Intracellular Na+,K+/H+ antiporters (NHXs) play central roles in maintaining ion homeostasis and pH control. Tonoplast-localized proteins NHX1 and NHX2 are critical for active K+ uptake into the vacuole, a process that is required to create osmotic potential for cell expansion and turgor regulation. These proteins are abundantly expressed in guard cells, where they contribute to stomata function.
Abstract
Intracellular NHX proteins are Na+,K+/H+ antiporters involved in K+ homeostasis, endosomal pH regulation, and salt tolerance. Proteins NHX1 and NHX2 are the two major tonoplast-localized NHX isoforms. Here, we show that NHX1 and NHX2 have similar expression patterns and identical biochemical activity, and together they account for a significant amount of the Na+,K+/H+ antiport activity in tonoplast vesicles. Reverse genetics showed functional redundancy of NHX1 and NHX2 genes. Growth of the double mutant nhx1 nhx2 was severely impaired, and plants were extremely sensitive to external K+. By contrast, nhx1 nhx2 mutants showed similar sensitivity to salinity stress and even greater rates of Na+ sequestration than the wild type. Double mutants had reduced ability to create the vacuolar K+ pool, which in turn provoked greater K+ retention in the cytosol, impaired osmoregulation, and compromised turgor generation for cell expansion. Genes NHX1 and NHX2 were highly expressed in guard cells, and stomatal function was defective in mutant plants, further compromising their ability to regulate water relations. Together, these results show that tonoplast-localized NHX proteins are essential for active K+ uptake at the tonoplast, for turgor regulation, and for stomatal function.
INTRODUCTION
Potassium (K+) is an essential macronutrient that fulfills important functions related to enzyme activation, osmotic adjustment and turgor generation, regulation of membrane electric potential, and cytoplasmic pH homeostasis. K+ is acquired by roots, redistributed among plant tissues and organs, and stored in large quantities inside vacuoles, and it is the most abundant inorganic cation in plants, comprising up to 10% of their dry weight (White and Karley, 2010). Most terrestrial plants are able to grow in a wide range of external K+ concentrations, from low micromolar to 10 to 20 mM levels (Rodríguez-Navarro, 2000). K+ uptake by plant roots is thought to be facilitated by two independent transport mechanisms with distinct kinetic parameters and selectivity (Epstein et al., 1963). The high-affinity, K+ selective, and saturable System 1 operates in the micromolar range and moves K+ into the cytosol of root cells against the electrochemical gradient. Electrophysiological evidence indicates that this pathway involves a H+:K+ symporter coupled to the activity of the plasma membrane H+-ATPase and is capable of driving K+ accumulation ratios in excess of 106-fold (Maathuis and Sanders, 1994). Molecular genetic approaches have implicated high-affinity K+ uptake permease (HAK/KUP) transporters in this process (Santa-María et al., 1997; Gierth et al., 2005; Rodríguez-Navarro and Rubio, 2006). The low-affinity pathway, or System 2, has the characteristics of channel-mediated transport and dominates K+ uptake at external K+ concentrations of above 0.5 to 1 mM and usual plasma membrane potentials of −120 to −200 mV. Channels of the Shaker family have been implicated in this passive K+ permeation through the plasma membrane downhill the K+ electrochemical gradient. In Arabidopsis thaliana, the voltage-gated K+-selective channel protein K+ Transporter1 (AKT1) has been shown to participate in K+ uptake by roots (Hirsch et al., 1998; Spalding et al., 1999; Xu et al., 2006). Stelar K+ Outward Rectifier is structurally similar to AKT1 but mediates K+ efflux in root stellar cells to facilitate K+ loading into the xylem (Gaymard et al., 1998). In Arabidopsis guard cells, where massive K+ fluxes mediate stomatal movements, stomatal opening driven by K+ entry occurs mainly through K+ channel in Arabidopsis thaliana 1 (KAT1) and KAT2, whereas stomatal closing is caused by K+ efflux through Gated Outwardly Rectifying K+, a channel activated by membrane depolarization (Ache et al., 2000; Kwak et al., 2001; Hosy et al., 2003; Lebaudy et al., 2010).
Compared with the plasma membrane, much less is known regarding K+ transport processes at the vacuole, although insights into the transport mechanisms and proteins involved in K+ fluxes across the tonoplast are now emerging. Two different proton pumps energize the tonoplast: the V-ATPase that is powered by ATP and the tonoplast-bound pyrophosphatase that hydrolyzes inorganic pyrophosphate (V-PPase). In most species and cell types, both H+ pumps generate pH gradients of 1 to 2 pH units (acidic inside) and an electrical charge (membrane potential) of 20 to 40 mV that is positive in the vacuolar lumen relative to the cytosol. This fact implies that positively charged K+ ions are excluded from the vacuole in K+-replete cells unless transport is coupled to an energy-dependent uptake mechanism, whereas efflux could be driven by channels that permeate K+ downhill its electrochemical gradient. Electrophysiological and genetic evidence has shown that upon a decrease in cytoplasmic K+, slow-activating vacuolar (SV) channels release K+ from vacuoles to assist in the homeostatic partition of K+ between the cytoplasmic and vacuolar pools (reviewed in Isayenkov et al., 2010; Hedrich and Marten, 2011). Several HAK/KUP transporters (Arabidopsis KUP4, KUP5, and KUP7) have also been found in the tonoplast, and they might catalyze K+ efflux from the vacuole below the electrochemical limits of passive permeation by channels; however, functional evidence is still lacking (Bañuelos et al., 2002; Jaquinod et al., 2007).
Since transport of K+ into the vacuole in K+-replete cells proceeds against the K+ electrochemical gradient, a K+/H+ antiporter energized by the pH gradient across the tonoplast was thought to achieve vacuolar K+ accumulation (Walker et al., 1996; Carden et al., 2003). Scattered evidence indicated that vacuolar Na+,K+/H+ antiporter (NHX)–type exchangers could serve this critical function in plant cells (Venema et al., 2002; Rodríguez-Rosales et al., 2008, 2009; Yoshida et al., 2009; Jiang et al., 2010; Leidi et al., 2010). Studies in transgenic tomato (Solanum lycopersicum) showed that overexpression of the vacuolar At NHX1 or the endosomal Le NHX2 Na+,K+/H+ exchangers led to greater K+ accumulation (Rodríguez-Rosales et al., 2008; Leidi et al., 2010). Recently, a reverse genetics approach in Arabidopsis has shown that NHX1 and NHX2 proteins sustain the intravacuolar K+ concentration and, in the process, regulate the vacuolar pH and facilitate cell expansion (Bassil et al., 2011b).
Because vacuoles occupy most of the intracellular volume in many plant cells and are the main cellular reservoir for K+, changes in tissue K+ concentration are largely a reflection of the dynamics of the vacuolar pool. By contrast, cytosolic K+ concentrations are tightly regulated through the integrated regulation of K+ uptake and efflux at the plasma membrane and K+ import and export at the tonoplast (Leigh, 2001; White and Karley, 2010). Cytosolic K+ concentration is thought to decline only when the vacuolar K+ reserve has been depleted below the thermodynamical equilibrium with the cytosolic pool (Walker et al., 1996). Conversely, surplus K+ is placed into the vacuole to maintain cytosolic K+ within narrow limits independently of K+ abundance in the growth medium. In K+-sufficient plants, the vacuolar pool plays a chief biophysical function, the lowering of osmotic potential to generate turgor and drive cell expansion. Rapid cell expansion relies on high mobility of the active osmoticum, and the highly permeant and abundant inorganic ion K+ fits this role. There are numerous examples of K+ fluxes driving cell expansion and organ movements (White and Karley, 2010), but nowhere is this critical function of the vacuolar K+ pool more apparent than in guard cells. The rapid accumulation and loss of K+ and anionic organic acids by guard cells, mostly in the vacuolar compartment, regulates the opening and closing of stomata and, thereby, gas exchange and transpiration. Stomatal opening starts with membrane hyperpolarization caused by H+-ATPases, which induces K+ uptake through inward-rectifying K+in channels (reviewed in Kim et al., 2010). Influx of K+, Cl−and NO3−the synthesis of malate increase turgor in the guard cell and induce stomatal opening. The rapid loss of K+ from guard cells during stomatal closure is started by anion release via anion channels, which causes plasma membrane depolarization and the opening of K+ channels that facilitate K+ efflux from the vacuole and across the plasma membrane. Among the solutes released from guard cells, >90% originate from vacuoles.
Here, we report that the two major isoforms of vacuolar-localized NHX proteins in Arabidopsis, NHX1 and NHX2, play a critical and redundant role in the accrual of K+ in the vacuole, which in turn impinges on the ability of plants to generate turgor and sustain osmotic regulation. Furthermore, we show that NHX1 and NHX2 genes are greatly expressed in guard cells of stomata, where they contribute to the regulation of stomatal function and transpiration.
RESULTS
Biochemical Similarities of NHX1 and NHX2 Exchangers
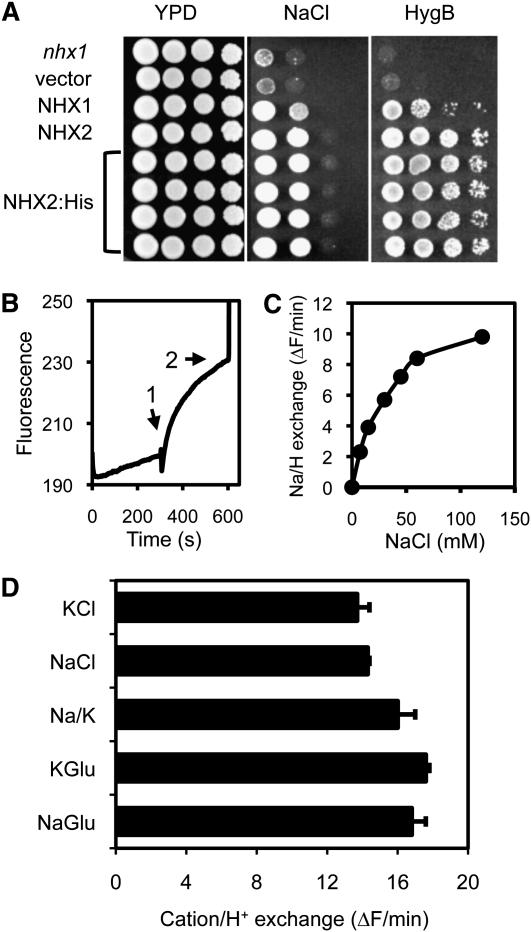
Six genes (NHX1 to NHX6) encoding NHX exchangers have been identified in the genomic sequence of Arabidopsis (Yokoi et al., 2002). RNA gel blotting and RT-PCR, used to determine the relative abundance of the NHX transcripts, showed that NHX1 and NHX2 transcripts were both abundant and widely distributed in plant tissues and that they accumulated in response to the same effectors (NaCl, KCl, LiCl, osmotic stress, and abscisic acid) (Yokoi et al., 2002; Aharon et al., 2003). At the protein level, NHX1 and NHX2 are the most closely related members of the NHX family in Arabidopsis (87.5% sequence identity) and are both localized in the tonoplast (Yokoi et al., 2002; Bassil et al., 2011b). NHX1 has been shown to mediate Na+/H+ and K+/H+ exchange with similar affinity (Venema et al., 2002; Yamaguchi et al., 2005; Hernández et al., 2009). To test whether NHX2 had similar transport properties as NHX1, NHX2 tagged with a hexa-His tag at the C terminus was expressed in budding yeast (Saccharomyces cerevisiae) cells and purified by Ni2+ affinity chromatography as previously described for NHX1 (Venema et al., 2002). Phenotypic complementation of a mutant nhx1 yeast strain showed that tagged NHX2:H6 was functional and indistinguishable from the untagged, wild-type protein (Figure 1A). The NHX2:H6 protein was purified and inserted in soybean (Glycine max) phospholipid vesicles formed in reconstitution buffer containing the pH indicator pyranine and (NH4)2SO4. Dilution of the proteoliposomes in reconstitution buffer without (NH4)2SO4 produced an instantaneous diminution of pyranine fluorescence due to leakage of NH3 and the subsequent internal acidification of the vesicles (Venema et al., 2002). Cation/proton exchange was initiated upon the addition of NaCl or KCl salts, and the rate of pH variation inside the vesicles was estimated from the change in pyranine fluorescence (Figure 1B). NaCl and KCl salts produced similar rates of fluorescence recovery. Replacing chloride salts with gluconate, a relatively impermeable anion, produced similar fluorescence recovery rates, demonstrating that cations were exchanged for intravesicular protons (Figure 1D). The small increase in net exchange with gluconate salts relative to chloride salts indicated that only a small fraction of protons that were transported out of the vesicles in the exchange reaction leaked back into the vesicles together with chloride anions. Importantly, mixing equal amounts of sodium and K+ gluconate salts produced the same exchange rate as either salt alone, demonstrating that NHX2 lacked significant discrimination between Na+ and K+. Exchange reactions catalyzed by NHX2 displayed saturation kinetics (Figure 1C) and the Km estimated for Na+ and K+ ions was ~35 mM (R2 = 0.999). Similar results have been obtained with NHX1 (Venema et al., 2002; Hernández et al., 2009; Leidi et al., 2010). Therefore, NHX1 and NHX2 appear to be biochemically equivalent Na+,K+/H+ exchangers.
Figure 1.
Biochemical Activity of NHX2.
(A) Complementation of the yeast nhx1 mutant (top), transformed with an empty vector, or to express NHX1, NHX2, or His-tagged NHX2 (four independent transformants), in Arg phosphate medium supplemented with 70 mM NaCl and yeast peptone dextrose (YPD) medium with 30 μg/mL hygromycin B (HygB).
(B) Typical exchange reaction in proteoliposomes. Arrows indicate the addition of substrate salts NaCl or KCl (1). SO4(NH4)2 was added to collapse the proton gradient and end the assay (2). Pyranine fluorescence at 510 nm is given in arbitrary units.
(C) Saturation kinetics of exchange rates catalyzed by NHX2 as a function of substrate (NaCl) concentration.
(D) Exchange rates using as substrate 45 mM sodium (NaGlu) or K+ (KGlu) gluconate salts, 22.5 mM each of these salts (Na/K), and 45 mM NaCl or KCl. Shown is the mean exchange rates of two independent assays.
Expression Pattern of NHX2
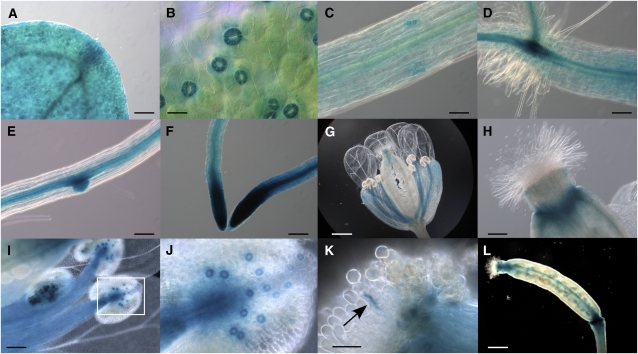
We next compared the gene expression pattern of NHX2 to that of NHX1. The latter gene has been shown to be expressed in nearly all tissues throughout plant development (Shi and Zhu, 2002; Apse et al., 2003). To determine the tissue expression pattern of NHX2, an ~3.1-kb promoter region upstream of the NHX2 start codon was fused with the β-glucuronidase (GUS) reporter gene, and the resulting construct, ProNHX2:GUS, was transformed into wild-type Arabidopsis plants. Three independent transgenic lines were assayed for GUS expression and they produced consistent patterns. GUS expression was detected at all developmental stages tested, from seed germination to flowering and seed setting. As depicted in Figure 2, histochemical GUS staining was detected, with varying intensity, in most tissues of Arabidopsis seedlings. In leaves and hypocotyls, GUS staining was stronger around the vasculature, in meristems, and in guard cells of the stomata (Figures 2A to 2D). In roots, the strongest GUS activity was detected in the vasculature, at the root tip, and at the points of emergence of secondary roots, but not in root hairs and the epidermis (Figures 2D to 2F). In flowers, GUS staining was restricted to the filament of the stamens, the ovarian stigma, mature pollen grains within the anthers, and the pollen tube (Figures 2G to 2K). Much weaker staining was seen in sepals, and it was associated with vascular tissues. No significant GUS activity was detected in petals (Figure 2G). Remarkably, the strongest GUS staining in flowers was also observed in stomata, including those in the anthers (Figure 2J). In immature siliques, GUS staining was restricted to the septum and to the silique tip and base (Figure 2L). This expression pattern strongly resembled that of NHX1 (Shi and Zhu, 2002), except at the tip of main roots, root hairs, and meristems, in which NHX1 and NHX2 promoter constructs showed opposite expression levels. Public microarray databases also indicate similar expression patterns of NHX1 and NHX2 genes and the significant greater abundance of their transcripts in guard cells of Arabidopsis (Winter et al., 2007). However, the various divergences regarding their expression patterns imply extensive overlapping, but not identical physiological functions of NHX1 and NHX2. The tissue expression pattern of NHX2 was also examined in seedlings after sodium, lithium, and sorbitol treatments that were reported to induce the accumulation of NHX2 mRNA (Yokoi et al., 2002), but no significant deviation from the pattern found under control conditions was observed, suggesting that stress environments upregulated expression without altering tissue specificity.
Figure 2.
NHX2 Promoter-GUS Expression Pattern in Transgenic Arabidopsis Plants.
(A) GUS activity detected in cotyledons.
(B) Strong GUS staining in guard cells of stomata in mature leaves.
(C) to (E) Preferential expression in the vasculature of the main stem (C) and roots (E). Note the strong expression in the root-shoot transition (D) and the point of emergence of secondary roots (E).
(F) Strong GUS staining in the root meristems.
(G) to (L) Expression in reproductive organs was greater in filaments of the stamen ([G] and [I]), stigma ([G] and [H]), mature grain pollen (I), and silique septum (L). Note the high expression in the stomata of anthers in (J), which is a close-up image of (I), and in the elongating pollen tube (marked by arrow) (K).
Bars = 20 μm in (K), 50 μm in (B), 100 μm in (A), (C), to (F), and (I), and 400 μm in (G) and (L).
Functional Redundancy of NHX1 and NHX2
Previous work showed that NHX1 and NHX2 are functionally redundant and that nhx1 and nhx2 single mutants have moderate disturbances in the germination rate, biomass production, and foliar area compared with wild-type plants (Apse et al., 2003; Bassil et al., 2011b). This is in agreement with the extensive overlapping expression pattern of NHX1 and NHX2 (Figure 2) (Shi and Zhu, 2002), identical subcellular localization of the proteins (Yokoi et al., 2002; Bassil et al., 2011b), and similar transport activity (Figure 1) (Venema et al., 2002). To investigate further the physiological role of vacuolar NHX proteins, we sought to produce homozygous null double mutants with alleles nhx1-2 and nhx2-1 carrying mutations within the coding regions of NHX1 and NHX2, respectively (Bassil et al., 2011b) (see Supplemental Figure 1A online). However, early attempts to produce double knockout plants with nhx1-2 nhx2-1 null alleles were unsuccessful. To circumvent this problem, we used another allele, nhx1-1, that carries the T-DNA insertion in the 5′-untranslated region of NHX1 (at nucleotide −114; see Supplemental Figure 1A online). Contrary to mutant nhx1-2, which failed to produce detectable levels of gene transcripts as determined by RNA gel blot and RT-PCR assays, mutant nhx1-1 showed a low, yet detectable, amount of NHX1 transcript, which accumulated slightly under salt stress (see Supplemental Figure 1B online). Mutant nhx2-1 produced a 5′-truncated mRNA that accumulated at greater levels than in wild-type plants (see Supplemental Figure 1C online).
Homozygous lines of genotype nhx1-1 (leaky allele) and nhx2-1 were crossed, and homozygous double mutants were identified by diagnostic PCR amplification of NHX1 and NHX2. Only two homozygous nhx1-1 nhx2-1 plants were found in the F2 population (n = 72), and these plants were severely stunted and failed to produce seeds. To increase the chances of isolating a larger number of double homozygous nhx1-1 nhx2-1 mutants, an F2 plant of genotype nhx1-1/nhx1-1 NHX2/nhx2-1 was self-pollinated, and homozygous mutants nhx1-1 nhx2-1 were recovered among the F3 progeny, albeit with a frequency lower than expected (7.7% instead of 25%, two out of 26 plants genotyped by diagnostic PCR), further indicating that not only the vegetative growth of double nhx1-1 nhx2-1 mutants was compromised, but also their viability. Two lines of genotype nhx1-1 nhx2-1 that produced seeds, L2 and L14, were selected for further study. Note that lines L2 and L14 share the same genotype and that they should be regarded as biological replicates. The absence of full-length NHX2 transcripts and the residual levels of NHX1 mRNA in these lines were confirmed by RT-PCR.
A modified Long Ashton mineral nutrient solution with 1 mM K+ and nominally free of Na+ and NH4+ (LAK medium) was designed to test the effects of K+ and Na+ on the growth of nhx1 nhx2 mutants. Avoidance of the high concentration of NH4+ in the Murashige and Skoog medium routinely used for Arabidopsis growth was important to prevent inhibition of K+ uptake at low external K+ concentrations (Spalding et al., 1999; Rubio et al., 2008). As recently described by Bassil et al. (2011b), plants of the nhx1-1 nhx2-1 genotype were smaller than the wild-type and single mutant plants when grown in soil under standard growth conditions and in hydroponic culture in LAK medium (see Supplemental Figure 2 online). Importantly, nhx1-1 nhx2-1 plants were prone to wilting under our regular growth conditions (day/night settings: 25/20 ± 2°C, 40/60% relative humidity, 14 h light), suggesting that failure to obtain double knockout nhx1-2 nhx2-1 plants might have been due to severely damaged water relations in the complete absence of NHX1 and NHX2 proteins. The strong expression of ProNHX2:GUS (Figure 2) and ProNHX1:GUS (Shi and Zhu, 2002) promoter fusions in guard cells lent further support to this hypothesis. Therefore, double mutant plants carrying the null alleles nhx1-2 and nhx2-1 were produced by self-pollination of a plant of genotype nhx1-2/nhx1-2 NHX2/nhx2-1 and homozygous nhx1-2 nhx2-1 plants were recovered under high relative humidity (above 60%). In these conditions, homozygous mutants nhx1-2 nhx2-1 were obtained with one-quarter frequency among the progeny (16 out of 44 plants, 3:1 segregation ratio with P < 0.05, χ2 test). Knockout plants displayed an extremely stunted growth, were highly sensitive to humidity fluctuations, and produced small siliques with few viable seeds (Figure 3). Because leaky mutants of genotype nhx1-1 nhx2-1 had an array of phenotypic disorders similar to but less extreme than those of the complete knockouts nhx1-2 nhx2-1, and they were easier to handle and propagate, subsequent experiments were done with the leaky lines L2 and L14 (nhx1-1 nhx2-1 genotype) except when indicated otherwise.
Figure 3.
Impaired Development of Double Null nhx1 nhx2 Mutant.
Depicted are inflorescences and rosettes of double knockout (KO) mutant plants of genotype nhx1-2 nhx2-1 and of wild-type Col-0 plants grown in hydroponic LAK medium. Note the diminutive siliques in the mutant plant.
Reduced NHX Activity in the nhx1 nhx2 Double Mutant
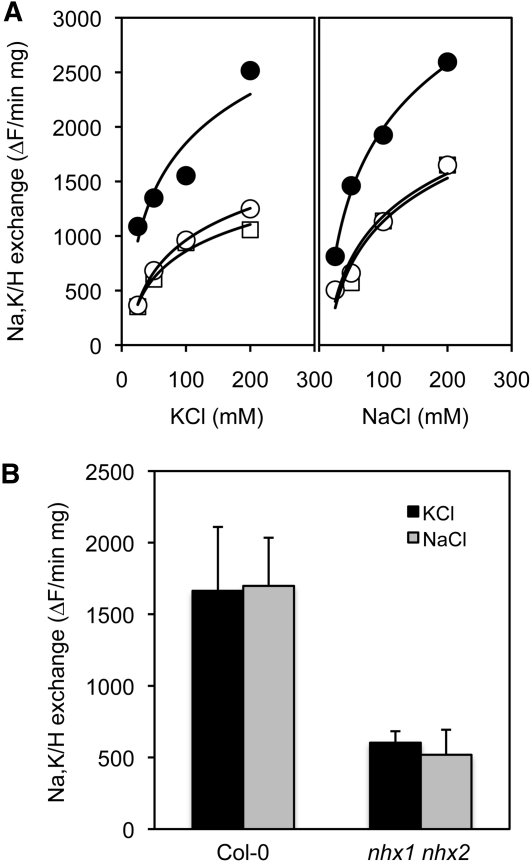
To quantitate the accumulated contribution of NHX1 and NHX2 proteins to Na+,K+/H+ exchange in the vacuole, K+/H+ and Na+/H+ antiporter activity was assayed in tonoplast vesicles from the leaves of wild-type and nhx1-1 nhx2-1 plants (lines L2 and L14) by following the relaxation of the pH gradient created by the V-type H+-ATPase. Calculation of initial rates of fluorescence recovery after the addition of KCl or NaCl showed that nhx1 nhx2 mutant lines L2 and L14 exhibited much lower Na+/H+ and K+/H+ exchange activity than wild-type plants (Figure 4A). At 50 mM NaCl or KCl, the nhx1 nhx2 mutants retained less than one-third the exchange activity of the wild type (Figure 4B), demonstrating that NHX1 and NHX2 account for a large proportion of the total monovalent cation exchange capacity of the tonoplast. Similar results were obtained with tonoplast vesicles isolated from roots. The commensurate reduction of exchange activity of Na+ and K+ ions is in agreement with the NHX activity previously determined for Arabidopsis NHX1 (Venema et al., 2002; Hernández et al., 2009) and NHX2 herein (Figure 1).
Figure 4.
Reduced Cation/Proton Exchange in nhx1 nhx2 Mutants.
(A) K+/H+ and Na+/H+ in tonoplast vesicles from leaves of Col-0 (black circles) and mutant lines L2 (white circles) and L14 (white squares) over a range of substrate cation concentrations.
(B) Average rates of cation exchange at 50 mM were significantly reduced in the mutant line L14 (nhx1 nhx2) relative to Col-0.
NHX1 and NHX2 Are Essential to K+ Homeostasis
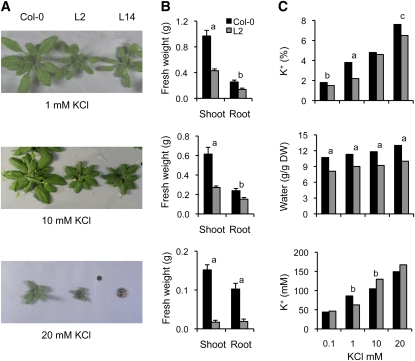
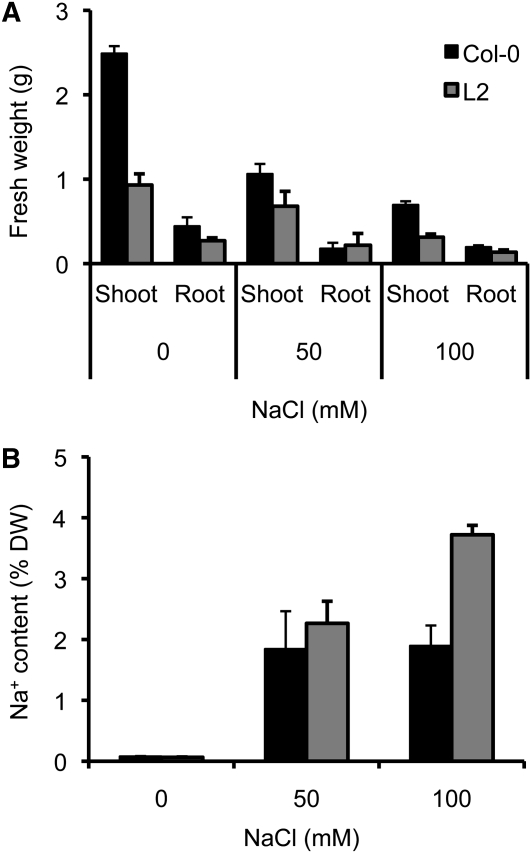
Growth of lines L2 and L14 nhx1 nhx2 mutant plants was progressively reduced relative to Columbia-0 (Col-0) at increasing K+ concentrations (from 0.1 to 20 mM K+) in hydroponic culture (Figure 5; see Supplemental Table 1 online; note that these two data sets belong to independent experiments). High KCl produced a striking toxicity to nhx1 nhx2 mutants that led to leaf desiccation and eventual plant death (Figure 5). Toxicity was specifically linked to K+ ions since 10 mM K2SO4 produced effects similar to 20 mM KCl. The knockout plants with null alleles nhx1-2 nhx2-1 grew poorly in all K+ regimes in hydroponic culture, but high KCl concentrations further aggravated growth impairment (see Supplemental Figure 2 online). Genetic complementation of line L14 with the cDNA of NHX2 under the 35S promoter partially restored growth parameters under various KCl regimes (see Supplemental Figure 2 online). In contrast with K+ ions, nhx1-1 nhx2-1 mutant plants did not show greater susceptibility to Na+ toxicity compared with the wild type (Figure 6). Notably, nhx1 nhx2 mutant plants accumulated more Na+ in their shoots than the wild type at 50 to 100 mM NaCl (Figure 6; see Supplemental Table 1 online).
Figure 5.
Sensitivity of nhx1 nhx2 Mutant Plants to External K+.
(A) One-week-old seedlings of Col-0 and of mutant line L2 grown in LAK medium with 1 mM KCl were transferred to fresh hydroponic LAK medium supplemented with the indicated concentrations of KCl. After 2 weeks, plants were collected and their shoot and root fresh weight were determined.
(B) Average fresh weight and sd values (n = 8 per line) of plants shown in (A). Differences between the mutant line and Col-0 were statistically significant at P < 0.01 (a) or P < 0.05 (b) by the Fisher’s LSD method. Mutant line 14 produced identical results to L2.
(C) Average K+ and water content in shoots of Col-0 and nhx1 nhx2 mutant plants of line L2 (n = 6 per line) grown as in (A) in hydroponic LAK medium with the indicated K+ concentrations. K+ content is given as a percent of dry weight (DW; top panel) and millimolar concentration (bottom panel). Water content is given in the middle panel. Statistical differences by the LSD method of mutant relative to the wild type are indicated by letters; a, P < 0.01; b, P < 0.05; c, P < 0.1. Line 14 produced identical results to L2.
Figure 6.
nhx1 nhx2 Mutants Are Not Sensitive to Sodium.
Plants of Col-0 and of nhx1-1 nhx2-1 mutant line L2 (n = 4 per line) were grown for 3 weeks in hydroponic LAK medium with 1 mM KCl and supplemented or not with 50 and 100 mM NaCl. Shown are average and sd values (n = 24)
(A) Fresh weight of shoots and roots of the wild type and mutant line L2.
(B) Sodium content in the shoot of plants after salinity treatment. DW, dry weight.
K+ concentration analyzed in leaf sap extracts and expressed on a tissue water basis (i.e., molar concentration) showed a significantly greater K+ concentration in nhx1-1 nhx2-1 mutants at high K+ in the hydroponic solution (Figure 5; see Supplemental Table 1 online). However, lower K+ concentrations were found on a dry weight basis (i.e., percentage of content) in shoots of nhx1 nhx2 double mutants in plants growing at various K+ concentrations (Figure 5; see Supplemental Table 1 online). Significant differences in shoot water content among lines explained this paradox, as K+ supply affects the water content of plants (Leigh and Wyn Jones, 1984). Plants of the nhx1 nhx2 genotype consistently accumulated less water in their shoots than the wild type, which translated into greater K+ mM concentrations, even though they had a lower K+ content on a dry matter basis (Figure 5; see Supplemental Table 1 online). In roots, where no differences in water content among lines were found, K+ concentrations were significantly lower in the double mutants except at 1 mM K+ (see Supplemental Table 1 online). Similar results were obtained with a complete knockout line of genotype nhx1-2 nhx2-1 (see Supplemental Figure 2 online), albeit growth reduction due to K+ toxicity was less evident than in the leaky nhx1-1 nhx2-1 lines because growth of nhx1-2 nhx2-1 plants was extremely impaired even at the lowest K+ concentration tested. At 20 mM KCl, shoots of the knockout plants had only half the K+ and water contents of the wild type (see Supplemental Figure 2 online).
Together, these results demonstrate that NHX1 and NHX2 play a major role in the accrual of K+ in shoot tissues of Arabidopsis, whereas they appeared to be dispensable for Na+ compartmentation. Compared with the wild type, Na+ accumulation proceeded to an even greater extent in the shoots of the nhx1 nhx2 mutant upon exposure to salinity stress (Figure 6).
Reduced Vacuolar K+ Pool Affected Turgor, Cell Expansion, and Stomatal Function
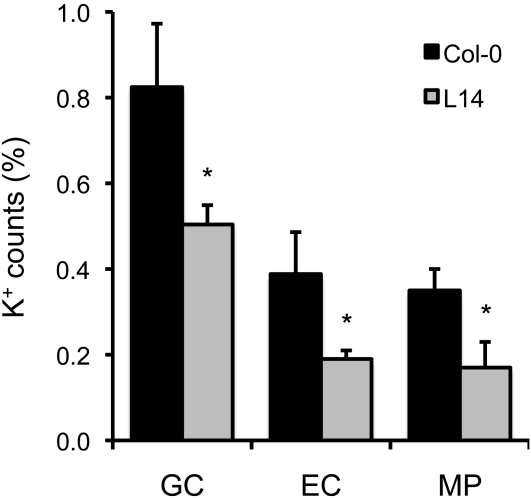
To estimate the size of the vacuolar K+ pool in the nhx1 nhx2 mutant, freeze-fractured leaves of L14 plants grown in LAK medium with 1 mM K+ were analyzed with a scanning electron microscope fitted with energy-dispersive x-ray spectroscopy (EDX). This technique analyzes the elemental composition of cells and cell compartments in plant tissues. The large vacuoles of mesophyll palisade cells of mutant plants showed fewer K+ counts than Col-0 (Figure 7). K+ counts were also significantly lower in guard cells of stomata of mutant plants, where both NHX1 and NHX2 show high expression levels (Figure 2; Shi and Zhu, 2002), as well as in epidermal subsidiary cells neighboring the stomata.
Figure 7.
Reduced Vacuolar K+ Content in the nhx1 nhx2 Mutant.
K+ content in the vacuoles of guard cells (GC), epidermal cells neighboring the stomata (EC), and in mesophyll palisade cells (MP) of leaves as determined by scanning electron microscopy/EDX. Shown are the average percentage and sd of K+ counts relative to total elemental counts. A minimum of 20 cells of each cell type per line was analyzed. Statistical differences by the LSD method (P < 0.05) of mutant line L14 relative to the wild type are indicated by asterisks.
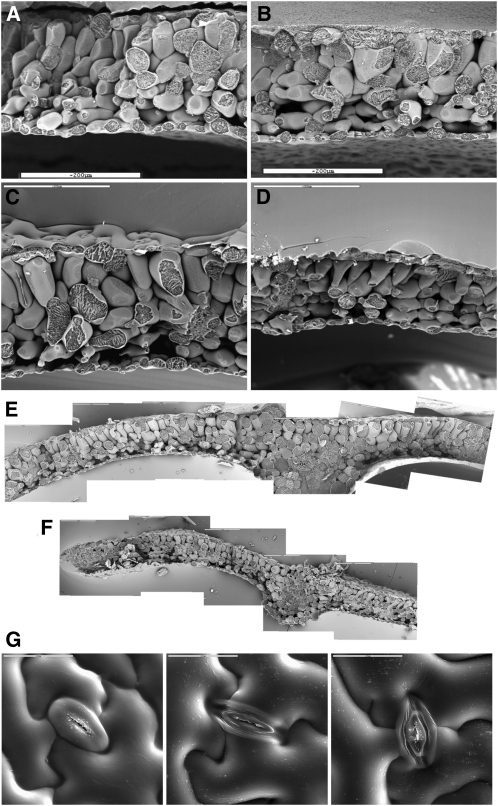
The uptake of K+ by plant cells, and its accumulation in vacuoles, is the primary driver for turgor generation and cell expansion (Leigh, 2001). Plants of lines L2 and L14 showed smaller leaf areas than Col-0 when grown at various K+ concentrations (see Supplemental Table 2 online). Specific leaf weight and succulence were also significantly reduced in the nhx1-1 nhx2-1 mutant plants, suggesting that leaf expansion was hindered by lower water uptake in the mutant (see Supplemental Table 2 online). In freeze-fractured sections for scanning electron microscopy, leaves from the nhx1-1 nhx2-1 leaky mutant (lines L2 and L14) presented a less developed spongy parenchyma when grown at 1 mM K+ (Figures 8A and 8B). At 10 mM K+, palisade cells were significantly smaller compared with the wild type and pyramidal in shape (Figures 8C and 8D). Smaller mesophyll cells resulted in a thinner leaf lamina in the nhx1-1 nhx2-1 mutant (Figures 8E and 8F), in agreement with the smaller specific leaf weight (see Supplemental Table 2 online). Notably, stomata of mutant lines showed aberrant morphology with thinner guard cells compared with the wild type, indicating that the lack of NHX1 and NHX2 function severely affected the capacity of guard cells to expand (Figure 8G).
Figure 8.
Impaired Leaf Cell Expansion in nhx1 nhx2 Mutants.
Freeze-fracture sections at scanning electron microscopy from leaves of Arabidopsis Col-0 and mutant plants from line L14 (nhx1-1 nhx2-1) grown in LAK medium for 2 weeks and then transferred to 1, 10, and 20 mM KCl for an additional 2-week period. Equivalent leaves from each plant were processed for scanning electron microscopy. Bars = 200 μM.
(A) Col-0 plants in 1 mM K+.
(B) L14 plants in 1 mM K+.
(C) Col-0 plants in 10 mM K+.
(D) L14 plants in 10 mM K+.
(E) Composition of serial pictures of a leaf from Col-0 plants grown for 2 weeks at 1 mM K+ and then transferred to 20 mM K+ for another 2 weeks.
(F) Composition of an equivalent leaf from a plant of mutant line L14 treated as in (E).
(G) Morphology of guard cells in stomata of wild-type, L2, and L14 plants.
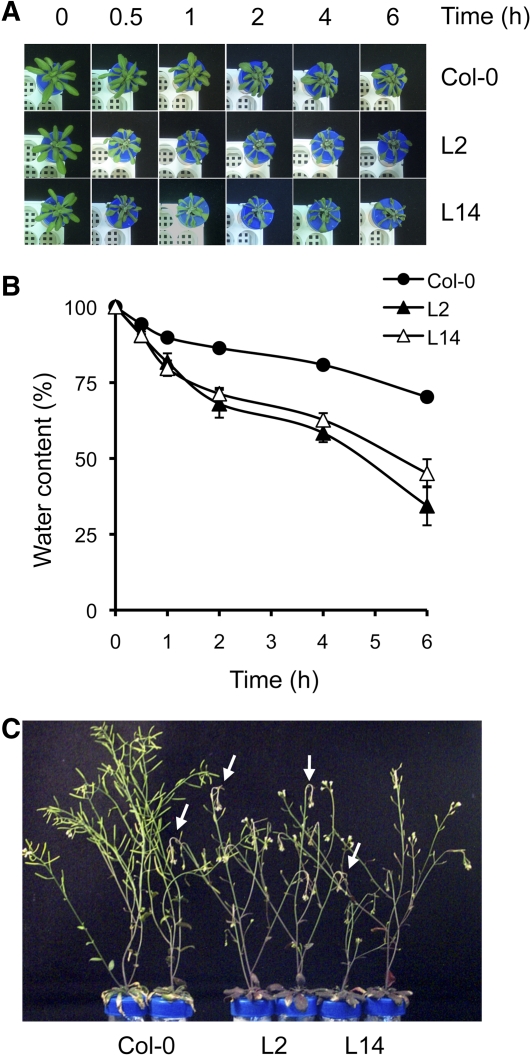
We next examined the ability of nhx1 nhx2 mutant plants to withstand osmotic stress. Three-week-old plants of single mutant lines (nhx1-2, nhx1-1, and nhx2-1) and double mutants nhx1-1 nhx2-1 were transferred individually to 50-mL test tubes adapted to hydroponic culture and filled with LAK medium (1 mM K+) supplemented with 20% (w/v) polyethylene glycol 6000 (PEG6000) (Figure 9A). Plants were weighted at the time of transfer and then at 0.5, 1, 2, 4, and 6 h afterward. Plants with genotype nhx1-1 nhx2-1 suffered a significantly greater water loss than the wild-type control (Figure 9B). Single mutant plants experienced an intermediate water loss. After treatment with PEG, wild-type and double mutant plants had their roots washed and were transferred to fresh LAK (1 mM K+) medium for recovery. Wild-type plants flowered and produced healthy siliques, whereas the mutant plants accumulated anthocyanins, their flower buds wilted, and they failed to produce siliques (Figure 9C).
Figure 9.
nhx1 nhx2 Mutants Are Sensitive to Osmotic Stress.
Wild-type (Col-0) and nhx1 nhx2 mutant lines L2 and L14 were grown individually in capped test tubes adapted to hydroponic culture in LAK medium with 1 mM KCl for 3 weeks and then transferred to LAK medium with 20% PEG6000. The fresh weight of plants (n = 4 per line) was determined at the indicated times. Single mutants with alleles nhx1-2, nhx1-1, and nhx2-1, carried in parallel, produced intermediate results that have been removed for simplicity.
(A) Visual appearance of dehydration symptoms during the assay.
(B) Fresh weight of plants from the onset of stress to completion of the hyperosmotic treatment. Data are presented as water content relative to initial values before treatment.
(C) Wild-type and mutant plants recovering from hyperosmotic stress. Arrows indicate wilted flower buds in the mutants. Note small siliques in the mutants.
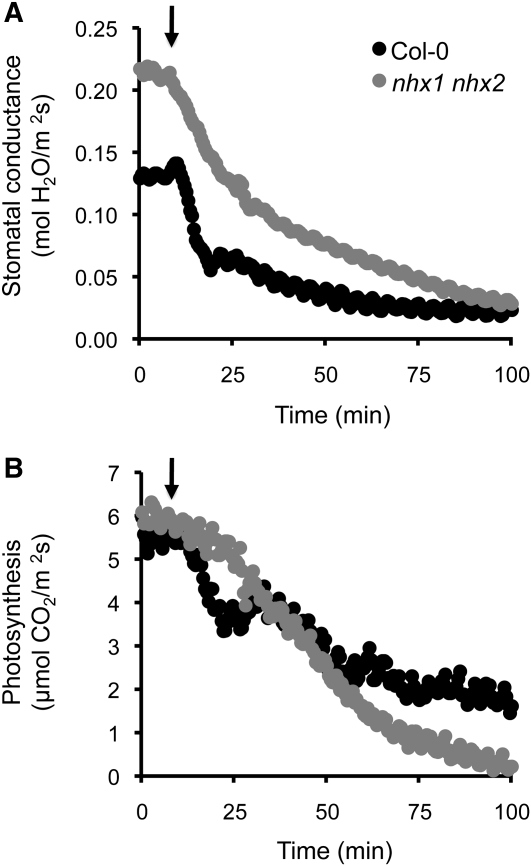
Since NHX1 and NHX2 were strongly expressed in guard cells, we examined stomatal conductance in nhx1-1 nhx2-1 mutants and its response to osmotic stress. The rates of transpiration and photosynthesis were recorded with an infrared gas analyzer in 3-week-old plants of wild-type Col-0 and of mutant lines L2 and L14 grown in hydroponic LAK medium. Without osmotic stress, the stomatal conductance was significantly greater (P < 0.05; n = 12) in the mutant lines (0.195 and 0.217 mol water m−2 s−1, respectively) than in the wild type (0.123 mol water m−2 s−1). However, the photosynthetic rates were similar (Col-0, 5.33 μmol CO2 m−2 s−1; L2, 5.18 μmol CO2 m−2 s−1; L14, 5.01 μmol CO2 m−2 s−1). For stress treatment, leaves in the chamber were allowed to equilibrate for at least 10 min and then plants were subjected to osmotic shock by 20% PEG6000 (arrows in Figure 10). In response to the hyperosmotic treatment, stomata of wild-type plants closed rapidly and reduced gas exchange by half in ~7 min (Figure 10A). By contrast, stomata from mutant plants responded slowly, took 25 min to reduce transpiration to 50%, and reached wild-type values only after 1.5 h. The slow response of mutant plants provoked the collapse of photosynthesis, whose rate dropped to negligible values after 1.5 h (Figure 10B). The experiment was repeated five more times with different mutant and control plants, and similar results were obtained. These data indicate that stomatal function and response to environmental clues are impaired in plants lacking NHX1 and NHX2.
Figure 10.
Stomatal Conductance under Osmotic Stress.
Wild-type (Col-0) and nhx1 nhx2 mutant line L2 plants were grown in hydroponic culture in LAK medium with 1 mM KCl for 3 weeks. The rates of transpiration and photosynthesis were recorded with an infrared gas analyzer. Leaves were allowed to equilibrate before treatment for at least 10 min and were then subjected to osmotic shock by treatment with 20% PEG6000 (arrows). Shown is a representative experiment of five repetitions with independent plants.
(A) Stomatal conductance.
(B) Photosynthetic rate.
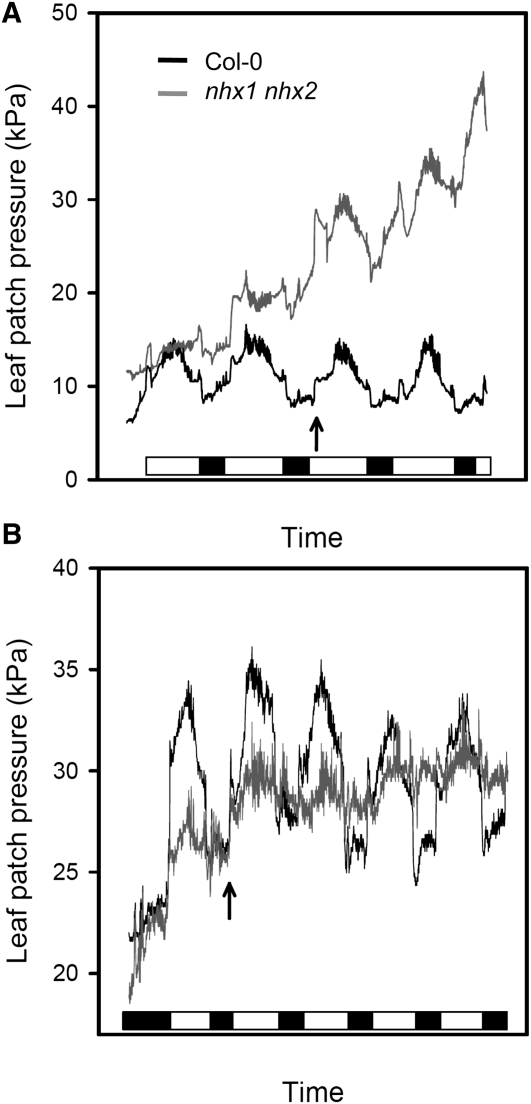
Stomatal action directly influences leaf turgor (Ache et al., 2010). To inspect leaf turgor using intact Arabidopsis leaves, we used a patch-clamp pressure probe to monitor turgor pressure noninvasively. Continuous recording of leaf turgor showed significant differences between the wild type and the nhx1-1 nhx2-1 mutant. Wild-type plants responded to the light stimulus with a sudden decrease in leaf turgor followed by turgor recovery when the light was off (Figure 11). These diurnal rhythms arise from stomatal opening and closing, which regulate the rates of transpiration (Ache et al., 2010). In plants growing in hydroponic culture at low (0.1 mM) and medium (1 mM) K+ concentrations, leaves from the mutant line showed a steady loss of turgor during the period of measurement. Moreover, turgor pressure oscillations in dark/light transitions were erratic in the mutant compared with the wild type. After raising the K+ concentration in the nutrient solution from 1 to 10 mM (arrow in Figure 11B), Col-0 plants showed a shift toward greater turgor pressures with clearly marked dark/light oscillations, whereas the mutant showed the opposite trend (i.e., moderate loss in average turgor while dark/light oscillations were essentially absent). These results are coherent with the inability of nhx1 nhx2 mutant plants to osmoregulate, a phenomenon that appears to be aggravated by high K+, since transfer to 10 mM external K+ clamped leaf turgor in the mutant and abrogated turgor oscillations in dark/light transitions.
Figure 11.
Daily Shifts in Leaf Turgor.
The turgor of leaves of wild-type Col-0 (black traces) and nhx1 nhx2 mutant line L14 (gray traces) growing in hydroponic culture in the greenhouse was measured with a patch-clamp pressure probe. Note that leaf turgor pressure and the pressure recorded by the probe are inversely proportional. The turgor pressure in the leaf patch is opposed to the magnetic pressure of the clamp, which is kept constant, and the pressure probe measures the difference between magnetic pressure and the relative turgor value. Thus, high pressure values mean lower leaf turgor pressure. Black and white boxes in the horizontal bars represent dark and light periods (8/16 h).
(A) Plants growing in 0.1 mM K+ were transferred to 1 mM K+ at the time indicated by the arrow.
(B) Plants were transferred from 1 to 10 mM K+ (arrow).
Cytosolic K+ Activity
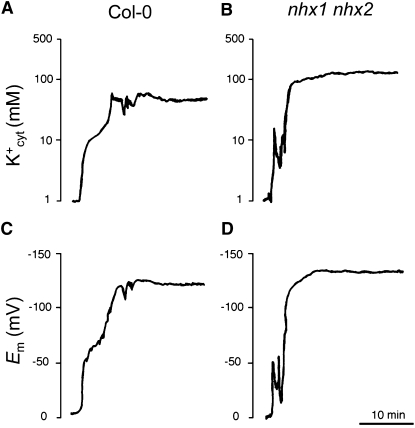
Having established that nhx1 nhx2 mutant plants had a reduced capacity to build up the vacuolar K+ pool, we next examined whether cytosolic K+ was also affected in the mutant. To measure the cytosolic K+ activity (K+cyt) and membrane potentials simultaneously, double-barreled K+-selective microelectrodes were used to impale epidermal root cells of 15-d-old control and mutant plants of genotype nhx1-2 nhx2-1 grown in LAK medium with 1 mM K+ (Figure 12). Cytosolic K+ activities were calculated from calibration curves (slopes were close to 49 mV/p K+). The average K+cyt was 75 ± 14 mM K+ (n = 5) in Col-0, which is similar to previous estimates in root cells of wild-type Arabidopsis (83 ± 4 mM) (Maathuis and Sanders, 1993). In the nhx1 nhx2 mutant, average K+cyt was 112 ± 17 mM K+, which is significantly different from wild-type values at P = 0.0086 (Student’s t test). By contrast, membrane potentials were not significantly different (P = 0.3123, Student’s t test) in the wild type (−122 ± 14 mV, n = 5) and nhx1 nhx2 mutant (−132 ± 14 mV, n = 5). These results indicate that the impaired K+/H+ exchange at the tonoplast of nhx1 nhx2 mutants elicited a significant increase of the cytosolic K+ pool.
Figure 12.
Higher Cytosolic K+ Concentrations in Roots of the nhx1 nhx2 Mutant.
Cytosolic K+ activities (a and b) and plasma membrane potentials (c and d) were directly measured by double-barreled K+-selective microelectrodes in single epidermal root cells of the wild type (a and c) and nhx1 nhx2 mutant line L2 (b and d). Shown are representative traces of five replicates.
Decreases in cytosolic K+ content are thought to be required for induction and development of high-affinity K+ uptake in Arabidopsis (Rubio et al., 2008). Three-week-old plants of Col-0 and mutant lines L2 and L14 growing in LAK hydroponic medium with 1 mM K+ where starved in K+-free medium for 3 d and then assayed for ion uptake rates by roots using rubidium (Rb+) at low (100 μM) concentration as a tracer for K+. Short-term (≤5 min) uptake rates showed small differences among lines that were not statistically significant, although roots from mutant plants achieved a lower net uptake of Rb+ than the wild type over time. Rb+ concentration in root tissues of Col-0 was 0.3 ± 0.04 (mM ± sd) after 20 min, but only 0.18 ± 0.02 in line L2 and 0.19 ± 0.07 in line L14. These results suggest that K+ uptake rates are somewhat reduced in the mutant lines, presumably as a consequence of greater K+cyt in the mutant.
DISCUSSION
The Essential Role of NHX Proteins in Creating the Vacuolar K+ Pool
K+ is the major ionic osmoticum in plant cells and occurs in two major pools, in the vacuole and in the cytosol. Cytosolic K+ plays essential roles as activator of biochemical processes, in the regulation of cytosolic pH, and in the fine-tuning of the plasma membrane electrical potential. These fundamental functions demand the maintenance of the cytosolic K+ concentration within narrow limits (75 to 100 mM), regardless of changes in K+ supply (Walker et al., 1996; Leigh, 2001). Since vacuolar K+ concentration closely follows K+ availability, the vacuolar pool is the largest and most dynamic reservoir. In this compartment, K+ functions as an osmoticum to generate turgor and drive cell expansion. Intracellular osmolytes reduce the cell water potential and give rise to a passive water influx, which in turn increases cell volume (Leigh and Wyn Jones, 1984). The lowest limit for vacuolar K+ concentration appears to be 10 to 20 mM, which is thought to reflect equilibrium with the cytosol at a maximum trans-tonoplast voltage of ~40 to 60 mV (Leigh, 2001). Greater concentrations of K+ inside the vacuole require active transport from cytosol to vacuole that could be achieved by a nonelectrogenic K+/H+ antiporter (Walker et al., 1996). Biochemical analyses have shown that tonoplast-localized NHX proteins catalyze the K+/H+ exchange with apparent affinity values (12 to 40 mM) that are below the lower limits of cytosolic K+ concentrations and are thus able to translocate K+ from the cytosol to the vacuole under regular physiological conditions (Figure 1) (Venema et al., 2002; Yamaguchi et al., 2005; Hernández et al., 2009). Recently, a reverse genetics approach has shown that the intravacuolar K+ concentration in the Arabidopsis nhx1 nhx2 mutant was only 30% of the wild type and that the vacuolar lumen was more acidic, presumably due to impaired K+/H+ exchange (Bassil et al., 2011b). Here, we showed that, indeed, the nhx1 nhx2 mutant had a significant threefold reduction in K+/H+ exchange in tonoplast vesicles compared with Col-0 plants (Figure 4) and a marked reduction in the amount of K+ stored in the vacuoles of leaf mesophyll cells, epidermal cells, and guard cells of stomata (Figure 7). Using a ratiometric fluorescence assay, Bassil et al. (2011b) found a reduction in vacuolar K+ in roots of the nhx1 nhx2 mutant. Together, these results indicate that NHX1 and NHX2 proteins account for the majority of the total K+/H+ exchange capacity in the vacuole of Arabidopsis. The transport activity still remaining in nhx1 nhx2 plants could be due to the presence of NHX3 and/or NHX4, which together with NHX1 and NHX2 constitute the class-I family of tonoplast-localized NHX proteins in Arabidopsis (Yokoi et al., 2002; Pardo et al., 2006). However, ProNHX3:GUS fusions were preferentially expressed in reproductive organs (Wang et al., 2007); thus, NHX3 is unlikely to contribute to Na+,K+/H+ exchange in leaves. By contrast, ProNHX4:GUS expression stained roots and vascular bundles in leaves (Wang et al., 2007), partially overlapping the expression of NHX1 and NHX2. The nonvacuolar, class-II NHX proteins, NHX5 and NHX6, localize in the Golgi and trans-Golgi network, where they may regulate endosomal pH (Bassil et al., 2011a). Other cation/proton exchangers that may add to the residual Na+,K+/H+ activity in the nhx1 nhx2 plant are Cation/H+ Exchanger (CHX) and K+ Efflux Antiporter (KEA) proteins (Pardo et al., 2006; Chanroj et al., 2012). Arabidopsis CHX proteins (28 members), which are also thought to mediate K+ transport and pH homeostasis, have been localized to the plasma membrane and various intracellular compartments, but not to the tonoplast so far (Chanroj et al., 2012). The KEA proteins (six isoforms in Arabidopsis) are thought to regulate K+ homeostasis in organelles (Chanroj et al., 2012). Thus, the available evidence strongly suggests that NHX1 and NHX2, the two most highly expressed members of the class-I group of NHX proteins, are also the main players in the active accumulation of K+ in the vacuole. It is worth noting that disruption of active accumulation of K+ in the vacuoles of nhx1 nhx2 plants resulted in greater retention of K+ in the cytosolic pool (Figure 12). This implies that, while the plasma membrane potential, which is negative inside the cell, drove the acquisition of extracellular K+, further transit to the vacuole was impeded by the tonoplast membrane potential, which is positive in the lumen relative to the cytoplasm, in the absence of an active transport mechanism that is capable of accumulating K+ against its electrochemical gradient. The converse situation was found in transgenic tomato expressing Arabidopsis NHX1, where enhanced recruitment of K+ into vacuoles occurred at the expense of a diminishing cytosolic pool (Leidi et al., 2010).
Plant cells expand by accumulating solutes, absorbing water, generating turgor pressure, and extending the cell wall. The vacuolar K+ pool plays a fundamental biophysical role and, jointly with other vacuolar osmolytes, drives osmotic changes and water movements (Leigh, 2001). Plants starved for K+ show, among other disorders, smaller sizes of aerial parts, decreased water content, reduced turgor, impaired stomatal regulation, and reduced transpiration (Mengel et al., 2001; White and Karley, 2010). All these symptoms are linked to the fundamental role that intracellular K+ plays as osmoticum. Arabidopsis mutants lacking NHX1 and NHX2 were smaller than control plants at all external K+ regimes, and this was more apparent in shoots than in roots (Figures 3 and 5). Shoot size was strictly correlated with the amount of NHX1 and NHX2 activity remaining. Whereas single mutants had near normal shoot development, leaky double mutants of the nhx1-1 nhx2-1 genotype were significantly smaller than the wild type, and complete knockout plants (nhx1-2 nhx2-1) were severely stunted (see Supplemental Figure 2 online). Supplemental Na+ could partially recover growth of the nhx1 nhx2 mutant, since nontoxic concentrations of Na+ may substitute for K+ as osmoticum (Rodríguez-Navarro, 2000; Bassil et al., 2011b). Reduced leaf size in nhx1 nhx2 mutant plants appeared to be a consequence of compromised cell expansion and not a reduction in the number of cells (Figure 8) (Bassil et al., 2011b). The relative growth rate of plant cells is a function of the internal hydrostatic or turgor pressure and the yield threshold and extensibility of the cell wall. Tissues of the nhx1 nhx2 mutant consistently showed reduced water contents, which correlated with K+ contents on a dry weight basis. This indicates that the diminished size of the vacuolar K+ pool hindered water uptake and that these plants were no longer able to supply the vacuole with sufficient amounts of K+ for the normal expansion of leaf cells (Figure 7). Accordingly, leaf turgor pressure measured with a leaf patch pressure probe was lower in the nhx1 nhx2 mutant plants than in control Col-0, and this difference increased steadily over time at low (0.1 to 1 mM) external K+ (Figure 11A). Transfer to 10 mM K+ stabilized leaf turgor, although daily shifts in leaf turgor due to light/dark transitions and stomatal function were dampened in the mutant plants. These findings are coherent with the prevailing view that K+ in the vacuolar pool acts as the major osmoticum driving water uptake and cell expansion. Consequently, NHX1 and NHX2, as key players in the creation of the vacuolar K+ pool, are important determinants of plant growth. Plants with compromised K+ acquisition also showed reduced sizes (Hirsch et al., 1998), but phenotypes under nonstarving conditions were not as dramatic as those found in the nhx1 nhx2 mutant plants.
How Are Na+ Ions Compartmentalized in the Vacuoles of nhx1 nhx2 Mutant Plants?
We have shown that Arabidopsis NHX1 overexpression in tomato imparted tolerance to NaCl, which was related to the preemptive accrual of K+ in vacuoles and improved K+ retention after stress imposition, but did not enhance the ability to compartmentalize toxic Na+ ions into the vacuole (Leidi et al., 2010). Similar findings were obtained by overexpression of the tomato protein NHX2 (Rodríguez-Rosales et al., 2008). The long-standing view is that, owing to the reduced volume of the apoplastic space, the principal if not the only line of defense of plant cells to avert cell injury by extracellular salt accumulation is to rely on the sequestration of salt inside the large central vacuoles. Thereby, plant cells avert ion toxicity and reduce their osmotic potential to facilitate water uptake (Oertli, 1968; Flowers et al., 1991; Munns, 2002). The discovery that vacuolar NHX proteins were capable of exchanging Na+ and H+ across the tonoplast led to the now widespread view that NHX proteins mediate this critical process in plants faced with a saline environment (Apse et al., 1999; Gaxiola et al., 1999; Blumwald, 2000; Quintero et al., 2000). However, this notion has been recently challenged based on the biochemistry of NHX proteins, which do not discriminate between Na+ and K+ or have a preference for K+ transport (Venema et al., 2002; Rodríguez-Rosales et al., 2009; Jiang et al., 2010). The lack of correlative evidence between greater salt tolerance and the enhancement of Na+ accumulation in different plant species overexpressing NHX proteins from various sources has also been pointed out (Rodríguez-Rosales et al., 2009; Jiang et al., 2010). Recently, Bassil et al. (2011b) have shown that NHX1 and NHX2 proteins play a comparatively greater role in K+ homeostasis than in Na+ sequestration. Here, we showed that mutant plants of genotype nhx1 nhx2 were extraordinarily sensitive to moderate KCl concentrations (10 to 20 mM) but they did not show greater susceptibility to NaCl compared with the wild type (Figure 6). In fact, salt-related growth retardation was proportionally less in the nhx1 nhx2 plants than in the wild type at 50 to 100 mM NaCl (Figure 6; see Supplemental Table 1 online), and the inclusion of moderate amounts of NaCl in the nutrient solution containing 20 mM K+ alleviated K+-associated toxicity symptoms (Bassil et al., 2011b). Notably, nhx1 nhx2 mutant plants accumulated more Na+ in their shoots than the wild type at 100 mM NaCl (Figure 6; see Supplemental Table 1 online). Together, these findings raise the questions of how Na+ gets compartmentalized into the vacuoles of Arabidopsis and which transport proteins underlie this process (Jiang et al., 2010). It is now apparent, at least in Arabidopsis, that ion transporters other than NHX1 and NHX2 mediate the influx of Na+ into the vacuolar lumen. Biochemical analyses suggest the potential operation of NHX1 and NHX2 as Na+/H+ antiporters in the tonoplast (Figure 4), but genetic evidence rules out any significant contribution of NHX1 and NHX2 in the compartmentation of Na+ (Figure 6). The budding yeast VNX1 protein, a member of the type II calcium exchange family, catalyzed Na+/H+ and K+/H+ exchange, but not Ca2+/H+ exchange, in vacuole-enriched fractions with a Km of 22.4 and 82.2 mM for Na+ and K+, respectively (Cagnac et al., 2007). Suggestions that members of the calcium/cation antiporter and CHX exchanger superfamilies may also mediate Na+/H+ exchange at the plant tonoplast have not been confirmed experimentally (Zhao et al., 2009; Chanroj et al., 2012). In this regard, it is intriguing that genetic inactivation of NHX1 and NHX2 reduced simultaneously Na+/H+ and K+/H+ exchange capacity in tonoplast vesicles and that no specific Na+/H+ exchange activity was unmasked by removing NHX1 and NHX2, while Na+ accumulation still proceeded under salinity stress (Figure 6). Electrophysiological studies have shown that nonselective SV channels permeate K+ and Na+ into the vacuolar compartment. Under salt stress, plant cells accumulate Na+ in the vacuole and release vacuolar K+ into the cytoplasm. SV channels are thought to mediate K+ release, but it appears that concomitant Na+ leakage from the vacuole is impeded as luminal Na+ blocks the SV channel in Arabidopsis (Ivashikina and Hedrich, 2005). In contrast with K+ ions, Na+ could not be released by SV channels even in the presence of a 150-fold gradient (lumen to cytoplasm). This property of the SV channel guarantees that K+ can shuttle across the vacuolar membrane while maintaining the Na+ stored in this organelle. However, since vacuoles of glycophytic plants may accumulate up to 80 mM Na+, cytosolic Na+ concentrations remain at 10 to 30 mM, and the tonoplast membrane potential is ~30 mV, positive in the lumen relative to the cytosol (Carden et al., 2003; Tester and Davenport, 2003), it appears unlikely that passive permeation by SV channels would account for meaningful accumulation of Na+ inside vacuoles. In summary, there is no likely candidate(s) yet to account for the Na+ accumulation that occurs in the absence of NHX1 and NHX2 in Arabidopsis.
NHX Proteins Facilitate Stomatal Movements
The nhx1 nhx2 mutant exhibited delayed stomatal closure and thus pronounced leaf turgor loss compared with the wild type. Regulation of stomatal aperture, preventing excess transpirational vapor loss, relies on turgor changes in two highly differentiated epidermal cells surrounding the pore, the guard cells. Increased guard cell turgor due to solute accumulation results in stomatal opening, whereas decreased guard cell turgor following solute release promotes stomatal closing. The main solutes involved in the osmoregulation process of guard cell are sucrose K+, and accompanying anions (malate and chloride), depending on the environmental conditions. K+ salts are highly mobile and energetically cheap solutes and, consequently, guard cells accumulate K+ salts in large quantities to open the stomata (MacRobbie, 2006). Arabidopsis CHX20 is a putative K+/H+ exchanger that appears to play a role in guard cell osmoregulation through K+ fluxes and possibly pH modulation (Padmanaban et al., 2007). However, CHX20 localizes to the endomembrane system and not to the tonoplast; thus, this protein has been suggested to associate with vesicles that traffic among various subcellular membranes.
Scanning electron microscopy/EDX data confirmed that guard cells produced significantly more K+ counts than neighboring subsidiary cells and mesophyll parenchyma cells (Figure 7), and this correlated with the expression of NHX1 and NHX2 in guard cells (Figure 2). Inactivation of both genes reduced by half the K+ content in the cells of leaves, including the guard cells, and compromised stomata function and development. Guard cells in mutant plants appeared wrinkled and much thinner than those in the wild type (Figure 8G), demonstrating that cell expansion is particularly hindered in the cell type with the most abundant expression of NHX1 and NHX2. Stomata, however, were not completely dysfunctional. Wild-type plants responded to an osmotic challenge in hydroponic culture with a rapid closure of stomata (Figure 10). Mutant plants nhx1 nhx2 responded more slowly and reached the reduced transpiration rates of the wild type only after 90 min of treatment, suggesting that the fast response mediated by rapid K+ fluxes was impaired in the mutant. In these experimental conditions, the stomatal conductance prior to stress imposition was higher in the mutant plants than in the wild type, suggesting that the closure of stomata is unconditionally impaired in the nhx1 nhx2 mutant line, perhaps due to the aberrant development of guard cells (Figure 8G).
Leaves undergo diurnal rhythms of day/night changes in turgor pressure that are a sensitive indicator of stomatal dynamics and plant water status. The pressure probe monitors the leaf turgor response that is directly associated with stomatal movements (Ache et al., 2010). In low K+, the nhx1 nhx2 line displayed daily rhythms of stomatal closure and opening that indicated some preservation of regulated stomatal function, but nonetheless the leaf turgor pressure decreased steadily over several days as the plants wilted (Figure 11). Supplementation with 10 mM K+ had the opposite effect and partially stabilized turgor in the leaf of nhx1 nhx2 mutants over a few days, while dampening the daily turgor shifts that remained unaffected in the wild-type plants. These observations are further evidence of dysfunctional stomatal regulation in nhx1 nhx2 mutant plants that is presumably linked to the reduced ability to compartmentalize K+ in the vacuoles of guard cells (Figure 7). Delayed stomatal closure and loss of leaf turgor was observed in Arabidopsis mutants lacking the SnRK protein kinase OPEN STOMATA1 and the SLOW EFFLUX ANION CHANNEL-ASSOCIATED1 (Ache et al., 2010). These proteins trigger membrane depolarization in guard cells in response to abscisic acid, followed by K+ release and stomata closing. By analogy with electrophysiological measurements demonstrating similar plasma membrane potentials in root cells of the wild type and nhx1 nhx2 mutant (Figure 12), the plasma membrane potential in guard cells is expected to remain at normal values even though the cytosolic K+ concentration might be higher in the nhx1 nhx2 plants.
In summary, NHX1 and NHX2 are vacuolar K+/H+ exchangers essential for active K+ uptake at the tonoplast, osmotic adjustment, and turgor regulation, and they play a unique role in stomata function.
METHODS
Growth Conditions and Physiological Determinations
Seeds of Arabidopsis thaliana ecotype Col-0 and the nhx mutant lines were stratified for 2 to 4 d at 4°C and then germinated at room temperature in plastic holders containing mineral wool imbibed in distilled water. Seedlings were transferred to 8-liter plastic containers for hydroponic culture. A modified Long Ashton mineral solution (Hewitt, 1966) with 1 mM K+ and nominally free of Na+ and NH4+ (LAK medium) was used as base solution for hydroponic cultures. The final composition of the LAK base solution was as follows: 1 mM KH2PO4, 2 mM Ca(NO3)2, 1 mM MgSO4, 30 μM H3BO3, 10 μM MnSO4, 1 μM ZnSO4, 1 μM CuSO4, 0.03 μM (NH4)6Mo7O24, and 100 μM Fe2+ as Sequestrene 138-Fe, pH ~5.3. In experiments with higher K+ levels (10 and 20 mM), supplemental K+ was added as K2SO4. For low (<1 mM) K+ medium, KH2PO4 was replaced by NaH2PO4 and K+ was added as K2SO4. For salinity treatments, Na+ was added as NaCl to the LAK medium. Hydroponic pots were incubated in a controlled growth chamber with the day/night regime of 25/20 ± 2°C, 40/60% relative humidity, 14/10 h illumination, and 250 μmol m−2 s−1 PAR.
To measure Na+ and K+ contents, salt treatments were administered to 2-week-old plants in hydroponic culture. For salinity treatments, NaCl was added at 25 mM increments every 12 h until reaching 50 and 100 mM Na+ in the nutrient solution. Typically, treatments were performed for two additional weeks before sampling unless specified otherwise. For ion content analyses, plants were separated into shoots and roots, and fresh weight was measured. Roots were washed thoroughly in tap water and blotted dry before weighing. Dry weight was also measured after drying samples at 70°C for 48 h in a forced-air oven to obtain water contents (grams of water per gram of dry weight). Na+ and K+ were extracted by autoclaving finely ground material and measured by atomic absorption spectrophotometry (Perkin-Elmer 1100B). For scanning electron microscopy/EDX analysis, pieces of freshly harvested, fully expanded leaves were processed as described previously (Leidi et al., 2010). Elemental analysis was performed focusing the excitation x-ray beam into the vacuoles exposed in fractured cells. The number and energy of the x-rays reemitted from the specimen were measured by an energy-dispersive spectrometer, and counts pertaining to C, O, K, Na, Ca, and Mg atoms were recorded. Results are presented as the percent of K+ and Na+ counts relative to total counts. Plant extracts obtained from the remaining parts of samples were used to measure ion concentrations by atomic absorption spectrophotometry. All statistical analyses were performed with the software package SPSS version 19.
Leaf gas exchange was determined using the open gas exchange system Li-6400 (LI-COR) equipped with the chamber head (Li-6400-40; LI-COR) that allowed full control of light, CO2, and humidity. Stomatal conductance (gs; mmol m−2 s−1) and the net photosynthetic rate (AN; μmol m−2 s−1) were measured in 3-week-old plants of the wild type and mutant line L2 grown hydroponically in LAK standard solution. Leaf responses to osmotic shock were recorded in fully expanded leaves, attached to the plant, under ambient CO2 and a saturating PPFD of 150 μmol m−2 s−1. Leaves were allowed to equilibrate under those conditions for at least 10 min and then subjected to osmotic shock by 20% PEG6000 in LAK medium. Measurements were recorded every 30 s over a period of 120 min. A total of 12 measurements for each genotype (six plants per line and two measurements per plant) were recorded.
Leaf turgor was monitored every 5 min by means of ZIM-patch clamp pressure probes (ZIM Plant Technology) attached to the youngest fully expanded leaves. This system allows noninvasive continuous recording of leaf turgor by means of an inversely and highly correlated value, the leaf patch clamp pressure (Zimmermann et al., 2008; Ache et al., 2010). Estimates of leaf water potential and osmotic potential were performed with a dew point microvoltmeter (HR 33T) and a C-52 sample chamber (Wescor) as described (Wullschleger and Oosterhuis, 1986). Leaf water contents were measured either by estimates of relative water contents using leaf discs (RWC = [fresh weight − dry weight/rehydrated weight − dry weight] * 100) or by measuring total tissue water content (water content = [leaf fresh weight – leaf dry weight]/leaf dry weight). Leaf area was determined by the program Δ-Scan (Scan Analytics) on real-size images of rosette leaves.
Genetic Methods
Mutant lines with T-DNA insertions were obtained from the SALK collection (SALK Institute). Alleles and SALK lines used in this work were SALK_065623 (nhx1-2), SALK_034001 (nhx1-1), and SALK_036114 (nhx2-1) (Alonso et al., 2003). Alleles are named as described by Bassil et al. (2011b). Insertion mutant information was obtained from the SIGnAL website (http://signal.salk.edu) and confirmed experimentally. Positions of T-DNA insertion sites are shown in Supplemental Figure 1A online. Mutant nhx1-2 has a T-DNA insertion at nucleotide +1246 relative to the start codon, whereas mutant nhx2-1 carries the insertion at nucleotide +2429. The T-DNA insertion in line SALK_034001 occurred at the 5′-untranslated region (nucleotide −114, allele nhx1-1) of NHX1. Homozygous mutant lines were identified by kanamycin resistance selection combined with diagnostic PCR screening with allele-specific primers designed to amplify wild-type or mutated loci.
To test mutant complementation in Arabidopsis, plasmid pGreen-AtNHX2 was constructed by subcloning the complete NHX2 coding region in XbaI-BamHI restriction sites of the vector pGreenII-35S-nos-Hyg (John Innes Centre; http://www.pgreen.ac.uk). The construct pGreenAtNHX2 was coelectroporated with pSoup vector into the Agrobacterium tumefaciens GV3101 strain, and the resulting bacterial clones were used to transform the nhx1-1 nhx2-1 Arabidopsis double mutant by the floral dipping method (Clough and Bent, 1998). Hygromycin-resistant T1 transgenic plants were selected on Murashige and Skoog agar medium supplemented with 20 μg/mL hygromycin B. Complementation tests were performed in LAK medium supplemented with a range of KCl concentrations.
For yeast mutant complementation and protein purification, a hexa-His tag was introduced at the 3′ end of the NHX2 cDNA by PCR using the primers 5′-GCCAGGATCCTCAGTGATGGTGATGGTGATGCGATCCACGAGGTTTACTAAGATCATGGCTGC-3′ and 5′-CACTCGAGGAAAGATGACAATGTTCGC-3′ and the complete open reading frame of NHX2 as template. The resulting in-frame fusion was sequenced and introduced into the XhoI-BamHI sites of plasmid pDR195, generating plasmid pDR195-NHX2:His6. The tagged protein NHX2:His6 was checked for complementation of the budding yeast (Saccharomyces cerevisiae) strain AXT3 (Δena1-4::HIS3, Δnha1::LEU2, and Δnhx1::TRP1) (Quintero et al., 2000) by the drop-test method using plates of Arg phosphate medium supplemented with 70 mM NaCl and yeast peptone dextrose medium supplemented with 30 mg/L hygromycin B.
Biochemical Methods
Isolation of tonoplast vesicles from rosette leaves and measurement of cation/proton exchange were as described (Barkla et al., 1999), except that fluorescence quenching of 9-amino-6-chloro-2-methoxy-acridine was used to monitor the formation and dissipation of pH gradients. Purified tonoplast vesicles (50 μg of protein) were added to a buffer containing 250 mM mannitol, 10 mM 1,3-bis[tris(hydroxymethyl)-methylamino]propane–MES, pH 8.0, 100 mM tetramethyl ammonium chloride, 3 mM MgSO4, and 1 μM 9-amino-6-chloro-2-methoxy-acridine (1 mL final volume). Fluorescence was recorded with a Hitachi fluorescence spectrophotometer (FL-2500) in a thermostated cell (26°C) at excitation and emission wavelengths of 415 and 485 nm, respectively (slit-width, 10 nm). The reaction mixture was stirred and maintained at 26°C throughout the transport assays. Formation of acid-inside pH gradients were started with the addition of 1.5 mM ATP–1,3-bis[tris(hydroxymethyl)-methylamino]propane, pH 8.0. When fluorescence stabilized, the initial rate of dissipation was measured after the addition of NaCl or KCl salts. Exchange rates are expressed as fluorescence recovery relative to fluorescence values prior to ATP addition (ΔF/Fmax), per minute and milligram of protein. Exchange rates were fitted to Michaelis-Menten kinetics using nonlinear regression with KALEIDAGRAPH (Synergy Software).
His-tagged Arabidopsis NHX2 was purified from yeast cells by Ni2+-affinity chromatography and inserted into artificial vesicles of soybean phospholipids as described (Venema et al., 2002). Measurement of NHX activity of His-tagged NHX2 was conducted in pyranine-loaded proteoliposomes as previously described for NHX1 (Venema et al., 2002). Proton efflux coupled to cation influx was monitored from the increase of pyranine fluorescence (463-nm excitation; 510-nm emission).
Histochemical Methods and Microscopy
A genomic DNA fragment upstream of the ATG start codon of NHX2 encompassing ~3.1 kb of the promoter region was fused with the GUS reporter gene in plasmid pBI101, and the resulting construct was introduced into wild-type Arabidopsis Col-0 plants. Three independent transgenic lines were assayed for GUS expression and they showed coherent results. Stress treatments were applied to seedlings by a 5-h incubation in 160 mM NaCl, 40 mM LiCl, or 320 mM sorbitol in half-strength Murashige and Skoog medium (Yokoi et al., 2002). Histochemical GUS analyses were performed as described (Jefferson et al., 1987). The GUS expression pattern was determined in young seedlings and organs of mature plants at different stages of development, including roots, rosette leaves, young flowers, and green siliques. Samples were incubated at 37°C for 18 h in GUS staining buffer (200 mM phosphate buffer, pH 7.2, 0.1% [v/v] Triton X-100, and 1 mM X-Gluc) and then placed for 5 h in 90% ethanol to remove chlorophyll. Photographs of GUS-stained tissues were taken using a Zeiss Axioskop microscope equipped with Nomarski optics and the Zeiss AxioVision software.
Electrophysiological Measurements
For electrophysiological experiments, Arabidopsis (Col-0 and the nhx1-2 nhx2-1 mutant line) was grown aseptically and vertically for 15 d on solid LAK medium with 1 mM K+ and 0.9% (w/v) Phytagel. Cytosolic K+ activities in epidermal root cells were directly measured by double-barreled K+-selective microelectrodes. Seedlings were mounted in plexiglass chambers (volume of ~1.1 mL), and continuous perfusion of the assay solution containing 2 mM CaCl2 and 0.1 mM KCl, pH 6.0 (10 mM MES-BisTris propane) was maintained at a constant flux rate of ~10 mL min−1. Microelectrode pretreatment and backfilling were as described previously (Leidi et al., 2010). The microelectrodes were filled with a K+-sensor cocktail containing K+ ionophore I (cocktail B, cat. No. 60398; Fluka, now part of Sigma-Aldrich) dissolved in a mixture of polyvinylchloride/tetrahydrofuran (40 mg mL−1) at a ratio of 30:70 (v/v) (Mithöfer et al., 2005). Impalements were performed in epidermal root cells at 5 mm from the apex. The signals from the K+-selective and voltage barrels were recorded and simultaneously subtracted by a high-impedance differential amplifier (FD223; World Precision Instruments). The difference was calibrated before and after experiments with different KCl solutions (from 1 to 500 mM KCl), maintained at a constant ionic strength by the addition of MgCl2 (Walker et al., 1995). Calibration curves showed slopes of around 49 mV/pK+. The impalements were stable for at least 20 min.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: NHX1 (At5g27150), NHX2 (At3g05030), NHX3 (At3g06370), NHX4 (At5g55470), NHX5 (At1g54370), and NHX6 (At1g79610). Notation of NHX genes is as described by Yokoi et al. (2002).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Molecular Genetics Analyses of nhx1 and nhx2 Mutants.
Supplemental Figure 2. Growth of Double Knockout Mutant nhx1-2 nhx2-1 in Different Potassium Regimes and upon Complementation with NHX2.
Supplemental Table 1. Shoot Growth and Water Content, and K+ and Na+ Concentrations in Shoots and Roots of Col-0 and nhx1-1 nhx2-1 Mutant Plants.
Supplemental Table 2. Leaf Area, Specific Leaf Weight, and Succulence of Col-0 and nhx1-1 nhx2-1 Mutant Plants.
Acknowledgments
We thank Francisco J. Quintero and Miguel A. Botella for helpful suggestions, Antonio Diaz Espejo for help with leaf gas exchange measurements, and Imelda Mendoza and Maria A. Parrado for technical assistance. This work was supported by grants from Ministerio de Ciencia e Innovación (cofinanced by the European Regional Development Fund) to J.M.P. (BIO2009-08641 and CSD2007-00057) and J.A.F. (CTM2011-30356). Z.A. was supported by the Junta de Ampliación de Estudios–Consejo Superior de Investigaciones Cientificas Fellowship Program. Maria A. Parrado and A.D.L. were supported by the Programa Nacional de Potenciacion de Recursos Humanos del Plan Nacional de Investigacion Cientifica.
AUTHOR CONTRIBUTIONS
V.B., E.O.L., Z.A., A.D.L. and B.C. performed research on the physiological, biochemical, and genetic data. L.R. and J.A.F. conducted the electrophysiological measurements. E.O.L., B.C., and J.M.P. designed the research and wrote the article.
References
- Ache P., Bauer H., Kollist H., Al-Rasheid K.A.S., Lautner S., Hartung W., Hedrich R. (2010). Stomatal action directly feeds back on leaf turgor: new insights into the regulation of the plant water status from non-invasive pressure probe measurements. Plant J. 62: 1072–1082 [DOI] [PubMed] [Google Scholar]
- Ache P., Becker D., Ivashikina N., Dietrich P., Roelfsema M.R., Hedrich R. (2000). GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K(+)-selective, K(+)-sensing ion channel. FEBS Lett. 486: 93–98 [DOI] [PubMed] [Google Scholar]
- Aharon G.S., Apse M.P., Duan S.L., Hua X.J., Blumwald E. (2003). Characterization of a family of vacuolar Na+/H+ antiporters in Arabidopsis thaliana. Plant Soil 253: 245–256 [Google Scholar]
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Apse M.P., Aharon G.S., Snedden W.A., Blumwald E. (1999). Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285: 1256–1258 [DOI] [PubMed] [Google Scholar]
- Apse M.P., Sottosanto J.B., Blumwald E. (2003). Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J. 36: 229–239 [DOI] [PubMed] [Google Scholar]
- Bañuelos M.A., Garciadeblas B., Cubero B., Rodríguez-Navarro A. (2002). Inventory and functional characterization of the HAK potassium transporters of rice. Plant Physiol. 130: 784–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkla B.J., Vera-Estrella R., Maldonado-Gama M., Pantoja O. (1999). Abscisic acid induction of vacuolar H+-ATPase activity in mesembryanthemum crystallinum is developmentally regulated. Plant Physiol. 120: 811–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E., Ohto M.A., Esumi T., Tajima H., Zhu Z., Cagnac O., Belmonte M., Peleg Z., Yamaguchi T., Blumwald E. (2011a). The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell 23: 224–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E., Tajima H., Liang Y.C., Ohto M.A., Ushijima K., Nakano R., Esumi T., Coku A., Belmonte M., Blumwald E. (2011b). The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23: 3482–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E. (2000). Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 12: 431–434 [DOI] [PubMed] [Google Scholar]
- Cagnac O., Leterrier M., Yeager M., Blumwald E. (2007). Identification and characterization of Vnx1p, a novel type of vacuolar monovalent cation/H+ antiporter of Saccharomyces cerevisiae. J. Biol. Chem. 282: 24284–24293 [DOI] [PubMed] [Google Scholar]
- Carden D.E., Walker D.J., Flowers T.J., Miller A.J. (2003). Single-cell measurements of the contributions of cytosolic Na(+) and K(+) to salt tolerance. Plant Physiol. 131: 676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanroj S., Wang G., Venema K., Zhang M.W., Delwiche C.F., Sze H. (February 14, 2012). Conserved and diversified gene families of monovalent cation/H+ antiporters from algae to flowering plants. Front. Plant Sci. 3 (online), doi/10.3389/fpls.2012.00025 [DOI] [PMC free article] [PubMed]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Epstein E., Rains D.W., Elzam O.E. (1963). Resolution of dual mechanisms of potassium absorption by barley roots. Proc. Natl. Acad. Sci. USA 49: 684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers T.J., Hajibagherp M.A., Yeo A.R. (1991). Ion accumulation in the cell walls of rice plants growing under saline conditions: Evidence for the Oertli hypothesis. Plant Cell Environ. 14: 319–325 [Google Scholar]
- Gaxiola R.A., Rao R., Sherman A., Grisafi P., Alper S.L., Fink G.R. (1999). The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc. Natl. Acad. Sci. USA 96: 1480–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard F., Pilot G., Lacombe B., Bouchez D., Bruneau D., Boucherez J., Michaux-Ferrière N., Thibaud J.B., Sentenac H. (1998). Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94: 647–655 [DOI] [PubMed] [Google Scholar]
- Gierth M., Mäser P., Schroeder J.I. (2005). The potassium transporter AtHAK5 functions in K(+) deprivation-induced high-affinity K(+) uptake and AKT1 K(+) channel contribution to K(+) uptake kinetics in Arabidopsis roots. Plant Physiol. 137: 1105–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R., Marten I. (2011). TPC1-SV channels gain shape. Mol. Plant 4: 428–441 [DOI] [PubMed] [Google Scholar]
- Hernández A., Jiang X., Cubero B., Nieto P.M., Bressan R.A., Hasegawa P.M., Pardo J.M. (2009). Mutants of the Arabidopsis thaliana cation/H+ antiporter AtNHX1 conferring increased salt tolerance in yeast: The endosome/prevacuolar compartment is a target for salt toxicity. J. Biol. Chem. 284: 14276–14285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt E.J. (1966). Sand and water culture methods used in the study of plant nutrition. In Technical Communications 22, 2nd ed, (London: Commonwealth Agricultural Bureau)
- Hirsch R.E., Lewis B.D., Spalding E.P., Sussman M.R. (1998). A role for the AKT1 potassium channel in plant nutrition. Science 280: 918–921 [DOI] [PubMed] [Google Scholar]
- Hosy E., et al. (2003). The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA 100: 5549–5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isayenkov S., Isner J.C., Maathuis F.J. (2010). Vacuolar ion channels: Roles in plant nutrition and signalling. FEBS Lett. 584: 1982–1988 [DOI] [PubMed] [Google Scholar]
- Ivashikina N., Hedrich R. (2005). K+ currents through SV-type vacuolar channels are sensitive to elevated luminal sodium levels. Plant J. 41: 606–614 [DOI] [PubMed] [Google Scholar]
- Jaquinod M., Villiers F., Kieffer-Jaquinod S., Hugouvieux V., Bruley C., Garin J., Bourguignon J. (2007). A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture. Mol. Cell. Proteomics 6: 394–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R.A., Kavanagh T.A., Bevan M.W. (1987). GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X.-Y., Leidi E.O., Pardo J.M. (2010). How do vacuolar NHX exchangers function in plant salt tolerance? Plant Signal. Behav. 5: 792–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.H., Böhmer M., Hu H., Nishimura N., Schroeder J.I. (2010). Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 61: 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J.M., Murata Y., Baizabal-Aguirre V.M., Merrill J., Wang M., Kemper A., Hawke S.D., Tallman G., Schroeder J.I. (2001). Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis. Plant Physiol. 127: 473–485 [PMC free article] [PubMed] [Google Scholar]
- Lebaudy A., Pascaud F., Véry A.A., Alcon C., Dreyer I., Thibaud J.B., Lacombe B. (2010). Preferential KAT1-KAT2 heteromerization determines inward K+ current properties in Arabidopsis guard cells. J. Biol. Chem. 285: 6265–6274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidi E.O., Barragán V., Rubio L., El-Hamdaoui A., Ruiz M.T., Cubero B., Fernández J.A., Bressan R.A., Hasegawa P.M., Quintero F.J., Pardo J.M. (2010). The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J. 61: 495–506 [DOI] [PubMed] [Google Scholar]
- Leigh R.A. (2001). Potassium homeostasis and membrane transport. J. Plant Nutr. Soil Sci. 164: 193–198 [Google Scholar]
- Leigh R.A., Wyn Jones R.G. (1984). A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 97: 1–13 [Google Scholar]
- Maathuis F.J.M., Sanders D. (1993). Energization of potassium uptake in Arabidopsis thaliana. Planta 191: 302–307 [Google Scholar]
- Maathuis F.J.M., Sanders D. (1994). Mechanism of high-affinity potassium uptake in roots of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 91: 9272–9276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie E.A. (2006). Control of volume and turgor in stomatal guard cells. J. Membr. Biol. 210: 131–142 [DOI] [PubMed] [Google Scholar]
- Mengel K., Kirkby E.A., Kosegarten H., Appel T. (2001). Principles of Plant Nutrition. (Dordrecht, The Netherlands: Kluwer Academic Publishers; ). [Google Scholar]
- Mithöfer A., Ebel J., Felle H.H. (2005). Cation fluxes cause plasma membrane depolarization involved in beta-glucan elicitor-signaling in soybean roots. Mol. Plant Microbe Interact. 18: 983–990 [DOI] [PubMed] [Google Scholar]
- Munns R. (2002). Comparative physiology of salt and water stress. Plant Cell Environ. 25: 239–250 [DOI] [PubMed] [Google Scholar]
- Oertli J. (1968). Extracellular salt accumulation, a possible mechanism of salt injury in plants. Agrochimica 12: 461–469 [Google Scholar]
- Padmanaban S., Chanroj S., Kwak J.M., Li X., Ward J.M., Sze H. (2007). Participation of endomembrane cation/H+ exchanger AtCHX20 in osmoregulation of guard cells. Plant Physiol. 144: 82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J.M., Cubero B., Leidi E.O., Quintero F.J. (2006). Alkali cation exchangers: Roles in cellular homeostasis and stress tolerance. J. Exp. Bot. 57: 1181–1199 [DOI] [PubMed] [Google Scholar]
- Quintero F.J., Blatt M.R., Pardo J.M. (2000). Functional conservation between yeast and plant endosomal Na(+)/H(+) antiporters. FEBS Lett. 471: 224–228 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Navarro A. (2000). Potassium transport in fungi and plants. Biochim. Biophys. Acta 1469: 1–30 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Navarro A., Rubio F. (2006). High-affinity potassium and sodium transport systems in plants. J. Exp. Bot. 57: 1149–1160 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Rosales M.P., Gálvez F.J., Huertas R., Aranda M.N., Baghour M., Cagnac O., Venema K. (2009). Plant NHX cation/proton antiporters. Plant Signal. Behav. 4: 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Rosales M.P., Jiang X., Gálvez F.J., Aranda M.N., Cubero B., Venema K. (2008). Overexpression of the tomato K+/H+ antiporter LeNHX2 confers salt tolerance by improving potassium compartmentalization. New Phytol. 179: 366–377 [DOI] [PubMed] [Google Scholar]
- Rubio F., Nieves-Cordones M., Alemán F., Martínez V. (2008). Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high-affinity range of concentrations. Physiol. Plant. 134: 598–608 [DOI] [PubMed] [Google Scholar]
- Santa-María G.E., Rubio F., Dubcovsky J., Rodríguez-Navarro A. (1997). The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell 9: 2281–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H.Z., Zhu J.K. (2002). Regulation of expression of the vacuolar Na+/H+ antiporter gene AtNHX1 by salt stress and abscisic acid. Plant Mol. Biol. 50: 543–550 [DOI] [PubMed] [Google Scholar]
- Spalding E.P., Hirsch R.E., Lewis D.R., Qi Z., Sussman M.R., Lewis B.D. (1999). Potassium uptake supporting plant growth in the absence of AKT1 channel activity: Inhibition by ammonium and stimulation by sodium. J. Gen. Physiol. 113: 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester M., Davenport R. (2003). Na+ tolerance and Na+ transport in higher plants. Ann. Bot. (Lond.) 91: 503–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema K., Quintero F.J., Pardo J.M., Donaire J.P. (2002). The Arabidopsis Na+/H+ exchanger AtNHX1 catalyzes low affinity Na+ and K+ transport in reconstituted liposomes. J. Biol. Chem. 277: 2413–2418 [DOI] [PubMed] [Google Scholar]
- Walker D.J., Leigh R.A., Miller A.J. (1996). Potassium homeostasis in vacuolate plant cells. Proc. Natl. Acad. Sci. USA 93: 10510–10514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D.J., Smith S.J., Miller A.J. (1995). Simultaneous measurement of intracellular pH and K+ or NO3- in barley root cells using triple-barreled, ion-selective microelectrodes. Plant Physiol. 108: 743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Li Y., Zhang Y., Yang C., Zheng N., Xie Q. (2007). Comparative expression analysis of three genes from the Arabidopsis vacuolar Na+/H+ antiporter (AtNHX) family in relation to abiotic stresses. Chin. Sci. Bull. 52: 1754–1763 [Google Scholar]
- White P., Karley A. (2010). Potassium. In Cell Biology of Metals and Nutrients, R.D. Hell and R.-R. Mendel, eds (Berlin/Heidelberg, Germany: Springer), pp. 199–224
- Winter D., Vinegar B., Nahal H., Ammar R., Wilson G.V., Provart N.J. (2007). An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S.D., Oosterhuis D.M. (1986). A rapid leaf-disc sampler for psychrometric water potential measurements. Plant Physiol. 81: 684–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Li H.D., Chen L.Q., Wang Y., Liu L.L., He L., Wu W.H. (2006). A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125: 1347–1360 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Aharon G.S., Sottosanto J.B., Blumwald E. (2005). Vacuolar Na+/H+ antiporter cation selectivity is regulated by calmodulin from within the vacuole in a Ca2+- and pH-dependent manner. Proc. Natl. Acad. Sci. USA 102: 16107–16112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi S., Quintero F.J., Cubero B., Ruiz M.T., Bressan R.A., Hasegawa P.M., Pardo J.M. (2002). Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J. 30: 529–539 [DOI] [PubMed] [Google Scholar]
- Yoshida K., Miki N., Momonoi K., Kawachi M., Katou K., Okazaki Y., Uozumi N., Maeshima M., Kondo T. (2009). Synchrony between flower opening and petal-color change from red to blue in morning glory, Ipomoea tricolor cv. Heavenly Blue. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 85: 187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Connorton J.M., Guo Y., Li X., Shigaki T., Hirschi K.D., Pittman J.K. (2009). Functional studies of split Arabidopsis Ca2+/H+ exchangers. J. Biol. Chem. 284: 34075–34083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann D., Reuss R., Westhoff M., Gessner P., Bauer W., Bamberg E., Bentrup F.W., Zimmermann U. (2008). A novel, non-invasive, online-monitoring, versatile and easy plant-based probe for measuring leaf water status. J. Exp. Bot. 59: 3157–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]