et al., 2001). Recently, phototropins were found to regulate the phototropic responses of the Arabidopsis inflorescence (Kagawa et al., 2009), and PHOT2 is required for light-dependent nuclear positioning and chloroplast positioning in darkness (Suetsugu et al. 2005; Iwabuchi et al., 2010).
Phototropins have two major functional domains: an N-terminal photosensory region that contains two structurally similar LOV domains (for light, oxygen, or voltage sensing), LOV1 and LOV2 (Huala et al. 1997), and a C-terminal Ser/Thr kinase that belongs to an AGC-type kinase superfamily (Christie 2007). Just downstream from LOV2 is an amphipathic α-helix (the Jα-helix), which may fold the protein in darkness, inhibiting its kinase activity (Harper et al., 2003). The photosensitivity of PHOT1 and PHOT2 is determined by their LOV domains (Aihara et al., 2008; Kaiserli et al., 2009), and LOV2 plays an essential role in photosensing (Christie et al., 2002; Cho et al., 2007).
Blue light perception activates autophosphorylation of multiple Ser residues in phototropins (Short et al., 1994; Salomon et al., 2003) that are essential for further signal transduction (Christie et al., 2002; Kinoshita et al., 2003; Kong et al., 2007; Inoue et al., 2008a, 2011). Essential phosphorylation sites in the activation loop of both PHOT1 and PHOT2 have been mapped (Inoue et al., 2008a; Sullivan et al., 2008; Inoue et al., 2011). Phosphorylation of these Ser residues may represent a common event for downstream signaling. Mutations of PHOT1 Ser-849 and Ser-851 or PHOT2 Ser-761 and Ser-763 reduce blue light responses mediated by PHOT1 and PHOT2, respectively (Inoue et al., 2011).
Phototropin signaling pathways may have diverged early, leading to distinct blue light responses, by associating with different interacting partners. Several phototropin-associating proteins have been identified that affect certain blue light responses (Kaiserli et al., 2009; Sullivan et al., 2009; Inoue et al., 2010; Tseng and Briggs 2010). For instance, Non-Phototropic Hypocotyl3 mediates phototropic responses (Motchoulski and Liscum, 1999); root phototropism 2 regulates PHOT1-mediated phototropism and stomatal opening (Inada et al., 2004); an auxin efflux transporter, ATP BINDING CASSETTE B19 (ABCB19), was identified as a substrate for PHOT1 kinase activity and inhibition of the efflux activity of ABCB19 by phosphorylation led to auxin redistribution involving the activity of PIN-FORMED3 (Christie et al., 2011; Ding et al., 2011); and members of PHYTOCHROME KINASE SUBSTRATE were found to function in PHOT1-mediated phototropism, leaf positioning, and leaf flattening responses (Lariguet et al., 2006; de Carbonnel et al., 2010). Previously, we reported that PHOT2 interacts with the A1 subunit of protein phosphatase 2A (PP2A) (Tseng and Briggs, 2010). Reduced PP2A activity, caused by pp2a a1 mutation (rcn1-1), prolonged the phosphorylation state of PHOT2 and enhanced the sensitivity of PHOT2-mediated phototropism and stomatal opening.
In the same search for PHOT2-interacting proteins, we also identified the 14-3-3 isoform λ. The 14-3-3 proteins are small acidic proteins, often encoded by multigene families, and are highly conserved and ubiquitously expressed in eukaryotes (Aitken 2006; Gardino et al., 2006; Li and Dhaubhadel, 2011). 14-3-3 proteins function as homodimers or heterodimers and bind a large number of different phosphorylated substrates to regulate a wide array of cellular signaling and physiological processes (Oecking and Jaspert, 2009; Gökirmak et al., 2010). The binding of 14-3-3 proteins to phosphorylated target proteins completes an important step in signal transduction. In Arabidopsis, 13 isoforms of 14-3-3 proteins have been identified (Rosenquist et al., 2000; Sehnke et al., 2002). Recent research has shown that members of the Arabidopsis 14-3-3 protein family are involved in several different signaling pathways (Oecking and Jaspert, 2009; Gökirmak et al., 2010). For instance, the μ and ε isoforms were found to regulate flowering and act in red light signaling (Mayfield et al., 2007). The interaction of 14-3-3 proteins with the BRASSINAZOLE-RESISTANT1 transcription factor was shown to be essential for brassinosteroid signal transduction (Bai et al., 2007; Gampala et al., 2007; Ryu et al., 2007). Of direct interest to this study, Arabidopsis PHOT1 was found to interact with 14-3-3 λ in yeast two-hybrid and in vitro assays (Sullivan et al., 2009), although no functional role for the binding was demonstrated. In general, there is sufficient redundancy among 14-3-3 proteins that single 14-3-3 mutants do not show a phenotype (Denison et al., 2011).
Our yeast two-hybrid studies suggested that the Arabidopsis 14-3-3 λ isoform might play a role in PHOT2-mediated blue light responses. To investigate any possible functional role of the Arabidopsis 14-3-3 λ isoform, we examined the following blue light responses of a phot1-5 14-3-3 λ double mutant: stomatal opening, chloroplast movement, and phototropism. We also investigated which PHOT2 domain was required for 14-3-3 λ binding and whether that interaction was essential to obtain a physiologically active PHOT2.
RESULTS
The Arabidopsis 14-3-3 λ Isoform Interacts with the PHOT2 Kinase Domain in Yeast
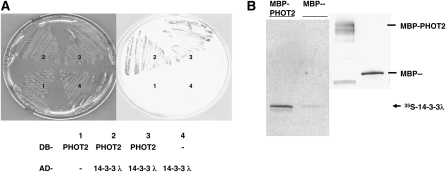
We identified a PHOT2-interacting clone encoding the Arabidopsis 14-3-3 λ protein from yeast two-hybrid screens as described by Tseng and Briggs (2010). Figure 1A shows that only yeast cells expressing both the 14-3-3 λ protein and PHOT2 activated the LacZ reporter system. 14-3-3 λ and PHOT2 also showed physical association in an in vitro pull-down assay (Figure 1B). More 35S-labeled 14-3-3 λ peptide became bound to the bacterial maltose binding protein (MBP)-PHOT2 fusion as determined by signal intensity than MBP alone (Figure 1B, left panel), indicating that 14-3-3 λ interacts directly with PHOT2 in vitro. The immunoblot was reacted with anti-MBP antibodies (Figure 1B, right panel).
Figure 1.
14-3-3 λ Interacts with PHOT2 in Yeast and in in Vitro Pull-Down Assays.
(A) Yeast colonies expressing different combinations of the DNA binding domain (DB-) and activation domain (AD-) of the yeast GAL4 two-hybrid system. Only yeast cells coexpressing the PHOT2 and 14-3-3 λ constructs (2 and 3) activated the LacZ reporter gene (blue color). 1, DB-PHOT2 + AD-; 2, original 14-3-3 λ-interacting clone; 3, cotransformation of the DB-PHOT2 and the rescued AD-14-3-3 λ; 4, DB- + AD-14-3-3 λ.
(B) Arabidopsis14-3-3 λ interacts with PHOT2 in in vitro pull-down assays. Equal OD280 readings of the affinity-purified bacterial MBP- and MBP-PHOT2 fusion protein were used to interact with [35S]Met-labeled 14-3-3 λ (Promega TNT coupling system). Proteins were resolved in SDS-PAGE and blotted. The immunoblot (right panel) was reacted with anti-MBP antibodies. The signals of the retained [35S]Met-labeled 14-3-3 λ by MBP- and MBP-PHOT2 were visualized by autoradiography (left panel). MBP-PHOT2 retained more [35S]Met-labeled 14-3-3 λ, as it had a stronger signal than the MBP fraction alone.
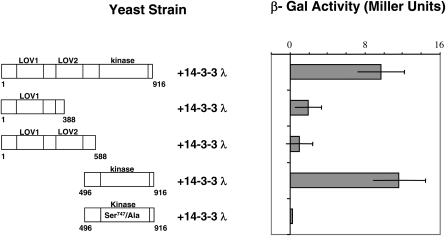
We also investigated which region of PHOT2 interacts with the 14-3-3 λ protein in the yeast two-hybrid system. We found that the C-terminal kinase region of PHOT2 interacted as strongly as the full-length PHOT2 with 14-3-3 λ (Figure 2; β-galactosidase [β-Gal]). Arabidopsis 14-3-3 λ interacted only weakly with the PHOT2 N-terminal region containing both LOV domains and with the shorter N-terminal region containing only the LOV1 domain.
Figure 2.
The PHOT2 Kinase Domain Interacts with 14-3-3 λ.
The β-Gal activity (LacZ reporter gene) was used to determine the strength of interaction between different domains of PHOT2 and 14-3-3 λ in liquid assays using o-nitrophenyl-β-d-galactopyranoside as a substrate. The PHOT2 kinase domain alone interacts as strongly as the full-length PHOT2. Little or no β-Gal activity was detected when the putative 14-3-3 λ recognition site that includes Ser-747 of the PHOT2 kinase domain was replaced with Ala. Representative data (mean ± sd; n = 3 yeast colonies) from two different experiments with similar results are shown. Numbers labeled on the representation of PHOT2 start from the initiation Met (1).
Ser-747 Is Essential for Binding the 14-3-3 λ Protein
The 14-3-3 proteins are known to associate with phosphopeptides by recognizing phosphoserine or phosphothreonine within certain binding motifs. Three consensus peptide sequences have been identified as 14-3-3 recognition sites (Yaffe et al., 1997; Fuglsang et al., 1999; Svennelid et al., 1999). In the PHOT2 kinase region, the amino acid sequence of resides 744 to 749, RSKSQP, resembled the mode-I (RSXpSXP) recognition site, with Ser-747 as a phosphorylation site. Although this particular Ser residue has not been reported to be phosphorylated in vivo (Inoue et al., 2011), nor has PHOT2 kinase activity in the yeast two-hybrid system been reported, we nevertheless hypothesized that Ser-747 may become phosphorylated within the yeast cells (Fuglsang et al., 1999) and that residues 744 to 749, RSKSQP with the Ser-747 phosphorylated, may serve as a 14-3-3 λ recognition site in the yeast two-hybrid assay. We tested this hypothesis by replacing the Ser-747 with Ala in the wild-type PHOT2 bait and monitored the activity of the LacZ reporter system in yeast cells expressing wild-type or mutated PHOT2 with the 14-3-3 λ interacting clone. The yeast cells expressing the Ser-747/Ala phot2 and 14-3-3 λ constructs had only weak activity in comparison with those expressing wild-type PHOT2, indicating that the Ser-747/Ala replacement in PHOT2 disrupted the interaction with 14-3-3 λ (Figure 2). This result was consistent with Ser-747 being required for the 14-3-3 λ recognition site. We cannot eliminate the possibility that Ser-747 may serve some structural function other than through its phosphorylation, needed for signal transmission. Given that it is in a 14-3-3 binding motif, however, we consider this possibility unlikely.
The 14-3-3 λ Mutation Impairs Stomatal Opening Mediated by PHOT2
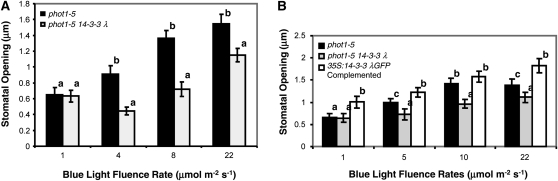
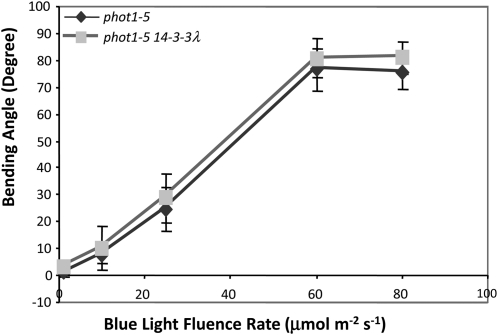
Considering the important roles of 14-3-3 proteins in Arabidopsis (DeLille et al., 2001), we investigated the possible role of the 14-3-3 λ isoform on several phototropin-mediated processes. Both functional PHOT1 and PHOT2 can independently regulate stomatal opening in response to blue light (Kinoshita et al., 2001). We first examined blue light–induced stomatal opening for the phot1-5 single and the phot1-5 14-3-3 λ double mutants (the 14-3-3 λ mutant contained a T-DNA insertion in the second intron; see Supplemental Figure 1 online). Stomata in the phot1-5 14-3-3 λ double mutant opened significantly less than those in the phot1-5 single mutant control after 2 h of blue light treatment (Figure 3A). At all fluence rates tested, stomatal opening in the phot1-5 14-3-3 λ double mutant was severely impaired. At the highest fluence rate tested (22 μmol m-2s−1), significant opening occurred, suggesting either that one or more other 14-3-3 proteins may be weakly redundant with 14-3-3 λ or that the low level of expression seen in the 14-3-3 λ mutant (se Supplemental Figure 1 online) was sufficient for a response at that fluence rate. Hence, we hypothesized that 14-3-3 λ is required for normal PHOT2-mediated stomatal opening in blue light.
Figure 3.
The 14-3-3 λ Mutation Reduces Stomatal Opening Mediated by PHOT2 in Response to Blue Light.
(A) Blue light fluence rate responses testing the stomatal opening mediated by functional PHOT2 only. The T-DNA insertion mutation of 14-3-3 λ caused reduced stomatal opening in response to blue light. Black bars, phot1-5 control; gray bars, phot1-5 14-3-3λ double mutant. Representative data from three different experiments with similar results are shown. Data represents mean ± se (n ≥ 75). Except for the lowest fluence rate, the stomatal aperture for the 14-3-3 λ mutant differed from the wild type with a P value below 0.05. Different letters denote significant differences between the 14-3-3 λ mutant and the control. All blue light treatments were performed with background red light at 50 μmol m−2 s−1.
(B) Complementation of the 14-3-3 λ T-DNA insertion mutation with 35S:14-3-3 λ-GFP restores blue light–induced stomatal opening mediated by PHOT2. Representative data from three different experiments with similar results are shown; data represent mean ± se (n ≥ 75). Except for the lowest fluence rate, the stomatal aperture for the 14-3-3 λ mutant differed from the 14-3-3 λ wild type with a P value below 0.05. Different letters denote significant differences between the 14-3-3 λ mutant and the control. The complemented line had significantly wider stomatal apertures than the 14-3-3 λ wild type (P < 0.05) at all blue light fluence rates except 10 μmol m−2 s−1. Black bars, phot1-5 control; gray bars, phot1-5 14-3-3 λ double mutant; white bars, phot1-5 14-3-3 λ double mutant complemented with 35S:14-3-3 λ-GFP by crossing. All blue light treatments were performed with background red light at 50 μmol m−2 s−1.
To test the hypothesis further, we complemented the mutation of the 14-3-3 λ mutant by crossing a 35S:14-3-3 λ-green fluorescent protein (GFP) line (Paul et al., 2005) into the phot1-5 14-3-3 λ double mutant background. Plants were selected that exhibited GFP fluorescence but lacked functional PHOT1. The stomatal responses were then examined under different blue light intensities as for Figure 3A. The results showed that the 35S:14-3-3 λ-GFP transgene rescued the stomatal opening response in the phot1-5 14-3-3 λ double mutant (Figure 3B). Indeed, the 35S:14-3-3 λ-GFP–complemented line actually had greater stomatal opening than the phot1-5 control at all of the fluence rates of blue light tested except 10 μμmol m−2 s−1.
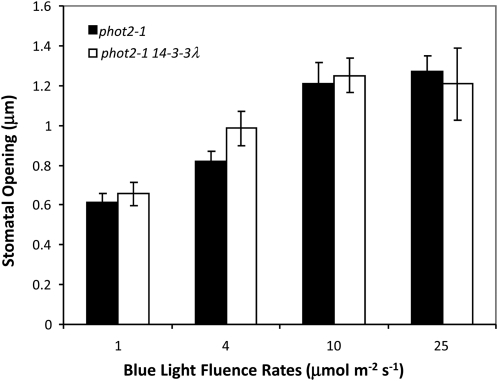
The 14-3-3 λ Mutation Had No Effect on Stomatal Opening Mediated by PHOT1
We next tested whether the 14-3-3 λ T-DNA mutation has any effect on the stomatal opening mediated by PHOT1. We compared the phot2-1 single mutant and the phot2-1 14-3-3 λ double mutant. As shown in Figure 4, the blue light responses of stomatal opening in both the phot2-1 single mutant and the phot2-1 14-3-3 λ double mutant were virtually identical at all of the fluence rates tested, indicating that 14-3-3 λ is likely not involved in regulating stomatal opening mediated by PHOT1. Kinoshita et al. (2001) had shown earlier that a phot1 phot2 double mutant lacked any blue light–induced stomatal opening.
Figure 4.
The 14-3-3 λ Mutation Has No Effect on Stomatal Opening Mediated by PHOT1.
Fluence rate responses for stomatal opening mediated by PHOT1. Both phot2-1 control and the phot2-1 14-3-3 λ double mutant showed no difference in stomatal opening in response to blue light. Representative data from two experiments with similar results are shown. Data represent mean ± se (n = 75). All blue light treatments were performed with background red light at 50 μmol m−2 s−1.
The 14-3-3 λ Mutation Had No Effect on the Phototropism Mediated by PHOT2
As the 14-3-3 λ mutation impaired PHOT2-mediated stomatal opening, we then examined the PHOT2-mediated phototropic responses of etiolated Arabidopsis seedlings carrying both phot1-5 and 14-3-3 λ mutations (i.e., only PHOT2 is functional). In fluence rate response experiments (Figure 5), the phot1-5 14-3-3 λ double mutant and the phot1-5 control responded identically to unilateral blue light, suggesting either that 14-3-3 λ plays no role in the phototropic responses mediated by PHOT2 or that functional redundancy of other members of the Arabidopsis 14-3-3 proteins can compensate for the 14-3-3 λ mutation.
Figure 5.
The 14-3-3 λ Mutation Does Not Affect Phototropism Mediated by PHOT2.
Fluence rate responses of phototropism mediated by PHOT2. The 14-3-3 λ mutation does not affect blue light sensitivity of phototropism mediated by PHOT2. Data represent mean ± se (n ≥ 22).
The 14-3-3 λ Mutation Has No Effect on Chloroplast Movements
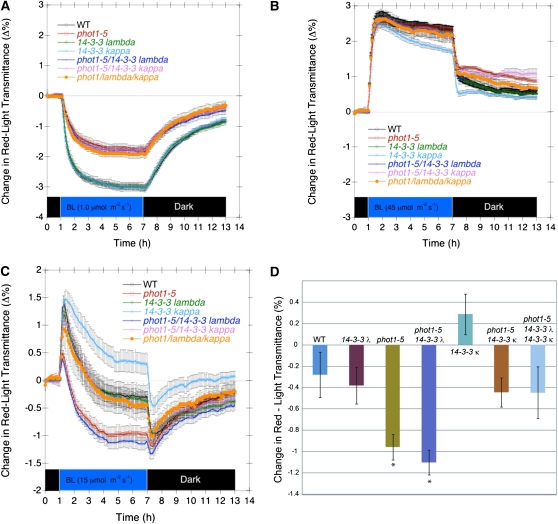
Given the known roles of the phototropins as photoreceptors for chloroplast movement (Sakai et al., 2001), we measured the effects of both the 14-3-3 λ and 14-3-3 κ mutations on chloroplast accumulation (PHOT1- and PHOT2-mediated) and avoidance (PHOT2-mediated only) in response to weak and strong blue light, respectively. The κ isoform was included as it is phylogenetically closest to the λ isoform (DeLille et al., 2001) and hence might be expected to have some redundancy with the λ isoform. As shown in Figure 6A, neither mutation, either alone or together, in a wild-type or phot1-5 background had any effect on chloroplast accumulation in low fluence rate blue light (1.0 μmol m−2 s−1). Likewise, neither mutation, either alone or together, had any effect on the chloroplast avoidance response in high fluence rate blue light (40 μmol m−2 s−1) (Figure 6B). At the intermediate fluence rate of 15 μmol m−2 s−1, however, the situation was more complex. With or without functional PHOT1, the 14-3-3 λ mutation was without effect. However, the 14-3-3 κ mutation reduced the extent to which the chloroplasts showed avoidance when they reached a steady state (Figures 6C and 6D).
Figure 6.
The 14-3-3 λ Mutation Does Not Affect Blue Light–Activated Chloroplast Movement in Leaves of Wild-Type or Mutant Arabidopsis Plants.
(A) Kinetics of chloroplast accumulation induced by 7 h of 1.0 μmol m−2 s−1 blue light and subsequent recovery in darkness. WT, wild type.
(B) Kinetics of chloroplast avoidance response induced by 7 h of 45 μmol m−2 s−1 blue light and subsequent recovery in darkness.
(C) Complex chloroplast response induced by 7 h of 15 μmol m−2 s−1 blue light and subsequent recovery in darkness. Rapid accumulation is reversed by a slower but dominating avoidance response.
(D) Steady state chloroplast positions following 7 h of blue light at 15 μmol m−2 s−1. With or without the phot1-5 mutant background, the 14-3-3 κ mutation reduces the extent of avoidance by roughly the same amount. Note that in the wild-type background, the result is a slight net accumulation response. Each data point represents the average of five to seven measurements of separate leaves. Error bars represent se. Measurements performed with the method described by de Carbonnel et al. (2010).
PHOT2 Ser-747 Is Required for Normal Stomatal Opening in Response to Blue Light
Since we found that Ser-747 in PHOT2 is required for a PHOT2-14-3-3 λ interaction in our yeast two-hybrid investigation, we then tested whether Ser-747 was required for PHOT2-mediated stomatal opening. In fava bean (Vicia faba) PHOT1, a specific Ser residue in the hinge region between LOV1 and LOV2 was found to serve as a recognition site for 14-3-3 protein binding. The phosphorylation of a Ser residue and subsequent 14-3-3 protein binding were thought to be key steps for the blue light opening response in stomata (Kinoshita et al., 2003). Replacing the Ser residue with Ala prevented 14-3-3 protein from binding with fava bean PHOT1a in far protein gel blot analysis. In Arabidopsis PHOT1, three Ser residues toward the N terminus, 350, 376, and 410, were found to bind 14-3-3 proteins (Inoue et al., 2008a; Sullivan et al., 2009). Based on these results, we attempted to identify a 14-3-3 binding site on PHOT2 relevant for the stomatal response.
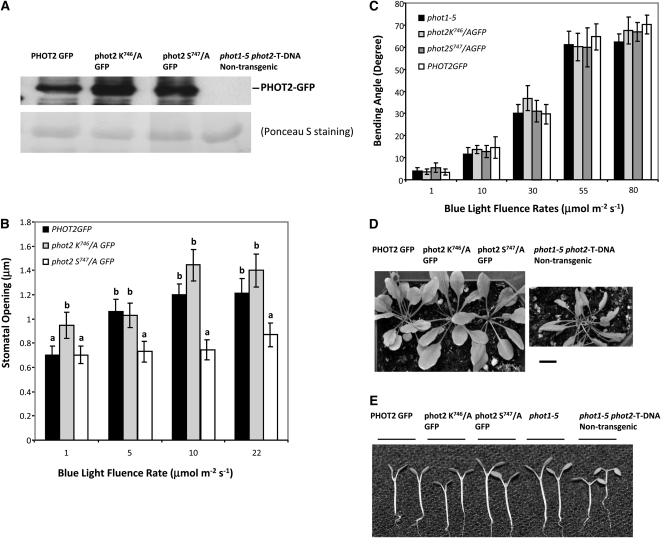
Our above results from yeast two-hybrid assays showed a scenario for the recognition of Arabidopsis PHOT2 by 14-3-3 λ that is different from those previously published. Although several phosphorylation sites in PHOT2 have been reported (Inoue et al., 2011), Ser-747, in a putative mode I recognition sequence, is not one of them. When Ala replaced this putative phosphorylated Ser-747 residue, there was little or no interaction between PHOT2 and 14-3-3 λ in the yeast two-hybrid system (Figure 2). We thus tested the possible physiological role of the Ser-747 residue by investigating the phenotypes of individual transformant plants of the phot1-5 phot2 double mutant (Mao et al., 2005), expressing PHOT2-GFP (Aihara et al., 2008), phot2-GFP with Ser-747/Ala replacement, and phot2-GFP with Lys-746/Ala replacement under the control of the native PHOT2 promoter (Figure 7). The expression level of individual transgenes was almost equal among transformants carrying a single transgene (Figure 7A). These three representative transgenic lines were selected for further characterizations (see Methods).
Figure 7.
Functional Analyses of the Ser-747 Residue of PHOT2.
(A) Immunoblot analysis of the expression levels of PHOT2 GFP, phot2 Lys-746/A-GFP, and phot2 Ser-747/A-GFP in the transformants. Approximately 30 μg (per well) of microsomal protein from each genotype was resolved in 7.5% SDS-PAGE and subjected to immunoblotting for PHOT2. The blot was then stained with Ponceau S solution to test for equal loading of protein samples.
(b) Phot2 Ser-747/Ala replacement reduces blue light responses of stomatal opening. Abaxial-leaf epidermal strips from Arabidopsis plants about 5 weeks old were tested for their relative sensitivity to blue light for stomatal opening. The phot2 Ser-747/A GFP transformant line exhibited reduced stomatal opening compared with the PHOT2 GFP and phot2 Lys-746/A GFP controls, suggesting that Ser-747 functioned as a major recognition site for 14-3-3 λ. Data represent mean ± se (n ≥ 75) from two different experiments with similar results. Except for the lowest fluence rate, both the PHOT2GFP and PHOT2GFP Lys-746/A transformants had apertures that were significantly greater than those of the PHOT2GFP Ser-747/A transformant (P < 0.05). Different letters denote aperture values for the phot2 Ser-747/A transformant significantly below those of the control. All blue light treatments were performed with background red light at 50 μmol m−2 s−1. All plants are in the double mutant background (phot1-5 phot2-T DNA insertion) and were kept under a 16-h-light/8-h-dark day/night cycle at 22°C.
(C) PHOT2 GFP, phot2 Lys-746/A GFP, and phot2 Ser-747/A GFP transformant lines exhibit sensitivity to blue light equal to that of the phot1-5 control. Representative data (mean ± se; n ≥ 22) from two experiments with similar results are shown.
(D) Arabidopsis transformant lines expressing PHOT2-GFP, phot2 Lys-746/A-GFP, and phot2 Ser-747/A GFP together with a nontransformant line, all ~6 weeks old, were investigated for the leaf-curling phenotype seen in the double mutant background (phot1-5 phot2-T DNA insertion); plants were grown at 22°C under a 16-h-light/8-h-dark day/night cycle. Leaves of all three transformant lines showed full expansion, but not the nontransformant. Bar = 1 cm.
(E) PHOT2-GFP, phot2 Lys-746/A-GFP, and phot2 Ser-747/A-GFP are equally functional in rescuing cotyledon positioning phenotype. No difference in cotyledon position was seen in the 8-d-old transformant seedlings expressing wild-type PHOT2-GFP, phot2 Lys-746/A-GFP, and phot2 Ser-747/A-GFP compared with the phot1-5 control. The phot1-5 phot2-T-DNA nontransgenic control exhibited a downward cotyledon-positioning phenotype. Seedlings were grown under a 16-h-light/8-h-dark day/night cycle at 22°C. Images were all taken 1 h after lights on.
We first looked at the stomatal responses in these three transgenic lines. Both the wild-type PHOT2-GFP transgene and the PHOT2-GFP with the Lys-746/Ala mutation rescued the stomatal phenotype. Both transgenic lines exhibited similarly increased stomatal opening in response to increasing blue light intensity (Figure 7B) and with no major differences in the final stomatal apertures. The final apertures reached were similar to those mediated by the wild-type PHOT2 controls shown in Figure 3. Hence, PHOT2-GFP protein with the Lys-746/Ala replacement functioned as well as the PHOT2-GFP control. However, the plants expressing phot2-GFP with the Ser-747/Ala replacement exhibited severely impaired stomatal opening in response to blue light at all blue light intensities tested (Figure 7B). Our results support the hypothesis that Arabidopsis 14-3-3 λ interacts with PHOT2 in vivo to mediate blue light–induced stomatal opening, and the PHOT2 amino acid sequence 744 to 749, RSKSQP, is required and serves as a recognition site for the 14-3-3 λ interaction, possibly through Ser-747 phosphorylation. However, some structural requirement for the Ser independent of phosphorylation is not eliminated. Asterisks denote significant differences.
PHOT2 Ser-747 Is Not Required for Phototropism, Leaf Flattening, or Cotyledon Positioning in a phot1 phot2 Mutant Background
We next inquired as to whether Ser-747 was required for other PHOT2-mediated physiological responses. When we tested their phototropic responses as a function of fluence rate, all three of the above transgenic lines exhibited a normal PHOT2 response (Figure 7C). Since the protein expression levels of these three transgenes were similar (Figure 7A), it is likely that neither Lys-746 nor Ser-747 is required for phototropic responses mediated by PHOT2. In light-grown plants, functional PHOT1 and PHOT2 can independently confer a fully expanded leaf phenotype (Sakamoto and Briggs, 2002). All three transgenic lines exhibited phenotypes with fully expanded leaves, compared with the curly leaves of the nontransformed phot1-5 phot2 T-DNA insertion double mutant (Figure 7D). They also exhibited normal cotyledon positioning (Figure 7E). Thus, phot2-GFP with either Ser-747 replaced by Ala or Lys-746 replaced by Ala rescued all three phenotypes of phot1-5 phot2.
DISCUSSION
Stomata serve as the main portal for the exchange of oxygen and carbon dioxide and the loss of water vapor. Multiple environmental stimuli together with endogenous hormones coordinate the activity of proton pumps and ion channels to regulate the reversible swelling and shrinking of the two guard cells and hence stomatal aperture (Kim et al., 2010). Blue light is one of the most effective environmental signals for inducing stomatal opening (Kinoshita and Hayashi, 2011). In Arabidopsis, PHOT1 and PHOT2 have been identified as the photoreceptors redundantly mediating stomatal opening induced by blue light (Kinoshita et al., 2001; Mao et al., 2005). On exposure to blue light, the guard cell plasma membrane H+-ATPase becomes activated and drives stomatal opening by generating an electrochemical gradient across the plasma membrane that drives the K+ uptake into the guard cells, leading to water uptake, swelling, and increased stomatal aperture.
A 14-3-3 protein has already been reported to be involved in stomatal opening, although a functional role in vivo has not been directly demonstrated. The Arabidopsis H+-ATPase binds a 14-3-3 protein to the penultimate Thr in its C-terminal regulatory domain (Fuglsang et al., 1999; Svennelid et al., 1999). Studies on fava bean suggest that it is this binding that activates the H+-ATPase (Kinoshita and Shimazaki, 1999, 2002). Biochemical evidence indicates that blue light ultimately induces phosphorylation of the H+ ATPase Thr, leading to the consequent binding of the 14-3-3 protein (Kinoshita et al., 2003). In addition, immunohistochemical studies have demonstrated that this blue light–dependent binding occurs in guard cell protoplasts (Ueno et al., 2005). Kinoshita et al. (2003) demonstrated blue light– and phosphorylation-dependent binding of a 14-3-3 protein to fava bean PHOT1 but did not carry out any functional studies. Thus, the possibility exists that a single isoform of a 14-3-3 protein binds both phototropin and the H+-ATPase, linking the two molecules in a functionally interactive conformation. However, extensive efforts to identify 14-3-3 λ–PHOT2 association by coimmunoprecipitation were not successful, making it not possible to address this hypothesis. Blue light was also found to inhibit the activity of plasma membrane anion channels. This inhibition was missing in the phot1 phot 2 double mutant (Marten et al., 2007). Hence, there could be other blue light–sensitive mechanisms involved in stomatal function.
Recent immunohistochemical data showed that phosphorylation of the C-terminal regulatory domain of the H+-ATPase in Arabidopsis is also phototropin dependent (Hayashi et al., 2011). However, it is not known whether phototropin excitation induces direct phosphorylation of the H+-ATPase, a phosphotransfer reaction from the phosphorylated phototropin to the H+-ATPase during direct association of the two proteins, or whether there are one or more intervening steps.
The interaction of the Arabidopsis 14-3-3 λ isoform with PHOT2 in yeast cells was dramatically reduced when the PHOT2 Ser-747 in a putative mode I 14-3-3 recognition site was replaced with an amino acid residue that cannot be phosphorylated (Figure 2). Likewise, transgenic plants carrying the same Ser-747/Ala mutation in PHOT2-GFP against a phot1-5 phot2 double mutant background had a severely impaired stomatal opening response (Figure 7). Thus, the interaction of a 14-3-3 protein both with an H+-ATPase and PHOT2 may be essential for PHOT2-mediated stomatal opening. Since the ATPase study was performed in fava bean (Ueno et al., 2005) and our work was with Arabidopsis, we do not know whether the 14-3-3 proteins involved are the same or different isoforms. However, we conclude that at least in Arabidopsis, 14-3-3 λ is required for normal blue light–induced stomatal opening mediated by PHOT2. Consistent with our observations, Arabidopsis 14-3-3 λ, when overexpressed in transgenic cotton (Gossypium hirsutum) plants, caused increased stomatal conductance (Yan et al., 2004), presumably the consequence of wider opening of the stomata. Moreover, the defective stomatal response to blue light in the 14-3-3 λ mutant was rescued by a 35S:14-3-3 λ-GFP line (Figure 3). Taken together, these results strongly suggest that the PHOT2–14-3-3 λ interaction is physiologically relevant. The current model for light activation of phototropin involves light-activated unfolding of the molecule to expose the various sites for phosphorylation and to activate the kinase (Christie, 2007). Presumably one of the Ser residues exposed is Ser-747. To date, however, there is not rigorous experimental evidence for this model. An open question is why the interaction in yeast does not show light dependency. It could be that a kinase in yeast can carry out the phosphorylation of the relevant Ser regardless of light condition. It could also be that the PHOT2 heterologously produce in yeast is slightly denatured, allowing for phosphorylation of the Ser independent of blue light. At present, this question is not resolved.
There appears to be a high level of specificity for the various phototropin-mediated signal transduction pathways in Arabidopsis (reviewed in Inoue et al., 2010). Of the three PHOT2-mediated blue light responses we have examined here (stomatal opening, phototropism, and chloroplast movement), only stomatal opening was impaired in a phot1-5 14-3-3 λ double mutant background. PHOT2-mediated phototropism (Figure 5), leaf flattening, cotyledon positioning (Figure 7), and chloroplast movement (Figure 6) were all normal. Furthermore, the phototropin–14-3-3 λ interaction required for the stomatal response was exclusive to PHOT2: The phot2-1 14-3-3 λ double mutant had a stomatal opening response in blue light no different from that of the phot2-1 single mutant (Figure 4). The latter case is similar to that with the phosphatase PP2A A1, which dephosphorylates and hence deactivates PHOT2: The rcn1 (pp2a a1) mutation that enhances PHOT2-mediated stomatal opening has no effect on PHOT1-mediated stomatal opening (Tseng and Briggs, 2010). At present, it is not possible to determine whether this PHOT1-PHOT2 difference reflects their requirement for a different 14-3-3 or phosphatase protein or because of a functional redundancy of these proteins for PHOT1 that is missing for PHOT2.
The 14-3-3 protein family itself is extensively involved in a myriad of process in plants (Denison et al., 2011). Given the broad range of processes affected by 14-3-3 binding, and the importance of these processes in virtually every phase of plant development, it is not surprising that there is extensive functional redundancy within this protein family. As a consequence, single mutants normally do not show any phenotype (Denison et al., 2011). Therefore, we found the highly specific role of 14-3-3 λ, limited to stomatal opening among PHOT2-mediated responses, and apparently not interacting with PHOT1 unexpected, especially given the findings of previous workers both in identifying phosphorylation sites (Inoue et al., 2008; Sullivan et al., 2008) and in demonstrating specific 14-3-3–phototropin interactions (Inoue et al., 2008; Sullivan et al., 2009). In this work, however, this physiological specificity provided a convenient tool to address a single PHOT2 function. PHOT1 was not involved and no redundant 14-3-3 protein could substitute for 14-3-3 λ.
The PHOT2–14-3-3 λ interaction identified from the yeast two-hybrid screening reflects a further level of interaction specificity. Approximately fivefold more 14-3-3 λ isoform clones were found interacting with PHOT2 than the κ isoform in yeast. The 14-3-3 κ isoform is the family member most closely related to 14-3-3 λ (DeLille et al., 2001; Sullivan et al., 2009), but the phot1-5 14-3-3 κ double mutant did not exhibit defects in stomatal opening (see Supplemental Figure 2 online). Different subcellular distribution of these two 14-3-3 proteins in guard cells (Paul et al., 2005) could account for the difference in role. The κ isoform had a small effect at an intermediate light intensity on the chloroplast movement in intact leaves that was activated only by PHOT2 and not PHOT1 (Figure 6). Whether this difference reflects a difference at the biochemical level or a difference in the tissue or subcellular distribution of these two 14-3-3 proteins remains to be determined.
Though the responses of stomatal opening in the phot1-5 14-3-3 λ double mutant were much reduced compared with the phot1-5 control, there was some opening induced by blue light at the higher intensities tested. It is not clear, however, whether this is because the 14-3-3 λ mutant is leaky (see Supplemental Figure 1 online), there is functional redundancy of other Arabidopsis 14-3-3 proteins, or if it is caused by responses mediated by other blue light photoreceptors, for instance, cryptochromes (Mao et al., 2005).
The nature of the PHOT2–14-3-3 λ interaction leading to the blue light responses of stomatal opening is not clear. Several Arabidopsis 14-3-3 proteins with different interaction affinity, including 14-3-3 λ, have been shown to bind to the C-terminal recognition site of a plasma membrane H+-ATPase (Rosenquist et al., 2000; Alsterfjord et al., 2004) in in vitro binding assays. Alternatively, the PHOT2–14-3-3 λ interaction may have an impact on some other pathway involving the ion channels on the tonoplast (Latz et al., 2007). In vitro assays showed that 14-3-3 λ has high interaction affinity for a phosphorylated two-pore K+ channel (TPK1) (Latz et al., 2007). The binding of 14-3-3 λ with TPK1 increased the open probability of the channel. However, the same study also demonstrated that 14-3-3 λ binding inhibited a slow-activating vacuolar (SV) channel. Both TPK1 and SV channels have been shown to be involved in the closure of stomata (Ward and Schroeder, 1994; Gobert et al., 2007). Unfortunately, those experiments did not investigate any possible blue light effect on these channels. It is not clear what effect the PHOT2–14-3-3 λ interaction might have on the regulation of the TPK1 and SV channels in vivo.
Autophosphorylation of phototropins on multiple Ser residues is essential for the downstream signal transduction (Christie et al., 2002; Kinoshita et al., 2003; Kong et al., 2007; Inoue et al., 2008a, 2011; Tseng and Briggs, 2010). Mutation analyses showed that substituting residues that could not be phosphorylated for either of two Ser residues in the activation loop of both PHOT1 and PHOT2 attenuated blue light responses mediated by both phototropins (Inoue et al., 2008a; Inoue et al., 2011). Phosphorylation of Ser residues in the activation loop represents key steps for downstream signaling.
Kinoshita et al. (2003) demonstrated binding of a 14-3-3 protein to the Vf-PHOT1a and Vf-PHOT1b phototropins in fava bean. However, in these two cases, the binding sites were at the upstream end of the linker between LOV1 and LOV2, nowhere near Ser-747. Sullivan et al. (2009) reported 14-3-3 λ binding to Arabidopsis PHOT1 (although they did not identify the residue). Therefore, the lack of any phenotype in the absence of 14-3-3 λ for PHOT1-mediated stomatal opening was unexpected. Also surprising is the fact that they failed to detect any PHOT2–14-3-3 λ-interaction. It is possible that this association is weak and transient; therefore, neither they nor we were able to detect it in vivo. The possible significance of the above-mentioned phototropin–14-3-3 protein interactions in fava bean and Arabidopsis is unknown as neither article reported any functional studies. It is still possible that different blue light–activated signal transduction pathways are activated by binding of different 14-3-3 isoforms at different unique phototropin sites.
Even though the Ser-747 residue of PHOT2 has not been reported to be phosphorylated (Inoue et al., 2011), we believe that it is a critical phosphorylation site and functions as a recognition site for interacting with 14-3-3 λ. In our analyses, the replacement of Ser-747 residue of PHOT2 with an Ala residue reduced stomatal opening in response to blue light, a result similar to the stomatal phenotype caused by a loss-of-function 14-3-3 λ mutation. We were able to detect the expression of Ser-747/Ala–mutated phot2 in the leaf tissue of the transgenic line that exhibited normal leaf expansion, cotyledon positioning, and phototropism. All of the results pointed to the fact that the Ser-747/Ala phot2 transgene was fully functionally expressed, except for the blue light–induced stomatal opening mediated by PHOT2. Furthermore, the Lys-746/Ala phot2 transgene control rescued the other four PHOT2-mediated responses that we investigated. Altogether, our results support the hypothesis that phosphorylated Ser-747 of PHOT2 interacts directly with and binds to 14-3-3 λ in vivo, an interaction required for and specific to the blue light–activated stomatal opening response.
The high degree of specificity of 14-3-3 λ in regulating only the stomatal response to blue light and just that response mediated by PHOT2 suggests that the signal transduction pathways for the many different responses may diverge very close to the photoreceptor itself. This divergence could be at the biochemical level, but it could also reflect the different tissue distributions of phototropins and 14-3-3 proteins or both. Future work will be required to sort these divergent pathways out.
METHODS
Plant Materials
Arabidopsis thaliana Columbia ecotype (gl1 background) was used in all studies. The 14-3-3 λ mutant (SALK-075219) was obtained from the ABRC and screened by PCR genotyping with the forward primer: 5′-TTTGAGCAATATGGG-3′ and reverse primer: 5′-TTCCGTTGCTCTCTGGTAAC-3′. The phot1-5 14-3-3 λ double mutant was constructed by genetic crossing. The F2 seedlings that did not curve toward blue light at a fluence rate of 1 μmol m−2 s−1 were selected and screened for the 14-3-3 λ T-DNA insertion and the phot1-5 mutation by PCR genotyping (Cho et al., 2007).
Complementation of the phot1-5 14-3-3 λ Double Mutant with 35S:14-3-3 λ-GFP Transgenic Lines
The 35S:14-3-3λ-GFP transgenic plants (Paul et al., 2005; gifts from Robert Ferl, University of Florida) were crossed to the phot1-5 14-3-3 λ double mutant. Three-day-old etiolated seedlings of the segregating F2 population were exposed to 1 μmol m−2 s−1 of unilateral blue light for 24 h. Seedlings that did not show curvature to the blue light were selected for PCR genotyping with the 14-3-3 λ-specific forward primer 5′-TTTGAGCAAAGGTTATGGG-3′ and the GFP-specific reverse primer 5′-CATGGCGGACTTAAAGAAGTC-3′ for the presence of the 35S:14-3-3λ-GFP transgene, and the same 14-3-3 λ–specific forward and reverse primers were used to confirm the 14-3-3 λ T-DNA insertion. The stomatal opening phenotypes were compared among the adult plants of the selected GFP lines and the nonfluorescent lines without backcrossing.
Phototropism
Seeds were hydrated at 4°C for 3 d, followed by 2-h red light treatment (two 20-W red fluorescent tubes; Sylvania F10T12/R filtered through one layer of Rohm and Haas #2444 red Plexiglas) on half-strength Murashige and Skoog salts with 0.8% agar. Hypocotyl curvatures were assayed as described in Han et al. (2008). Blue light sources used for phototropism experiments were two fluorescent lamps (Philips F20T12/CW), a Kodak Carousel 4400 slide projector, in both cases passing through a blue Plexiglas filter (Rohm and Haas #2424), or from a band of blue-emitting diodes (470 nm). After unilateral illumination with blue light, plates of seedlings were scanned and analyzed with NIH Image 1.62 software. All the data points are the average of at least 22 seedlings. Similar results were seen in at least two different experiments.
Chloroplast Movement
Chloroplast movement was assessed photometrically. Briefly, Arabidopsis plants were grown for 5 to 7 weeks under short-day conditions (10 h light) and fertilized weekly with a solution of 20-20-20. Prior to the experiment, Arabidopsis plants were dark-acclimated overnight (~16 h of darkness). In the morning, rosette leaves were excised under red light and placed on a system of photometric light-emitting diode (LED) arrays as described by de Carbonnel et al. (2010). Chloroplast movement was followed by monitoring the amount of red light transmission at 5-min intervals. Five to seven leaves of each genotype were used in each chloroplast movement assay.
Yeast Two-Hybrid Screening
We obtained the yeast two-hybrid system and the Arabidopsis λ-ACT cDNA expression library (Kim et al., 1997) from the ABRC (stock number CD4-22). Arabidopsis phot2 cDNA (either full length or truncated for the domain analysis) was amplified with forward primer 5′-GCTCTAGAGCCATGGAGAGGCCAAGAGCCC-3′ and reverse primer 5′-AACTGCAGTTAGAAGAGGTCAATGTCCAAG-3′ and subcloned to the NcoI and PstI sites of the pAS1-CYH2 plasmid. The Ser-747 residue of the phot2 bait construct was replaced with Ala by site-directed mutagenesis (Quick Change II XL; Stratagene), using the forward primer 5′-GCAAACGAAGGAGATCCAAAGCTCAACCACTACCC-3′ and the reverse primer 5′-GGGTAGTGGTTGAGCTTTGGATCTCCTTCGTTTGC-3′, following the manufacturer’s instructions. Yeast transformation and library screening were as described (Tseng et al., 2004). The yeast colonies expressing different combination of bait and prey constructs were analyzed for the LacZ reporter gene with paper-lifting methods. Whatman filter paper discs (#1) were used to transfer yeast colonies from the media, then briefly dipped in liquid nitrogen for 10 s; subsequently, the color reaction was performed by imbibing the paper discs in a solution containing 60 mM Na2HPO4 7H2O, 40 mM NaH2PO4 H2O, 10 mM KCl, 1 mM MgSO4, 38.6 mM 2-mercaptomethanol, and 0.334 mg of X-Gal, at 30°C. Quantitation of the β-Gal activity was conducted using o-nitrophenyl-β-d-galactopyranoside as a substrate. Representative data from one of three different experiments are shown. Three colonies from the yeast strains were assayed. Bars indicate sd.
RT-PCR
RT-PCR was performed as described by Cho et al. (2007) to determine the expression level of the 14-3-3λ RNA message in the phot1-5 and phot1-5 14-3-3λ double mutants. Mature leaf tissues from plants about 5 weeks old (16 h light/8 h dark, 22°C) were harvested for total RNA extraction. Approximately 5 μg of total RNA was used for the first-strand cDNA synthesis. Gene-specific primers (14-3-3λ forward primer, 5′-TTTGAGCAAAGGTTATGGG-3′, and reverse primer, 5′-TTCCGTTGCTCTCTGGTAAC-3′; UBQ10 primers, 5′-AACTTTCTCTCAATTCTCTCTACC-3′ and 5′-CTTCTTAAGCATAACAGAGACGAG-3′) were used to amplify a fragment of the 14-3-3λ and UBQ10 cDNA for 30 PCR cycles. DNA gel blots were hybridized with the corresponding probes. The hybridization signals were analyzed with Typhoon Trio (Amersham Bioscience). The exposure time was in the linear range of the signal intensity. Representative data from two different experiments with similar results are shown.
In Vitro Protein Interaction Assays
The fusion protein of bacterial MBP and Arabidopsis PHOT2 (MBP-PHOT2) was constructed and purified as described by Tseng and Briggs (2010). In vitro binding assays were conducted as described (Tseng et al., 2001). The yeast clone of 14-3-3 λ was in vitro transcribed and translated in the T7 TNT system (Promega) with [35S]Met (Amersham). Approximately equal amounts (equal OD280) of MBP and MBP-PHOT2 fusion proteins were used to interact with [35S]Met-labeled 14-3-3 λ peptide. After interaction and extensive washes, the interacting proteins were eluted, resolved by SDS-PAGE, and blotted. Electrophoresis was performed on a precast 4 to 20% gel (Bio-Rad). The signal was detected by autoradiography. Anti-MBP antibodies (NEN Biolabs) were used to monitor loading of the MBP and MBP-PHOT2 fusion proteins.
Generation of Transgenic Plants
We obtained the PHOT2 transgenic plasmid, Pp2:P2G-nosT/pPZP211 (Aihara et al., 2008), from Akira Nagatani (Kyoto University). The Stratagene Quick Change II XL site-directed mutagenesis kit was used to replace the Lys-746 residue and the Ser-747 residue with Ala using the following primer sets: Lys-746/Ala forward, 5′-GCAAACGAAGGAGATCCGCTAGTCAACCACTACCC-3′, and reverse, 5′-GGGTAGTGGTTGACTAGCGGATCTCCTTCGTTTGC-3′; and Ser-747/Ala forward, 5′-GCAAACGAAGGAGATCCAAAGCTCAACCACTACCC-3′, and the reverse, 5′-GGGTAGTGGTTGAGCTTTGGATCTCCTTCGTTTGC-3′. The resulting plasmids were used to transform the phot1-5 phot2-TDNA insertion double mutant (Mao et al., 2005), as described (Sakamoto and Briggs, 2002). Segregating seedlings of the T1 generation were screened under 20 μmol m−2 s−1 of blue light for 8 h. Seedlings showing curvature to the blue light treatment were selected for PCR genotyping with PHOT2-specific primer (5′-CCCCTCCAAAATCGTTTGTCTG-3′) and GFP primer (5′-CATGGCGGACTTAAAGAAGTC-3′). Transformant lines carrying single transgenes, confirmed by PCR genotyping, were used for further studies.
Measurement of Stomatal Aperture
Measurements of stomatal aperture (the maximum distance between guard cell inner walls) were performed as described by Tseng and Briggs (2010). Briefly, abaxial epidermal strips were made from ~5-week-old light-grown plants (16 h light/8 h dark, 22°C) under dim red light. Epidermal strips were floated in buffer containing 5 mM MES, pH 6.5, 50 mM KCl, and 0.1 mM CaCl2 in the dark for 1 h and then treated with blue light (468-nm blue LED; fluence rates as indicated) with superimposed red light (668-nm red LED; 50 μmol m−2 s−1) for 2 h (E-30 LED chamber; Percival Scientific). Different fluence rates of blue light were obtained by adjusting the output from the LED arrays. Red light was used in these experiments to maintain an adequate level of photosynthesis to drive stomatal opening (Zeiger and Field, 1982). At the end of the light treatments, epidermal strips were fixed in 95% ethanol (Loftfield, 1921). Images of stomata were captured microscopically and analyzed with NIH Image 1.62 software. More than 75 stomata were analyzed for each treatment.
Immunoblot Blot Analysis
Leaves of ~5-week-old plants were used for membrane protein extraction. Plants were kept under 16 h light/8 h dark at 22°C. Leaf tissue were taken and extracted at 4°C in extraction buffer and processed as described (Cho et al., 2007). The 100,000g pellet was resuspended in 1× phosphorylation buffer (Cho et al., 2007) containing 37.5 mM Tris HCl buffer, pH 7.5, 5.3 mM MgSO4, 150 mM NaCl, 1 mM EGTA, 1 mM DTT, 0.5% Triton X-100, and 1× protease inhibitor (Roche). Protein concentrations were determined by the BCA protein assay kit (Pierce). Approximately 30 μg of microsomal membrane proteins was used for each gel lane. Sample sets were resolved in SDS-PAGE and then blotted, and PHOT2-specific antibodies (Cho et al., 2007) were used for detection.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: PHOT1, At3g45780; PHOT2, At5g58140; 14-3-3 λ, At5g10450; and 14-3-3 κ, At5g65430.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Expression Levels of 14-3-3 λ Analyzed by RT-PCR in Wild-Type and Mutant Arabidopsis Plants.
Supplemental Figure 2. The 14-3-3 κ Mutation Does Not Affect Stomatal Opening Mediated by PHOT2 in Response to Blue Light.
Acknowledgments
We thank Akira Nagatani (Kyoto University) for providing the PHOT2 transgenic plasmid, Pp2:P2G-nosT/pPZP211, Robert J. Ferl (University of Florida) for the gift of 35S:14-3-3 λ-GFP transgenic lines, and William Eisinger (Santa Clara University) and David Nelson (University of Georgia) for critical reading of the manuscript. The research was supported by National Science Foundation Grant 0843617 to W.R.B. and 0848083 to R.P.H. We are grateful for this support.
AUTHOR CONTRIBUTIONS
T.-S.T. carried out the experimental work except for the chloroplast studies. C.W. carried out the chloroplast experiments. R.P.H. supervised the chloroplast experiments and assisted with the final writing. T.-S.T. and W.R.B. wrote the article.
References
- Aihara Y., Tabata R., Suzuki T., Shimazaki K., Nagatani A. (2008). Molecular basis of the functional specificities of phototropin 1 and 2. Plant J. 56: 364–375 [DOI] [PubMed] [Google Scholar]
- Aitken A. (2006). 14-3-3 proteins: A historic overview. Semin. Cancer Biol. 16: 162–172 [DOI] [PubMed] [Google Scholar]
- Alsterfjord M., Sehnke P.C., Arkell A., Larsson H., Svennelid F., Rosenquist M., Ferl R.J., Sommarin M., Larsson C. (2004). Plasma membrane H(+)-ATPase and 14-3-3 isoforms of Arabidopsis leaves: Evidence for isoform specificity in the 14-3-3/H(+)-ATPase interaction. Plant Cell Physiol. 45: 1202–1210 [DOI] [PubMed] [Google Scholar]
- Bai M.Y., Zhang L.Y., Gampala S.S., Zhu S.W., Song W.Y., Chong K., Wang Z.Y. (2007). Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice. Proc. Natl. Acad. Sci. USA 104: 13839–13844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.Y., Tseng T.S., Kaiserli E., Sullivan S., Christie J.M., Briggs W.R. (2007). Physiological roles of the light, oxygen, or voltage domains of phototropin 1 and phototropin 2 in Arabidopsis. Plant Physiol. 143: 517–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie J.M. (2007). Phototropin blue-light receptors. Annu. Rev. Plant Biol. 58: 21–45 [DOI] [PubMed] [Google Scholar]
- Christie J.M., Swartz T.E., Bogomolni R.A., Briggs W.R. (2002). Phototropin LOV domains exhibit distinct roles in regulating photoreceptor function. Plant J. 32: 205–219 [DOI] [PubMed] [Google Scholar]
- Christie J.M., et al. (2011). phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol. 9: e1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carbonnel M., Davis P., Roelfsema M.R., Inoue S., Schepens I., Lariguet P., Geisler M., Shimazaki K., Hangarter R., Fankhauser C. (2010). The Arabidopsis PHYTOCHROME KINASE SUBSTRATE2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol. 152: 1391–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLille J.M., Sehnke P.C., Ferl R.J. (2001). The Arabidopsis 14-3-3 family of signaling regulators. Plant Physiol. 126: 35–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison F.C., Paul A.L., Zupanska A.K., Ferl R.J. (2011). 14-3-3 proteins in plant physiology. Semin. Cell Dev. Biol. 22: 720–727 [DOI] [PubMed] [Google Scholar]
- Ding Z., Galván-Ampudia C.S., Demarsy E., Łangowski Ł., Kleine-Vehn J., Fan Y., Morita M.T., Tasaka M., Fankhauser C., Offringa R., Friml J. (2011). Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis. Nat. Cell Biol. 13: 447–452 [DOI] [PubMed] [Google Scholar]
- Djouani-Tahri B., Christie J.M., Sanchez-Ferandin S., Sanchez F., Bouget F.Y., Corellou F. (2011). A eukaryotic LOV-histidine kinase with circadian clock function in the picoalga Ostreococcus. Plant J. 65: 578–588 [DOI] [PubMed] [Google Scholar]
- Folta K.M., Spalding E.P. (2001). Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J. 26: 471–478 [DOI] [PubMed] [Google Scholar]
- Fuglsang A.T., Visconti S., Drumm K., Jahn T., Stensballe A., Mattei B., Jensen O.N., Aducci P., Palmgren M.G. (1999). Binding of 14-3-3 protein to the plasma membrane H(+)-ATPase AHA2 involves the three C-terminal residues Tyr(946)-Thr-Val and requires phosphorylation of Thr(947). J. Biol. Chem. 274: 36774–36780 [DOI] [PubMed] [Google Scholar]
- Gampala S.S., et al. (2007). An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev. Cell 13: 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardino A.K., Smerdon S.J., Yaffe M.B. (2006). Structural determinants of 14-3-3 binding specificities and regulation of subcellular localization of 14-3-3-ligand complexes: A comparison of the X-ray crystal structures of all human 14-3-3 isoforms. Semin. Cancer Biol. 16: 173–182 [DOI] [PubMed] [Google Scholar]
- Gobert A., Isayenkov S., Voelker C., Czempinski K., Maathuis F.J. (2007). The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc. Natl. Acad. Sci. USA 104: 10726–10731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh C.H., Jang S., Jung S., Kim H.S., Kang H.G., Park Y.I., Bae H.J., Lee C.H., An G. (2009). Rice phot1a mutation reduces plant growth by affecting photosynthetic responses to light during early seedling growth. Plant Mol. Biol. 69: 605–619 [DOI] [PubMed] [Google Scholar]
- Gökirmak T., Paul A.L., Ferl R.J. (2010). Plant phosphopeptide-binding proteins as signaling mediators. Curr. Opin. Plant Biol. 13: 527–532 [DOI] [PubMed] [Google Scholar]
- Han I.S., Tseng T.-S., Eisinger W., Briggs W.R. (2008). Phytochrome A regulates the intracellular distribution of phototropin 1-green fluorescent protein in Arabidopsis thaliana. Plant Cell 10: 2835–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper S.M., Neil L.C., Gardner K.H. (2003). Structural basis of a phototropin light switch. Science 301: 1541–1544 [DOI] [PubMed] [Google Scholar]
- Hayashi M., Inoue S.I., Takahashi K., Kinoshita T. (2011). Immunohistochemical detection of blue light-induced phosphorylation of the plasma membrane H+-ATPase in stomatal guard cells. Plant Cell Physiol. 52: 1238–1248 [DOI] [PubMed] [Google Scholar]
- Huala E., Oeller P.W., Liscum E., Han I.S., Larsen E., Briggs W.R. (1997). Arabidopsis NPH1: A protein kinase with a putative redox-sensing domain. Science 278: 2120–2123 [DOI] [PubMed] [Google Scholar]
- Inada S., Ohgishi M., Mayama T., Okada K., Sakai T. (2004). RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. Plant Cell 16: 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Kinoshita T., Takemiya A., Doi M., Shimazaki K. (2008). Leaf positioning of Arabidopsis in response to blue light. Mol. Plant 1: 15–26 [DOI] [PubMed] [Google Scholar]
- Inoue S., Matsushita T., Tomokiyo Y., Matsumoto M., Nakayama K.I., Kinoshita T., Shimazaki K. (2011). Functional analyses of the activation loop of phototropin2 in Arabidopsis. Plant Physiol. 156: 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Takemiya A., Shimazaki K. (2010). Phototropin signaling and stomatal opening as a model case. Curr. Opin. Plant Biol. 13: 587–593 [DOI] [PubMed] [Google Scholar]
- Inoue S.-I., Kinoshita T., Matsumoto M., Nakayama K.I., Doi M., Shimazaki K.-I. (2008a). Blue light-induced autophosphorylation of phototropin is a primary step for signaling. Proc. Natl. Acad. Sci. USA 105: 5626–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi K., Minamino R., Takagi S. (2010). Actin reorganization underlies phototropin-dependent positioning of nuclei in Arabidopsis leaf cells. Plant Physiol. 152: 1309–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Kimura M., Wada M. (2009). Blue light-induced phototropism of inflorescence stems and petioles is mediated by phototropin family members phot1 and phot2. Plant Cell Physiol. 50: 1774–1785 [DOI] [PubMed] [Google Scholar]
- Kagawa T., Sakai T., Suetsugu N., Oikawa K., Ishiguro S., Kato T., Tabata S., Okada K., Wada M. (2001). Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kaiserli E., Sullivan S., Jones M.A., Feeney K.A., Christie J.M. (2009). Domain swapping to assess the mechanistic basis of Arabidopsis phototropin 1 receptor kinase activation and endocytosis by blue light. Plant Cell 21: 3226–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Harter K., Theologis A. (1997). Protein-protein interactions among the Aux/IAA proteins. Proc. Natl. Acad. Sci. USA 94: 11786–11791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.H., Böhmer M., Hu H., Nishimura N., Schroeder J.I. (2010). Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 61: 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Doi M., Suetsugu N., Kagawa T., Wada M., Shimazaki K. (2001). Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Emi T., Tominaga M., Sakamoto K., Shigenaga A., Doi M., Shimazaki K.-I. (2003). Blue-light- and phosphorylation-dependent binding of a 14-3-3 protein to phototropins in stomatal guard cells of broad bean. Plant Physiol. 133: 1453–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Hayashi Y. (2011). New insights into the regulation of stomatal opening by blue light and plasma membrane H(+)-ATPase. Int. Rev. Cell Mol. Biol. 289: 89–115 [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Shimazaki K. (1999). Blue light activates the plasma membrane H(+)-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 18: 5548–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Shimazaki K. (2002). Biochemical evidence for the requirement of 14-3-3 protein binding in activation of the guard-cell plasma membrane H+-ATPase by blue light. Plant Cell Physiol. 43: 1359–1365 [DOI] [PubMed] [Google Scholar]
- Kong S.-G., Kinoshita T., Shimazaki K.-I., Mochizuki N., Suzuki T., Nagatani A. (2007). The C-terminal kinase fragment of Arabidopsis phototropin 2 triggers constitutive phototropin responses. Plant J. 51: 862–873 [DOI] [PubMed] [Google Scholar]
- Krauss U., Minh B.Q., Losi A., Gärtner W., Eggert T., von Haeseler A., Jaeger K.E. (2009). Distribution and phylogeny of light-oxygen-voltage-blue-light-signaling proteins in the three kingdoms of life. J. Bacteriol. 191: 7234–7242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariguet P., Schepens I., Hodgson D., Pedmale U.V., Trevisan M., Kami C., de Carbonnel M., Alonso J.M., Ecker J.R., Liscum E., Fankhauser C. (2006). PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc. Natl. Acad. Sci. USA 103: 10134–10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz A., Becker D., Hekman M., Müller T., Beyhl D., Marten I., Eing C., Fischer A., Dunkel M., Bertl A., Rapp U.R., Hedrich R. (2007). TPK1, a Ca(2+)-regulated Arabidopsis vacuole two-pore K(+) channel is activated by 14-3-3 proteins. Plant J. 52: 449–459 [DOI] [PubMed] [Google Scholar]
- Li X., Dhaubhadel S. (2011). Soybean 14-3-3 gene family: Identification and molecular characterization. Planta 233: 569–582 [DOI] [PubMed] [Google Scholar]
- Loftfield J.V.G. (1921). The behavior of stomata. In Carnegie Institution of Washington Publication 314 (Washington, DC: Carnegie Institution of Washington; ), pp. 7–104 [Google Scholar]
- Mao J., Zhang Y.C., Sang Y., Li Q.H., Yang H.Q. (2005). From The Cover: A role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening. Proc. Natl. Acad. Sci. USA 102: 12270–12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten H., Hedrich R., Roelfsema M.R. (2007). Blue light inhibits guard cell plasma membrane anion channels in a phototropin-dependent manner. Plant J. 50: 29–39 [DOI] [PubMed] [Google Scholar]
- Mayfield J.D., Folta K.M., Paul A.L., Ferl R.J. (2007). The 14-3-3 proteins mu and upsilon influence transition to flowering and early phytochrome response. Plant Physiol. 145: 1692–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motchoulski A., Liscum E. (1999). Arabidopsis NPH3: A NPH1 photoreceptor-interacting protein essential for phototropism. Science 286: 961–964 [DOI] [PubMed] [Google Scholar]
- Oecking C., Jaspert N. (2009). Plant 14-3-3 proteins catch up with their mammalian orthologs. Curr. Opin. Plant Biol. 12: 760–765 [DOI] [PubMed] [Google Scholar]
- Paul A.L., Sehnke P.C., Ferl R.J. (2005). Isoform-specific subcellular localization among 14-3-3 proteins in Arabidopsis seems to be driven by client interactions. Mol. Biol. Cell 16: 1735–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist M., Sehnke P., Ferl R.J., Sommarin M., Larsson C. (2000). Evolution of the 14-3-3 protein family: Does the large number of isoforms in multicellular organisms reflect functional specificity? J. Mol. Evol. 51: 446–458 [DOI] [PubMed] [Google Scholar]
- Ryu H., Kim K., Cho H., Park J., Choe S., Hwang I. (2007). Nucleocytoplasmic shuttling of BZR1 mediated by phosphorylation is essential in Arabidopsis brassinosteroid signaling. Plant Cell 19: 2749–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Kagawa T., Kasahara M., Swartz T.E., Christie J.M., Briggs W.R., Wada M., Okada K. (2001). Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K., Briggs W.R. (2002). Cellular and subcellular localization of phototropin 1. Plant Cell 14: 1723–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon M., Knieb E., von Zeppelin T., Rüdiger W. (2003). Mapping of low- and high-fluence autophosphorylation sites in phototropin 1. Biochemistry 42: 4217–4225 [DOI] [PubMed] [Google Scholar]
- Sehnke P.C., DeLille J.M., Ferl R.J. (2002). Consummating signal transduction: the role of 14-3-3 proteins in the completion of signal-induced transitions in protein activity. Plant Cell 14(suppl.): S339–S354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short T.W., Porst M., Palmer J., Fernbach E., Briggs W.R. (1994). Blue light induces phosphorylation at seryl residues in a pea (Pisum sativum L.) plasma membrane protein. Plant Physiol. 104: 1317–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N., Kagawa T., Wada M. (2005). An auxilin-like J-domain protein, JAC1, regulates phototropin-mediated chloroplast movement in Arabidopsis. Plant Physiol. 139: 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S., Thomson C.E., Kaiserli E., Christie J.M. (2009). Interaction specificity of Arabidopsis 14-3-3 proteins with phototropin receptor kinases. FEBS Lett. 583: 2187–2193 [DOI] [PubMed] [Google Scholar]
- Sullivan S., Thomson C.E., Lamont D.J., Jones M.A., Christie J.M. (2008). In vivo phosphorylation site mapping and functional characterization of Arabidopsis phototropin 1. Mol. Plant 1: 178–194 [DOI] [PubMed] [Google Scholar]
- Svennelid F., Olsson A., Piotrowski M., Rosenquist M., Ottman C., Larsson C., Oecking C., Sommarin M. (1999). Phosphorylation of Thr-948 at the C terminus of the plasma membrane H(+)-ATPase creates a binding site for the regulatory 14-3-3 protein. Plant Cell 11: 2379–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz T.E., et al. (2007). Blue-light-activated histidine kinases: two-component sensors in bacteria. Science 317: 1090–1093 [DOI] [PubMed] [Google Scholar]
- Takahashi F., Yamagata D., Ishikawa M., Fukamatsu Y., Ogura Y., Kasahara M., Kiyosue T., Kikuyama M., Wada M., Kataoka H. (2007). AUREOCHROME, a photoreceptor required for photomorphogenesis in stramenopiles. Proc. Natl. Acad. Sci. USA 104: 19625–19630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng T.-S., Briggs W.R. (2010). The Arabidopsis rcn1-1 mutation impairs dephosphorylation of Phot2, resulting in enhanced blue light responses. Plant Cell 22: 392–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng T.-S., Salomé P.A., McClung C.R., Olszewski N.E. (2004). SPINDLY and GIGANTEA interact and act in Arabidopsis thaliana pathways involved in light responses, flowering, and rhythms in cotyledon movements. Plant Cell 16: 1550–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng T.-S., Swain S.M., Olszewski N.E. (2001). Ectopic expression of the tetratricopeptide repeat domain of SPINDLY causes defects in gibberellin response. Plant Physiol. 126: 1250–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K., Kinoshita T., Inoue S.-I., Emi T., Shimazaki K.-I. (2005). Biochemical characterization of plasma membrane H+-ATPase activation in guard cell protoplasts of Arabidopsis thaliana in response to blue light. Plant Cell Physiol. 46: 955–963 [DOI] [PubMed] [Google Scholar]
- Ward J.M., Schroeder J.I. (1994). Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell 6: 669–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe M.B., Rittinger K., Volinia S., Caron P.R., Aitken A., Leffers H., Gamblin S.J., Smerdon S.J., Cantley L.C. (1997). The structural basis for 14-3-3:phosphopeptide binding specificity. Cell 91: 961–971 [DOI] [PubMed] [Google Scholar]
- Yan J., He C., Wang J., Mao Z., Holaday S.A., Allen R.D., Zhang H. (2004). Overexpression of the Arabidopsis 14-3-3 protein GF14 lambda in cotton leads to a “stay-green” phenotype and improves stress tolerance under moderate drought conditions. Plant Cell Physiol. 45: 1007–1014 [DOI] [PubMed] [Google Scholar]
- Zeiger E., Field C. (1982). Photocontrol of the functional coupling between photosynthesis and stomatal conductance in the intact leaf. Plant Physiol. 70: 370–375 [DOI] [PMC free article] [PubMed] [Google Scholar]