Using electron and optical microscopy techniques, including electron tomography, this work characterizes the thylakoid membranes in plastids of the shoot apex. It shows that the maturation state of the thylakoids is not uniform within the shoot apical meristem and that plastids either acquire or lose thylakoid membranes depending on the position and lineage of the cells in which they are found.
Abstract
Chloroplasts of higher plants develop from proplastids, which are undifferentiated plastids that lack photosynthetic (thylakoid) membranes. In flowering plants, the proplastid-chloroplast transition takes place at the shoot apex, which consists of the shoot apical meristem (SAM) and the flanking leaf primordia. It has been believed that the SAM contains only proplastids and that these become chloroplasts only in the primordial leaves. Here, we show that plastids of the SAM are neither homogeneous nor necessarily null. Rather, their developmental state varies with the specific region and/or layer of the SAM in which they are found. Plastids throughout the L1 and L3 layers of the SAM possess fairly developed thylakoid networks. However, many of these plastids eventually lose their thylakoids during leaf maturation. By contrast, plastids at the central, stem cell–harboring region of the L2 layer of the SAM lack thylakoid membranes; these appear only at the periphery, near the leaf primordia. Thus, plastids in the SAM undergo distinct differentiation processes that, depending on their lineage and position, lead to either development or loss of thylakoid membranes. These processes continue along the course of leaf maturation.
INTRODUCTION
Higher plant chloroplasts possess one of the most complex lamellar systems found in cells—the thylakoid membrane network. The sac-like paired membranes of this network host the protein complexes that carry out the light reactions of oxygenic photosynthesis and provide a medium for energy transduction. In plants, the thylakoid membranes are organized into an intricate three-dimensional (3D) network uniquely characterized by the differentiation into two distinct morphological domains: stacks of multiple, tightly appressed layers, called grana, which are connected to each other by nonappressed membrane regions, called stroma lamellae. Accompanying, and in fact driving, the differentiation in structure is a functional segregation manifested in the asymmetric distribution of the major photosynthetic complexes between the two membrane domains. Photosystem II (PSII) and its major antenna complex, light-harvesting complex II (LHCII) are localized primarily to the grana, whereas photosystem I and ATP synthase are concentrated in unstacked regions of the network, namely, the stroma lamellae, grana end membranes, and grana margins (Mustárdy, 1996; Anderson, 1999; Albertsson, 2001; Chow et al., 2005; Mullineaux, 2005; Anderson et al., 2008; Nevo et al., 2009).
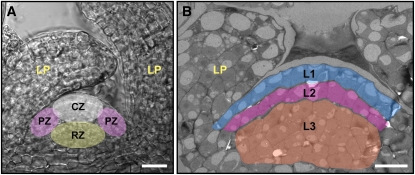
The mature, functional chloroplasts of plants develop from proplastids, which are small, undifferentiated plastids that contain little or no thylakoids or photosynthetic complexes. In flowering plants (angiosperms), the transition from proplastids to photosynthetically competent chloroplasts takes place at the vegetative shoot apex, which consists of a dome-like layered structure, called the shoot apical meristem (SAM), and flanking leaf primordia (Figure 1). At the tip of the SAM is the central zone (CZ), a region that contains a small group of stem cells that are the source of all of the aerial parts of the plant. Surrounding the CZ is the peripheral zone (PZ), from which the leaves emerge. The rib zone, found beneath the CZ, provides the cells for the internal tissues of the stem and leaves (Figure 1A). The SAM is also divided into clonally distinct layers (Figure 1B), each of which generates different parts of the leaf. The two outer one-cell-thick layers, L1 and L2, give rise to the epidermis and outer mesophyll, respectively, with the latter constituting the primary photosynthetic tissue of the leaf. Beneath them is the L3 layer, or corpus, a multilayer of cells that makes up the inner bulk of the SAM and contributes cells for the inner mesophyll and vasculature (Tilney-Bassett, 1986; Steeves and Sussex, 1989; Furner and Pumfrey, 1992; Irish and Sussex, 1992; Telfer and Poethig, 1994).
Figure 1.
The Vegetative Shoot Apex.
(A) Transmission microscope image of a longitudinal section of the Arabidopsis vegetative shoot apex, which consists of the SAM (colored regions) and leaf primordia (LP). The SAM is divided into three radial zones (marked schematically): The CZ contains a small group of stem cells that give rise to all of the aboveground parts of the plant; the PZ is the site of leaf initiation; and the rib zone (RZ) forms the inner leaf and stem tissues.
(B) Electron micrograph of the SAM and a young leaf primordium. The three clonally distinct layers of the SAM, L1 (blue), L2 (pink), and L3 (orange), give rise to the epidermis, outer mesophyll, and inner mesophyll and vasculature of the leaf, respectively. The same color coding is used for the tomograms shown in Figures 2 to 4.
Bars = 20 μm in (A) and 10 μm in (B).
Albeit being the site for initiation of thylakoid network biogenesis, the SAM has been mostly studied in relation to ontogenetic processes involved in organ development and the vegetative-to-reproductive transition (reviewed in Barton, 2010; Ha et al., 2010). It is generally believed that the SAM contains only proplastids and thus lacks thylakoids and chlorophyll binding proteins (López-Juez and Pyke, 2005; Fleming, 2006a, 2006b; Sakamoto et al., 2008). By contrast, in cells of the adjacent primordial leaves, differentiated, photosynthetically active chloroplasts are already found. A developmental gradient was thus predicted to exist between these two regions of the shoot apex.
In this study, we applied different microscopic techniques to characterize the maturation state of the thylakoid membrane in plastids dispersed throughout the shoot apex of Arabidopsis thaliana. In contrast with the accepted view, we found that the \SAM, including its stem cell–containing region, the CZ, is neither null nor uniform with respect to thylakoid maturation. We also found that, depending on their position within the SAM, plastids undergo different developmental processes that can lead to either acquisition or loss of thylakoid membranes. These processes are not restricted to the SAM, but continue along the course of leaf development.
RESULTS
Electron Microscope Tomography of Plastids in the Shoot Apex
In Arabidopsis, the adult vegetative SAM has a diameter of ~60 μm, and the shoot apex can be visualized within hundreds of microns. Thus, it is an ideal model system for studying thylakoid membrane biogenesis and differentiation at high resolution, as they can be monitored within single longitudinal sections by electron microscopy (Figure 1). Such sections of samples prepared by cryoimmobilization, freeze substitution, and resin embedding were used for determining the 3D structure of the thylakoid membranes in the shoot apex by dual-axis transmission electron microscopy (TEM) and scanning-TEM tomography (EMT). The three clonal layers of the SAM are easily distinguishable (Figure 1B), and cell lineages can also be partially traced in the leaf primordia. Examination of plastids along each layer from the center of the SAM toward the leaf is in a way equivalent to monitoring sequential time points. For the L1 and L2 layers, in which cells divide exclusively anticlinally (perpendicular to the SAM surface), this is more straightforward than for the L3 layer, in which cells divide both anticlinally and periclinally (parallel to the SAM surface). For the analysis, we acquired ~30 tomograms of plastids distributed throughout the SAM and flanking primordial leaves. A schematic map showing the position of these plastids accompanies Figures 2 to 5 and Supplemental Figure1 online.
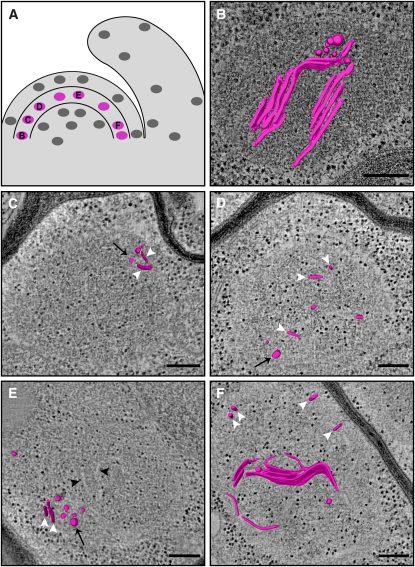
Figure 2.
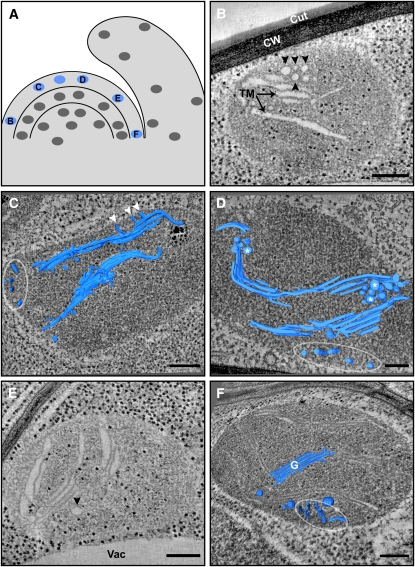
Plastids in the Outer Layer (L1) of the SAM Contain Fairly Developed Thylakoid Membrane Networks.
(A) Schematic drawing of the shoot apex, with marked positions of the tomograms (blue) shown in (B) to (F).
(B) A 12-nm-thick tomographic slice of a plastid from the PZ. The thylakoid membranes (TM), some of which are arranged in stacked layers, are seen as white strips. Black arrowheads denote PG-resembling inclusions, which appear as electron translucent bodies. The inclusions are organized into clusters (see asterisks in [D] and Supplemental Figure 3 online); the small black particles dispersed throughout the plastid and cytoplasm are ribosomes. Cut, cuticule; CW, cell wall.
(C) and (D) 3D models generated by segmentation of the thylakoid membranes, overlaid on 9-nm-thick (C) and 5-nm-thick (D) tomographic slices of plastids from the CZ. The thylakoids appear to connect to the envelope via multiple membrane protrusions ([C]; arrowheads). Raw tomographic slices of the plastids shown in (C) and (D), and in panels of Figures 3 to 5 in which 3D models appear as overlays, are presented in Supplemental Figure 7 online.
(E) A 12-nm-thick tomographic slice of a plastid from the CZ-PZ boundary. Vac, vacuole. One inclusion is marked (arrowhead). A tilted view of this tomogram (see Supplemental Figure 3C online) shows that this inclusion is, in fact, part of a cluster.
(F) Partial 3D model depicting a mature-like granum body (G), overlaid on a 14-nm-thick tomographic slice of a PZ-located plastid. A cluster of tubular structures located near the plastid envelope is also observed (white outline; see also [C] and [D]).
Bars = 200 nm in (B), (C), (E), and (F) and 100 nm in (D).
Figure 5.
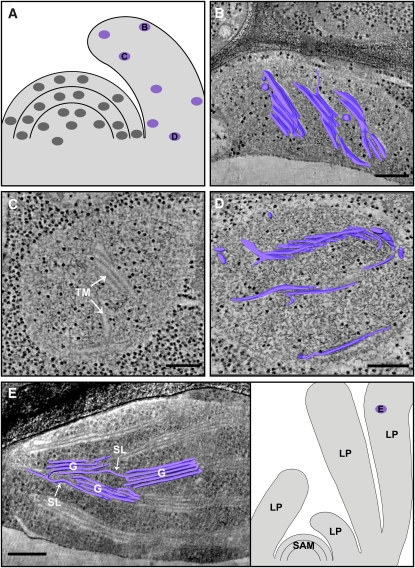
Thylakoid Membranes in the Leaf Primordia.
(A) Schematic drawing of the shoot apex, with marked positions of the tomograms (purple) shown in (B) to (D).
(B) and (D) 3D models of the thylakoid membranes present in young leaf primordial plastids, overlaid on 11-nm-thick tomographic slices.
(C) A 14-nm-thick tomographic slice of a plastid from the inner tissue of the leaf; a granum-like structure of four thylakoids is apparent (TM; top arrow). The extent of maturation of the thylakoids is similar in plastids found at the outer (B) or inner ([C] and [D]) layers of the leaf primordia. It is also similar to plastids found in the L1 and L3 layers of the SAM (Figures 2 and 3).
(E) A partial 3D model (left panel) of thylakoid membranes in a plastid from a more mature primordial leaf (right panel). Clearly segregated granal (G) and interconnecting stroma lamellar (SL) domains, reminiscent of those present in mature thylakoid networks, are evident. LP, leaf primordia.
Bars = 200 nm.
Plastids in the L1 and L3 Layers Contain Small Thylakoid Networks
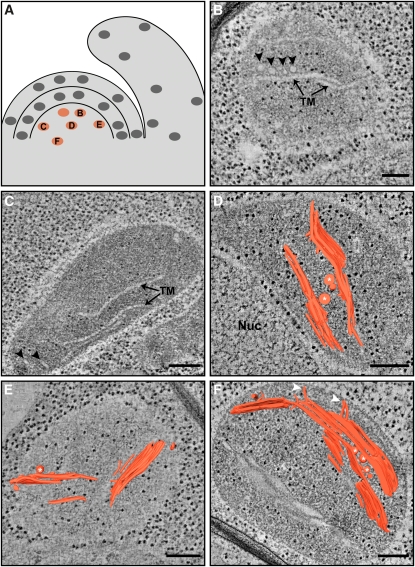
The outermost layer of the SAM, L1, generates the leaf epidermis, which is primarily a nonphotosynthetic tissue. Nonetheless, and in contrast with the common belief that plastids in the SAM lack thylakoids, plastids in L1 contain small, yet partially differentiated, thylakoid networks (Figure 2) that display characteristic features of mature thylakoids (see below). Moving along this layer from the CZ to the PZ, no significant changes in network size or maturation are apparent. Fairly developed thylakoid networks are also observed in the corpus (L3) (Figure 3), whose contribution to the leaf photosynthetic tissue is limited to part of the inner spongy mesophyll (cells in L3 also give rise to the vasculature of the shoot) (Furner and Pumfrey, 1992; Irish and Sussex, 1992). Note, though, that some of the plastids at the topmost layer of L3 (Figure 3B), just below the L2 layer, are less mature than those located in other regions of L3 and than those of L1.
Figure 3.
Developed Thylakoid Membrane Structures Are Also Seen in the Corpus (L3) of the SAM.
(A) Schematic drawing of the shoot apex, with marked positions of the tomograms (orange) shown in (B) to (F).
(B) and (C) Shown are 20- and 12-nm-thick tomographic slices, respectively. Thylakoid membranes (TM), some of which are stacked, PG-resembling inclusions ([B]; arrowheads) and peripheral tubules ([C]; arrowheads) are apparent.
(D) to (F) 3D models of thylakoid networks overlaid on 9-nm-thick ([D] and [E]) and 11-nm-thick (F) tomographic slices. Note the concentration of ribosomes between the plastid and the nucleus (Nuc), which are in tight association with each other (D). The inclusions are found in proximity to the thylakoid membranes ([D] to [F]; white asterisks). Membrane extensions emanating from the thylakoids toward the plastid envelope are also observed ([F]; white arrowheads; see Supplemental Figure 3 online). Since the thylakoids in young plastids are not all parallel to each other like those of mature chloroplasts, some of them appear to be wider or swollen ([F]; bottom unsegmented membranes), as they are not viewed orthogonally to the slice shown and not necessarily because they are inherently expanded.
Bars = 200 nm.
Plastids at the Center of the L2 Layer Lack Thylakoid Membranes
Most of the photosynthetic tissue, including all of the palisade (upper) mesophyll, all of the outer spongy and part of the inner spongy mesophyll (at the leaf perimeter), are generated by the subepidermal layer of the SAM, the L2 layer. In spite of this, and in contrast with the L1 and L3 layers, some plastids in L2 do not possess thylakoid networks. At the stem cell–harboring zone (CZ) of L2, plastids lack thylakoid membrane assemblies and contain only some vesicular structures and tubules and, occasionally, primordial lamellar structures (Figures 4C to 4E; see Supplemental Figures 1B and 1C online). However, only ~20 to 30 μm away, at the periphery of this layer, where cells are recruited to form the leaves, thylakoid membranes resembling those found in L1 and L3 are present (Figures 4B and 4F; see Supplemental Figure 1D online).
Figure 4.
A Gradient of Thylakoid Development Exists within the L2 Layer.
(A) Schematic drawing of the shoot apex, with marked positions of the tomograms (pink) shown in (B) to (F).
(B) to (F) 3D models of thylakoid membranes, primordial lamellar structures, and tubules, overlaid on 10-nm-thick ([B], [E], and [F]), 12-nm-thick (C), and 9-nm-thick (D) tomographic slices. Plastids found at the CZ or the CZ-PZ boundary lack thylakoid networks and possess only some vesicles (arrows in [C] to [E]; see Supplemental Figures 3F and 3G online), tubules (white arrowheads in [C] to [F]), and a few, immature, short lamellae (black arrowheads; [E]). By contrast, those found at the periphery of this layer ([B] and [F]) contain thylakoid networks that are similar to those of the L1 and L3 layers. Additional plastids from L2 are shown in Supplemental Figure 1 online.
Bars = 200 nm.
Thylakoid Networks in the SAM Exhibit Features of Mature Networks
Albeit small and not fully differentiated, the thylakoid membranes found in the L1 and L3 layers, and even those present at the periphery of the L2 layer, exhibit many features of the mature network. As mentioned in the Introduction, the hallmark of mature higher plant thylakoid networks is its morphological segregation into appressed grana and unstacked stroma lamellar membranes domains (Figure 5E). The grana layers are connected to each other and to the stroma lamellae via two different connections: membrane bifurcations, in which a stroma lamellar sheet splits to form two parallel granum layers, and membrane bridges that join adjacent stacked layers to each other (Shimoni et al., 2005; Nevo et al., 2009; Daum et al., 2010; Adam et al., 2011).
These characteristics, although often in rudimentary form, are observed in the thylakoid membrane networks, when present, in plastids of the SAM. We observed stacking of the membranes (up to five layers), sometimes closely resembling the structure of mature grana. In most of the stacked regions, however, the layers do not fully overlap with each other as they vary in length or are shifted with respect to each other along their long axis. Membrane forks are likewise observed quite frequently, but not always at the edges of the stacked regions (see Supplemental Figures 2A to 2C online), as is the case in mature thylakoids. The third basic feature, bridges that connect adjacent membrane layers in the stacks, is also present (see Supplemental Figures 2D to 2F online). This structural motif is seen even when only two stacked layers are present (see Supplemental Figure 2D online), indicating that it is formed early during granum formation. Unconnected membrane domains present during the early stages of network formation eventually fuse to each other, leading, along with the bifurcations and interlayer connections, to a continuous network that encloses a single lumen (e.g., Figure 5E). Such interconnectedness is an essential property of thylakoid membranes as it provides an uninterrupted medium for the movement of both water- and lipid-soluble molecules throughout the entire network (Mustárdy, 1996; Mustárdy and Garab, 2003; Shimoni et al., 2005).
We also note that some of the thylakoids in plastids of the SAM (as well as of young leaf primordia) appear to be swollen (e.g.,Figure 2B). Besides geometrical considerations (see legend of Figure 3), this distended morphology likely reflects a genuine property of the thylakoids at these early developmental stages. This notion is supported by the fact that thylakoids in more mature chloroplasts that are found within the same sections exhibit the characteristic morphology (Figures 5E and 7C), with a thickness similar to that of fully developed thylakoids. In addition, we recently analyzed thylakoid membranes in frozen-hydrated leaf sections or, as used in this study, in samples prepared by high-pressure freezing and freeze substitution and found that the two methods yield comparable results (Kirchhoff et al., 2011). It is thus highly unlikely that preparation-related artifacts were the cause of swelling of the thylakoids. At present, we do not know what underlies this swollen appearance in young plastids, but differences in lipid or protein/pigment composition with respect to mature thylakoid membranes are likely candidates.
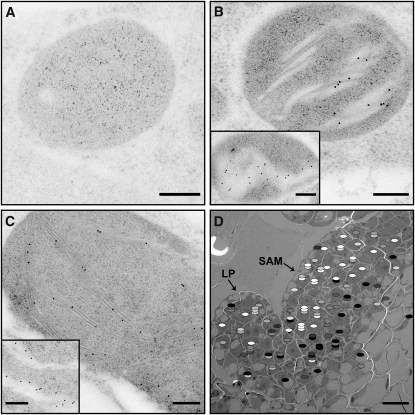
Figure 7.
Immunogold Labeling of OEC33 in the Shoot Apex.
(A) to (C) Images of plastids from the CZ of the L2 layer (A), the L3 periphery (B), and of a chloroplast from a relatively mature leaf primordium (C), labeled with an antibody against subunit 33 of the OEC. No labels are present in the plastid from the center of L2 ([A]; the small dark spots seen in the image are stain particles), consistent with the absence of thylakoid membranes in this region. By contrast, multiple labels, localized to the thylakoid membranes, are seen in the plastids from the L3 layer (B) and the primordial leaf (C). Labeling is more pronounced in sections made along the thylakoid sheet plane (insets in [B] and [C]), which expose the luminal compartment, where the OEC resides. Bars = 200 nm.
(D) Map summarizing the label density of plastids of the SAM and a young leaf primordium. Each ellipse represents one plastid and stacked ellipses represent plastids belonging to the same cell. The density of label is represented in grayscale (from white [low] to black [high]) and normalized to that of older leaf chloroplasts present in the same section. As no averaging was made, and because the number of plastids and labels was relatively small, the data are noisy. Nevertheless, the pattern emerging is consistent with the structural and chlorophyll fluorescence data. LP, leaf primordia. Bar = 10 μm.
Additional Features of Plastids in the SAM
In addition to the characteristic membrane elements of mature chloroplasts, several features that may have a role in the formation of the thylakoid membranes were observed. The first are short protrusions that emanate from the thylakoid membranes toward the plastid inner envelope membrane (arrowheads, Figures 2C and 3F; see Supplemental Figures 3A and 3B online). While we were not able to see the actual fusion of the protrusions with the envelope membranes, due to the low contrast of the latter in the tomograms, we believe that they connect the thylakoids to the envelope membranes. This is because they always appear to be in close proximity to the envelope and because we observed similar connections in mature chloroplasts, where fusion was evident (Shimoni et al., 2005). Such connections may serve as a route for the transfer of lipids from the inner envelope membrane to the thylakoid membranes during biogenesis.
The second feature we observed is clusters of inclusions that highly resemble plastoglobules (PGs) (Figures 2B and 3B, arrowheads; see Supplemental Figures 3C to 3E online). PGs are lipoprotein particles that participate in lipid and other metabolic processes and play various roles in thylakoid membrane homeostasis, protection against photooxidative damage, and degradation during senescence and formation of chromoplasts (reviewed in Bréhélin et al., 2007; Bréhélin and Kessler, 2008). In our samples, the inclusions are electron translucent, which is how PGs appear in samples prepared by high-pressure freezing and freeze substitution (Zechmann et al., 2005; Austin et al., 2006). This is in contrast with samples prepared by chemical fixation, in which PGs are heavily stained and therefore have a dark appearance. In plastids of L1 and L3, we observed numerous inclusions, 25 to 65 nm in diameter, mostly organized in clusters that are found in close proximity to the thylakoid membranes and that sometimes contain up to 15 bodies. The inclusions in the clusters are connected to each other through narrow extensions (see Supplemental Figure 3D online), which also appear to join them to the thylakoids. It has been previously shown that PGs form similar interconnected clusters that are fused to the thylakoid membranes in greening and senescing leaf chloroplasts (Austin et al., 2006), as opposed to their appearance in unstressed mature chloroplasts, where they are mostly observed as single entities. We also observed inclusions in the L2 layer, but only in plastids that contain thylakoid membranes.
Infrequently, we observed isolated vesicular bodies in plastids of the SAM, which appeared to be distinct from the inclusions described above. The vesicles were in proximity to the plastid periphery (Figure 4E; see Supplemental Figures 3F and 3G online), suggesting that they may participate in transport of lipids, proteins, or pigments from the chloroplast envelope to the developing thylakoid membranes.
Another feature observed in plastids found in all regions of the SAM, including the undifferentiated ones in the CZ of the L2 layer, which lack thylakoid networks, is distinctive tubular structures. These tubules, ~20 nm in diameter, are mostly found at the periphery of the plastids, between the thylakoid membranes (when present) and the plastid envelope (e.g. Figure 2D; see Supplemental Figures 3F and 3G online). In some cases, several tubules are clustered together but are not always oriented in the same direction (Figures 2C, 2D, and 2F). Tubular structures similar to these, termed microtubule-like or plastid microtubules, have been observed previously in algae and plant plastids (Pickett-Heaps, 1968; Lawrence and Possingham, 1984; Cheniclet and Carde, 1988; Artus et al., 1990; Kutík, 1992). They were proposed to be involved in processes associated with plastid growth and differentiation, though neither the exact roles they play in these processes nor their composition are known. Nonetheless, their presence in the most undifferentiated plastids, at the center of the L2 layer (Figures 4C to 4E), suggests that they function during very early stages of chloroplast development.
The Development of Thylakoid Membranes in Leaf Primordia Is Gradated
In very young leaf primordia, which are 50 to 100 μm in length and are curved over the SAM (defined as stage 1; Carland and McHale, 1996) (Figure 1B), plastids are similar in size to those of the SAM. The thylakoid membranes in these plastids exhibit the same structural features and appear to be developed to a similar extent as those of the L1 and L3 layers of the SAM (Figure 5). This is true both for plastids found at the outer epidermal layer (Figure 5B) and for ones at the inner tissues of the leaf (Figures 5C and 5D), whose origin is from the L1 and L2/L3 layers of the SAM, respectively. Hence, thylakoid maturation appears to be halted or slowed down in young leaf primordia.
Analysis of tomograms from more developed leaf primordia (~200 to 500 μm, defined as stage 3; Carland and McHale, 1996), in which some of the features characteristic of mature leaf tissues are apparent (e.g., vacuolated cells, adaxial-abaxial polarity, and venation), reveals quite mature thylakoid networks that occupy a large fraction of the chloroplast volume. The membranes are organized into distinct grana and stroma lamellar domains (Figure 5E). Nevertheless, even these quite mature chloroplasts still have to grow and their membranes have to proliferate before they become typical mesophyll chloroplasts.
Plastids of the SAM or of young leaf primordia that were analyzed by EMT were subjected to a morphometric analysis to determine whether thylakoid membrane presence and/or fraction volume are related to plastid size. The cross-section area of the plastids in these regions varied between ~0.5 and ~1 μm2 and was not correlated to the location of the plastids within the shoot apex. We likewise did not detect any correlation between the size and the presence of thylakoid membranes. When present, the membranes were found to occupy a small fraction of the (probed) organelle volume, ranging from 2 to 6%, and no differences were apparent between plastids located in the different regions of the SAM or in (young) primordial leaves. For comparison, the fractional volume occupied by thylakoid membranes in mature chloroplasts is ~60% (Kubínová and Kutík, 2007).
Large-Scale Analysis of Plastids in Thin-Section Micrographs
To complement the EMT analyses, which for practical reasons could not be performed on a large number of cells in the shoot apex, we also performed an extensive analysis of plastids in thin-section micrographs. This analysis encompassed ~300 plastids dispersed throughout the SAM and young leaf primordia. The results are summarized in Supplemental Figure 4 online, which shows the fraction of plastids that contain thylakoid membranes, defined by the presence of one or more stacked lamellae. Of the 15 plastids analyzed in the L2 CZ, only one complied with this criterion. By contrast, over 50% of the plastids located at the L2 periphery had one or more stacked lamellae. For plastids in L1, L3, and the leaf primordia, the percentage was higher, ranging between 75 and 90. Albeit the obvious limitation associated with the analysis of projection images, the above results are fully consistent with the picture derived from the EMT data, namely, that plastids in the L1 and L3 layers of the SAM, as well as at the L2 periphery, possess thylakoid membrane networks, whereas those within the CZ region of L2 are generally devoid of them. This notion is further supported by the results described in the next section.
Chlorophyll and Photosynthetic Proteins in the Shoot Apex
The maturation of thylakoid membranes is contingent on the presence of photosynthetic proteins, of which the most abundant are chlorophyll binding proteins that constitute the core and antenna complexes of the two photosystems. Aside from being functionally sensible, this dependence has a clear structural origin, which relates to the unique lipid composition of the thylakoid membrane. About half of the lipids that constitute this membrane are monogalactosyldiacylglycerol (MGDG) molecules (Block et al., 1983; Kobayashi et al., 2009). On their own, MGDG molecules tend to arrange into inverted hexagonal (HII) phases rather than bilayered membrane structures (Epand, 1998; Lee, 2000). Only in the presence of certain stabilizing proteins do these lipids form planar lamellae. In agreement with this, mixing of MGDG with LHCII, the major antenna complex of PSII and the most abundant protein in thylakoid membranes, at increasing protein/lipid ratios, resulted in the gradual transition of the HII phase into bilayered lamellar structures (Simidjiev et al., 2000). LHCII also plays a decisive role in the formation of grana by promoting membrane appression of neighboring layers (Simidjiev et al., 2000; Nevo et al., 2009; Adam et al., 2011).
To visualize chlorophyll fluorescence in the shoot apex, we examined slices of fresh, unfixed tissue by confocal microscopy. Due to the spiral phyllotactic arrangement of the leaves in Arabidopsis (Kuhlemeier, 2007), leaves of distinct ages are captured within longitudinal sections of different plants (Figure 6; see Supplemental Figure 5 online). The chlorophyll fluorescence images reveal that, as opposed to the current notion, the SAM is not pigment-less. Chlorophyll is found all along the L1 layer and in all layers of the PZ (Figures 6B and 6D; see Supplemental Figure 5B online). Fluorescence emission spectra recorded under the microscope were typical of PSII (see Supplemental Figure 6 online). This is expected as the fluorescence emitted by thylakoid membranes at room temperature originates predominantly from this complex (Govindjee, 1995). Consistent with the EMT data, the only region of the SAM that is essentially devoid of chlorophyll is part of the CZ, which encompasses cells of L2 and, seemingly, a few cells at the topmost layer of L3 (Figure 6B; see Supplemental Figure 5B online). All these cells reside within the stem cell niche, which extends four cell layers down from the tip of the SAM (Yadav et al., 2009; see Discussion). By inference from the cells present in the CZ of L2, the uppermost, CZ-located cells of L3, whose thylakoids were found to be less mature in the EMT analysis, may be the ones that reach the inner spongy mesophyll of the leaf, which is photosynthetic.
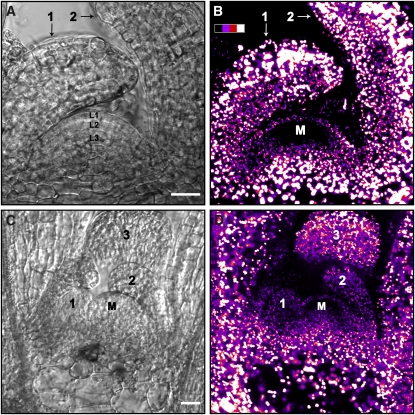
Figure 6.
Chlorophyll Fluorescence in the Shoot Apex.
Transmission ([A] and [C]) and confocal chlorophyll fluorescence ([B] and [D]) images of the vegetative shoot apex. (A) plus (B) and (C) plus (D) are pairs of images at the same magnification. The fluorescence is shown in pseudocolor from black (low) to white (high). The SAM (M) exhibits chlorophyll fluorescence in most of its parts, with the exception of a region that includes cells of L2 and the uppermost layer of L3, within the CZ ([B] and [D]; see Supplemental Figure 5B online). Emission spectra recorded under the microscope reveal that the emitted fluorescence is characteristic of PSII-associated chlorophyll (see Supplemental Figure 6 online). The fluorescence pattern and intensity in very young leaf primordia (leaves 1 and 2 in [D]; see leaf B in Supplemental Figure 5 online) is similar to that of the L1 and (inner) L3 layers of the SAM. In older leaf primordia (leaves 1 and 2 in [B] and leaf 3 in [D]), the fluorescence intensity is significantly higher than that of the SAM. Also note that the intensity is higher in the abaxial (facing away from SAM) side of the leaf (B). Bars = 20 μm.
The youngest leaf primordia observed (leaves 1 and 2 in Figures 6C and 6D) are similar in size and shape (beginning to curve and curved over the SAM dome up to its midpoint, respectively) to the youngest leaf primordia that we analyzed by EMT (Figure 1B). The chlorophyll fluorescence intensity of these young leaves is comparable to that found in the L1 and L3 layers of the SAM (Figure 6D; see also the young leaf primordium in Supplemental Figure 5B online). This is in agreement with the finding that the thylakoid membranes of young leaf primordia are developed to a similar extent as those of the SAM. In the images taken of another sample, older leaf primordia are visible (1 and 2 in Figures 6A and 6B). These leaves, in which the adaxial-abaxial polarity is already evident (polarity is established immediately after separation of the leaf from the SAM surface; Eshed et al., 2004), exhibit a higher chlorophyll fluorescence intensity than the SAM and youngest leaves. This is also true for leaf 3 in Figure 6D, which is seen in transverse section, as it is folded over the SAM. It is also apparent that the fluorescence intensity is higher at the abaxial side (far from the SAM surface) of these primordia (Figure 6B).
As mentioned above, in mature leaves, most epidermal cells (aside from stomatal guard cells; Zeiger et al., 1981) do not contain mature chloroplasts. Nonetheless, we do observe chlorophyll fluorescence in the epidermal layer of primordial leaves. In fact, in very young leaf primordia, there is no difference in the fluorescence pattern between the inner and outer (epidermal) tissues (leaf 1 in Figure 6D and leaf in Supplemental Figure 5B online). As the leaves mature, differences between plastids of the epidermis and those of the inner leaf tissues become apparent, as in the inner leaf tissues the plastids are bigger and exhibit higher chlorophyll fluorescence intensity (see Supplemental Figures 5C and 5D online). Following further maturation, when the stomata are fully formed, chlorophyll fluorescence is observed almost exclusively in their guard cells (right inset in Supplemental Figure 5A online). Thus, the loss of chlorophyll binding proteins and, by inference, of thylakoid membranes along the course of leucoplast differentiation in the epidermis, occurs at relatively late stages of leaf development.
To complement the chlorophyll fluorescence imaging and spectroscopy studies, we also probed the presence of the luminal PSII protein, OEC33 (a subunit of the oxygen-evolving complex), by immunogold labeling. This protein was chosen, following an extensive screen of >20 antibodies against different photosynthetic proteins, because its antibody gave the most sensitive and specific labeling, which was crucial for this study. Representative images of plastids from the SAM and a relatively developed primordial leaf are shown in Figures 7A to 7C. As can be seen, the plastid from L2, in which thylakoid membranes are not visible, is not labeled (Figure 7A). By contrast, labeling is observed on the thylakoid membranes of a plastid from the peripheral L3 region (Figure 7B) and on the stacked membranes of the leaf chloroplast (Figure 7C). In some cases, plastids were sectioned along the plane of the thylakoid sheet, exposing large parts of the thylakoid lumen to the section surface. As the OEC resides in the lumen, labeling of OEC33 is more pronounced in these sections (insets in Figures 7B and 7C).
Figure 7D shows a map generated for the label density of plastids distributed throughout the SAM and adjacent young leaf primordium. Each ellipse represents one plastid and is shown at the coordinates of the cell in which it is found. The ellipses are depicted in grayscale according to their label density, normalized to that of mature leaf chloroplasts present within the same section. The map indicates that the central region of the SAM, encompassing cells of the L2 layer and some of the adjacent L3 cells, contains less OEC33 (and likely, less PSII complexes) than the surrounding regions, including the L1 layer, most of the L3 layer, and the leaf primordium. This pattern mirrors the data derived from the EMT and chlorophyll fluorescence studies, in spite of the high variability associated with this assay, which is imposed by the small number of shoot apical cells present in the section.
DISCUSSION
Using electron tomography, chlorophyll fluorescence, and immunoelectron microscopy analyses, we monitored the proplastid-chloroplast transition in the shoot apex of Arabidopsis. We found that plastids within the SAM are not homogeneous with respect to the extent of maturation of their thylakoid membranes. Plastids in the L1 layer and most of the plastids in the L3 layer of the SAM possess fairly developed thylakoid networks and chlorophyll binding proteins regardless of their position within the layer. The finding that plastids in the L1 layer (which forms the epidermis) have developed thylakoid membranes implies that most of them eventually lose their thylakoids during leaf maturation. By contrast, in the L2 layer, only plastids found at the PZ possess thylakoid networks, whereas those at the CZ lack them. We also found that networks present in young leaf primordia are similar to those observed in the SAM (when present) but become larger and more differentiated in older developing leaves. Our data therefore reveal that plastids in the SAM undergo different developmental processes culminating in the formation of mature thylakoid networks or their complete loss; these processes are not confined to the SAM and young leaf primordia but continue along the course of leaf development.
Comparison with Other Models of Thylakoid Membrane Development
The formation of thylakoid networks has been studied extensively by deetiolation, in which dark-grown plants are subjected to light and their leaves examined at different time points thereafter (reviewed in Vothknecht and Westhoff, 2001; López-Juez and Pyke, 2005; Solymosi and Schoefs, 2010; Adam et al., 2011). During this process, etioplasts, which contain prolamellar bodies (PLBs) and perforated lamellae called prothylakoids, are converted into functional chloroplasts that possess fully mature thylakoid networks. PLBs contain many of the building blocks of the thylakoid membrane, including MGDG, the chlorophyll precursor protochlorophyllide, enzymes involved in chlorophyll and carotenoid biosynthesis, and proteins involved in photosynthetic light reactions (Lonosky et al., 2004; Kleffmann et al., 2007; Blomqvist et al., 2008; Kanervo et al., 2008). This transition is therefore quite different from the one occurring at the shoot apex (from the L2 to the leaf primordia), both in its starting point (PLBs and prothylakoid-containing etioplasts versus thylakoid-less proplastids) and its end point (fully mature versus partially mature networks).
Thylakoid membrane formation was also studied in grass leaves e.g., (Leech et al., 1973; Brangeon and Mustárdy, 1979), in which a linear gradient of thylakoid maturation exists as one moves from the leaf base to its tip. In this model, like in the shoot apex, the proplastid-to-chloroplast transition is monitored. Here, network biogenesis begins with a parental sheet, which splits into several layers. At the next stage, grana emerge at perforation sites within the lamellae. These primordial grana continue to grow and differentiate and finally granum bodies present on different lamellar sheets fuse with each other to form the mature network (Brangeon and Mustárdy, 1979). By contrast, most of the membrane networks in the SAM, when present, appear as miniature versions of the mature, interconnected network and possess all of its basic structural characteristics, albeit in elementary form. Nonetheless, elongated lamellar sheets reminiscent of those seen at the initial stages of thylakoid membrane formation in grasses were observed in some plastids of the SAM (Figures 3B and 3C; see Supplemental Figure 1D online). In all of the developmental models, construction of the mature form of the network necessitates that separate (unconnected) membrane bodies fuse with each other, possibly through overgrown granal domains, as proposed by Brangeon and Mustárdy (1979).
Trafficking of Molecules to the Thylakoids during Network Ontogeny
The buildup of thylakoid membranes requires that lipids, proteins, and pigments be shuttled to and assembled or incorporated at the sites of construction. All the lipids that constitute the thylakoid membranes arrive from the plastid envelope membranes, where the last stages of their biosynthesis take place (reviewed in Benning, 2009). In principle, transport of lipids from the inner envelope to the thylakoids can be performed either by vesicular transport or by direct thylakoid-envelope connections. Such connections and/or inner envelope invaginations and vesicles have been observed in developing and cold-treated leaves (Brangeon and Mustárdy, 1979; Carde et al., 1982; Morré et al., 1991) and in thylakoid biogenesis mutants (e.g., Tanz et al., 2012), providing support for the existence of the two modes of trafficking. In plastids of the SAM, we observed protrusions that emanate from the thylakoids and appear to join them to the envelope membrane. As noted before, such thylakoid-envelope connections have also been observed in mature chloroplasts, but only infrequently (Shimoni et al., 2005). These connections may therefore be a transient means for efficiently transferring lipids, proteins, or pigments during biogenesis, after which they disassemble. However, given that our data do not conclusively demonstrate that the thylakoid membranes are indeed fused to the plastid inner envelope membrane, this point remains speculative. Serendipitously, we also observed vesicular structures near the plastid periphery that may also serve in the trafficking of material from the plastid envelope to the thylakoids.
Development and Loss of Thylakoid Membranes in the Shoot Apex and Leaves
The defined contribution of the different layers of the SAM to the different tissues of the leaf dictates that cells originating from these layers undergo discrete developmental pathways. Plastids within cells developing into specific tissues should differentiate accordingly. It may be expected that the extent of plastid differentiation within progenitor (stem) cells reflect the identity of the tissue they are destined to form. However, we find that, in the SAM, this rationale does not hold (Figure 8). While the L2 layer is the progenitor of the major photosynthetic tissue of the leaf, plastids in its central region lack chlorophyll binding proteins and assembled thylakoids. Notably, this region only partially overlaps the stem cell–containing region of the SAM, which is defined by CLAVATA3 expression and includes central cells of L1, L2, and the two upper layers of L3 (Yadav et al., 2009). Thus, thylakoid networks are found within stem cells of both L1 and L3 (at the fourth cell layer from the SAM tip). They are also present at other regions of L1 and L3 despite the fact that these layers do not significantly contribute to the formation of the photosynthetic leaf tissues and many of the plastids derived from these layers eventually lose their thylakoid membranes.
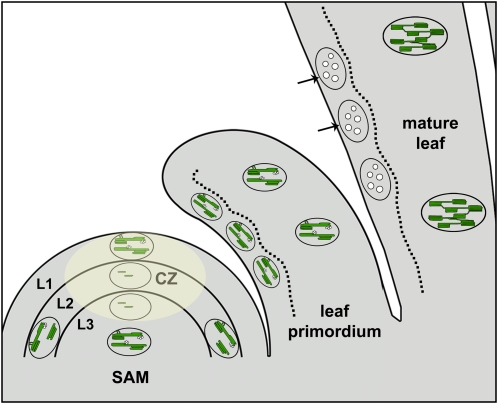
Figure 8.
Thylakoid Membrane Biogenesis and Loss in Arabidopsis.
Plastids in the L1 layer and most of the corpus (L3) of the SAM possess fairly developed thylakoid membranes, although both of these layers contribute relatively little to the formation of the photosynthetic tissues of the leaves. On the other hand, within the CZ, plastids of the L2 layer (and a few cells at the top layer of L3), which gives rise to the primary photosynthetic tissue of the leaf, are true proplastids that lack thylakoid membranes. Upon arrival to the periphery, near the interface with the leaf primordia, these plastids acquire thylakoid networks. In young leaf primordia, thylakoid development is slowed down; networks here resemble the ones found in the SAM. Thylakoid membrane networks exhibiting the typical interconnected granal and stroma lamellar domains become apparent only at later stages, in older primordial leaves (data not shown). The epidermis in mature leaves contains leucoplasts (arrows), aside from its stomatal guard cells, which possess functional chloroplasts (data not shown). Thus, most plastids that originate from the L1 layer of the SAM, which possess developed thylakoid membranes, eventually lose them as the epidermis matures.
The differences between the layers of the SAM (see also Spencer et al., 2005) have to be set during the initial formation or maturation of the SAM. The SAM arises from three distinct cell layers during the torpedo stage of embryogenesis (Barton and Poethig, 1993; Aida et al., 1999). A recent work showed that, at this stage, chloroplast-containing cells are present just below the region of the SAM, but not within cells that form the SAM (Tejos et al., 2010). Thus, the point at which cells of the SAM acquire thylakoid membranes and chlorophyll binding proteins likely takes place sometime between the end of embryogenesis and the establishment of a young seedling. The time frame is likely to be a few days after germination, during which the SAM takes on its mature form (Medford et al., 1992).
The most undeveloped plastids that we observed, genuine proplastids, were present in the CZ of the L2 layer. Upon arrival at a certain position in the PZ, near the interface with the leaf primordia, these proplastids begin to synthesize chlorophyll and assemble thylakoid membranes. This indicates that there exists either a signal(s) responsible for repressing thylakoid formation at the CZ of the L2 layer or one promoting it at the PZ. A great deal is known about the signaling networks that operate within the SAM, especially ones relating to its maintenance and organ formation. These networks are quite complex, involving the activities of many genes and hormones (recently reviewed in Barton, 2010; Ha et al., 2010; Moon and Hake, 2011). However, we currently do not know which signal(s) corresponds to the pattern of thylakoid development within the SAM. Insights into these may be gained from studies of mutants deficient in proteins suspected to play a role in thylakoid membrane formation or of mutants in proteins involved in SAM maintenance and differentiation.
Our results indicate that the processes that govern the biogenesis and differentiation of the thylakoid membranes are not restricted to the SAM but rather continue throughout the course of leaf development. This is also true for the loss of thylakoids in most of the plastids that end up in the leaf epidermis. This layer is generated by the outer SAM layer (L1), which, as shown here, contains plastids with quite developed thylakoid networks. In mature leaves, the epidermal layer, for the most part, contains small, nonphotosynthetic plastids called leucoplasts. Most plastids that originate from L1 should therefore lose their thylakoid membranes. We nevertheless observed chlorophyll fluorescence in cells of the epidermis in leaf primordia at several different developmental stages, indicating that the loss of thylakoids takes place at a later point of leaf maturation. The exception in the epidermis is the stomatal guard cells, which do possess functional chloroplasts. The development of stomata takes place, however, at leaf developmental stages that are later than the ones examined in this work (Larkin et al., 1996; Donnelly et al., 1999).
With its plastids generally considered to lack chlorophyll and thylakoid membranes, the vegetative SAM is believed to be heterotrophic (Fleming, 2006a). However, our data show that plastids in the L1 and L3 layers, as well as at the PZ of L2, possess PSII-associated chlorophyll and thylakoid membranes. As chlorophyll biosynthesis in higher plants is a light-dependent process, it is clear that a sufficient amount of light reaches the SAM; this is in spite of the fact that the leaf primordia are curled over it. In this respect, it has recently been shown that light plays a role in leaf initiation and positioning in the tomato (Solanum lycopersicum) shoot apex (Yoshida et al., 2011). Moreover, as noted in the Results section, construction of the mature, appressed form of the thylakoid membranes is contingent on the presence the PSII major antenna complex, LHCII. It is thus possible that those plastids in the SAM that have thylakoid networks are photosynthetically active and therefore may fulfill the metabolic needs of the SAM, including of the heterotrophic cells found at the CZ of L2 and at the uppermost layer of L3.
METHODS
Plants
Arabidopsis thaliana (ecotype Columbia-0) were grown under short-day conditions (10/14-h light/dark cycles) at 22°C and 80 μmol photons∙m−2∙s−1. For all experiments, seedlings were harvested 2 weeks after sowing, embedded in 7% low-melting-point agarose (gelling temperature ~25°C; Pronadisa), and subsequently sectioned using a vibrating tissue slicer (OTS-4000, EMS; or VT1000S, Leica Microsystems) to isolate the shoot apex region.
Confocal Microscopy and Spectral Imaging
Fresh plant tissue slices (~100 μm thick) were imaged with IX71/81-based Olympus FluoView 500/1000 confocal laser scanning microscopes, using a 0.75–numerical aperture (NA) ×20, oil immersion 1.3-NA ×40, or 1.35-NA ×60 objectives. Fluorescence images were acquired following excitation with a He-Ne laser (λex = 633 nm; FluoView 500) or diode lasers (λex = 442 or 638 nm). Emission was collected using a long-pass filter (>660 nm; FluoView 500) or at 651 to 751 nm (FluoView 1000) using a spectral detector. Differential interference contrast was used for all transmitted light images. Spectral images were recorded (FluoView 1000) at 4 μs/pixel using an emission bandwidth of 4 nm and step size of 3 nm.
Electron Microscopy
Plants were infiltrated with 15% dextran (~40 kD; Fluka) to fill intercellular airspaces prior to embedding in agarose. Vibratome sections (~100 μm thick) containing the shoot apex were then high-pressure frozen, freeze substituted, embedded, sectioned, and poststained as described (Nevo et al., 2007), with the addition of one step in which samples were incubated with propylene oxide for 30 min to aid resin infiltration. Thin sections (60 to 80 nm) were examined using an FEI Tecnai T12 TEM operating at 120 kV. For EMT, semithick sections (150 to 250 nm) were decorated with 10-nm colloidal gold markers on both sides prior to poststaining. Dual-tilt series were acquired with an FEI Tecnai F20, equipped with a field emission gun operating at 200 kV and a Gatan Tridiem energy filter, at bright-field TEM, or at bright-field or dark-field scanning TEM modes. Images were acquired at 1° to 1.5° intervals over the maximal range of ±70°. For TEM mode, a (4k × 4k pixel) TemCam F415 CCD camera (TVIPS) and the SerialEM program for automated tilt series collection (Mastronarde, 2005) were used. For STEM modes, a Fischione high-angle annular dark-field detector or an FEI bright-field STEM detector and the FEI Xplore3D software were used for image acquisition. Tomograms were collected from nine different sections of three independent shoot apex samples. Alignment, 3D reconstruction, and segmentation were performed using the IMOD image-processing package (Kremer et al., 1996) and the Amira software (Visage Imaging).
Immunoelectron Microscopy
Cryo-fixed samples were freeze substituted in dry acetone containing 0.2% uranyl acetate and 0.1% glutaraldehyde as described (Nevo et al., 2007), gradually infiltrated with increasing concentrations of Lowicryl HM-20, and polymerized under UV. Sections (100 nm thick) on formvar-coated nickel mesh grids were blocked (0.5% BSA, 0.2% Gly, and 0.5% gelatin in PBS) for 30 min and subsequently incubated with a rabbit OEC33 antibody (Itzhaki et al., 1998) diluted 1:200 or with blocking solution (as negative control) for 2 h at room temperature. Grids were then were rinsed several times with PBS containing 0.2% Gly, incubated with 10-nm-gold-conjugated goat anti-rabbit for 30 to 45 min, rinsed, and dried. Samples were visualized using an FEI Spirit Tecnai operating at 120 kV, and images were acquired with an FEI 2k × 2k Eagle charge-coupled device camera. Analysis of the labeling data was done using macros written under the Fiji program (National Institutes of Health). For each image, the background label density was subtracted from that of the plastid. The label density of plastids found within the SAM and young leaf primordia were normalized to that of mature leaf chloroplasts found within the same section.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. A Gradient of Thylakoid Membrane Development Exists within the L2 Layer.
Supplemental Figure 2. Membrane Bifurcations and Interlayer Connections in Thylakoid Membranes of the SAM.
Supplemental Figure 3. Thylakoid-Envelope Connections, Inclusions, Vesicles, and Tubules in Plastids of the SAM.
Supplemental Figure 4. Analysis of Plastids of the Shoot Apex in Thin-Section Micrographs.
Supplemental Figure 5. Plastids in the Epidermis of Young Leaf Primordia Contain Chlorophyll Binding Proteins.
Supplemental Figure 6. Fluorescence Emission Spectra of Cells in the Shoot Apical Meristem and Young Leaf Primordia.
Supplemental Figure 7. Tomographic Slices of Plastids Shown (with Overlaid 3D Models) in Figures 2 to 5.
Acknowledgments
We thank Alexander Goldshmidt (Weizmann Institute of Science) for his help with sample sectioning, David Mastronarde (University of Colorado) for his continuous support with the IMOD image-processing package, and Robert Brandt (Visage Imaging) for his assistance with the Amira software. We also thank Naomi Ori and Leor Williams (Hebrew University of Jerusalem) and Ruti Kapon and Onie Tsabari (Weizmann Institute of Science) for stimulating discussions and for critically reading the manuscript. The electron microscopy studies were conducted at the Irving and Cherna Moskowitz Center for Nano and Bio-Nano Imaging at the Weizmann Institute of Science. This work was supported by a grant from the F.I.R.S.T. (Bikura) program of the Israel Science Foundation (No. 1282/09; Z.A. and Z.R.). Z.A. thanks additional support from the Israel Science Foundation (No. 385/08) and the U.S.–Israel Binational Agricultural Research and Development Fund (Grant 4228- 09). Financial support from the Israel Science Foundation (No. 1005/07), from the Dr. Josef Cohn Minerva Center for Biomembrane Research, and from the Carolito Stiftung to Z.R. is acknowledged.
AUTHOR CONTRIBUTIONS
Z.A. and Z.R. share senior authorship for this article. D.C. designed and carried out the experiments. V.K., R.N., and E.S. assisted with the experiments. All authors contributed to data analysis and writing of the article. Z.A. and Z.R. supervised the project.
References
- Adam Z., Charuvi D., Tsabari O., Knopf R.R., Reich Z. (2011). Biogenesis of thylakoid networks in angiosperms: Knowns and unknowns. Plant Mol. Biol. 76: 221–234 [DOI] [PubMed] [Google Scholar]
- Aida M., Ishida T., Tasaka M. (1999). Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: Interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 126: 1563–1570 [DOI] [PubMed] [Google Scholar]
- Albertsson P. (2001). A quantitative model of the domain structure of the photosynthetic membrane. Trends Plant Sci. 6: 349–358 [DOI] [PubMed] [Google Scholar]
- Anderson J.M. (1999). Insights into the consequences of grana stacking of thylakoid membranes in vascular plants: A personal perspective. Aust. J. Plant Physiol. 26: 625–639 [Google Scholar]
- Anderson J.M., Chow W.S., De Las Rivas J. (2008). Dynamic flexibility in the structure and function of photosystem II in higher plant thylakoid membranes: The grana enigma. Photosynth. Res. 98: 575–587 [DOI] [PubMed] [Google Scholar]
- Artus N.N., Ryberg M., Sundqvist C. (1990). Plastid microtubule-like structures in wheat are insensitive to microtubule inhibitors. Physiol. Plant. 79: 641–648 [DOI] [PubMed] [Google Scholar]
- Austin J.R., II, Frost E., Vidi P.A., Kessler F., Staehelin L.A. (2006). Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 18: 1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M.K. (2010). Twenty years on: The inner workings of the shoot apical meristem, a developmental dynamo. Dev. Biol. 341: 95–113 [DOI] [PubMed] [Google Scholar]
- Barton M.K., Poethig R.S. (1993). Formation of the shoot apical meristem in Arabidopsis thaliana: An analysis of development in the wild-type and in the shoot meristemless mutant. Development 119: 823–831 [Google Scholar]
- Benning C. (2009). Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annu. Rev. Cell Dev. Biol. 25: 71–91 [DOI] [PubMed] [Google Scholar]
- Block M.A., Dorne A.J., Joyard J., Douce R. (1983). Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. II. Biochemical characterization. J. Biol. Chem. 258: 13281–13286 [PubMed] [Google Scholar]
- Blomqvist L.A., Ryberg M., Sundqvist C. (2008). Proteomic analysis of highly purified prolamellar bodies reveals their significance in chloroplast development. Photosynth. Res. 96: 37–50 [DOI] [PubMed] [Google Scholar]
- Brangeon J., Mustárdy L. (1979). The ontogenic assembly of intra-chloroplast lamallae viewed in 3-dimensions. Biol. Cell. 36: 71–80 [Google Scholar]
- Bréhélin C., Kessler F. (2008). The plastoglobule: A bag full of lipid biochemistry tricks. Photochem. Photobiol. 84: 1388–1394 [DOI] [PubMed] [Google Scholar]
- Bréhélin C., Kessler F., van Wijk K.J. (2007). Plastoglobules: Versatile lipoprotein particles in plastids. Trends Plant Sci. 12: 260–266 [DOI] [PubMed] [Google Scholar]
- Carde J.P., Joyard J., Douce R. (1982). Electron microscopic studies of envelope membranes from spinach plastids. Biol. Cell 44: 315–324 [Google Scholar]
- Carland F.M., McHale N.A. (1996). LOP1: A gene involved in auxin transport and vascular patterning in Arabidopsis. Development 122: 1811–1819 [DOI] [PubMed] [Google Scholar]
- Cheniclet C., Carde J.P. (1988). Differentiation of leucoplasts: Comparative transition of proplastids to chloroplasts or leucoplasts in trichomes of Stachys lanata leaves. Protoplasma 143: 74–83 [Google Scholar]
- Chow W.S., Kim E.H., Horton P., Anderson J.M. (2005). Granal stacking of thylakoid membranes in higher plant chloroplasts: The physicochemical forces at work and the functional consequences that ensue. Photochem. Photobiol. Sci. 4: 1081–1090 [DOI] [PubMed] [Google Scholar]
- Daum B., Nicastro D., Austin J., II, McIntosh J.R., Kühlbrandt W. (2010). Arrangement of photosystem II and ATP synthase in chloroplast membranes of spinach and pea. Plant Cell 22: 1299–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly P.M., Bonetta D., Tsukaya H., Dengler R.E., Dengler N.G. (1999). Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev. Biol. 215: 407–419 [DOI] [PubMed] [Google Scholar]
- Epand R.M. (1998). Lipid polymorphism and protein-lipid interactions. Biochim. Biophys. Acta 1376: 353–368 [DOI] [PubMed] [Google Scholar]
- Eshed Y., Izhaki A., Baum S.F., Floyd S.K., Bowman J.L. (2004). Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development 131: 2997–3006 [DOI] [PubMed] [Google Scholar]
- Fleming A. (2006a). Metabolic aspects of organogenesis in the shoot apical meristem. J. Exp. Bot. 57: 1863–1870 [DOI] [PubMed] [Google Scholar]
- Fleming A.J. (2006b). The co-ordination of cell division, differentiation and morphogenesis in the shoot apical meristem: A perspective. J. Exp. Bot. 57: 25–32 [DOI] [PubMed] [Google Scholar]
- Furner I.J., Pumfrey J.E. (1992). Cell fate in the shoot apical meristem of Arabidopsis thaliana. Development 115: 755–764 [Google Scholar]
- Govindjee (1995). Sixty-three years since Kautsky: Chlorophyll a fluorescence. Aust. J. Plant Physiol. 22: 131–160 [Google Scholar]
- Ha C.M., Jun J.H., Fletcher J.C. (2010). Shoot apical meristem form and function. Curr. Top. Dev. Biol. 91: 103–140 [DOI] [PubMed] [Google Scholar]
- Irish V.F., Sussex I.M. (1992). A fate map of the Arabidopsis embryonic shoot apical meristem. Development 115: 745–753 [Google Scholar]
- Itzhaki H., Naveh L., Lindahl M., Cook M., Adam Z. (1998). Identification and characterization of DegP, a serine protease associated with the luminal side of the thylakoid membrane. J. Biol. Chem. 273: 7094–7098 [DOI] [PubMed] [Google Scholar]
- Kanervo E., Singh M., Suorsa M., Paakkarinen V., Aro E., Battchikova N., Aro E.M. (2008). Expression of protein complexes and individual proteins upon transition of etioplasts to chloroplasts in pea (Pisum sativum). Plant Cell Physiol. 49: 396–410 [DOI] [PubMed] [Google Scholar]
- Kirchhoff H., Hall C., Wood M., Herbstová M., Tsabari O., Nevo R., Charuvi D., Shimoni E., Reich Z. (2011). Dynamic control of protein diffusion within the granal thylakoid lumen. Proc. Natl. Acad. Sci. USA 108: 20248–20253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleffmann T., von Zychlinski A., Russenberger D., Hirsch-Hoffmann M., Gehrig P., Gruissem W., Baginsky S. (2007). Proteome dynamics during plastid differentiation in rice. Plant Physiol. 143: 912–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Nakamura Y., Ohta H. (2009). Type A and type B monogalactosyldiacylglycerol synthases are spatially and functionally separated in the plastids of higher plants. Plant Physiol. Biochem. 47: 518–525 [DOI] [PubMed] [Google Scholar]
- Kremer J.R., Mastronarde D.N., McIntosh J.R. (1996). Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116: 71–76 [DOI] [PubMed] [Google Scholar]
- Kubínová L., Kutík J. (2007). Surface density and volume density measurements of chloroplast thylakoids in maize (Zea mays L.) under chilling conditions. Photosynthetica 45: 481–488 [Google Scholar]
- Kuhlemeier C. (2007). Phyllotaxis. Trends Plant Sci. 12: 143–150 [DOI] [PubMed] [Google Scholar]
- Kutík J. (1992). Microtubule-like structures in developing wheat-leaf chloroplasts. Biol. Plantarum 34: 355–357 [Google Scholar]
- Larkin J.C., Young N., Prigge M., Marks M.D. (1996). The control of trichome spacing and number in Arabidopsis. Development 122: 997–1005 [DOI] [PubMed] [Google Scholar]
- Lawrence M.E., Possingham J.V. (1984). Observations of microtubule-like structures within spinach plastids. Biol. Cell 52: 77–82 [Google Scholar]
- Lee A.G. (2000). Membrane lipids: It’s only a phase. Curr. Biol. 10: R377–R380 [DOI] [PubMed] [Google Scholar]
- Leech R.M., Rumsby M.G., Thomson W.W. (1973). Plastid differentiation, acyl lipid, and fatty acid changes in developing green maize leaves. Plant Physiol. 52: 240–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonosky P.M., Zhang X., Honavar V.G., Dobbs D.L., Fu A., Rodermel S.R. (2004). A proteomic analysis of maize chloroplast biogenesis. Plant Physiol. 134: 560–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Juez E., Pyke K.A. (2005). Plastids unleashed: Their development and their integration in plant development. Int. J. Dev. Biol. 49: 557–577 [DOI] [PubMed] [Google Scholar]
- Mastronarde D.N. (2005). Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152: 36–51 [DOI] [PubMed] [Google Scholar]
- Medford J.I., Behringer F.J., Callos J.D., Feldmann K.A. (1992). Normal and abnormal development in the Arabidopsis vegetative shoot apex. Plant Cell 4: 631–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J., Hake S. (2011). How a leaf gets its shape. Curr. Opin. Plant Biol. 14: 24–30 [DOI] [PubMed] [Google Scholar]
- Morré D.J., Selldén G., Sundqvist C., Sandelius A.S. (1991). Stromal low temperature compartment derived from the inner membrane of the chloroplast envelope. Plant Physiol. 97: 1558–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux C.W. (2005). Function and evolution of grana. Trends Plant Sci. 10: 521–525 [DOI] [PubMed] [Google Scholar]
- Mustárdy L. (1996). Development of thylakoid membrane stacking. In Oxygenic Photosynthesis: The Light Reactions, Ort D.R., (Dordrecht, Boston, London: Kluwer Academic Publishers; ), pp. 59–68 [Google Scholar]
- Mustárdy L., Garab G. (2003). Granum revisited. A three-dimensional model—where things fall into place. Trends Plant Sci. 8: 117–122 [DOI] [PubMed] [Google Scholar]
- Nevo R., Charuvi D., Chuartzman S.G., Shimoni E., Tsabari O., Reich Z. (2009). Architecture and plasticity of thylakoid membrane networks. In Lipids in Photosynthesis, Wada H., Murata N., (The Netherlands, Springer-Verlag; ), pp. 295–328 [Google Scholar]
- Nevo R., Charuvi D., Shimoni E., Schwarz R., Kaplan A., Ohad I., Reich Z. (2007). Thylakoid membrane perforations and connectivity enable intracellular traffic in cyanobacteria. EMBO J. 26: 1467–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett-Heaps J.D. (1968). Microtubule-like structures in the growing plastids or chloroplasts of two algae. Planta 81: 193–200 [DOI] [PubMed] [Google Scholar]
- Sakamoto W., Miyagishima S., Jarvis P. (2008). Chloroplast biogenesis: Control of plastid development, protein import, division and inheritance. The Arabidopsis Book 6: e0110, doi/10.1199/tab.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni E., Rav-Hon O., Ohad I., Brumfeld V., Reich Z. (2005). Three-dimensional organization of higher-plant chloroplast thylakoid membranes revealed by electron tomography. Plant Cell 17: 2580–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simidjiev I., Stoylova S., Amenitsch H., Javorfi T., Mustardy L., Laggner P., Holzenburg A., Garab G. (2000). Self-assembly of large, ordered lamellae from non-bilayer lipids and integral membrane proteins in vitro. Proc. Natl. Acad. Sci. USA 97: 1473–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solymosi K., Schoefs B. (2010). Etioplast and etio-chloroplast formation under natural conditions: The dark side of chlorophyll biosynthesis in angiosperms. Photosynth. Res. 105: 143–166 [DOI] [PubMed] [Google Scholar]
- Spencer D., White R.G., Wildman S.G. (2005). Distribution of chlorophyll-bearing organelles in the shoot apex of a range of dicotyledonous plants. Protoplasma 225: 185–190 [DOI] [PubMed] [Google Scholar]
- Steeves T.A., Sussex I.M. (1989). Patterns in Plant Development. (Cambridge, UK: Cambridge University Press; ). [Google Scholar]
- Tanz S.K., Kilian J., Johnsson C., Apel K., Small I., Harter K., Wanke D., Pogson B., Albrecht V. (2012). The SCO2 protein disulphide isomerase is required for thylakoid biogenesis and interacts with LCHB1 chlorophyll a/b binding proteins which affects chlorophyll biosynthesis in Arabidopsis seedlings. Plant J. 69: 743–754 [DOI] [PubMed] [Google Scholar]
- Tejos R.I., Mercado A.V., Meisel L.A. (2010). Analysis of chlorophyll fluorescence reveals stage specific patterns of chloroplast-containing cells during Arabidopsis embryogenesis. Biol. Res. 43: 99–111 [PubMed] [Google Scholar]
- Telfer A., Poethig R.S. (1994). Leaf development in Arabidopsis. In Arabidopsis, Meyerowitz E.M., Somerville C.R., (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; ), pp. 379–401 [Google Scholar]
- Tilney-Bassett R.A. (1986). Plant Chimeras. (London: Edward Arnold; ). [Google Scholar]
- Vothknecht U.C., Westhoff P. (2001). Biogenesis and origin of thylakoid membranes. Biochim. Biophys. Acta 1541: 91–101 [DOI] [PubMed] [Google Scholar]
- Yadav R.K., Girke T., Pasala S., Xie M., Reddy G.V. (2009). Gene expression map of the Arabidopsis shoot apical meristem stem cell niche. Proc. Natl. Acad. Sci. USA 106: 4941–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Mandel T., Kuhlemeier C. (2011). Stem cell activation by light guides plant organogenesis. Genes Dev. 25: 1439–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechmann B., Müller M., Zellnig G. (2005). Effects of different fixation and freeze substitution methods on the ultrastructural preservation of ZYMV-infected Cucurbita pepo (L.) leaves. J. Electron Microsc. (Tokyo) 54: 393–402 [DOI] [PubMed] [Google Scholar]
- Zeiger E., Armond P., Melis A. (1981). Fluorescence properties of guard cell chloroplasts: Evidence for linear electron transport and light-harvesting pigments of photosystems I and II. Plant Physiol. 67: 17–20 [DOI] [PMC free article] [PubMed] [Google Scholar]