Plant sterols and steroid hormones brassinosteroids (BRs) are essential for plant growth, reproduction, and plant response to various abiotic and biotic stresses. In this review, we discuss the potential and strategies for enhancing yield in crops by genetically altering sterol and BR metabolism and signaling or by exogenously applying BRs and known inhibitors of their pathways.
Abstract
Plant sterols and steroid hormones, the brassinosteroids (BRs), are compounds that exert a wide range of biological activities. They are essential for plant growth, reproduction, and responses to various abiotic and biotic stresses. Given the importance of sterols and BRs in these processes, engineering their biosynthetic and signaling pathways offers exciting potentials for enhancing crop yield. In this review, we focus on how alterations in components of sterol and BR metabolism and signaling or application of exogenous steroids and steroid inhibitors affect traits of agronomic importance. We also discuss areas for future research and identify the fine-tuning modulation of endogenous BR content as a promising strategy for crop improvement.
INTRODUCTION
In the last decade, important advances have been made in elucidating the metabolism (biosynthesis and catabolism) and signaling pathways of sterols and brassinosteroids (BRs) as well as their importance for plant growth and development, in both model plants and crops (Schaller, 2004; Choe, 2006; Clouse, 2011; Williams, 2011). Both sterols and BRs control several traits of agronomic importance, such as plant growth, photosynthesis, architecture, and flowering time. Therefore, manipulation of the sterol and/or BR content or their perception has the potential to improve intrinsic seed yield significantly. Remarkably, sterols and BRs are also capable of increasing plant tolerance/resistance to a wide range of biotic and abiotic stresses, such as drought, salinity, heat, cold, virus infection, and pathogen attack (Divi and Krishna, 2009). Furthermore, altering the endogenous levels of sterols can have a beneficial impact on the nutritional quality of crops (Schaller, 2004).
Two strategies have been employed for sterol- and BR-mediated yield increase: exogenous application of sterol/BR analogs and/or their inhibitors and genetic manipulation of their activities. Both approaches have delivered promising results for seed yield and stress tolerance enhancement in a variety of crop species. High cost of synthetic BR molecules and variability of the results have discouraged the use of exogenous BR in agriculture and horticulture (Khripach et al., 2000; Gomes, 2011). By contrast, modulation of the endogenous sterol and BR levels by genetic engineering represents an efficient strategy for improving crop yield in a uniform and predictable manner (Divi and Krishna, 2009).
This review article focuses on the potential to increase plant yield and stress tolerance through engineering sterol and BR levels and sensitivity (Figures 1 and 2). We provide an exhaustive list of genes involved in plant sterol (phytosterols) and BR metabolic and signaling pathways and their respective loss- or gain-of-function phenotypes in both Arabidopsis thaliana and crops (see Supplemental Tables 1 and 2 online). We also summarize the physiological effects of application of sterols, BRs, or inhibitors of their synthesis on plant growth, development, and stress tolerance (see Supplemental Table 3 online). Finally, we discuss future research and strategies to modifiy the sterol and BR pathways for crop improvement.
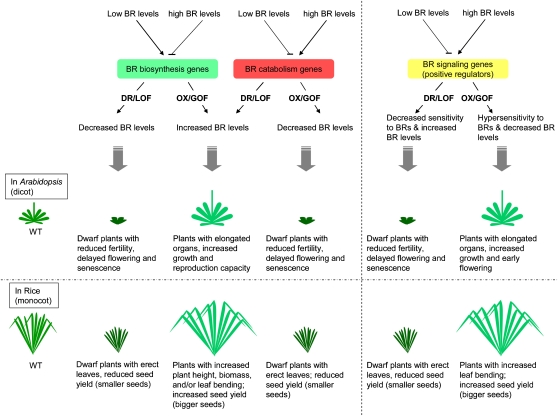
Figure 1.
BR Homeostasis and Signaling Mechanisms and Their Effects on Plant Growth and Development in Arabidopsis (Dicot) and Rice (Monocot).
Low levels of BRs promote BR biosynthesis. Conversely, under high BR levels, the signaling of BRs is active and the synthesis of BRs is repressed through BR signaling–mediated transcriptional negative feedback regulation. Downregulation of BR biosynthetic enzymes or overexpression of BR inactivation enzymes leads to decreased levels of the end product of BR synthesis, whereas downregulation of the positive regulators or overexpression of the negative regulators of the BR signaling pathway also decreases BR levels as well as increasing the sensitivity to BRs. On the contrary, BR levels are increased by overexpression of a BR biosynthetic enzyme (rate-limiting step)/BR signaling positive regulator or by downregulation of a BR catabolic enzyme/BR signaling negative regulator. A decrease in plant endogenous BR levels generally results in dwarfism, shorter petiole (in Arabidopsis)/more erect leaves (in rice), and reduced fertility. By contrast, Arabidopsis or rice plants with increased endogenous BR levels generally display improved plant growth and reproduction capacity. To date, the impact of altering endogenous BR content on the plant tolerance to abiotic and biotic stresses remains unclear. DR, downregulation; GOF, gain of function; LOF, loss of function; OX, overexpression.
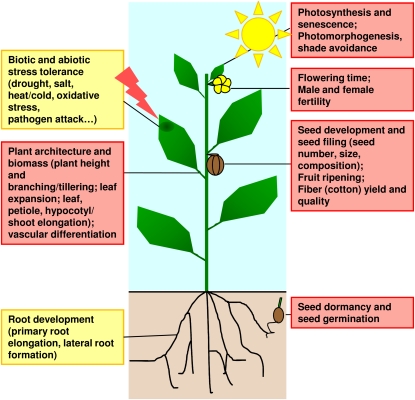
Figure 2.
Actions of BRs in Regulating Plant Development and Traits of Agronomic Importance in Model Plant Species and Crops.
Positive effects of increased BR content or signaling are boxed in red. Mixed effects are boxed in orange. Dose-dependent effects are observed on root development (positive effects at low concentrations and negative effects at high concentrations). Overall effects of BRs on the plant biotic and abiotic stress tolerance remain unclear to date.
PLANT STEROIDS AND VEGETATIVE GROWTH
Seed Dormancy and Germination
Regulation of the seed germination rate is key for a high seedling establishment, resulting in weed control and efficient crop production, especially under suboptimal growth conditions (Finch-Savage and Leubner-Metzger, 2006). BRs positively regulate seed germination in Arabidopsis. Exogenous BR application rescues the low germination phenotype of gibberellin mutants, and the seed germination of BR-related mutants is more sensitive to inhibition by abscisic acid (ABA) than the wild type (Steber and McCourt, 2001; Xue et al., 2009). Recent evidence suggests that the antagonistic effect of BRs on seed germination is partially mediated through the phosphatidylethanolamine binding protein MOTHER OF FT AND TFL1 (MFT) because application of BRs to loss-of-function mft mutants did not antagonize the inhibitory effect of ABA (Xi and Yu, 2010). The ABA inhibition of germination was overcome by overexpressing the Arabidopsis DWARF4 (DWF4) biosynthetic gene under the control of a seed-specific oleosin promoter in Arabidopsis (Divi and Krishna, 2010). Additional evidence for the involvement of BRs in seed dormancy and germination is provided by the Arabidopsis HYDROXYSTEROID DEHYDROGENASE1 (HSD1) gene that encodes a putative enzyme involved in BR synthesis. Seeds from transgenic Arabidopsis plants overexpressing HSD1 display a reduced dormancy compared with that of the wild type (Li et al., 2007; Baud et al., 2009). It remains to be determined whether this physiological effect correlates with an increased endogenous BR content in the transgenic seeds.
Recently, sterols have been proposed to have a positive function on seed germination. Seeds of transgenic Brassica juncea plants overexpressing the HMG-COA SYNTHASE gene had a higher sterol content and germinated earlier than those of the wild type (Wang et al., 2012).
Altogether, these results show that sterols and BRs play a role in promoting seed germination, both under normal and stress conditions. Therefore, modification of the sterol and BR contents in seeds might have important agronomical applications.
Plant Architecture and Biomass
Plant architecture is defined as the three-dimensional organization of the plant. For the aerial part, this includes plant height, branching/tillering pattern, foliar arrangement and morphology, and reproductive organ structure. Plant architecture is a trait of major agronomic importance as it has a strong effect on harvest index and grain yield potential (Reinhardt and Kuhlemeier, 2002). In the field, crops are generally grown at high planting density and with high nitrogen input, two factors that promote stem elongation and lodging. To ensure high yield and avoid lodging under these conditions, cereal crops with semidwarf and/or erect leaf phenotypes are highly desired (Van Camp, 2005). Semidwarf varieties of rice (Oryza sativa) and wheat (Triticum aestivum) with enhanced yield and resistance to lodging are at the basis of the green revolution (Athwal, 1971). Green biomass is another important trait, especially in energy crops (Xie and Peng, 2011).
BR-deficient and BR-insensitive mutants of Arabidopsis are generally dwarfed with shorter petioles and hypocotyls. By contrast, BR catabolic mutants and transgenic plants overexpressing the BR biosynthetic genes or positive regulators of BR signaling generally display increased growth and elongated organ phenotypes, including larger rosettes/taller plants with elongated leaves and longer petioles (Figure 1; see Supplemental Table 1 online). BR-deficient and BR-signaling mutants of other dicotyledonous (dicot) plant species, such as pea (Pisum sativum) and tomato (Solanum lycopersicum; formerly Lycopersicon esculentum) mutants of the BRASSINOSTEROID INSENSITIVE1 (BRI1) gene encoding the BR receptor, also present a dwarf phenotype (Bishop and Koncz, 2002) (Figure 1; see Supplemental Table 2 online). Similar phenotypes are observed in monocotyledonous (monocot) species. The shortened hypocotyl and leaf petiole of BR-deficient or BR-insensitive Arabidopsis mutants are mirrored in the respective rice mutants by shortened internodes and more erect leaves due to a reduced lamina joint inclination (e.g., Arabidopsis bri1 compared with O. sativa bri1 [Osbri1/d61] mutants) (Bishop and Koncz, 2002; Nakamura et al., 2006). Conversely, the elongated organ phenotype of BR mutant/transgenic plants in Arabidopsis translates in mutant/transgenic rice plants with increased leaf bending (e.g., the dominant Arabidopsis suppressor of phyb-4 7 [sob7]-D versus the rice bending lamina2 [bla2] mutant phenotype) (Turk et al., 2005; Park et al., 2006). Leaf angle is an important trait in cereal crops because it allows higher density planting and therefore can have a major impact on biomass and grain yield per hectare (Sakamoto et al., 2006). The molecular and cellular mechanisms by which BRs regulate lamina joint inclination remain unclear. Reduced leaf angle in the rice BR-deficient and BR-insensitive mutants is caused by an elongation failure in the abaxial lamina joint cells. This feature has been used as a marker in screens for BR-related mutants with mild phenotypes (Hong et al., 2004). Differential expression of one or several component(s) of the BR pathways may explain why some tissues are more sensitive than others to changes in BR levels and responses.
Given the relatively high level of transferability of architectural traits controlled by BRs between dicots and monocots (for instance, dwarfism and petiole length in Arabidopsis versus dwarfism and leaf lamina joint inclination in rice), a good transferability for these traits is also expected among monocot species. With the exception of rice, very little is known about BR biosynthetic and signaling pathways and the effect of manipulating them in monocot species. One of the few BR mutants identified in monocot species other than rice is the semidwarf uzu mutant of barley (Hordeum vulgare), which carries a mutation in the Hv BRI1 gene (Chono et al., 2003). In maize (Zea mays), a reduced expression of the BR biosynthetic gene Zm DWF1 results in dwarfism (Tao et al., 2004).
In addition to plant height and leaf morphology, BRs can also influence plant branching/tillering, as illustrated by the phenotypes of Arabidopsis and rice transgenic plants overexpressing DWF4 (Choe et al., 2001; Wu et al., 2008). In rice, alteration of BR-related gene expression also affects panicle architecture as observed, for example, in the rice ebisu dwarf d2/cytochrome P450 (cyp90d2) mutant (Hong et al., 2003). As plant height is among the most important biomass yield components (Salas Fernandez et al., 2009), and the BR-deficient and BR-insensitive mutants have dwarf phenotypes, BRs clearly have the potential to strongly affect plant biomass yield. Transgenic Arabidopsis, tobacco (Nicotiana tabacum), rapeseed (Brassica napus), and rice plants overexpressing the At/Zm DWF4 or At HSD1 genes have a higher biomass yield than the wild-type plants (Choe et al., 2001; Li et al., 2007; Wu et al., 2008). Under high planting density conditions, the semidwarf Osdwf4 mutants also display an increased (~40% of aboveground) biomass compared with the wild-type plants (Sakamoto et al., 2006).
Both sterols and BRs affect vascular tissue development. Vascular tissues are crucial in plant growth and development because they ensure the movement of water, nutrients, and photoassimilates throughout the plant and provide it with support/mechanical strength. BR-deficient mutants generally display abnormal patterns in vascular differentiation that are characterized by overproliferation of phloem cells and underproliferation of xylem cells (Caño-Delgado et al., 2004, 2010). Consistent with an involvement of BRs in xylem development, treatment with the BR biosynthetic inhibitor brassinazole (Brz) hampers the development of secondary xylem in cress plants (Lipidium sativum) (Nagata et al., 2001). In addition, BRs modulate the number of vascular bundles by promoting early procambial divisions, whereas periodic auxin maxima control their positioning (Ibañes et al., 2009; Fàbregas et al., 2010). Among the BR genes involved in vascular tissue development are BRI1 and its close homologs BRI1-LIKE1 (BRL1), BRL2, and BRL3. In Arabidopsis, both BRL1 and BRL3 are functional BR receptor proteins, whereas BRL2/VASCULAR HIGHWAY1 is not (Caño-Delgado et al., 2004; Ceserani et al., 2009). Orthologs of BRI1 and BRL genes also exist in rice and other monocots, but their role in vascular development has not been established yet. Their root-specific expression patterns in rice suggest that their function might differ from that of their Arabidopsis orthologs (Morillo and Tax, 2006).
Sterols also play a role in vascular tissue development. Analysis of the Arabidopsis sterol biosynthetic mutants hydra1 (hyd1) and fackel/hydra2 (fk/hyd2) has revealed that sterols are required to regulate the correct localization of the auxin efflux transporters, the PIN-FORMED proteins, and, hence, the correct auxin distribution necessary for normal vascular development (Pullen et al., 2010).
Photomorphogenesis
Shade avoidance is a set of responses also called shade avoidance syndrome (SAS) that plants display when their leaves are subjected to the shade of their own leaves or leaves from another plant. SAS is an important determinant of plant architecture and seed and biomass yields. Attenuation of SAS constitutes a breeding target for seed yield increase, especially for crop varieties grown at a high planting density. Conversely, enhancement of SAS to increase green biomass production at the expense of grain yield is of interest for the development of crop varieties cultivated for lignocellulosic-based/second-generation biofuel production (Kebrom and Brutnell, 2007).
Implication of BRs in response to shade stimuli is evidenced by the induction of many BR-related genes under shade conditions (Kozuka et al., 2010). Several transcription factors involved in SAS are regulated by BRs or control the expression of BR signaling pathway components and BR-regulated genes (Bou-Torrent et al., 2008; Sorin et al., 2009; Crocco et al., 2011). BRs are required for SAS responses to blue light depletion and to a low red:far-red light ratio. Petioles of the Arabidopsis weak mutant allele of BRI1, bri1-301, and of the BR-deficient mutant rotundifolia3 (rot3)/cyp90c1 display reduced responsiveness to blue light attenuation and end-of-day far-red light, respectively (Kozuka et al., 2010; Keller et al., 2011). Moreover, the Arabidopsis BR inactivation enzyme CYP72B1 modulates the developmental switch from skotomorphogenesis (plant development in the dark) to photomorphogenesis (plant development in the presence of light), mainly through far-red light–dependent modulation of BR levels (Turk et al., 2003).
Several lines of evidence point toward interactions between light and BR signaling. First, some BR-induced genes are involved in light responses, and BRASSINAZOLE-RESISTANT1 (BZR1) and BRI1-EMS-SUPPRESSOR1 (BES1)/BZR2, two key transcription factors of the BR signaling pathway bind to several of them (Sun et al., 2010; Yu et al., 2011). Notably, BZR1 is able to repress the expression of the GATA TRANSCRIPTION FACTOR2, a positive regulator of photomorphogenesis (Luo et al., 2010). Second, many of the Arabidopsis BR-deficient and BR-insensitive mutant seedlings display a deetiolation phenotype in the dark (Chory et al., 1991; Li et al., 1996; Szekeres et al., 1996). These observations suggest that BRs act as negative regulators of the deetiolation process. In support of this hypothesis, the expression levels of BR biosynthetic genes in Arabidopsis are higher in dark-grown than in light-grown seedlings (Symons et al., 2002). In addition, BR deficiency stimulates the expression of light-responsive genes and photomorphogenesis, whereas brassinolide (BL) treatment suppresses it (Song et al., 2009). However, direct measurements of endogenous BR levels revealed no correlation with these gene expression changes, neither in Arabidopsis nor in other species, such as pea and rice. In fact, BR levels were lower in dark-grown Arabidopsis seedlings than in light-grown controls (Symons et al., 2008; Symons and Reid, 2008).
Photosynthesis and Senescence
Photosynthesis is the main source of carbon assimilation in plants. Photoassimilate production may be enhanced by increasing either the photosynthetic efficiency or the plant total photosynthetic capacity (e.g., by delaying leaf senescence) (Van Camp, 2005). The prospect of increasing the photosynthetic efficiency for crop improvement has received much attention over the last few years with the discovery that generally crop yields are enhanced by a CO2-induced increase in leaf photosynthesis (Long et al., 2006).
Transgenic rice plants overexpressing the rice DWF4/CYP90B1 gene or its close homologs from Arabidopsis and maize under the control of a promoter active in stem, roots, and leaves (but not in seeds) displayed an increased seed yield and CO2 uptake, suggesting an enhanced photosynthetic efficiency (Wu et al., 2008). Surprisingly, the dwf4 mutants of rice (semidwarf, erect leaf phenotype) also had higher photosynthetic rates and seed yield under high planting density than the wild types, possibly due to their more erect leaves casting less shadow on lower leaves (Sakamoto et al., 2006). The gain-of-function phosphorylation Arabidopsis mutant allele bri1 Y831F (a Tyr-to-Phe substitution at position 831) also displayed increased rates of photosynthesis (Oh et al., 2011).
In agreement with their effect on photosynthesis, BRs activate the RUBISCO ACTIVASE protein in cucumber (Cucumis sativus) (Xia et al., 2009a). BRZ-INSENSITIVE PALE GREEN2, a Brz-induced gene encoding a chloroplast protein necessary for normal chloroplast biogenesis in Arabidopsis (Komatsu et al., 2010), provides further evidence for a role of BRs in the control of photosynthesis. Moreover, BES1/BZR2 has been shown to prevent chloroplast development in darkness by repressing the expression of GOLDEN2-LIKE1 (GLK1) and GLK2, two transcription factors that function redundantly to promote chloroplast development (Fitter et al., 2002; Waters et al., 2008, 2009; Yu et al., 2011).
Delayed leaf senescence, or a stay-green phenotype, is generally considered a desired characteristic in crops and constitutes a target for improvement of crop productivity (Horton, 2000). Leaf senescence is a complex process controlled by environmental as well as internal factors, such as irradiance, nitrogen and carbon status, and hormonal control. BRs may play a role in promoting leaf senescence because (1) several of the BR biosynthetic and signaling mutants, such as de-etiolated2 (det2) and bri1, display a delayed senescence phenotype (Clouse and Sasse, 1998), and (2) exogenous BR treatment induces leaf senescence in several plant species, such as mung bean (Vigna radiata) and wheat (He et al., 1996; Sağlam-Çağ, 2007). To date, the molecular mechanism of BR action on leaf senescence is still unknown.
Root Development
Roots are an important determinant of crop productivity given their role in water and nutrient uptake from the soil. BRs exert both positive and negative effects on root growth, depending on the applied concentration: Root growth is stimulated by low concentrations and inhibited by high concentrations of exogenous BR (Müssig et al., 2003). Many of the Arabidopsis sterol- and BR-deficient mutants display reduced root growth and lateral root formation (Bao et al., 2004), suggesting a positive role of sterols and BRs on root development at their physiological concentrations. The length and number of lateral roots are reduced in BR mutants of pea as well (Ferguson et al., 2005). It has also been established that BRs interact with auxin signaling to promote lateral root growth (Bao et al., 2004) and negatively regulate jasmonate inhibition of root growth in Arabidopsis (Huang et al., 2010). Several sterol and BR mutants also display alterations in root hair formation (Pérez-Pérez et al., 2002; Souter et al., 2002). Interestingly, the root hair phenotype of the Arabidopsis sterol biosynthesis mutant fk was partially rescued by inhibiting the auxin and ethylene signaling but not by applying exogenous sterols and BRs, suggesting that sterols are required for correct auxin and ethylene signaling (Souter et al., 2002). More recently, the local distribution of structural sterols has been proposed to be involved in both the initiation and tip growth of root hairs by regulating the vesicular trafficking and plasma membrane properties of root cells (Ovečka et al., 2010). By contrast, BRs are required to maintain position-dependent fate specification and to control meristem size in Arabidopsis roots by promoting the cell cycle progression (Kuppusamy et al., 2009; González-García et al., 2011; Gudesblat and Russinova, 2011; Hacham et al., 2011).
PLANT STEROIDS AND REPRODUCTION
Flowering Time
Another very important agronomical trait is the flowering time. Floral induction is a complex developmental process that requires the integration of endogenous signals (including hormones) and environmental cues (especially temperature and photoperiod) to ensure that flowering occurs under appropriate environmental conditions (Srikanth and Schmid, 2011). Plants that flower late tend to have high total seed production as a result of extended vegetative growth phase and source strength. However, delayed flowering in crops is generally undesirable (Van Camp, 2005).
In many BR mutants, the flowering time is delayed, suggesting a role for BRs in the control of this trait (Chory et al., 1991; Li and Chory, 1997; Azpiroz et al., 1998; Li et al., 2010). In support of this hypothesis, BRI1-mediated signaling promotes flowering in Arabidopsis by repressing the expression of the MADS box transcription factor FLOWERING LOCUS C (FLC) (Domagalska et al., 2007). Histone acetylation is dramatically increased at the FLC locus in the double mutant between bri1 and the late-flowering autonomous-pathway mutant luminidependens, suggesting that chromatin remodeling may be involved in the BR regulation of flowering. Interactions between BES1/BZR2 and the chromatin remodeling factors EARLY FLOWERING6 and RELATIVE OF EARLY FLOWERING6, two Jumonji domain–containing histone demethylases, might provide a molecular link between BRs and flowering time (Clouse, 2008; Yu et al., 2008). Recently, the role of BRs in regulating the flowering time has been shown to depend on its interaction with gibberellin (Domagalska et al., 2010).
Male and Female Fertility
Seed production in flowering plants relies on the formation of male and female gametophytes in the reproductive organs and is regulated by various external and internal cues. Many of the BR-deficient and BR-insensitive mutants display reduced male fertility (Ye et al., 2010). Systematic phenotypic analysis of the male reproductive organs of these mutants has revealed defects in anther development and in pollen number, viability, and release efficiency. These phenotypes have been associated with abnormal tapetal and microspore development; consistently, the expression of several genes involved in pollen wall formation and in tapetal and microspore development was reduced in Arabidopsis BR-related mutants. Moreover, BES1/BZR2 directly binds to the promoter of genes encoding transcription factors required for anther and pollen development, such as SPOROCYTELESS/NOZZLE, DEFECTIVE IN MERISTEM DEVELOPMENT AND FUNCTION1, ABORTED MICROSPORES, MALE STERILITY1 (MS1), and MS2 (Ye et al., 2010).
In addition to their role in male fertility, BRs may also play a role in female reproductive organ development. Mutation in the BR biosynthetic enzyme CYP85A2 enhances the phenotype of the Arabidopsis mutant seuss, which is defective in ovule and gynoecial development (Nole-Wilson et al., 2010). In addition, the CYP85A1 gene is expressed in the developing female gametophyte and cyp85a1 mutants exhibit a semisterile phenotype (Pérez-España et al., 2011). Fertility is also reduced in many BR-deficient rice mutants, such as leaf and tiller angle increased controller mutants (Wang et al., 2008). However, it remains to be determined whether the same function as the one established for BRs in male and female gametophyte development in Arabidopsis also applies to monocot species. Recently, the dwarf maize mutant nana plant1 that carries a loss-of-function mutation in the BR biosynthetic enzyme DET2 was found to display feminized male flowers (Hartwig et al., 2011). Feminized inflorescences were also observed in the dwarf brassinosteroid-dependent1 (brd1) maize mutant plants defective in a C6 oxidase BR biosynthetic enzyme (Makarevitch et al., 2012), suggesting an important role of BRs in the control of sex determination in maize.
Seed Development and Seed Filling
Seed yield is the most important agronomical trait in grain crops, and extensive efforts are made to enhance it, under both optimal and suboptimal conditions, especially in the three major cereal crops worldwide, namely, maize, wheat, and rice. In rice, the yield potential consists of four main components: grain weight (highly controlled by genetic factors), grain number per panicle, panicle number per plant (closely associated with tiller number per plant), and proportion of filled grains (strongly affected by environmental factors) (Sakamoto and Matsuoka, 2008). Concerning yield improvement in the major cereal crops, increased seed number is preferred over increased seed size or seed weight to limit the possible alterations in taste, cooking, and chalkiness properties often seen with larger, heavier grains (Fitzgerald et al., 2009).
There is convincing evidence for a role for BRs in plant seed production. Although in Arabidopsis detailed reports on seed yield and seed characteristics of BR-related mutants and transgenic plants are scarce, a few examples reveal BR effects on these traits. The Arabidopsis dwf5 mutant produced irregularly shaped, wrinkled seeds (Choe et al., 2000) similar to the seeds of the equivalent lk mutant in pea (Nomura et al., 2004, 2007). Overexpression of DWF4 in Arabidopsis increased seed weight per plant by ~60%, mainly due to more seeds produced than in the wild type as a result of an elevated number of branches and siliques (Choe et al., 2001). Likewise, an increased silique number and seed yield were observed in transgenic Arabidopsis plants overexpressing the HSD1 gene (Li et al., 2007).
Reports on BR-deficient or BR-insensitive mutants and transgenic plants with altered seed characteristics and/or seed yield are more numerous in rice. Knockout or downregulation of BR biosynthetic genes and positive regulators of the BR signaling pathway in rice, generally result (except in case of gene redundancy) in sterility and/or strongly reduced seed yield due to smaller and rounder seeds. Such phenotypes are observed in loss-of-function mutants of CYP90D2, DWF1/BRD2, DWARF11 (D11/CYP724B1), BRD1, BRI1, and HETEROTRIMERIC G PROTEIN α SUBUNIT1 (Ashikari et al., 1999; Mori et al., 2002; Hong et al., 2003, 2005; Tanabe et al., 2005; Morinaka et al., 2006; Oki et al., 2009). By contrast, rice transgenic plants overexpressing the transcription factor BRASSINOSTEROID UPREGULATED1, a positive regulator of BR signaling, have enlarged seeds (Tanaka et al., 2009). In rice, overexpression of the maize, rice, or Arabidopsis DWF4/CYP genes under the control of a promoter that is active in stems, leaves, and roots, but not in seeds, also positively affected seed yield. The transgenic rice plants had ~15 to 44% higher grain yield per plant than that of the wild-type plants due to more numerous and bigger seeds both under greenhouse and field conditions (Wu et al., 2008). By contrast, overexpression of Os DWF4 under the control of a medium constitutive promoter in rice led to a reduction in both growth and seed production (Reuzeau et al., 2005), highlighting the importance of promoters for the success of a transgene.
The molecular mechanisms by which seed size is regulated by BRs remain to be determined. Possibly, they might involve known effects of BRs on cell division, elongation, and differentiation. Alternatively, the effects of BRs on seed size might be driven by an enhanced seed filling caused by an increased carbon flux, as suggested by the analyses of transgenic rice plants overexpressing At DWF4/Zm CYP (Wu et al., 2008).
Dose-dependent, tissue/organ-specific phenotypic effects of BRs are observed in allelic series of the Os bri1 mutants. Whereas null mutant alleles display a very severe dwarfism and are sterile, weak mutant alleles are semidwarf, have an erect leaf phenotype, and remain fertile. Although when planted at a high density, the weakest mutant alleles display a high grain number, no seed yield increase was observed because of the smaller grain size (Morinaka et al., 2006). Taking into account that leaf lamina joints are more sensitive to altered BR synthesis/signaling than other tissues/organs, such as seeds, rice plants with an erect leaf phenotype and no negative effect on seed size were obtained by using a partial suppression strategy (Morinaka et al., 2006). It was estimated that such a phenotype would lead to ~30% increase in grain yield under high planting density. A similar partial suppression strategy has also been applied to the rice BRI1-ASSOCIATED RECEPTOR KINASE1 (Os BAK1) gene encoding a BRI1 coreceptor (Li et al., 2009). These results illustrate the usefulness of obtaining allelic series of genes of interest and/or of carrying out partial suppression strategies.
Gene duplication may also be exploited for crop improvement. For instance, the rice ebisu dwarf d2 mutant, defective in the CYP90D2 gene encoding one of the enzymes of BR synthesis, is severely dwarfed, produces smaller seeds, and has reduced seed yield (Hong et al., 2003). In Arabidopsis, two CYP90 genes function redundantly in the same step. Whereas the single mutants of each of these genes have a weak and subtle phenotype, the At cyp90c1 cyp90d1 double mutant displays a severe dwarf phenotype similar to the rice d2 mutant (Ohnishi et al., 2006). Conversely, in Arabidopsis, one DWF4 gene seems to be sufficient, while in rice two genes, namely, Os DWF4 and Os DWF4L1/D11, are necessary to perform the same function. Hence, the At dwf4 null mutant shows severe dwarfism (Azpiroz et al., 1998), whereas in rice, each of the Osdwf4 and Osdwf4l1/d11 single mutants have weak phenotypes regarding dwarfism and leaf erectness, and only the Osdwf4dwf4l1/d11 double mutant displays a severe dwarf phenotype (Sakamoto et al., 2006). The particularly high degree of gene duplication in cereal genomes suggests that selective inactivation of some BR-related gene family members could be more widely exploited as a strategy to alter the plant architecture in a subtle manner.
To date, little is known regarding the consequences of altering the endogenous BR level on the seed composition (carbohydrates, proteins, fats, and minerals). Experiments involving exogenous BR application on wheat and soybean (Glycine max) plants and seeds suggest that BRs might significantly affect it (Janeczko et al., 2009, 2010). In rice, treatment with BRs at anthesis increased starch content in kernels (Fujii and Saka, 2001). Additional studies on the BR effects on seed composition are needed if the BR pathway components are to be manipulated for grain crop improvement.
Fiber Production
In cotton (Gossypium herbaceum), fiber yield and quality are the most important traits. Cotton fibers consist almost exclusively of cellulose grown in a protective capsule (boll) around the seeds to aid their dispersal. Besides being a major constituent of cotton fiber, cellulose is a biopolymer that contributes to cell wall formation during cell elongation and expansion. A crucial role for cellulose synthesis has been established for phytosterols (Schrick et al., 2004) and, more recently, also for BRs. BR-deficient or BR-insensitive Arabidopsis mutants contain less cellulose than wild-type controls, and the expression of the cellulose synthase genes is regulated by BES1/BZR2 (Xie et al., 2011).
There are indications that careful manipulation of the BR content may significantly improve cotton fiber production. Constitutive and ectopic overexpression of DET2 in cotton led to shortened branches, sterility, and boll abortion. By contrast, overexpression of DET2 under the control of a seed coat–specific promoter resulted in fertile plants with 22.6 and 10.7% increase in fiber number and length, respectively (Luo et al., 2007). Besides demonstrating an important role for BRs in cotton fiber yield, this study emphasizes that promoter selection for transgene expression is critical and that alteration in BR levels must be optimal (both spatially and in terms of expression level) to produce a desirable phenotype. Overall, these results and others suggest that fine-tuning of the BR production and responses (through organ/tissue-specific expression and/or moderate increase of gene expression) is a very promising, yet largely underused, strategy for crop improvement.
Fruit Ripening
BRs also have a role in stimulating fruit ripening. In grape berry (Vitis vinifera), the ripening period was associated with an increase in catasterone levels. In addition, exogenous BR application enhanced the rate of berry ripening, whereas the inhibition of BR synthesis by application of Brz significantly delayed it (Symons et al., 2006). BRs are also implicated in the ripening of tomato fruits. Application of BRs to tomato pericarp discs increased the lycopene and carbohydrate contents and lowered the chlorophyll and ascorbic acid levels. This BR-induced fruit ripening has been associated with increased ethylene production (Vardhini and Rao, 2002).
PLANT STEROIDS AND STRESS TOLERANCE
In addition to their impact on many plant growth and developmental processes, BRs play a role in biotic and abiotic stress tolerance, including disease resistance and tolerance against drought, salt, heat, cold, hypoxia, pesticides, and heavy metals. Numerous studies have shown that exogenous BRs enhance plant stress tolerance (Krishna, 2003; Bajguz and Hayat, 2009; Gomes, 2011) (see Supplemental Table 3 online). In this section, we will focus on the effects of altering gene expression and endogenous content of BRs on the plant tolerance to stresses. Evidence for a role of phytosterols in stress tolerance is also included.
Salt and Drought Stress Tolerance
Water availability and high salt concentrations are the most limiting abiotic factors for crop productivity. Whereas many studies demonstrate a positive effect of BR application on plant tolerance to salt and drought stresses (Krishna, 2003; Bajguz and Hayat, 2009; Gomes, 2011), only a few studies have been performed to evaluate the effects of altered endogenous BR content on these traits. Overexpression of At HSD1 in Arabidopsis increased tolerance to salt stress (Li et al., 2007). By contrast, the semidwarfed barley uzu mutant that is defective in BRI1 gene displayed reduced tolerance to this stress (Chono et al., 2003). Similarly, seeds and seedlings ofthe Arabidopsis BR-deficient mutant det2-1 and the BR signaling gain-of-function and semidominant mutant brassinosteroid-insensitive2-1 (bin2-1), defective in the GLYCOGEN SYNTHASE KINASE3 (GSK3)/Shaggy-like protein kinase BIN2, were more sensitive to salt stress than those of the wild type (Zeng et al., 2010). Conversely, the rice loss-of-function gsk1 mutant carrying a T-DNA insertion in the ortholog of the Arabidopsis BIN2 gene displayed an increased tolerance to both salt and drought stresses when compared with the wild type (Koh et al., 2007).
Although the results described above point toward a clear effect of BRs on the plant salt and drought stress tolerance, the molecular mechanisms involved in these processes remain largely unknown. BRs might affect plant drought tolerance by controlling the density and/or opening of stomata. The density or clustering of stomata per leaf area is increased in the Arabidopsis BR-deficient mutant constitutive photomorphogenic dwarf (cpd/cyp90a1) (Schlüter et al., 2002) as well as in the sterol biosynthetic mutant dwf7-3/ste1-4/bul1-1 (Catterou et al., 2001). Recently, RNA interference–mediated disruption of the rice SQUALENE SYNTHASE (SQS) gene has been reported to reduce the stomatal conductance and to improve drought tolerance at both the vegetative and reproductive stages (Manavalan et al., 2012). Given that SQS catalyzes the first reaction of the isoprenoid metabolic pathway committed to sterol synthesis, these results suggest that decreased sterol and BR content might enhance stress tolerance and confirm the potential of manipulating plant steroid content to increase crop yield under stress conditions. The apparent contradictions between some of the results presented above highlight the need for more research in this area.
Another possible molecular mechanism that links BRs with abiotic stress acclimation/tolerance involves endoplasmic reticulum signaling. Regulated intramembrane proteolysis (RIP) is a conserved stress response mechanism in eukaryotes. In plants, RIP is implicated in responses to protein misfolding, salt, and heat stresses. Stress-mediated increase in the translocation to the nucleus of two bZIP transcription factors associated with this process (namely, bZIP17 and bZIP28) activated BR signaling that was required for acclimation to stresses (salt, heat, and protein glycosylation inhibition) (Che et al., 2010).
Thermotolerance (Heat and Cold Stresses)
Thermal (heat and cold) stresses have a high impact on seed yield. Crops are particularly sensitive to thermal stresses during their reproductive stages (Zinn et al., 2010). Exogenous BR treatment has been shown to significantly enhance plant tolerance to both heat and cold stresses (Dhaubhadel et al., 1999, 2002; Kagale et al., 2007; Xia et al., 2009b). By contrast, a few studies have been performed to study the effect of altering endogenous BR contents on plant thermotolerance and their results are somehow contradictory. Overexpression of At DWF4 gene in Arabidopsis seeds led to transgenic seedlings that were more tolerant to cold stress than wild-type seedlings (Divi et al., 2010). The barley uzu mutant displays an enhanced resistance to lodging, but it is also less tolerant to cold (Chono et al., 2003). By contrast, the Arabidopsis bri1-9 mutant has an increased tolerance to cold compared with the wild-type controls, whereas the transgenic plants overexpressing At BRI1 have the opposite phenotype. These results correlated with an increased expression of stress-inducible genes and transcription factors regulating them in the bri1-9 mutant compared with the BRI1-overexpressing plants (Kim et al., 2010). Data in yeast (Saccharomyces cerevisiae) also suggest a role for sterols in stress tolerance (Swan and Watson, 1998). It remains to be determined whether phytosterols also affect tolerance to stresses in plants.
Oxidative Stress Tolerance
Reactive oxygen species play a role in both plant growth and development and stress responses. Reactive oxygen species are generated in response to many abiotic and biotic stresses, including drought, salt, cold, heat, heavy metal exposure, high light, nutrient deprivation, and pathogen attack.
Results of BR treatments and studies of the Arabidopsis det2 mutant reveal a BR function in the plant responses to oxidative stresses (Bajguz and Hayat, 2009; Xia et al., 2009b, 2011). The det2 mutant is insensitive to the negative growth effect of very low O2. This enhanced oxidative stress resistance has been associated with a constitutive increase in superoxide dismutase activity and in catalase transcript levels, suggesting that long-term BR deficiency results in a constant in vivo physiological stress in the det2 mutant (Cao et al., 2005). These results imply that endogenous BR levels are negatively correlated with the plant tolerance to stress. By contrast, BR levels positively correlated with an increased tolerance to photooxidative stress in cucumber plants treated with 24-epibrassinolide (EBL) and Brz (Xia et al., 2009b). Most likely, exogenous BRs contribute to stress tolerance via the production of antioxidants that protect cells from damage. BR treatment also improved the activity of the photosynthetic machinery and antioxidant defense system in two tomato cultivars exposed to increasing levels of cadmium in the soil (Hasan et al., 2011). These data highlight some differences in the effect between BR application and manipulation of the endogenous BR level and/or between plant species.
Phytosterols also seem to be involved in the plant responses to oxidative stresses. Transgenic Brassica juncea plants overexpressing the positive regulator of the sterol synthesis, the enzyme 3-hydroxy-3-methylglutaryl-CoA synthase, displayed reduced hydrogen peroxide–induced cell death (Wang et al., 2012).
Pathogen Resistance
Pathogen attacks are major limiting factors of crop productivity. In the evolutionary arms race between plants and their pathogens, plants have evolved a highly sophisticated defense system in which plant hormones play a pivotal role. The hormones salicylic acid, jasmonate, and ethylene are well known regulatory signals of the plant’s immune response, and pathogens can antagonize it by affecting its hormone homeostasis. More recently, other plant hormones, including BRs, have been implicated in plant defense mechanisms (Bari and Jones, 2009; Pieterse et al., 2009).
When applied exogenously, BRs induce resistance against a broad range of diseases, notably against fungal and bacterial pathogens in tobacco and in rice. In rice, treatment with BL protected the plant against rice blast and bacterial rice blight diseases, whereas in tobacco, it enhanced resistance against the bacterial pathogen Pseudomonas syringae and the fungal pathogen Odium species (Nakashita et al., 2003).
Another important aspect of the plant protective action of BRs is their capacity to improve virus resistance. Treatment of tobacco plants with BL reduced infection by the Tobacco mosaic virus (Nakashita et al., 2003). Similarly, potato (Solanum tuberosum) cuttings cultured in a medium containing BL, EBL, or 28-homobrassinolide were more resistant to viral infection through all developmental stages (Khripach et al., 2000). BR analogs also possess biological effects on insects. More particularly, BRs can influence ecdysteroid-dependent steps of insect development (Zullo and Adam, 2002).
Besides its role as coreceptor of BRI1, BAK1/SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE3 (SERK3) is involved in a pathogen-sensing pathway and in cell death responses (Kemmerling et al., 2007). The interaction of BAK1/SERK3 with receptors that recognize PATHOGEN-ASSOCIATED MOLECULAR PATTERNS (PAMPs), such as bacterial flagellin (flg22), initiates innate immunity responses (Chinchilla et al., 2007; Heese et al., 2007; Roux et al., 2011; Schwessinger et al., 2011). Recently, a role for BAK1/SERK3 in herbivory-induced defense responses has been established (Yang et al., 2011). However, the role of BAK1/SERK3 in biotic stress responses seems independent of its function in BR signaling (Kemmerling et al., 2007). In this regard, it is interesting to note that the tomato golden mosaic virus pathogenicity protein C4 interacted with the Arabidopsis BIN2 in yeast two-hybrid assays (Piroux et al., 2007), suggesting that BR signaling components other than BAK1 may be directly involved in the plant responses to biotic stresses. Recently, a direct role for endogenous BRs in pathogen resistance has been established with the discovery that an increase or decrease in endogenous BRs compromises PAMP-induced responses in Arabidopsis (Belkhadir et al., 2012). In addition, BRs were also shown to inhibit PAMP-triggered immune signaling independently of BAK1 (Albrecht et al., 2012). Importantly, results of these two studies also suggested the existence of a tradeoff in which BR-mediated growth directly antagonizes innate immune signaling.
Elevated phytosterol content in B. juncea plants increases resistance to Botrytis cinerea (Wang et al., 2012). This sterol-enhanced pathogen resistance may be of a physical nature because cell membranes are an important physical barrier against pathogen infection and sterols are known to play a role in membrane rigidity and permeability (Hartmann, 1998). It is also interesting to note that treatment with exogenous ergosterol, the main sterol of most fungi, promoted the expression of a number of defense genes in tobacco and grape and enhanced the production of resveratrol and the tolerance against the fungal pathogen B. cinerea in grape (Laquitaine et al., 2006; Lochman and Mikes, 2006).
Heavy Metal Stress and Herbicide and Pesticide Tolerance
High concentrations of metals, including those essential for growth, have a toxic effect on plant metabolism. Numerous lines of evidence in different crops show that BRs interfere with the uptake of heavy metals and promote their detoxification, notably by enhancing the production of antioxidant enzymes and the accumulation of Pro (Bajguz and Hayat, 2009; Hasan et al., 2011).
Pesticides that include herbicides, fungicides, and insecticides play a major role in agriculture by reducing crop yield losses, but these molecules can also have a negative effect on the crop and can be detrimental to human health and the environment. BRs have been shown to reduce the damages caused by pesticides by accelerating their catabolism, consequently reducing their residual levels in the plants. Hence, BRs may be promising molecules suitable for application on crops to diminish the risks and deleterious effects associated with the exposure of humans, crops, and the environment to agrochemical substances (Bajguz and Hayat, 2009; Xia et al., 2009c). To date, nothing is known about the impact of altering endogenous BR levels on the plant responses to heavy metal and agrochemical stresses.
OTHER FUNCTIONS AND AGRONOMICAL APPLICATIONS OF PLANT STEROIDS
Effects of BRs on Plant Metabolism
A first piece of evidence suggesting that alteration of the BR content may affect the plant primary metabolism came from the analysis of Arabidopsis BR-deficient cpd mutants and antisense CPD transgenic plants. Both genotypes display significantly reduced starch content that is associated with altered activities of starch enzymes (Schlüter et al., 2002). In addition, the two Arabidopsis BETA-AMYLASE7 (BAM7) and BAM8 proteins, also named BZR-BAM because they display similarity to BZR1 in their N-terminal domain, have been shown to bind to a cis-regulatory element resembling a BR-responsive element. These BZR-BAM proteins regulate the expression of many BR-regulated genes, and alteration of their expression causes phenotypes similar to BR-deficient and BR-insensitive mutants. Hence, BZR1-BAM proteins appear to control plant growth and developmental processes through crosstalk with the BR signaling and potentially in response to metabolic signals (Reinhold et al., 2011).
BRs have also been implicated in the stimulation of the source-to-sink flow of assimilates in rice. More particularly, transgenic rice plants overexpressing the At DWF4/Zm CYP gene had enhanced CO2 assimilation, increased glucose pools in the flag leaves, and elevated glucose assimilation to starch in the seeds, leading to improved grain filling and larger seeds (Wu et al., 2008). In addition, Arabidopsis EXORDIUM-LIKE1, a BR-regulated gene, has recently been shown to be involved in the carbon starvation response, suggesting a link between BRs and the control of plant growth and development under low carbon availability (Schröder et al., 2011). Moreover, Arabidopsis bri1 Y831F mutant plants have been found to have an enhanced starch accumulation as well as increased levels of Suc and several amino acids, most prominently Gly and Pro (Oh et al., 2011). Additional evidence for a link between BR and carbohydrate metabolism concerns the Arabidopsis brassinosteroid, light, and sugar1 mutant. In addition to BR-deficient-like phenotypes (deetiolation in darkness, dwarfism, and compact rosette and short roots), this mutant displayed sugar hypersensitivity that can be rescued by BR application (Laxmi et al., 2004). Lastly, fruits of the tomato dx mutant that do not express a functional CYP85A1/DWARF (D) BR biosynthetic enzyme showed reduced level of starch and sugar and elevated levels of amino acids (Lisso et al., 2006).
Evidence also supports a role for BR in nitrogen metabolism. The transthyretin-like protein, evolutionarily related to allantoin synthase that is involved in purine ring metabolism (Werner and Witte, 2011), interacted with BRI1 in a yeast two-hybrid assay (Nam and Li, 2004). Moreover, the erect leaves in BR-deficient rice not only increased light capture for photosynthesis, but also enhanced nitrogen storage for grain filling (Sinclair and Sheehy, 1999). Lastly, BRs are involved in the regulation of symbiotic nitrogen fixation in legumes as revealed by BR-deficient mutants of pea that display a reduced nodule organogenesis (Ferguson et al., 2005). BRs also controlled the number of nodules in soybean (Terakado et al., 2005) and in common bean (Phaseolus vulgaris) (Upreti and Murti, 2004). It would be interesting to determine whether altered endogenous BR contents affect other plant primary and secondary metabolism processes.
Nutritional Quality of Crops and Medicinal Application of Phytosterols and BRs
Alterations in sterol composition in plants may lead to improved agricultural varieties destined for food and feed. Notably, phytosterol profiles can be manipulated to influence the levels of other metabolites, including some not suitable for industrial processing and/or mammalian consumption. For example, potato tubers can contain high amounts of the compound glycoalkaloids (TGAs), which may be toxic to humans. Therefore, new commercial varieties must have low levels of TGAs (Friedman, 2006). Cholesterol is present in relatively high amounts in potato and is presumably the biosynthetic precursor of TGAs. Overexpression of the soybean rate-limiting sterol synthesis gene STEROL METHYLTRANSFERASE1 in potato decreased the levels of TGAs in leaves and tubers by 41 and 63%, respectively. Levels of free cholesterol, a nonalkylated sterol, were also decreased (Arnqvist et al., 2003). Hence, engineering of phytosterol profiles offers the opportunity to reduce the amount of TGA metabolites unsuitable in crops for animal/human nutrition.
Phytosterols have been identified also as important blood cholesterol lowering agents in humans (Moreau et al., 2002; Quílez et al., 2003). Therefore, conjugated forms of phytosterols (steryl-esters and stanol-esters) have been incorporated into food products, such as margarine and milk derivatives (Schaller, 2004). In addition to their beneficial role in lowering cholesterol levels, a relatively high consumption of phytosterols may also enhance the immune function and the activity of antioxidant enzymes, reducing oxidative stress in humans (Bouic, 2001). As dietary components, phytosterols have been suggested to offer protection against many common cancers, such as prostate, breast, and colon cancer (Awad and Fink, 2000; Bradford and Awad, 2007; Woyengo et al., 2009). Such beneficial effects of phytosterols are expected to increase research efforts to engineer the phytosterol synthesis pathway in crop plants.
Recent studies indicate that BRs also have valuable properties for human health, including antiviral and anticancer activities. The BR analogs 28-homocastasterone and EBL inhibit cell growth in a dose-dependent manner in breast and prostate cancer cell lines without affecting the normal nontumor cell growth, suggesting that BRs may be promising leads as potential anticancer drugs (Malíková et al., 2008; Steigerová et al., 2010). Furthermore, BR analogs have been reported to have antiviral activities (Wachsman et al., 2000, 2002; Castilla et al., 2005; Romanutti et al., 2007).
USE OF EXOGENOUS STEROIDS AND STEROID INHIBITORS IN AGRICULTURE
Evidence shows that exogenous BRs can enhance vegetative growth, reproduction, and tolerance to a wide range of abiotic and biotic stresses in both model plant species and crops. Opposite phenotypic effects are generally obtained when applying inhibitors of plant sterols and BR synthesis in both model plants and crops (see Supplemental Table 3 online).
When applying exogenous sterols, BRs or their inhibitors to plants, several parameters need to be considered, including concentration, application mode, and application time (Gomes, 2011). Notably, the concentration used is not always compatible with the endogenous BR concentrations in plants, leading to confusing effects. Information is often lacking in the literature on the time lapse between the BR application and the observed physiological manifestation of the effects and on their duration. The type of BR compound applied is also very important when studying the physiological effects. To date, only three BR compounds, EBL, 28-homobrassinolide, and BL, have been largely used. Much remains to be discovered on the effect of application of other BR intermediates and conjugates on the phenotypes of different plant species. In particular, it would be essential to investigate the specific action of the different compounds. Even when all the parameters mentioned above are considered, it appears that the responsiveness to BRs can vary significantly depending on plant species, developmental stage, organ/tissue, and environmental conditions (Gomes, 2011). This variability in the results, together with the high cost of synthetic BR analogs, has discouraged the use of exogenous BRs in agriculture and horticulture (Khripach et al., 2000). By contrast, modulation of endogenous BR levels by altering the expression of genes involved in the BR pathways represents a promising strategy for improving crops yield in a more uniform and predictable manner (Divi and Krishna, 2009).
CONCLUSION AND PERSPECTIVES
Plant architecture, reproduction capacity, and tolerance to various abiotic and biotic stresses are among the agronomically important traits controlled by phytosterols and BRs. The prospective agronomical applications of the research on phytosterols and BRs require the consideration of several criteria: trait penetrance (the frequency with which identical genotypes display similar phenotypes), trait transferability (the likelihood of an improved trait of interest to be transferred to other crop species), performance across traits, and environmental conditions. To date, information is scarce on sterol and BR pathways in plants other than Arabidopsis and rice. Therefore, the potential exists to identify BR mutants/transgenics with improved productivity in agronomically important crops such as rice, maize, wheat, soybean, potato, poplar (Populus spp), banana (Musa spp), and other fruit crops. Preliminary results indicate a good level of transferability between dicots and monocots regarding the effects of BRs on plant architectural traits. Improving the predictability of trait transferability from model to crop species as well as between crops is highly desirable for future agrobiotechnological applications. In this regard, performing comparative genomics and organ/tissue-specific gene expression analyses to identify conservation and divergence in sterol and BR pathway components across plant species should be very useful.
Phytosterols and BRs affect seed yield and stress tolerance. The nutritional and seed qualities also constitute important traits to take into account. Whereas there is evidence of positive effects on human health of increased phytosterol content in several crops, the impact of modified endogenous phytosterol and/or BR content on the nutritional quality of seeds has yet to be determined. In contrast with the homogenous growth conditions found in a greenhouse, plants in the field are constantly challenged by changing growth conditions and a wide range of stresses. New plant varieties therefore need to be able to produce increased seed yields, under both optimal and suboptimal conditions (Van Camp, 2005). Although treatment with BRs has positive effects on plant tolerance to a wide range of stresses, it remains unclear whether increased or decreased endogenous BR levels and BR sensitivity in mutant and transgenic plants overall lead to either enhanced or reduced stress tolerance. Possibly, these effects depend on plant species, stress, and BR concentrations. Different compositions in endogenous sterol and BR pools might also lead to specific stress responses. Furthermore, the molecular mechanisms by which BRs control plant stress responses remains largely unknown, although it is clear that BRs exert their antistress effects both independently and via interaction with other hormones (Divi et al., 2010). Dose-dependent analyses on BR mutants and transgenic plants and comparative studies between different plant model species and crops exposed to various biotic and abiotic stresses would need to be performed before sterols and BRs can be used to increase crop stress tolerance.
As changes in BR content cause a mix of positive and negative effects on traits of agronomic importance (e.g., increased leaf erectness, delayed senescence associated with delayed flowering time, reduced seed yield, and increased seed size associated with increased leaf bending in rice), it is of the utmost importance to determine how one BR effect could be uncoupled from another. In this regard, the results obtained with the allelic series of bri1 mutants in rice are promising because they hint at a possibility of obtaining BR-specific and dosage-dependent, tissue-specific responses (Morinaka et al., 2006; Oh et al., 2011). Alternatively, the use of tissue-specific or inducible promoters also represents a promising strategy for increasing crop yield (Luo et al., 2007; Wu et al., 2008). Overall, the current most encouraging approach to obtain crops with increased seed yield under both optimal and stress conditions is the fine-tuned modulation of the endogenous BR contents and/or signaling pathways via partial downregulation and/or overexpression under the control of a conditional promoter driving gene expression in specific organs/tissues, at specific developmental stages, or in response to specific environmental conditions. Given the generally observed positive effect of BRs on agronomically important traits, moderate increase of early BR signaling gene expression/protein activities associated with increased endogenous BR levels appears to be a promising strategy.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table 1. Arabidopsis Plant Steroid-Related Genes and Phenotypes of the Mutants and Transgenic Plants.
Supplemental Table 2. Modified Expression of Plant Steroid-Related Genes in Crops and the Resulting Phenotypic Effects.
Supplemental Table 3. Effects of Exogenous Application of Steroid Compounds and Inhibitors on Plant Growth, Reproduction, and Stress Tolerance.
Acknowledgments
We thank Jan De Meutter, Rocío Sánchez-Fernández, Fernando Ferreira, Marieke Louwers, and Hatem Rouached for careful reading and critical comments on the manuscript as well as Martine De Cock and Annick Bleys for help in preparing it. This work was supported by a grant from the European Union FP7 framework program (BRAVISSIMO; Grant PITN-GA-2008-215118).
AUTHOR CONTRIBUTIONS
C.V., E.R., and C.R. wrote the article.
References
- Albrecht C., Boutrot F., Segonzac C., Schwessinger B., Gimenez-Ibanez S., Chinchilla D., Rathjen J.P., de Vries S.C., Zipfel C. (2012). Brassinosteroids inhibit pathogen-associated molecular pattern-triggered immune signaling independent of the receptor kinase BAK1. Proc. Natl. Acad. Sci. USA 109: 303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnqvist L., Dutta P.C., Jonsson L., Sitbon F. (2003). Reduction of cholesterol and glycoalkaloid levels in transgenic potato plants by overexpression of a type 1 sterol methyltransferase cDNA. Plant Physiol. 131: 1792–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikari M., Wu J., Yano M., Sasaki T., Yoshimura A. (1999). Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the α-subunit of GTP-binding protein. Proc. Natl. Acad. Sci. USA 96: 10284–10289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athwal D.S. (1971). Semidwarf rice and wheat in global food needs. Q. Rev. Biol. 46: 1–34 [DOI] [PubMed] [Google Scholar]
- Awad A.B., Fink C.S. (2000). Phytosterols as anticancer dietary components: Evidence and mechanism of action. J. Nutr. 130: 2127–2130 [DOI] [PubMed] [Google Scholar]
- Azpiroz R., Wu Y., LoCascio J.C., Feldmann K.A. (1998). An Arabidopsis brassinosteroid-dependent mutant is blocked in cell elongation. Plant Cell 10: 219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajguz A., Hayat S. (2009). Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 47: 1–8 [DOI] [PubMed] [Google Scholar]
- Bao F., Shen J., Brady S.R., Muday G.K., Asami T., Yang Z. (2004). Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol. 134: 1624–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R., Jones J.D.G. (2009). Role of plant hormones in plant defence responses. Plant Mol. Biol. 69: 473–488 [DOI] [PubMed] [Google Scholar]
- Baud S., et al. (2009). Regulation of HSD1 in seeds of Arabidopsis thaliana. Plant Cell Physiol. 50: 1463–1478 [DOI] [PubMed] [Google Scholar]
- Belkhadir Y., Jaillais Y., Epple P., Balsemão-Pires E., Dangl J.L., Chory J. (2012). Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proc. Natl. Acad. Sci. USA 109: 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G.J., Koncz C. (2002). Brassinosteroids and plant steroid hormone signaling. Plant Cell 14(suppl.): S97–S110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouic P.J.D. (2001). The role of phytosterols and phytosterolins in immune modulation: A review of the past 10 years. Curr. Opin. Clin. Nutr. Metab. Care 4: 471–475 [DOI] [PubMed] [Google Scholar]
- Bou-Torrent J., Roig-Villanova I., Galstyan A., Martínez-García J.F. (2008). PAR1 and PAR2 integrate shade and hormone transcriptional networks. Plant Signal. Behav. 3: 453–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford P.G., Awad A.B. (2007). Phytosterols as anticancer compounds. Mol. Nutr. Food Res. 51: 161–170 [DOI] [PubMed] [Google Scholar]
- Caño-Delgado A., Lee J.-Y., Demura T. (2010). Regulatory mechanisms for specification and patterning of plant vascular tissues. Annu. Rev. Cell Dev. Biol. 26: 605–637 [DOI] [PubMed] [Google Scholar]
- Caño-Delgado A., Yin Y., Yu C., Vafeados D., Mora-García S., Cheng J.-C., Nam K.H., Li J., Chory J. (2004). BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 131: 5341–5351 [DOI] [PubMed] [Google Scholar]
- Cao S., Xu Q., Cao Y., Qian K., An K., Zhu Y., Hu B., Zhao H., Kuai B. (2005). Loss-of-function mutations in DET2 gene lead to an enhanced resistance to oxidative stress in Arabidopsis. Physiol. Plant. 123: 57–66 [Google Scholar]
- Castilla V., Larzábal M., Sgalippa N.A., Wachsman M.B., Coto C.E. (2005). Antiviral mode of action of a synthetic brassinosteroid against Junin virus replication. Antiviral Res. 68: 88–95 [DOI] [PubMed] [Google Scholar]
- Catterou M., Dubois F., Schaller H., Aubanelle L., Vilcot B., Sangwan-Norreel B.S., Sangwan R.S. (2001). Brassinosteroids, microtubules and cell elongation in Arabidopsis thaliana. I. Molecular, cellular and physiological characterization of the Arabidopsis bull mutant, defective in the Δ7-sterol-C5-desaturation step leading to brassinosteroid biosynthesis. Planta 212: 659–672 [DOI] [PubMed] [Google Scholar]
- Ceserani T., Trofka A., Gandotra N., Nelson T. (2009). VH1/BRL2 receptor-like kinase interacts with vascular-specific adaptor proteins VIT and VIK to influence leaf venation. Plant J. 57: 1000–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che P., Bussell J.D., Zhou W., Estavillo G.M., Pogson B.J., Smith S.M. (2010). Signaling from the endoplasmic reticulum activates brassinosteroid signaling and promotes acclimation to stress in Arabidopsis. Sci. Signal. 3: ra69. [DOI] [PubMed] [Google Scholar]
- Chinchilla D., Zipfel C., Robatzek S., Kemmerling B., Nürnberger T., Jones J.D.G., Felix G., Boller T. (2007). A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Choe S. (2006). Brassinosteroid biosynthesis and inactivation. Physiol. Plant. 126: 539–548 [Google Scholar]
- Choe S., Fujioka S., Noguchi T., Takatsuto S., Yoshida S., Feldmann K.A. (2001). Overexpression of DWARF4 in the brassinosteroid biosynthetic pathway results in increased vegetative growth and seed yield in Arabidopsis. Plant J. 26: 573–582 [DOI] [PubMed] [Google Scholar]
- Choe S., Tanaka A., Noguchi T., Fujioka S., Takatsuto S., Ross A.S., Tax F.E., Yoshida S., Feldmann K.A. (2000). Lesions in the stero Δ7 reductase gene of Arabidopsis cause dwarfism due to a block in brassinosteroid biosynthesis. Plant J. 21: 431–443 [DOI] [PubMed] [Google Scholar]
- Chono M., Honda I., Zeniya H., Yoneyama K., Saisho D., Takeda K., Takatsuto S., Hoshino T., Watanabe Y. (2003). A semidwarf phenotype of barley uzu results from a nucleotide substitution in the gene encoding a putative brassinosteroid receptor. Plant Physiol. 133: 1209–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J., Nagpal P., Peto C.A. (1991). Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell 3: 445–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse S.D. (2008). The molecular intersection of brassinosteroid-regulated growth and flowering in Arabidopsis. Proc. Natl. Acad. Sci. USA 105: 7345–7346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse S.D. (2011). Brassinosteroid signal transduction: From receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 23: 1219–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse S.D., Sasse J.M. (1998). Brassinosteroids: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49: 427–451 [DOI] [PubMed] [Google Scholar]
- Crocco C.D., Holm M., Yanovsky M.J., Botto J.F. (2011). Function of B-BOX proteins under shade. Plant Signal. Behav. 6: 101–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaubhadel S., Browning K.S., Gallie D.R., Krishna P. (2002). Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. Plant J. 29: 681–691 [DOI] [PubMed] [Google Scholar]
- Dhaubhadel S., Chaudhary S., Dobinson K.F., Krishna P. (1999). Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol. Biol. 40: 333–342 [DOI] [PubMed] [Google Scholar]
- Divi U.K., Krishna P. (2010). Overexpression of the brassinosteroid biosynthetic gene AtDWF4 in Arabidopsis seeds overcomes abscisic acid-induced inhibition of germination and increases cold tolerance in transgenic seedlings. J. Plant Growth Regul. 29: 385–393 [Google Scholar]
- Divi U.K., Krishna P. (2009). Brassinosteroid: A biotechnological target for enhancing crop yield and stress tolerance. New Biotechnol. 26: 131–136 [DOI] [PubMed] [Google Scholar]
- Divi U.K., Rahman T., Krishna P. (2010). Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol. 10: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalska M.A., Sarnowska E., Nagy F., Davis S.J. (2010). Genetic analyses of interactions among gibberellin, abscisic acid, and brassinosteroids in the control of flowering time in Arabidopsis thaliana. PLoS ONE 5: e14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalska M.A., Schomburg F.M., Amasino R.M., Vierstra R.D., Nagy F., Davis S.J. (2007). Attenuation of brassinosteroid signaling enhances FLC expression and delays flowering. Development 134: 2841–2850 [DOI] [PubMed] [Google Scholar]
- Fàbregas N., Ibañes M., Caño-Delgado A.I. (2010). A systems biology approach to dissect the contribution of brassinosteroid and auxin hormones to vascular patterning in the shoot of Arabidopsis thaliana. Plant Signal. Behav. 5: 903–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B.J., Ross J.J., Reid J.B. (2005). Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea. Plant Physiol. 138: 2396–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch-Savage W.E., Leubner-Metzger G. (2006). Seed dormancy and the control of germination. New Phytol. 171: 501–523 [DOI] [PubMed] [Google Scholar]
- Fitter D.W., Martin D.J., Copley M.J., Scotland R.W., Langdale J.A. (2002). GLK gene pairs regulate chloroplast development in diverse plant species. Plant J. 31: 713–727 [DOI] [PubMed] [Google Scholar]
- Fitzgerald M.A., McCouch S.R., Hall R.D. (2009). Not just a grain of rice: The quest for quality. Trends Plant Sci. 14: 133–139 [DOI] [PubMed] [Google Scholar]
- Friedman M. (2006). Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric. Food Chem. 54: 8655–8681 [DOI] [PubMed] [Google Scholar]
- Fujii S., Saka H. (2001). Distribution of assimilates to each organ in rice plants exposed to a low temperature at the ripening stage, and the effect of brassinolide on the distribution. Plant Prod. Sci. 4: 136–144 [Google Scholar]
- Gomes M.M.A. (2011). Physiological effects related to brassinosteroid application in plants. In Brassinosteroids: A Class of Plant Hormone, Hayat S., Ahmad A., (Dordrecht, The Netherlands: Springer; ), pp. 193–242 [Google Scholar]
- González-García M.-P., Vilarrasa-Blasi J., Zhiponova M., Divol F., Mora-García S., Russinova E., Caño-Delgado A.I. (2011). Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development 138: 849–859 [DOI] [PubMed] [Google Scholar]
- Gudesblat G.E., Russinova E. (2011). Plants grow on brassinosteroids. Curr. Opin. Plant Biol. 14: 530–537 [DOI] [PubMed] [Google Scholar]
- Hacham Y., Holland N., Butterfield C., Ubeda-Tomas S., Bennett M.J., Chory J., Savaldi-Goldstein S. (2011). Brassinosteroid perception in the epidermis controls root meristem size. Development 138: 839–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann M.-A. (1998). Plant sterols and the membrane environment. Trends Plant Sci. 3: 170–175 [Google Scholar]
- Hartwig T., Chuck G.S., Fujioka S., Klempien A., Weizbauer R., Potluri D.P.V., Choe S., Johal G.S., Schulz B. (2011). Brassinosteroid control of sex determination in maize. Proc. Natl. Acad. Sci. USA 108: 19814–19819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S.A., Hayat S., Ahmad A. (2011). Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere 84: 1446–1451 [DOI] [PubMed] [Google Scholar]
- He Y.-J., Xu R.-J., Zhao Y.-J. (1996). Enhancement of senescence by epibrassinolide in leaves of mung bean seedling. Acta Phytophysiol. Sinica 22: 58–62 [Google Scholar]
- Heese A., Hann D.R., Gimenez-Ibanez S., Jones A.M.E., He K., Li J., Schroeder J.I., Peck S.C., Rathjen J.P. (2007). The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl. Acad. Sci. USA 104: 12217–12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z., Ueguchi-Tanaka M., Fujioka S., Takatsuto S., Yoshida S., Hasegawa Y., Ashikari M., Kitano H., Matsuoka M. (2005). The rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone. Plant Cell 17: 2243–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z., Ueguchi-Tanaka M., Matsuoka M. (2004). Brassinosteroids and rice architecture. J. Pestic. Sci. 29: 184–188 [Google Scholar]
- Hong Z., Ueguchi-Tanaka M., Umemura K., Uozu S., Fujioka S., Takatsuto S., Yoshida S., Ashikari M., Kitano H., Matsuoka M. (2003). A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome P450. Plant Cell 15: 2900–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P. (2000). Prospects for crop improvement through the genetic manipulation of photosynthesis: morphological and biochemical aspects of light capture. J. Exp. Bot. 51: 475–485 [DOI] [PubMed] [Google Scholar]
- Huang Y., Han C., Peng W., Peng Z., Xiong X., Zhu Q., Gao B., Xie D., Ren C. (2010). Brassinosteroid negatively regulates jasmonate inhibition of root growth in Arabidopsis. Plant Signal. Behav. 5: 140–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañes M., Fàbregas N., Chory J., Caño-Delgado A.I. (2009). Brassinosteroid signaling and auxin transport are required to establish the periodic pattern of Arabidopsis shoot vascular bundles. Proc. Natl. Acad. Sci. USA 106: 13630–13635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeczko A., Biesaga-Kościelniak J., Dziurka M. (2009). 24-Epibrassinolide modifies seed composition in soybean, oilseed rape and wheat. Seed Sci. Technol. 37: 625–639 [Google Scholar]
- Janeczko A., Biesaga-Kościelniak J., Oklešt’ková J., Filek M., Dziurka M., Szarek-Łukaszewska G., Kościelniak J. (2010). Role of 24-epibrassinolide in wheat production: Physiological effects and uptake. J. Agron. Crop Sci. 196: 311–321 [Google Scholar]
- Kagale S., Divi U.K., Krochko J.E., Keller W.A., Krishna P. (2007). Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 225: 353–364 [DOI] [PubMed] [Google Scholar]
- Kebrom T.H., Brutnell T.P. (2007). The molecular analysis of the shade avoidance syndrome in the grasses has begun. J. Exp. Bot. 58: 3079–3089 [DOI] [PubMed] [Google Scholar]
- Keller M.M., Jaillais Y., Pedmale U.V., Moreno J.E., Chory J., Ballaré C.L. (2011). Cryptochrome 1 and phytochrome B control shade-avoidance responses in Arabidopsis via partially independent hormonal cascades. Plant J. 67: 195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerling B., et al. (2007). The BRI1-associated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr. Biol. 17: 1116–1122 [DOI] [PubMed] [Google Scholar]
- Khripach V., Zhabinskii V., De Groot A. (2000). Twenty years of brassinosteroids: Steroidal plant hormones warrant better crops for the XXI century. Ann. Bot. (Lond.) 86: 441–447 [Google Scholar]
- Kim S.Y., Kim B.H., Lim C.J., Lim C.O., Nam K.H. (2010). Constitutive activation of stress-inducible genes in a brassinosteroid-insensitive 1 (bri1) mutant results in higher tolerance to cold. Physiol. Plant. 138: 191–204 [DOI] [PubMed] [Google Scholar]
- Koh S., Lee S.-C., Kim M.-K., Koh J.H., Lee S., An G., Choe S., Kim S.-R. (2007). T-DNA tagged knockout mutation of rice OsGSK1, an orthologue of Arabidopsis BIN2, with enhanced tolerance to various abiotic stresses. Plant Mol. Biol. 65: 453–466 [DOI] [PubMed] [Google Scholar]
- Komatsu T., et al. (2010). The chloroplast protein BPG2 functions in brassinosteroid-mediated post-transcriptional accumulation of chloroplast rRNA. Plant J. 61: 409–422 [DOI] [PubMed] [Google Scholar]
- Kozuka T., Kobayashi J., Horiguchi G., Demura T., Sakakibara H., Tsukaya H., Nagatani A. (2010). Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol. 153: 1608–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna P. (2003). Brassinosteroid-mediated stress responses. J. Plant Growth Regul. 22: 289–297 [DOI] [PubMed] [Google Scholar]
- Kuppusamy K.T., Chen A.Y., Nemhauser J.L. (2009). Steroids are required for epidermal cell fate establishment in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 106: 8073–8076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laquitaine L., Gomès E., François J., Marchive C., Pascal S., Hamdi S., Atanassova R., Delrot S., Coutos-Thévenot P. (2006). Molecular basis of ergosterol-induced protection of grape against Botrytis cinerea: Induction of type I LTP promoter activity, WRKY, and stilbene synthase gene expression. Mol. Plant Microbe Interact. 19: 1103–1112 [DOI] [PubMed] [Google Scholar]
- Laxmi A., Paul L.K., Peters J.L., Khurana J.P. (2004). Arabidopsis constitutive photomorphogenic mutant, bls1, displays altered brassinosteroid response and sugar sensitivity. Plant Mol. Biol. 56: 185–201 [DOI] [PubMed] [Google Scholar]
- Li D., Wang L., Wang M., Xu Y.-Y., Luo W., Liu Y.-J., Xu Z.-H., Li J., Chong K. (2009). Engineering OsBAK1 gene as a molecular tool to improve rice architecture for high yield. Plant Biotechnol. J. 7: 791–806 [DOI] [PubMed] [Google Scholar]
- Li F., Asami T., Wu X., Tsang E.W.T., Cutler A.J. (2007). A putative hydroxysteroid dehydrogenase involved in regulating plant growth and development. Plant Physiol. 145: 87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Chory J. (1997). A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90: 929–938 [DOI] [PubMed] [Google Scholar]
- Li J., Li Y., Chen S., An L. (2010). Involvement of brassinosteroid signals in the floral-induction network of Arabidopsis. J. Exp. Bot. 61: 4221–4230 [DOI] [PubMed] [Google Scholar]
- Li J., Nagpal P., Vitart V., McMorris T.C., Chory J. (1996). A role for brassinosteroids in light-dependent development of Arabidopsis. Science 272: 398–401 [DOI] [PubMed] [Google Scholar]
- Lisso J., Altmann T., Müssig C. (2006). Metabolic changes in fruits of the tomato dx mutant. Phytochemistry 67: 2232–2238 [DOI] [PubMed] [Google Scholar]
- Lochman J., Mikes V. (2006). Ergosterol treatment leads to the expression of a specific set of defence-related genes in tobacco. Plant Mol. Biol. 62: 43–51 [DOI] [PubMed] [Google Scholar]
- Long S.P., Zhu X.-G., Naidu S.L., Ort D.R. (2006). Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 29: 315–330 [DOI] [PubMed] [Google Scholar]
- Luo M., Xiao Y., Li X., Lu X., Deng W., Li D., Hou L., Hu M., Li Y., Pei Y. (2007). GhDET2, a steroid 5α-reductase, plays an important role in cotton fiber cell initiation and elongation. Plant J. 51: 419–430 [DOI] [PubMed] [Google Scholar]
- Luo X.-M., et al. (2010). Integration of light- and brassinosteroid-signaling pathways by a GATA transcription factor in Arabidopsis. Dev. Cell 19: 872–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarevitch I., Thompson A., Muehlbauer G.J., Springer N.M. (2012). Brd1 gene in maize encodes a brassinosteroid C-6 oxidase. PLoS ONE 7: e30798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malíková J., Swaczynová J., Kolár Z., Strnad M. (2008). Anticancer and antiproliferative activity of natural brassinosteroids. Phytochemistry 69: 418–426 [DOI] [PubMed] [Google Scholar]
- Manavalan L.P., Chen X., Clarke J., Salmeron J., Nguyen H.T. (2012). RNAi-mediated disruption of squalene synthase improves drought tolerance and yield in rice. J. Exp. Bot. 63: 163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau R.A., Whitaker B.D., Hicks K.B. (2002). Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 41: 457–500 [DOI] [PubMed] [Google Scholar]
- Mori M., Nomura T., Ooka H., Ishizaka M., Yokota T., Sugimoto K., Okabe K., Kajiwara H., Satoh K., Yamamoto K., Hirochika H., Kikuchi S. (2002). Isolation and characterization of a rice dwarf mutant with a defect in brassinosteroid biosynthesis. Plant Physiol. 130: 1152–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillo S.A., Tax F.E. (2006). Functional analysis of receptor-like kinases in monocots and dicots. Curr. Opin. Plant Biol. 9: 460–469 [DOI] [PubMed] [Google Scholar]
- Morinaka Y., Sakamoto T., Inukai Y., Agetsuma M., Kitano H., Ashikari M., Matsuoka M. (2006). Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice. Plant Physiol. 141: 924–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müssig C., Shin G.-H., Altmann T. (2003). Brassinosteroids promote root growth in Arabidopsis. Plant Physiol. 133: 1261–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata N., Asami T., Yoshida S. (2001). Brassinazole, an inhibitor of brassinosteroid biosynthesis, inhibits development of secondary xylem in cress plants (Lepidium sativum). Plant Cell Physiol. 42: 1006–1011 [DOI] [PubMed] [Google Scholar]
- Nakamura A., et al. (2006). The role of OsBRI1 and its homologous genes, OsBRL1 and OsBRL3, in rice. Plant Physiol. 140: 580–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashita H., Yasuda M., Nitta T., Asami T., Fujioka S., Arai Y., Sekimata K., Takatsuto S., Yamaguchi I., Yoshida S. (2003). Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J. 33: 887–898 [DOI] [PubMed] [Google Scholar]
- Nam K.H., Li J. (2004). The Arabidopsis transthyretin-like protein is a potential substrate of BRASSINOSTEROID-INSENSITIVE1. Plant Cell 16: 2406–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nole-Wilson S., Rueschhoff E.E., Bhatti H., Franks R.G. (2010). Synergistic disruptions in seuss cyp85A2 double mutants reveal a role for brassinolide synthesis during gynoecium and ovule development. BMC Plant Biol. 10: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T., et al. (2004). Brassinosteroid deficiency due to truncated steroid 5α-reductase causes dwarfism in the lk mutant of pea. Plant Physiol. 135: 2220–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T., Ueno M., Yamada Y., Takatsuto S., Takeuchi Y., Yokota T. (2007). Roles of brassinosteroids and related mRNAs in pea seed growth and germination. Plant Physiol. 143: 1680–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M.-H., Sun J., Oh D.H., Zielinski R.E., Clouse S.D., Huber S.C. (2011). Enhancing Arabidopsis leaf growth by engineering the BRASSINOSTEROID INSENSITIVE1 receptor kinase. Plant Physiol. 157: 120–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T., Szatmari A.-M., Watanabe B., Fujita S., Bancos S., Koncz C., Lafos M., Shibata K., Yokota T., Sakata K., Szekeres M., Mizutani M. (2006). C-23 hydroxylation by Arabidopsis CYP90C1 and CYP90D1 reveals a novel shortcut in brassinosteroid biosynthesis. Plant Cell 18: 3275–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki K., Inaba N., Kitagawa K., Fujioka S., Kitano H., Fujisawa Y., Kato H., Iwasaki Y. (2009). Function of the α subunit of rice heterotrimeric G protein in brassinosteroid signaling. Plant Cell Physiol. 50: 161–172 [DOI] [PubMed] [Google Scholar]
- Ovečka M., Berson T., Beck M., Derksen J., Šamaj J., Baluška F., Lichtscheidl I.K. (2010). Structural sterols are involved in both the initiation and tip growth of root hairs in Arabidopsis thaliana. Plant Cell 22: 2999–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W., Kim H.B., Kim W.T., Park P.B., An G., Choe S. (2006). Rice bending lamina 2 (bla2) mutants are defective in a cytochrome P450 (CYP734A6) gene predicted to mediate brassinosteroid catabolism. J. Plant Biol. 49: 469–476 [Google Scholar]
- Pérez-España V.H., Sánchez-León N., Vielle-Calzada J.-P. (2011). CYP85A1 is required for the initiation of female gametogenesis in Arabidopsis thaliana. Plant Signal. Behav. 6: 321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pérez J.M., Ponce M.R., Micol J.L. (2002). The UCU1 Arabidopsis gene encodes a SHAGGY/GSK3-like kinase required for cell expansion along the proximodistal axis. Dev. Biol. 242: 161–173 [DOI] [PubMed] [Google Scholar]
- Pieterse C.M.J., Leon-Reyes A., Van der Ent S., Van Wees S.C.M. (2009). Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 5: 308–316 [DOI] [PubMed] [Google Scholar]
- Piroux N., Saunders K., Page A., Stanley J. (2007). Geminivirus pathogenicity protein C4 interacts with Arabidopsis thaliana shaggy-related protein kinase AtSKη, a component of the brassinosteroid signalling pathway. Virology 362: 428–440 [DOI] [PubMed] [Google Scholar]
- Pullen M., Clark N., Zarinkamar F., Topping J., Lindsey K. (2010). Analysis of vascular development in the hydra sterol biosynthetic mutants of Arabidopsis. PLoS ONE 5: e12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quílez J., García-Lorda P., Salas-Salvadó J. (2003). Potential uses and benefits of phytosterols in diet: Present situation and future directions. Clin. Nutr. 22: 343–351 [DOI] [PubMed] [Google Scholar]
- Reinhardt D., Kuhlemeier C. (2002). Plant architecture. EMBO Rep. 3: 846–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold H., Soyk S., Šimková K., Hostettler C., Marafino J., Mainiero S., Vaughan C.K., Monroe J.D., Zeeman S.C. (2011). β-Amylase-like proteins function as transcription factors in Arabidopsis, controlling shoot growth and development. Plant Cell 23: 1391–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuzeau C., Pen J., Frankard V., de Wolf J., Peerbolte R., Broekaert W., Van Camp W. (2005). TraitMill™: A discovery engine for identifying yield enhancement genes in cereals. Mol. Plant Breed. 5: 753–759 [Google Scholar]
- Romanutti C., Castilla V., Coto C.E., Wachsman M.B. (2007). Antiviral effect of a synthetic brassinosteroid on the replication of vesicular stomatitis virus in Vero cells. Int. J. Antimicrob. Agents 29: 311–316 [DOI] [PubMed] [Google Scholar]
- Roux M., Schwessinger B., Albrecht C., Chinchilla D., Jones A., Holton N., Malinovsky F.G., Tör M., de Vries S., Zipfel C. (2011). The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23: 2440–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sağlam-Çağ S. (2007). The effect of epibrassinolide on senescence in wheat leaves. Biotechnol. Biotechnol. Equip. 21: 63–65 [Google Scholar]
- Sakamoto T., Matsuoka M. (2008). Identifying and exploiting grain yield genes in rice. Curr. Opin. Plant Biol. 11: 209–214 [DOI] [PubMed] [Google Scholar]
- Sakamoto T., et al. (2006). Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat. Biotechnol. 24: 105–109 [DOI] [PubMed] [Google Scholar]
- Salas Fernandez M.G., Becraft P.W., Yin Y., Lübberstedt T. (2009). From dwarves to giants? Plant height manipulation for biomass yield. Trends Plant Sci. 14: 454–461 [DOI] [PubMed] [Google Scholar]
- Schaller H. (2004). New aspects of sterol biosynthesis in growth and development of higher plants. Plant Physiol. Biochem. 42: 465–476 [DOI] [PubMed] [Google Scholar]
- Schlüter U., Köpke D., Altmann T., Müssig C. (2002). Analysis of carbohydrate metabolism of CPD antisense plants and the brassinosteroid-deficient cbb1 mutant. Plant Cell Environ. 25: 783–791 [Google Scholar]
- Schrick K., Fujioka S., Takatsuto S., Stierhof Y.-D., Stransky H., Yoshida S., Jürgens G. (2004). A link between sterol biosynthesis, the cell wall, and cellulose in Arabidopsis. Plant J. 38: 227–243 [DOI] [PubMed] [Google Scholar]
- Schröder F., Lisso J., Müssig C. (2011). EXORDIUM-LIKE1 promotes growth during low carbon availability in Arabidopsis. Plant Physiol. 156: 1620–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B., Roux M., Kadota Y., Ntoukakis V., Sklenar J., Jones A., Zipfel C. (2011). Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet. 7: e1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair T.R., Sheehy J.E. (1999). Erect leaves and photosynthesis in rice. Science 283: 1456–145710206873 [Google Scholar]
- Song L., Zhou X.-Y., Li L., Xue L.-J., Yang X., Xue H.-W. (2009). Genome-wide analysis revealed the complex regulatory network of brassinosteroid effects in photomorphogenesis. Mol. Plant 2: 755–772 [DOI] [PubMed] [Google Scholar]
- Sorin C., Salla-Martret M., Bou-Torrent J., Roig-Villanova I., Martínez-García J.F. (2009). ATHB4, a regulator of shade avoidance, modulates hormone response in Arabidopsis seedlings. Plant J. 59: 266–277 [DOI] [PubMed] [Google Scholar]
- Souter M., Topping J., Pullen M., Friml J., Palme K., Hackett R., Grierson D., Lindsey K. (2002). hydra mutants of Arabidopsis are defective in sterol profiles and auxin and ethylene signaling. Plant Cell 14: 1017–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth A., Schmid M. (2011). Regulation of flowering time: All roads lead to Rome. Cell. Mol. Life Sci. 68: 2013–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steber C.M., McCourt P. (2001). A role for brassinosteroids in germination in Arabidopsis. Plant Physiol. 125: 763–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigerová J., Oklešt’ková J., Levková M., Rárová L., Kolář Z., Strnad M. (2010). Brassinosteroids cause cell cycle arrest and apoptosis of human breast cancer cells. Chem. Biol. Interact. 188: 487–496 [DOI] [PubMed] [Google Scholar]
- Sun Y., et al. (2010). Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev. Cell 19: 765–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan T.M., Watson K. (1998). Stress tolerance in a yeast sterol auxotroph: Role of ergosterol, heat shock proteins and trehalose. FEMS Microbiol. Lett. 169: 191–197 [DOI] [PubMed] [Google Scholar]
- Symons G.M., Davies C., Shavrukov Y., Dry I.B., Reid J.B., Thomas M.R. (2006). Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiol. 140: 150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons G.M., Reid J.B. (2008). Brassinosteroids, de-etiolation and the re-emerging art of plant hormone quantification. Plant Signal. Behav. 3: 868–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons G.M., Schultz L., Kerckhoffs L.H.J., Davies N.W., Gregory D., Reid J.B. (2002). Uncoupling brassinosteroid levels and de-etiolation in pea. Physiol. Plant. 115: 311–319 [DOI] [PubMed] [Google Scholar]
- Symons G.M., Smith J.J., Nomura T., Davies N.W., Yokota T., Reid J.B. (2008). The hormonal regulation of de-etiolation. Planta 227: 1115–1125 [DOI] [PubMed] [Google Scholar]
- Szekeres M., Németh K., Koncz-Kálmán Z., Mathur J., Kauschmann A., Altmann T., Rédei G.P., Nagy F., Schell J., Koncz C. (1996). Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85: 171–182 [DOI] [PubMed] [Google Scholar]
- Tanabe S., Ashikari M., Fujioka S., Takatsuto S., Yoshida S., Yano M., Yoshimura A., Kitano H., Matsuoka M., Fujisawa Y., Kato H., Iwasaki Y. (2005). A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 17: 776–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., et al. (2009). BRASSINOSTEROID UPREGULATED1, encoding a helix-loop-helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice. Plant Physiol. 151: 669–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Zheng J., Xu Z., Zhang X., Zhang K., Wang G. (2004). Functional analysis of ZmDWF1, a maize homolog of the Arabidopsis brassinosteroids biosynthetic DWF1/DIM gene. Plant Sci. 167: 743–751 [Google Scholar]
- Terakado J., Fujihara S., Goto S., Kuratani R., Suzuki Y., Yoshida S., Yoneyama T. (2005). Systemic effect of a brassinosteroid on root nodule formation in soybean as revealed by the application of brassinolide and brassinazole. Soil Sci. Plant Nutr. 51: 389–395 [Google Scholar]
- Turk E.M., Fujioka S., Seto H., Shimada Y., Takatsuto S., Yoshida S., Denzel M.A., Torres Q.I., Neff M.M. (2003). CYP72B1 inactivates brassinosteroid hormones: An intersection between photomorphogenesis and plant steroid signal transduction. Plant Physiol. 133: 1643–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk E.M., et al. (2005). BAS1 and SOB7 act redundantly to modulate Arabidopsis photomorphogenesis via unique brassinosteroid inactivation mechanisms. Plant J. 42: 23–34 [DOI] [PubMed] [Google Scholar]
- Upreti K.K., Murti G.S.R. (2004). Effects of brassinosteroids on growth, nodulation, phytohormone content and nitrogenase activity in French bean under water stress. Biol. Plant. 48: 407–411 [Google Scholar]
- Van Camp W. (2005). Yield enhancement genes: seeds for growth. Curr. Opin. Biotechnol. 16: 147–153 [DOI] [PubMed] [Google Scholar]
- Vardhini B.V., Rao S.S.R. (2002). Acceleration of ripening of tomato pericarp discs by brassinosteroids. Phytochemistry 61: 843–847 [DOI] [PubMed] [Google Scholar]
- Wachsman M.B., López E.M.F., Ramirez J.A., Galagovsky L.R., Coto C.E. (2000). Antiviral effect of brassinosteroids against herpes virus and arenaviruses. Antivir. Chem. Chemother. 11: 71–77 [DOI] [PubMed] [Google Scholar]
- Wachsman M.B., Ramirez J.A., Galagovsky L.R., Coto C.E. (2002). Antiviral activity of brassinosteroids derivatives against measles virus in cell cultures. Antivir. Chem. Chemother. 13: 61–66 [DOI] [PubMed] [Google Scholar]
- Wang H., Nagegowda D.A., Rawat R., Bouvier-Navé P., Guo D., Bach T.J., Chye M.-L. (2012). Overexpression of Brassica juncea wild-type and mutant HMG-CoA synthase 1 in Arabidopsis up-regulates genes in sterol biosynthesis and enhances sterol production and stress tolerance. Plant Biotechnol. J. 10: 31–42 [DOI] [PubMed] [Google Scholar]
- Wang L., Xu Y., Zhang C., Ma Q., Joo S.-H., Kim S.-K., Xu Z., Chong K. (2008). OsLIC, a novel CCCH-type zinc finger protein with transcription activation, mediates rice architecture via brassinosteroids signaling. PLoS ONE 3: e3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M.T., Moylan E.C., Langdale J.A. (2008). GLK transcription factors regulate chloroplast development in a cell-autonomous manner. Plant J. 56: 432–444 [DOI] [PubMed] [Google Scholar]
- Waters M.T., Wang P., Korkaric M., Capper R.G., Saunders N.J., Langdale J.A. (2009). GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell 21: 1109–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A.K., Witte C.-P. (2011). The biochemistry of nitrogen mobilization: Purine ring catabolism. Trends Plant Sci. 16: 381–387 [DOI] [PubMed] [Google Scholar]
- Williams M.E. (November 22, 2011). Brassinosteroids: Plant steroid hormones. Teaching Tools in Plant Biology: Lecture Notes. The Plant Cell (online), doi/10.1105/tpc.110.tt0910.
- Woyengo T.A., Ramprasath V.R., Jones P.J.H. (2009). Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 63: 813–820 [DOI] [PubMed] [Google Scholar]
- Wu C.Y., et al. (2008). Brassinosteroids regulate grain filling in rice. Plant Cell 20: 2130–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi W., Yu H. (2010). MOTHER OF FT AND TFL1 regulates seed germination and fertility relevant to the brassinosteroid signaling pathway. Plant Signal. Behav. 5: 1315–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X.-J., Huang L.-F., Zhou Y.-H., Mao W.-H., Shi K., Wu J.-X., Asami T., Chen Z., Yu J.-Q. (2009a). Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 230: 1185–1196 [DOI] [PubMed] [Google Scholar]
- Xia X.-J., Wang Y.-J., Zhou Y.-H., Tao Y., Mao W.-H., Shi K., Asami T., Chen Z., Yu J.-Q. (2009b). Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 150: 801–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X.J., Zhang Y., Wu J.X., Wang J.T., Zhou Y.H., Shi K., Yu Y.L., Yu J.Q. (2009c). Brassinosteroids promote metabolism of pesticides in cucumber. J. Agric. Food Chem. 57: 8406–8413 [DOI] [PubMed] [Google Scholar]
- Xia X.-J., Zhou Y.-H., Ding J., Shi K., Asami T., Chen Z., Yu J.-Q. (2011). Induction of systemic stress tolerance by brassinosteroid in Cucumis sativus. New Phytol. 191: 706–720 [DOI] [PubMed] [Google Scholar]
- Xie G., Peng L. (2011). Genetic engineering of energy crops: A strategy for biofuel production in China. J. Integr. Plant Biol. 53: 143–150 [DOI] [PubMed] [Google Scholar]
- Xie L., Yang C., Wang X. (2011). Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in Arabidopsis. J. Exp. Bot. 62: 4495–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L.-W., Du J.-B., Yang H., Xu F., Yuan S., Lin H.-H. (2009). Brassinosteroids counteract abscisic acid in germination and growth of Arabidopsis. Z. Naturforsch. C. 64: 225–230 [DOI] [PubMed] [Google Scholar]
- Yang D.-H., Hettenhausen C., Baldwin I.T., Wu J. (2011). BAK1 regulates the accumulation of jasmonic acid and the levels of trypsin proteinase inhibitors in Nicotiana attenuata’s responses to herbivory. J. Exp. Bot. 62: 641–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Zhu W., Li L., Zhang S., Yin Y., Ma H., Wang X. (2010). Brassinosteroids control male fertility by regulating the expression of key genes involved in Arabidopsis anther and pollen development. Proc. Natl. Acad. Sci. USA 107: 6100–6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Li L., Li L., Guo M., Chory J., Yin Y. (2008). Modulation of brassinosteroid-regulated gene expression by jumonji domain-containing proteins ELF6 and REF6 in Arabidopsis. Proc. Natl. Acad. Sci. USA 105: 7618–7623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Li L., Zola J., Aluru M., Ye H., Foudree A., Guo H., Anderson S., Aluru S., Liu P., Rodermel S., Yin Y. (2011). A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J. 65: 634–646 [DOI] [PubMed] [Google Scholar]
- Zeng H., Tang Q., Hua X. (2010). Arabidopsis brassinosteroid mutants det2-1 and bin2-1 display altered salt tolerance. J. Plant Growth Regul. 29: 44–52 [Google Scholar]
- Zinn K.E., Tunc-Ozdemir M., Harper J.F. (2010). Temperature stress and plant sexual reproduction: Uncovering the weakest links. J. Exp. Bot. 61: 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullo M.A.T., Adam G. (2002). Brassinosteroid phytohormones: Structure, bioactivity and applications. Braz. J. Plant Physiol. 14: 143–181 [Google Scholar]