Abstract
Background
Acute mesenteric ischemia is still fatal in 50% to 70% of cases. This consensus paper was written with the participation of physicians from all of the involved specialties for the purpose of improving outcomes. Mesenteric ischemia must be recognized as a vascular emergency requiring rapid and efficient clinical evaluation and treatment.
Methods
We reviewed pertinent literature that was retrieved by a PubMed search on the terms “mesenteric ischemia” AND “arterial” OR “venous” OR “clinical presentation” OR “diagnosis” OR “therapy” OR “surgery” OR “ interventional radiology.” Our review also took account of the existing guidelines of the American College of Cardiology/American Heart Association. Intensive discussions among the participating physicians, representing all of the specialties involved in the management of mesenteric ischemia, led to the creation of this interdisciplinary paper.
Results
Biphasic contrast-enhanced computerized tomography is the diagnostic tool of choice for the detection of arterial or venous occlusion. If non-occlusive mesenteric ischemia is suspected, angiography should be performed, with the option of intraarterial pharmacotherapy to induce local vasodilation. Endovascular techniques have become increasingly important in the treatment of arterial occlusion. Embolic central mesenteric artery occlusion requires surgical treatment; surgery is also needed in case of peritonitis. Portal-vein thrombosis can be treated by local thrombolysis through a transhepatically placed catheter. This should be done within 3 to 4 weeks of the event to prevent later complications of portal hypertension.
Conclusion
Rapid diagnosis (within 4 to 6 hours of symptom onset) and interdisciplinary cooperation in the provision of treatment are required if the poor outcome of this condition is to be improved.
The mortality rate of acute mesenteric ischemia (AMI) is 50% to 70% and has remained at this high level for decades (1). The reasons for this are on the one hand insufficient understanding of its clinical picture in differential diagnosis of abdominal pain, when it is not considered, and on the other hand an unacceptable time delay before treatment even when a diagnosis of AMI is considered (2). This is often caused by the time-consuming use of inappropriate diagnostic procedures. As a result, even when mesenteric infarction is suspected diagnosis takes an average of 7.9 hours, and treatment another 2.5 hours before mesenteric reperfusion is achieved (3). Even a warm ischemia time of 6 hours leads to disintegration of the mucosal barriers with translocation. This is followed by morphological alterations of the intestinal wall.
While in total only around 1% of all patients with acute abdomen have AMI, AMI is the cause of acute abdomen in up to 10% of patients aged over 70. The following are predisposing risk factors: heart failure, atrial fibrillation, coronary heart disease (CHD), arterial hypertension, and peripheral arterial occlusion (4, 5) (Box).
Box. Clinical manifestation, risk factors, and classification of acute mesenteric ischemia.
Arterial occlusive
Sudden occlusion of the superior mesenteric artery by an embolus or thrombus in patients with preexisting wall alterations
Predisposition:
Cardiac arrhythmia, particularly atrial fibrillation
Coronary heart disease, clinical status following myocardial infarction
Peripheral arterial occlusive disease (PAOD)
Clinical manifestations:
Sudden-onset abdominal pain
Pain-free interval approximately 6 to 12 hours after symptom onset
Subsequent gangrene of the intestine with peritonitis
Arterial nonocclusive
Ischemia caused by reduction in cardiac output with reactive vessel spasm mesenterically
Predisposition:
Clinical status following heart surgery with extracorporeal circulation, particularly with complicated disease course
Long-term hemodialysis
Digitalis medication
Clinical manifestations:
In responsive patients: increasing abdominal pain
In intubated patients: abdominal distension, increase in inflammatory parameters, signs of sepsis
Venous
Thrombosis of the mesenteric-portal axis
Predisposition:
Paraneoplasia
Pancreatitis, pancreatic carcinoma
Congenital thrombophilia (e.g. AT III deficiency, protein C deficiency, protein S deficiency)
HCC (hepatocellular carcinoma) with macrovascular invasion
Clinical manifestations:
Dependent on severity of thrombosis
Often nonspecific abdominal complaints lasting several days
Venous infarction with peritonitis in a minority of cases
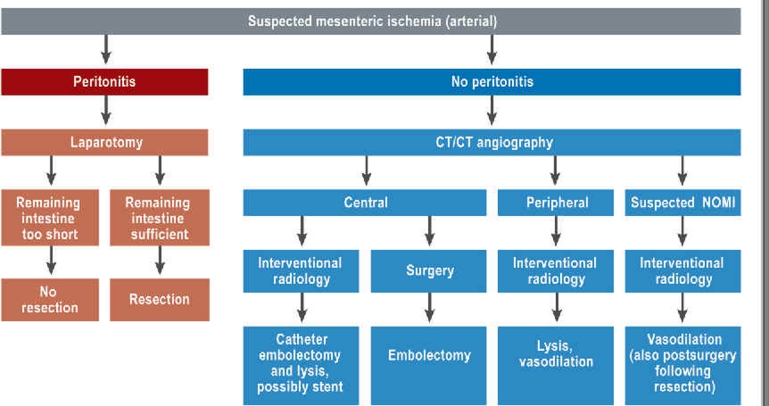
The aim of this consensus paper is to describe the emergency nature of this disorder and to outline a procedure that might reduce its high mortality rate (Figure 1). The analysis contains a review of the literature performed using PubMed and the term “mesenteric ischemia” in line with the guidelines of the American College of Cardiology (ACC)/American Heart Association (AHA). This was the basis for comprehensive coordination between the involved specialties, in order to develop an interdisciplinary concept.
Figure.
Procedure for diagnosing and treating acute mesenteric ischemia
NOMI: nonocclusive mesenteric ischemia
Occlusive mesenteric ischemia
Pathophysiology and clinical features
The superior mesenteric artery (SMA) forms a vulnerable functional terminal vascular bed running from the central collateral blood supply to the mobile convolute of the small intestine. It is affected in 85% of all cases of enteric ischemia. Within 6 hours, an acute complete disruption to intestinal blood supply leads to irreversible mucosal ischemia with cellular energy loss and leucocyte infiltration, accompanied by the formation of oxygen radicals (6).
Clinically the initial stage, characterized by sudden-onset, cramp-like abdominal pain, is followed after approximately 3 to 6 hours by a deceptive pain-free interval caused by a decline in intramural pain receptors as a result of sustained underperfusion of the intestinal wall. Subsequently, the mucosal barriers collapse, with bacterial translocation and gangrene of the wall of the intestine with peritonitis resulting from bacterial infiltration, ileus, sepsis, and multiorgan failure.
Parameters for prognosis
The decisive predictive factors for AMI progression are time to diagnosis and to enteric revascularization, location and etiology of AMI, and patient age and comorbidities. Thus the mortality rate rises from 0% to 10% with swift treatment to 50% to 60% with delays of 6 to 12 hours and 80% to 100% with delays of more than 24 hours after symptom onset (7). Turning to location, peripheral AMI is associated with lower mortality rates than central occlusion, as a result of better collateral growth capacity. Surprisingly, the prognosis of the nonocclusive form of AMI is worse than that of the occlusive form, because as a result of its uncharacteristic clinical presentation the former is often diagnosed too late and so causes irreversible damage.
Among the many serum parameters that have been investigated there is no sufficiently sensitive or specific marker to guarantee diagnosis of AMI. The diagnostic significance of serum lactate is generally overestimated. Although AMI mortality is associated with high lactate serum values, a normal serum lactate value does not rule AMI out (8). Increased D-dimer levels are equally nonspecific (9).
Diagnosis
AMI must be diagnosed urgently. The optimum time for imaging differs for the acute occlusive and acute nonocclusive forms of mesenteric ischemia (Box).
When acute occlusive mesenteric ischemia is suspected, biphasic contrast-enhanced computed tomography with three-dimensional multiplanar reconstruction (MPR-CT) is the diagnostic tool of choice. MPR-CT should include the whole abdomen, in both the arterial and the venous phases. Pragmatically, it can be assumed that the widespread use of initial abdominal plain film does not lead to any significant time delay, and the possibility of detecting free abdominal air can yield substantial benefits for differential diagnosis between mesenteric ischemia and hollow organ perforation. If abdominal plain film cannot reliably distinguish between mesenteric ischemia and other clinical pictures, computed tomography (CT) must be used immediately in addition (Figure 2). It is essential to define the suspected diagnosis when requesting diagnostic imaging. Both to save time and to achieve better representation of alterations in the intestinal wall, oral contrast should not be used. After administration of intravenous contrast substance, an arterial and a venous phase are routinely performed. The venous phase is essential for the diagnosis of mesenteric venous thrombosis. The universal availability and the quality of multidetector computed tomography (MDCT) allows sufficient enhancement of the mesenteric vessels. Three-dimensional reconstruction by CT angiography accurately depicts the mesenteric vascular anatomy. As a result, catheter angiography is becoming less popular (10). In addition to examining the wall of the intestine, the main advantages of multidetector CTA over catheter angiography are its use in ruling out other disorders during differential diagnosis of AMI. The sensitivity and specificity of MPR-CT are 93% and 100% respectively; its positive and negative predictive values are between 94% and 100% (11).
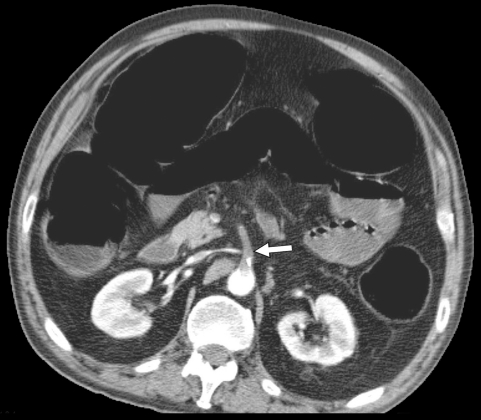
Figure 2.
Computed tomography following IV contrast administration (arterial phase) with evidence of occlusion of the superior mesenteric artery close to its origin, by an embolus (arrow). Distension of the represented portions of the intestine as a sign of paralytic ileus
Because mesenteric ischemia generally leads to distension of the intestinal loops, ultrasound should not be used for examination (Class III recommendation, level of evidence C, according to ACC/AHA guidelines). Although magnetic resonance imaging (MRI) can theoretically be used for diagnosis, computed tomography should be preferred because of the time it saves.
Treatment
AMI is a vascular emergency with comparable urgency to myocardial infarction or apoplexy. If treated during its initial stage, its mortality rate is less than 30%. If treatment is begun more than 6 to 8 hours after symptom onset, however, the mortality rate increases exponentially (6).
Basic intensive care treatment
Every patient with AMI should concomitantly receive emergency diagnosis and treatment according to the principles of intensive care. The first procedure is intravascular fluid replacement to stabilize hemodynamics, as volume displacement to the ischemic portions of the intestines and general endothelial disintegration occur within a few hours. In order to prevent exacerbation of thromboembolic occlusion, immediate anticoagulation should be performed using 5000 IU heparin IV followed by perfusor-directed application at an initial dose of 20 000 IU heparin/24 hours. Antibiotic treatment must be started concomitantly (e.g. second-generation cephalosporin plus metronidazole).
Endovascular techniques
The recommendations for treatment provided below are based on retrospective cohort studies with populations of between 67 and 76 patients (12– 14) and one case study (15). Because of patients’ high comorbidity rate (average age above 68 years) and the major access trauma of open vascular reconstruction, and thanks to the advances in endovascular techniques, indirect catheter techniques using transfemoral or transaxillary access should be favored when possible (12). The filigrane catheter and stent technologies available today can often be used instead of open vascular reconstruction. Choice and applicability of method are determined by the cause of AMI and the condition of the patient (Table). In particular, clinical manifestations of peritonitis or evidence of pre-existing gangrene of the intestine, which can be identified using computed tomography on the basis of gas in the wall of the intestine or the portal venous system (portal gas), rule out percutaneous endovascular therapies and are a direct indication for surgery.
Table. Endovascular treatment for AMI.
| Treatment | Indication | Level of evidence |
| Arterial | ||
| Transfemoral aspiration embolectomy | Large embolus close to outlet | IIa, C |
| IA continuous drug perfusion (papaverine, prostavasin, heparin) | Peripheral embolism with no peritonitis | IIa, C |
| nonocclusive ischemia | ||
| Local fibrinolysis (rt-PA) | Peripheral embolism with no peritonitis | IIa, C |
| as diagnostic technique for cases of stenosis | ||
| Stent PTA | Stenoses and occlusions close to outlet | I, B |
| Portal venous (transjugular transhepatic) | ||
| Portal venous rechanneling | Ischemia of the wall of the intestine in cases of mesenteric/portal venous thrombosis | IV |
| Portal decompression (TIPS) | Portal hypertension with venous ischemia of the wall of the intestine caused by congestion | IV |
AMI: acute mesenteric ischemia; IA: intraarterial; rt-PA: recombinant tissue plasminogen activator; PTA: percutaneous transluminal angioplasty; TIPS, transjugular intrahepatic portosystemic stent shunt
Endovascular treatment includes the possibility of angiographically-directed catheter-aspiration embolectomy and/or catheter lysis with recombinant tissue plasminogen activator (rt-PA), urokinase, or pharmacotherapy with prostaglandin E1 (16). In such cases, fractionation of the thrombus using a guide wire increases the surface area that comes into contact with the fibrinolytic agent and so speeds up dissolution of the thrombus. The aim is to reopen the main arterial branches of the SMA, as this will allow even remaining occlusion to be well compensated for as a result of good collateral growth.
If fibrinolysis and/or pharmacotherapy reveal changes in the vessel wall, stent PTA (percutaneous transluminal angioplasty) via the femoral artery can rechannel arteriosclerotic vessel occlusions or stenoses or at least facilitate a bridging reperfusion across the affected area (Figure 3). If the abdomen is open, this possibility is offered by retrograde catheterization of the peripheral SMA with intervention in the central segment (15).
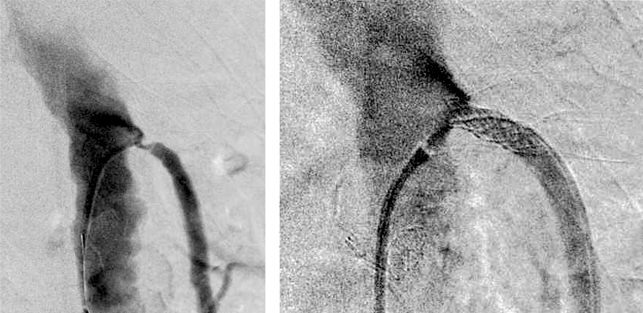
Figure 3.
Stent PTA of the superior mesenteric artery following fibrinolysis of a thrombotic occlusion. PTA: percutaneous transluminal angioplasty
Options for surgical treatment
Immediate surgical intervention remains the treatment of choice for central occlusion of the SMA, failure of endovascular treatment, or peritonitis. Cooperation between visceral and vascular surgeons is essential for this. Treatment is guided by the principle of arterial reperfusion before intestinal resection is considered. From a vascular point of view, mastery of embolectomy as well as reconstruction techniques for visceral arteries is required (13). Curing abdominal infection, on the other hand, involves identifying and resecting irreversibly ischemic portions of the intestine. Because damage to the inner mucosal layer is usually considerably more extensive than can be identified externally, continuity resections of the intestine must be considered very carefully. If relative ischemia of nonresected portions of the intestine is underestimated and an anastomosis is created, this results in failure of the anastomosis and a dramatically increased mortality rate (14). In case of doubt, the ends of the intestine classified as worth preserving should be placed outside the abdominal wall, as this offers a more favorable prognosis. It also allows endoscopic follow-up.
If any preserved portions of the intestine have uncertain reperfusion, second-look surgery must be performed within 12 hours of initial surgery. In addition, repeat exploration is indicated if the patient’s condition fails to stabilize.
If resection of larger portions of the intestine is necessary, the following minimum remaining intestinal lengths must be respected (17):
100 cm for terminal jejunostomy (colon removed)
65 cm for jejunocolic anastomosis (colon retained)
35 cm for jejunoileal anastomosis with retention of the ileocecal region.
Failure to respect these minimum values leads to short bowel syndrome. This requires long-term parenteral nutrition and possibly a small intestine transplant. Depending on the patient’s comorbidities and age, the aim of treatment may then need to be changed (to palliative care).
Postreperfusion phase
Intensive care must be continued until all infection parameters return to normal values and all organ function is stable. In particular, the end products of infection and ischemia and bacterial translocation can lead to a septic clinical picture in the longer term, and long-term artificial ventilation and dialysis are therefore not uncommon. In the initial postoperative phase it is particularly important to be vigilant for the effects of ischemia/reperfusion on abdominal, cardiac, pulmonary, and renal function, due to the risk of secondary organ failure. Subsequent follow-up consists of duplex ultrasound examination of the intestinal arteries and elimination of relevant risk factors.
Nonocclusive mesenteric ischemia
Clinical manifestations
Nonocclusive mesenteric ischemia (NOMI) is mesenteric underperfusion with reactive vascular spasm. It affects various portions of the intestine to varying extents, up to and including gangrene. The mortality rate of NOMI has been described at the constantly high level of 50% to 70% in recent years, which highlights the need for prompt diagnosis and treatment (18). NOMI occurs most frequently in two very different scenarios:
Long-term hemodialysis: caused by fluid loss with hypovolemia. After the onset of abdominal pain, volume replacement alone cannot interrupt intestinal vascular spasm.
Clinical status following heart surgery with extracorporeal circulation: NOMI manifests after approximately 0.5% to 1% of all heart operations (8, 19). Etiology depends on multiple factors. On the one hand, a reduction in cardiac output or a perioperative hypotonic phase can lead to vascular constriction of the splanchnic flow as a classical shock reaction. On the other hand, heart-lung machines can also contribute to reduced splanchnic blood flow. A generalized inflammatory reaction, laminar blood flow with no significant difference between systolic and diastolic pressure, and vasopressors that must be administered for heart surgery all play a role (20). Other risk factors for NOMI include patient-related factors such as age, limited left ventricular pump function, peripheral vascular and cerebrovascular diseases, and renal failure; and surgical factors such as bypass time, and the need for an intraaortic balloon pump. Diagnosis of NOMI is complicated by the fact that initial symptoms are usually nonspecific and often begin during an unstable phase when patients are frequently receiving sedation, analgesia, and/or artificial ventilation. Increased lactate levels are not conclusive, because they can also be observed after surgery involving heart-lung machines even without mesenteric ischemia (21).
Diagnosis and treatment
The first step in cases of suspected NOMI is catheter angiography (digital subtraction angiography, DSA) (Class I recommendation, level of evidence B, according to ACC/AHA guidelines), as the treatment of choice is the selective application of vasodilators into the SMA (PGE1 alprostadil 20 µg bolus, followed by perfusor-directed 60 to 80 µg/24 hour; alternative: PGI2 epoprostenol 5 to 6 ng/kg/min heparin IV 20 000 IU/24 hour). This can successfully interrupt generalized vascular spasm. For patients with suspected NOMI who generally require catecholamine therapy, vasodilators are largely catabolized as they pass through the liver, so no systemic effect or resulting neutralization of the effect of catecholamine is to be expected. Follow-up angiography is used to verify the efficiency of vasodilation (Figure 4). Surgery is required only when there is clinical evidence of peritonitis or for intubated patients with secondary disruption to organ function. It is indicated only for the resection of irreversibly damaged portions of the intestine.
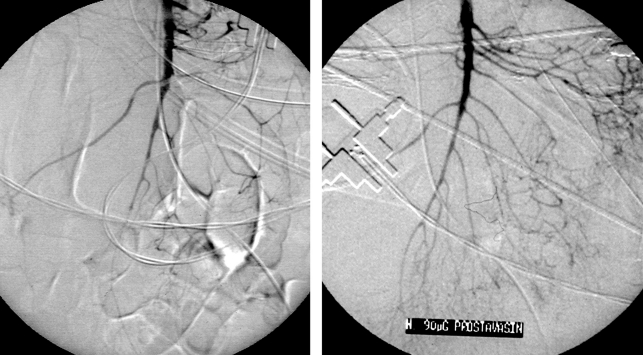
Figure 4.
NOMI following heart surgery before and after intraarterial prostavasin infusion. NOMI: nonocclusive mesenteric ischemia
Venous thrombosis
In most cases, venous thrombosis only leads to irreversible damage to the intestinal wall if it is centrally located and affects several areas downstream (22). Thrombosis of the superior mesenteric vein alone is also usually effectively compensated for by sufficient collateral vessel formation, even in central segments. In contrast, additional complete thrombosis of the portal vein leads to venous infarction of portions of the small intestine, of varying severity.
Clinical manifestation is often nonspecific and frequently does not lead to targeted diagnosis for several days. Biphasic contrast-enhanced CT with three-dimensional MPR reconstruction (MPR-CT) is the diagnostic tool of choice for imaging venous thrombosis, thickened walls of the intestine, and ascites. To prevent intestinal gangrene, the technique of choice is rechanneling via transjugular transhepatic access, possibly implanting a stent-shunt (TIPS, transjugular intrahepatic portosystemic stent shunt) (23, 24). For septic thromboses, systemic antibiotic treatment must be administered first, as rechanneling of the portal venous system can cause microbes in the thrombus to be released into the bloodstream. For peritonitis requiring laparotomy, a catheter can be transmesenterically placed before or in the thrombus intraoperatively, for subsequent local lysis. rt-PA 2 mg/hour is administered for 2 to 3 days, with daily angiographic monitoring. Just a few hours after thrombosis, the venous endothelium has undergone inflammatory alterations. Surgical thrombectomy is therefore performed only in exceptional circumstances, as there is a high repeat thrombosis rate.
If the portal vein is affected, endovascular rechanneling is always recommended, even if the patient presents after a delay (1 to 3 weeks) and intestinal perfusion appears to be clinically compensated for. In these cases, long-term life-limiting secondary complications of portal hypertension, particularly varices of the esophagus, fundus, and corpus with upper gastrointestinal bleeding, must be prevented.
Conclusion
The mortality rate of mesenteric arterial ischemia remains high, making it necessary to review the diagnostic and therapeutic approach to it. Mesenteric infarction is a vascular emergency and must be considered as such by physicians. Time-saving and consequent treatment are the decisive factors in prognosis. Emergency diagnosis must involve biphasic contrast-enhanced CT only, as this allows all treatment options to be deduced swiftly. Endovascular treatment techniques should be preferred, in order to place less of an additional burden on patients, most of whom have multiple morbidities. Immediate laparotomy must be performed for acute abdomen. Swift revascularization of the intestine is the primary aim of treatment. Irreversibly damaged portions of the intestine must be resected.
Key Messages.
The mortality rate of mesenteric ischemia remains high. This is due to delays in diagnosis.
Before hospital treatment, or at the latest upon emergency admission, the patient’s risk profile must lead to suspected diagnosis.
As soon as diagnosis is suspected, mesenteric ischemia must be treated as a vascular emergency, comparable to myocardial infarction.
One of the main sources for potential time-saving is targeted diagnosis. In cases of suspected occlusive mesenteric ischemia (arterial or venous), biphasic contrast-enhanced CT must be performed. If nonocclusive mesenteric ischemia is suspected, catheter angiography with intraarterial infusion of vasodilators must be performed.
In patients with multiple morbidities, interventional radiology options for rechanneling must be explored promptly. In cases of peritonitis, irreversibly damaged portions of the intestine must be resected, with surgical revascularization. Venous thrombosis is treated with transjugular transhepatic catheter lysis.
Acknowledgments
Translated from the original German by Caroline Devitt, MA.
PD Dr. Wiest of the Department of Internal Medicine, University of Regensburg and Prof. Dr. J. Hoffmann of the Surgical Clinic and Policlinic, Ludwig-Maximilians-Universität, Munich, deserve special thanks for their collaboration in the writing of this manuscript. They worked on important aspects of the internal medicine and surgery sections.
Footnotes
Conflict of interest statement
Prof. Jauch has received reimbursement of expenses for conventions and continuing education from Pfizer, lecture fees from Artellas, and payment for research projects from Fresenius, Novartis, and Pfizer.
The other authors declare that no conflict of interest exists.
References
- 1.Acosta S. Epidemiology of mesenteric vascular disease: clinical implications. Semin Vasc Surg. 2010;23:4–8. doi: 10.1053/j.semvascsurg.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Kortmann B, Klar E. Warum wird die mesenteriale Ischämie zu spät erkannt? Zentralbl Chir. 2005;130:223–226. doi: 10.1055/s-2005-836544. [DOI] [PubMed] [Google Scholar]
- 3.Luther B, Moussazadeh K, Müller BT, et al. Die akute mesenteriale Ischämie - unverstanden oder unheilbar? Zentralbl Chir. 2002;127:674–684. doi: 10.1055/s-2002-33574. [DOI] [PubMed] [Google Scholar]
- 4.Dahlke MH, Asshoff L, Popp FC, et al. Mesenteric ischemia-outcome after surgical therapy in 83 patients. Dig Surg. 2008;25:213–219. doi: 10.1159/000140692. [DOI] [PubMed] [Google Scholar]
- 5.Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91:17–27. doi: 10.1002/bjs.4459. [DOI] [PubMed] [Google Scholar]
- 6.Luther B. Intestinale Durchblutungsstörungen. Mesenterialinfarkt, Angina abdominalis, Therapieoptionen, Prognosen. Steinkopff Verlag Darmstadt. 2001 [Google Scholar]
- 7.Paes E, Vollmar JF, Hutschenreiter S, Schoenberg MH, Schölzel E. Diagnostik und Therapie des akuten Mesenterialinfarktes. Chir Gastroenterol. 1990;6:473–480. [Google Scholar]
- 8.Filsoufi F, Rahmanian PB, Castillo JG, Scurlock C, Legnani PE, Adams DH. Predictors and outcome of gastrointestinal complications in patients undergoing cardiac surgery. Ann Surg. 2007;246:323–329. doi: 10.1097/SLA.0b013e3180603010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akyildiz H, Akcan A, Oztürk A, Sozuer E, Kucuk C, Karahan I. The correlation of the D-dimer test and biphasic computed tomography with mesenteric computed tomography angiography in the diagnosis of acute mesenteric ischemia. Am J Surg. 2009;197:429–433. doi: 10.1016/j.amjsurg.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Kellow ZS, MacInnes M, Kurzencwyg D, et al. The role of abdominal radiography in the evaluation of the nontrauma emergency patient. Radiology. 2008;248:887–893. doi: 10.1148/radiol.2483071772. [DOI] [PubMed] [Google Scholar]
- 11.Aschoff AJ, Stuber G, Becker BW, et al. Evaluation of acute mesenteric ischemia: accuracy of biphasic mesenteric multi-detector CT angiography. Abdom Imaging. 2009;34:345–357. doi: 10.1007/s00261-008-9392-8. [DOI] [PubMed] [Google Scholar]
- 12.Arthurs ZM, Titus J, Bannazadeh M, et al. A comparison of endovascular revascularization with traditional therapy for the treatment of acute mesenteric ischemia. J Vasc Surg. 2011;53:698–704. doi: 10.1016/j.jvs.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 13.Park WM, Cherry KJ, Jr, Chua HK, et al. Current results of open revascularization for chronic mesenteric ischemia: a standard for comparison. J Vasc Surg. 2002;35:853–859. doi: 10.1067/mva.2002.123753. [DOI] [PubMed] [Google Scholar]
- 14.Unalp HR, Atahan K, Kamer E, Yasa H, Tarcan E, Onal MA. Prognostic factors for hospital mortality in patients with acute mesenteric ischemia who undergo intestinal resection due to necrosis. Ulus Travma Acil Cerrahi Derg. 2010;16:63–70. [PubMed] [Google Scholar]
- 15.Pisimisis GT, Oderich GS. Technique of hybrid retrograde superior mesenteric artery stent placement for acute-on-chronic mesenteric ischemia. Ann Vasc Surg. 2011;25(132):e7–e1. doi: 10.1016/j.avsg.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Schoots IG, Levi MM, Reekers JA, Lameris JS, van Gulik TM. Thrombolytic therapy for acute superior mesenteric artery occlusion. J Vasc Interv Radiol. 2005;16:317–329. doi: 10.1097/01.RVI.0000141719.24321.0B. [DOI] [PubMed] [Google Scholar]
- 17.Messing B, Crenn P, Beau P, Boutron-Ruault MC, Rambaud JC, Matuchansky C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999;117:1043–1050. doi: 10.1016/s0016-5085(99)70388-4. [DOI] [PubMed] [Google Scholar]
- 18.Björck M, Wanhainen A. Nonocclusive mesenteric hypoperfusion syndromes: recognition and treatment. Semin Vasc Surg. 2010;23:54–64. doi: 10.1053/j.semvascsurg.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Mangi AA, Christison-Lagay ER, Torchiana DF, Warshaw AL, Berger DL. Gastrointestinal complications in patients undergoing heart operation: an analysis of 8709 consecutive cardiac surgical patients. Ann Surg. 2005;241:895–901. doi: 10.1097/01.sla.0000164173.05762.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benk C, Klemm R, Schaller S, Brehm K, Schlensak C, Beyersdorf F. Was der Herzchirurg schon immer über die Herz-Lungen-Maschine wissen wollte. Zeitschrift für Herz-, Thorax-und Gefäßchirurgie. 2008;22:237–244. [Google Scholar]
- 21.Klotz S, Vestring T, Rotker J, Schmidt C, Scheld HH, Schmid C. Diagnosis and treatment of nonocclusive mesenteric ischemia after open heart surgery. Ann Thorac Surg. 2001;72:1583–1586. doi: 10.1016/s0003-4975(01)03179-4. [DOI] [PubMed] [Google Scholar]
- 22.Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med. 2010;15:407–418. doi: 10.1177/1358863X10379673. [DOI] [PubMed] [Google Scholar]
- 23.Goykhman Y, Ben-Haim M, Rosen G, et al. Transjugular intrahepatic portosystemic shunt: current indications, patient selection and results. Isr Med Assoc J. 2010;12:687–691. [PubMed] [Google Scholar]
- 24.Sasaki S, Ueda N, Nakano T, Urade M. Portal and superior mesenteric venous thrombosis treated with thrombolytic therapy via the superior mesenteric artery and vein. Nippon Shokakibyo Gakkai Zasshi. 2011;108:59–67. [PubMed] [Google Scholar]