Abstract
In mammals, circadian and daily rhythms influence nearly all aspects of physiology, ranging from behavior to gene expression. Functional molecular clocks have been described in the murine spleen and splenic NK cells. The aim of our study was to investigate the existence of molecular clock mechanisms in other immune cells. Therefore, we measured the circadian changes in gene expression of clock genes (Per1, Per2, Bmal1, and Clock) and clock-controlled transcription factors (Rev-erbα and Dbp) in splenic enriched macrophages, dendritic cells, and B cells in both mice entrained to a light-dark cycle and under constant environmental conditions. Our study reveals the existence of functional molecular clock mechanisms in splenic macrophages, dendritic cells, and B cells.
Keywords: Mouse splenic macrophages, dendritic cells, B cells possess functional circadian molecular clocks
1. Introduction
Organisms from cyanobacteria to humans have evolved mechanisms for adapting to environmental changes (e.g. seasonal, temperature, and light intensity) in order to optimize their survival and improve fitness (Bell-Pedersen et al., 2005). The anticipation of daily light changes manifests as near 24-hour oscillations in such biological processes as gene expression and behavior. In mammals, these circadian (daily) rhythms, are set by the master clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus (Ralph et al., 1990). The SCN is entrained by external light sensed by ganglion cells in the retina and then produces a multitude of neural and hormonal signals that influence sleep, activity, and set the peripheral oscillators throughout the body (Berson, 2007; Buijs et al., 2003; Reppert and Weaver, 2002). In higher organisms, external cues are unable to reach peripheral cell oscillators; therefore the SCN has to entrain the peripheral clocks through neural and endocrine (e.g. glucocorticoids) pathways (Buijs et al., 2003). Peripheral oscillators are believed to orchestrate cell-specific circadian physiology and metabolic programs (Hastings et al., 2003).
The circadian molecular clock mechanism is a transcription-translation-based negative feedback loop comprised of the core clock genes Per (Per1, Per2, and Per3), Cry (Cry1 and Cry2), Clock, and Bmal1. CLOCK and BMAL1 heterodimerize and activate transcription of the Per and Cry genes via E-box mediated binding in their promoter region. The resulting PER and CRY proteins heterodimerize and interact with the CLOCK-BMAL1 complex thereby inhibiting their own transcription (Reppert and Weaver, 2002). A second negative-feedback loop exists involving REV-ERBα, which some suggest is not an essential component of the clock but rather adds robustness to circadian oscillations as it is a strong repressor of Bmal1 transcription (Preitner et al., 2002). The entire cycle takes approximately 24 h to complete.
Circadian rhythms affect several aspects of mammalian physiology, ranging from maintaining the sleep/wake cycle and influencing feeding behavior to regulating cell-cycle progression (Saper et al., 2005). It is becoming increasingly evident that the circadian clock also has a profound influence on immune function as it has been shown that the relative and absolute number of immune cells in the spleen as well as total splenocytes experience circadian fluctuations throughout the daily cycle (Keller et al., 2009). Previous studies have shown clock genes are rhythmically expressed in immune tissues such as murine lymph nodes, bone marrow and spleen (Arjona and Sarkar, 2006; Chen et al., 2000; Keller et al., 2009). Circadian oscillations of clock genes have also been discovered at the cellular level in immune cells such as murine splenic natural killer cells and peritoneal macrophages along with serum entrained bone marrow derived mast cells (Arjona and Sarkar, 2005; Hayashi et al., 2007; Keller et al., 2009; Wang et al.).
The core molecular clock controls expression of clock-controlled genes (i.e. genes whose expression is dependent on the circadian oscillator) via direct transcriptional activation (e.g. CLOCK/BMAL1 binding to E-boxes in the promoter region) or indirectly through clock-controlled transcriptional regulators such as DBP (Ueda et al., 2005). It has previously been shown that the molecular clock plays a role in immune cell function as cytolytic factors and cytokines fluctuate with a daily rhythm in rat NK cells and phagocytosis is regulated by the molecular clock in mouse peritoneal macrophages (Arjona and Sarkar, 2005; Hayashi et al., 2007). Therefore, the presence of a circadian molecular clock in immune cells suggests it is involved in immune cell function. Consequently, we examined the presence of molecular clock mechanisms in splenic macrophages, dendritic and B cells. We measured circadian oscillations in expression of five clock genes (Per1, Per2, Bmal1, Clock, and Rev-erbα) and Dbp (a clock-controlled transcription factor) in enriched macrophages, dendritic and B cells from the mouse spleen over the daily light-dark cycle and under constant darkness.
2. Materials and methods
2.1. Animals
Eight week-old C57BL/6J male mice (Jackson ImmunoResearch Laboratories) were fed rodent chow ad libitum, maintained under constant environmental conditions and entrained to a 12 h light/12 h dark cycle (light period from 7:00 a.m. to 7:00 p.m.) for 2 weeks before experiments. For light-dark (LD) experiments, animals were euthanized at 10:00 a.m., 2:00 p.m., 6:00 p.m., 10:00 p.m., 2:00 a.m., and 6:00 a.m. These time points correspond with Zeitgeber times (ZT) 3, 7, 11, 15, 19 and 23, respectively. For experiments under dark-dark (DD) conditions, animals were entrained to the LD cycle as described above then held in constant darkness for 3 days prior to animals being euthanized at 10:00 a.m., 2:00 p.m., 6:00 p.m., 10:00 p.m., 2:00 a.m., and 6:00 a.m., which correspond to Circadian times (CT) 3, 7, 11, 15, 19 and 23, respectively. Tissues were immediately collected for further processing as described below. During the study, animal care and treatment complied with National Institutes of Health policy, were in accordance with institutional guidelines, and were approved by the Yale Animal Institutional Animal Care and Use Committee.
2.2. Cell isolation and purification
Spleens were collected in RPMI 1640 (Invitrogen Life Technologies) supplemented with 10% FBS and placed on ice. To obtain homogeneous splenocyte suspensions, each spleen was homogenized in 1 ml of RPMI, filtered through a 70 μM nylon cell strainer (BD Biosciences) and spun down for 5 min at 1,200 rpm. The supernatant was removed and cells were resuspended in 3 ml red cell lysing buffer (Sigma) and incubated for 10 min at room temperature. 7 ml of PBS were added to the cell suspension and cells were spun down for 5 min at 1,200 rpm. The supernatant was removed, cells were resuspended in 3 ml PBS and aliquoted into separate tubes for labeling with microbeads and subsequent cell separation. CD11b, CD11c and CD19 mouse microbeads (Miltenyi Biotec) were used to label splenic macrophages, dentritic cells and B cells, respectively. The labeling was followed according to manufacturers instructions and the autoMACS™ Pro Separator (Miltenyi Biotec) was utilized for the magnetic cell separation. The purity of the enriched fractions was assessed by flow cytometry using FITC-conjugated CD11b, CD11c and CD19 antibodies (Miltenyi Biotech). The enrichment method consistently yielded a purity of approximately 90, 60 and 80% for CD11b, CD11c and CD19 positive cells, respectively. Immediately after separation, enriched cells were lysed in appropriate buffers for later RNA extraction.
2.3. RNA extraction and quantitative PCR
RNA from enriched cells was isolated using the RNeasy Plus-Micro kit (Qiagen). cDNA was synthesized using the high capacity cDNA reverse transcription kit (Applied Biosystems). Due to low expression levels and a limited amount of cDNA, the TaqMan PreAmp Master Mix Kit (Applied Biosystems) was used in conjunction with TaqMan Gene Expression Assays (Applied Biosystems). The procedure was followed according to manufacturers instructions. Relative quantitation of mRNA levels was performed by quantitative PCR via TaqMan Gene Expression Assays (Applied Biosystems) using a real-time PCR 7500 Fast System (Applied Biosystems). Analyses were performed using the standard curve method with β-actin as the normalizing endogenous control.
2.4. Statistical analysis
To statistically assess the rhythmicity of the data, we performed JTK_CYCLE analysis (Hughes et al., 2010) implemented in R (x64 v2.12.1). Input data was formatted as six time points collected every four hours over one complete day. For LD experiments, n varied between 8 and 15 for each replicate; for DD experiments n varied between 3 and 5 for each replicate. All R scripts are available on demand. Data were also analyzed using GraphPad Prism 4.0b (GraphPad). One-way ANOVA with the Dunnett’s posttest was used to assess differences between means at different time points. Values are means ± standard error. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Canonical clock genes oscillate in enriched macrophages
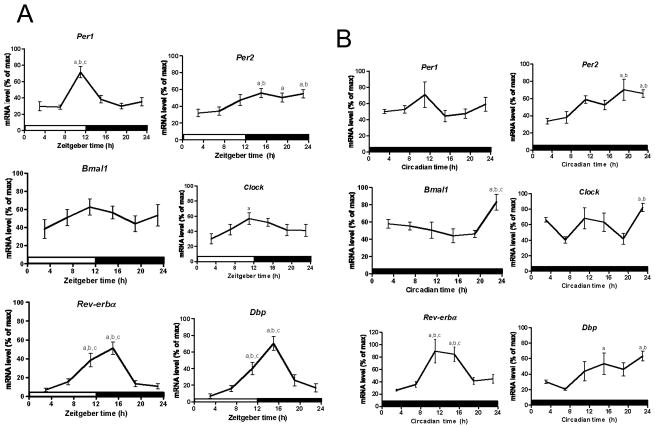
One of the signature features that define circadian oscillations is that the rhythm can be synchronized by an external time cue (Zeitgeber) such as the light-dark (LD) cycle. Therefore, in order to determine if splenic macrophages possess functional circadian clocks in vivo, mice were entrained to a LD cycle for two weeks and quantitative PCR was used to assess mRNA levels from enriched splenic macrophages every four hours over the daily LD cycle. Per1 mRNA levels peaked at ZT13 (pJTK_CYCLE < 0.01; Table 1) and were consistently low from ZT15 to ZT7 (F (5,54) = 11.45, pANOVA < 0.0001; Fig. 1A; Table S1). Per2 mRNA levels were lowest at ZT3, gradually increasing throughout the day and remaining consistently high from ZT15 to ZT23 (F (5,77) = 3.94, pANOVA = 0.0031; Fig. 1A; Table S1), peaking at ZT19 (pJTK_CYCLE < 0.001; Table 1). The expression profiles for Bmal1 (F (5,54) = 0.85, pANOVA = 0.52) and Clock (F (5,54) = 1.77, pANOVA = 0.13) were very similar, with slight expression peaks at ZT13 (pJTK_CYCLE = 0.43; Table 1) and ZT15 (pJTK_CYCLE < 0.05; Table 1), respectively, however, the expression peak for Bmal1 was not significantly different from the lowest level (Table 1; Fig. 1A). The expression levels of Rev-erbα (F (5,81) = 14.31, pANOVA < 0.0001; Fig. 1A; Table S1) and Dbp (F (5,54) = 14.78, pANOVA < 0.0001; Fig. 1A; Table S1) both increased at ZT11, peaking at ZT15 (pJTK_CYCLE < 0.0001; Table 1), and where consistently low from ZT19 to ZT7. We then examined gene expression levels under free running conditions (i.e. constant darkness) (DD). For these data mRNA level was plotted over Circadian time instead of Zeitgeber time since the mice were not subjected to an external time cue (i.e., light) for three days (see methods section). Per1 mRNA levels were highest at CT3 (pJTK_CYCLE = 1.0; Table 1) but it was not significantly higher than the nadir (F (5,22) = 1.48, pANOVA = 0.24; Fig. 1B; Table S1). Per2 expression levels were highest from CT19 to CT23 (F (5,18) = 5.18, pANOVA = 0.004; Fig. 1B; Table S1), peaking at CT19 (pJTK_CYCLE < 0.05; Table 1). Bmal1 mRNA displayed steady low levels From CT3 to CT19, with an expression peak at CT23 (F (5,23) = 4.26, pANOVA = 0.0069; Fig. 1B; Table S1). Clock mRNA levels, like Bmal1, also peaked at CT23 but also had higher levels from CT11 to CT15, although not significantly different from the lowest level (F (5,20) = 5.42, pANOVA = 0.0026; Fig. 1B; Table S1). However, according to JTK_CYCLE analyses, neither transcripts for Bmal1 (pJTK_CYCLE = 0.16) nor Clock (pJTK_CYCLE = 0.50) are cycling (Table 1). The mRNA oscillations for Rev-erbα (F (5,20) = 10.0, pANOVA < 0.0001; Fig. 1B) and Dbp (F (5,23) = 3.42, pANOVA = 0.019; Fig. 1B) showed maxima at CT15 (pJTK_CYCLE < 0.01;Table 1) and CT21 (pJTK_CYCLE < 0.01;Table 1), respectively.
Table 1.
JTK_CYCLE statistical analyses of the rhythmicity of clock genes in splenic macrophages, dendritic cells, and B cells under LD and DD conditions.
| Cell type | Condition | Gene | p valuea | Time of peak expressionb | Amplitude |
|---|---|---|---|---|---|
| Macrophages | LD | Per1 | < 0.01 | 13 | 7.9 |
| Per2 | < 0.001 | 19 | 12.7 | ||
| Bmal1 | = 0.43 | 13 | 9.1 | ||
| Clock | < 0.05 | 15 | 8.5 | ||
| Rev-erbα | < 0.0001 | 15 | 14.6 | ||
| Dbp | < 0.0001 | 15 | 20.9 | ||
| DD | Per1 | = 1.0 | 3 | 2.7 | |
| Per2 | < 0.05 | 19 | 16.7 | ||
| Bmal1 | = 0.16 | 3 | 12.9 | ||
| Clock | = 0.50 | 1 | 8.1 | ||
| Rev-erbα | < 0.01 | 15 | 17.6 | ||
| Dbp | < 0.01 | 21 | 19.1 | ||
| Dendritic cells | LD | Per1 | < 0.001 | 13 | 11.0 |
| Per2 | < 0.05 | 21 | 10.1 | ||
| Bmal1 | < 0.01 | 5 | 6.1 | ||
| Clock | = 1.0 | 9 | 1.1 | ||
| Rev-erbα | < 0.0001 | 15 | 25.2 | ||
| Dbp | < 0.0001 | 17 | 28.0 | ||
| DD | Per1 | < 0.01 | 17 | 9.8 | |
| Per2 | < 0.0001 | 21 | 32.8 | ||
| Bmal1 | = 0.16 | 1 | 7.7 | ||
| Clock | = 0.06 | 21 | 8.9 | ||
| Rev-erbα | < 0.0001 | 17 | 16.5 | ||
| Dbp | < 0.0001 | 17 | 21.0 | ||
| B cells | LD | Per1 | < 0.05 | 17 | 3.9 |
| Per2 | < 0.0001 | 19 | 11.8 | ||
| Bmal1 | < 0.0001 | 15 | 7.1 | ||
| Clock | = 1.0 | 15 | 1.5 | ||
| Rev-erbα | < 0.0001 | 15 | 16.5 | ||
| Dbp | < 0.01 | 15 | 13.0 | ||
| DD | Per1 | = 0.07 | 1 | 4.2 | |
| Per2 | = 0.15 | 1 | 9.1 | ||
| Bmal1 | = 0.85 | 5 | 2.0 | ||
| Clock | < 0.01 | 11 | 3.6 | ||
| Rev-erbα | < 0.0001 | 13 | 15.0 | ||
| Dbp | < 0.01 | 15 | 10.2 |
p value generated from JTK_CYCLE and adjusted for multiple comparisons.
Calculated from JTK_CYCLE output LAG (the number of hours past the first time point that a wave peaks) plus 3.
Fig. 1.
Circadian rhythms of mRNA levels in macrophages enriched from mouse spleen. Daily oscillations in canonical clock gene expression in macrophages enriched from mouse spleen collected every 4 h (ZT3/CT3 = 10 a.m.) (A) under LD conditions and (B), under DD conditions. Relative mRNA levels at each time point were determined by qPCR and calculated as the percentage of the maximum value over the 24-h period. Data are mean ± SEM of 14 or 15 animals per time point, compiled from 3 independent experiments under LD conditions (except n = 10 for Per1, Clock, and Dbp, compiled from 2 independent experiments) and 4 or 5 animals per time point under DD conditions. a, Significantly different (p < 0.05) from the lowest value; b, significantly different (p < 0.05) from the second lowest value; c, significantly different (p < 0.05) from the third lowest value. Open bar indicates light period, while colored bar indicates dark period.
3.2. Canonical clock genes oscillate in enriched dendritic cells
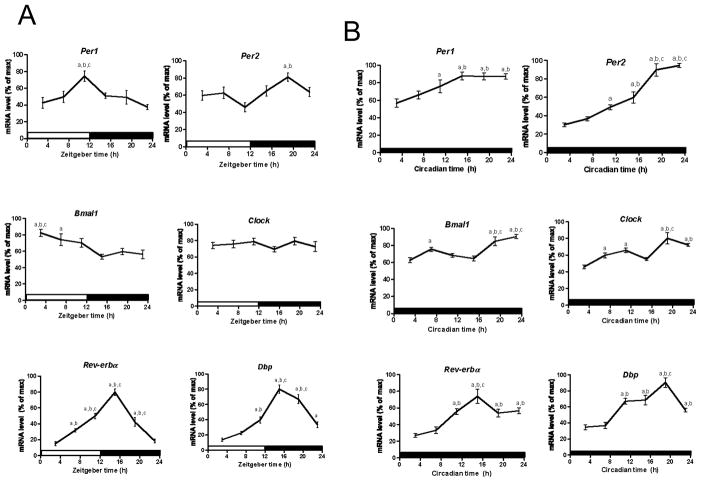
We next wanted to determine whether the molecular clock is functional in splenic dendritic cells in vivo. Therefore, we performed qPCR to assess mRNA levels in enriched dendritic cells isolated every four hours over the LD cycle. Per1 mRNA levels peaked at ZT13 (pJTK_CYCLE < 0.001; Table 1) and were minimum from ZT23 to ZT7 (F (5,51) = 4.65, pANOVA = 0.0014; Fig. 2A; Table S2). Per2 expression levels were lowest from ZT3 to ZT11 (F (5,54) = 4.09, pANOVA = 0.0032; Fig. 2A; Table S2) and gradually increased through the night, peaking at ZT21 (pJTK_CYCLE < 0.05; Table 1). Bmal1 mRNA levels were highest in the early morning, peaking at ZT5 (pJTK_CYCLE < 0.05; Table 1), and steadily decreased throughout the day and were maintained at a consistently low level from ZT15 to ZT23 (F (5,51) = 5.09, pANOVA = 0.0007; Fig. 2A; Table S2). Clock mRNA levels failed to display statistically significant daily oscillations (pJTK_CYCLE = 1.0; Table 1; F (5,50) = 070, pANOVA = 0.62; Fig. 2A; Table S2). Rev-erbα mRNA levels peaked at ZT15 (p JTK_CYCLE < 0.0001; Table 1) and were minimum from ZT23 to ZT3 (F (5,80) = 48.79, pANOVA < 0.0001; Fig. 2A; Table S2). Dbp expression levels were lowest from ZT3 to ZT7 and highest between ZT15 to ZT19 (p JTK_CYCLE < 0.0001; Table 1; F (5,53) = 37.51, pANOVA < 0.0001; Fig. 2A; Table S2). We also observed daily oscillations in gene expression under free running conditions (DD). Per1 (F (5,21) = 8.16, pANOVA = 0.0002; Fig. 2B) and Per2 (F (5,23) = 40.57 pANOVA < 0.0001; Fig. 2B) mRNA levels were both lowest at CT3 and gradually increased throughout the subjective day and night. Per1 mRNA levels peaked at CT17 (pJTK_CYCLE < 0.01; Table 1) and remained consistently high to CT23 (F (5,21) = 8.16 pANOVA = 0.0002; Fig. 2B; Table S2), while Per2 levels peaked at CT21 (pANOVA < 0.0001; Table 1). According to the JTK_CYCLE analyses, the mRNA levels of the positive clock genes, Bmal1 (pJTK_CYCLE = 0.16; Table 1) and Clock (pJTK_CYCLE = 0.06; Table 1) do not undergo circadian cycling. Rev-erbα (F (5,22) = 12.34, pANOVA < 0.0001; Fig. 2B; Table S2) and Dbp (F (5,21) = 21.42, pANOVA < 0.0001; Fig. 2B; Table S2) mRNA levels were both lowest between CT3 and CT7, and were consistently high from CT11 to CT23, both peaking at CT17 (pJTK_CYCLE < 0.0001; Table 1).
Fig. 2.
Circadian rhythms of mRNA levels in dendritic cells enriched from mouse spleen. Daily oscillations in canonical clock gene expression in dendritic cells enriched from mouse spleen collected every 4 h (ZT3/CT3 = 10 a.m.) (A) under LD conditions and (B) under DD conditions. Relative mRNA levels at each time point were determined by qPCR and calculated as the percentage of the maximum value over the 24-h period. Data are mean ± SEM of 9 or 10 animals per time point, compiled from 2 independent experiments under LD conditions (except n = 13 or 15 for Rev-erbα, compiled from 3 independent experiments) and 4 or 5 animals per time point under DD conditions. a, Significantly different (p < 0.05) from the lowest value; b, significantly different (p < 0.05) from the second lowest value; c, significantly different (p < 0.05) from the third lowest value. Open bar indicates light period, while colored bar indicates dark period.
3.3. Canonical clock genes oscillate in enriched B cells
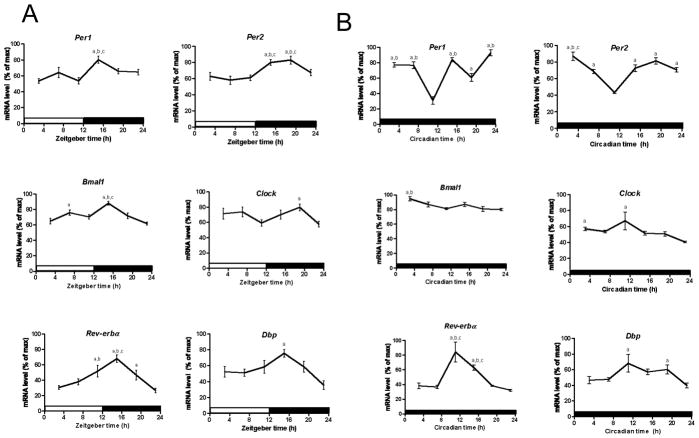
We also examined whether there are functional clocks in splenic B cells by examining in vivo mRNA levels of canonical clock genes over the daily LD cycle and under constant conditions (DD). mRNA levels were assessed by qPCR in enriched splenic B cells. Per1 mRNA levels were lowest from ZT3 to ZT11 (F (5,51) = 5.14, pANOVA = 0.0007; Fig. 3A; Table S3) and were highest at ZT17 (pJTK_CYCLE < 0.05; Table 1). Per2 mRNA levels were lowest from ZT3 to ZT11 and were consistently high from ZT15 to ZT19 (F (5,81) = 6.88, pANOVA <0.0001; Fig. 3A; Table S3), peaking at ZT19 (pJTK_CYCLE < 0.0001; Table 1). Bmal1 expression peaked at ZT15 (pJTK_CYCLE < 0.0001; Table 1) and was minimal at ZT23 (F (5,77) = 9.28, pANOVA < 0.0001; Fig. 3A; Table S3), while Clock expression did not undergo significant daily oscillations according to JTK_CYCLE (pJTK_CYCLE = 1.0; Table 1). Rev-erbα mRNA levels peaked at ZT15 (pJTK_CYCLE < 0.0001; Table 1) and were consistently low from ZT23 to ZT7 (F (5,80) = 10.29, pANOVA < 0.0001; Fig. 3A; Table S3), while Dbp peaked at ZT15 (pJTK_CYCLE < 0.01; Table 1) and was lowest at ZT23 (F (5,45) = 3.98, pANOVA = 0.0045; Fig. 3A; Table S3). Under constant conditions, according to the JTK_CYCLE analyses, Per1 (pJTK_CYCLE = 0.07), Per2 (pJTK_CYCLE = 0.15) and Bmal1 (pJTK_CYCLE = 0.85) transcripts do not undergo significant daily oscillations (Table 1). Clock expression levels were highest between CT3 and CT11, peaking at CT11 (pJTK_CYCLE < 0.01), and lowest at CT23 (F (5,23) = 4.03, pANOVA = 0.009; Fig. 3B; Table S3). Circadian oscillations of Rev-erbα were seen as mRNA levels were highest from CT11 to CT15 (pJTK_CYCLE < 0.0001; Table 1) and were consistently low from CT19 until CT7 (F (5,18) = 16.10, pANOVA < 0.0001; Fig. 3B; Table S3). We observed elevated expression levels in Dbp from CT11 to CT19, peaking at CT15 (pJTK_CYCLE < 0.01; Table 1) and consistently low levels from CT23 to CT7 (F (5,21) = 3.48, pANOVA = 0.019; Fig. 3B; Table S3).
Fig. 3.
Circadian rhythms of mRNA levels in B cells enriched from mouse spleen. Daily oscillations in canonical clock gene expression in B cells enriched from mouse spleen collected every 4 h (ZT3/CT3 = 10 a.m.) (A) under LD conditions and (B) under DD conditions. Relative mRNA levels at each time point were determined by qPCR and calculated as the percentage of the maximum value over the 24-h period. Data are mean ± SEM of 14 or 15 animals per time point, compiled from 3 independent experiments under LD conditions (except n = 9 or 10 for Per1, Clock, and Dbp, compiled from 2 independent experiments) and 4 or 5 animals per time point under DD conditions. a, Significantly different (p < 0.05) from the lowest value; b, significantly different (p < 0.05) from the second lowest value; c, significantly different (p < 0.05) from the third lowest value. Open bar indicates light period, while colored bar indicates dark period.
4. Discussion
Daily rhythms have been shown to influence an ever-increasing number of biological processes. The immune system is no exception as functional clocks have been discovered in both immunological tissues and cells (Arjona and Sarkar, 2005; Hayashi et al., 2007; Keller et al., 2009). Here we demonstrate that splenic macrophages, dendritic cells, and B cells possess functional molecular clocks as each exhibit daily oscillations in mRNA abundance of canonical clock genes under both LD and DD conditions.
Clock gene expression rhythms observed under LD conditions in enriched splenic macrophages were similar to what has previously been shown in murine peripheral tissues and cells. Per1 mRNA levels peaked approximately six hours before those of Per2 (Table 1), which coincides with the end of the light period (Hayashi et al., 2007; Kennaway et al., 2003). Per2 expression levels gradually increase throughout the day, culminating in a longer acrophase than that seen with Per1 (Fig. 1A) (Kennaway et al., 2003). Bmal1 transcript levels, which are typically expressed in an antiphasic manner compared to Per2 levels (Arjona and Sarkar, 2005; Hughes et al., 2009; Kennaway et al., 2003; Oishi et al., 1998), did not show robust oscillations (Table ; Fig. 1A). Rev-erbα and Dbp are clock-controlled transcription factors that demonstrate a similar robust oscillation in transcript levels that peak around the end of the light period (Kennaway et al., 2003; Yagita and Okamura, 2000). Their rhythm typically coincides with Per1 expression levels (Arjona and Sarkar, 2005; Kennaway et al., 2003), which has been demonstrated in peritoneal macrophages and splenic NK cells (Arjona and Sarkar, 2005; Hayashi et al., 2007). These findings are consistent with what is observed in splenic macrophages as the acrophase for Rev-erbα and Dbp is only two hours after that of Per1 peaking in the early subjective night (Table 1; Fig. 1A). In order to demonstrate these rhythms are truly circadian and not simply driven by the light-dark cycle, we examined clock gene expression under constant conditions (DD). According to the JTK_CYCLE analyses, Per1, Bmal1, and Clock did not undergo significant circadian oscillations (Table 1). However, the genes that were rhythmic according to JTK_CYCLE (Per2, Reverbα, and Dbp), displayed many of the same properties as those previously seen in peritoneal macrophages under DD conditions (Keller et al., 2009). Per2 levels gradually increase throughout the day, peaking late in the subjective night at CT19, which corresponds to the expression peak under LD conditions (ZT19) (Table 1; Fig. 1B; Table S1). mRNA levels of Rev-erbα oscillate in a similar fashion to what was observed under LD conditions (Fig 1B). Expression levels of Rev-erbα under both LD and DD conditions (Table 1; Fig. 1A and B) peak at ZT15 and CT15, respectively, which is approximately four hours later than what has been shown in peritoneal macrophages (Hayashi et al., 2007; Keller et al., 2009). Dbp mRNA levels increase throughout the subjective day (Fig. 1B; Table S1), peaking at CT21 (Table 1), which differs from previous findings that peak levels coincide with an increase in Per1 levels and its rhythm is in phase to that of Rev-erbα (Damiola et al., 2000; Keller et al., 2009; Yagita and Okamura, 2000), as we found Dbp to peak six hours after that of Rev-erbα (Table 1).
Next we examined clock gene expression rhythms under LD conditions in enriched splenic DCs. Per1 and Per2 expression patterns (Fig. 2A) were similar to what we observed in splenic macrophages (Fig. 1A; Table 1) and what has previously been shown in murine splenic NK cells (Arjona and Sarkar, 2005). Bmal1 expression oscillates in an antiphasic manner compared to the Per genes, which is consistent with what has previously been shown in murine tissues, peritoneal macrophages and splenic NK cells (Arjona and Sarkar, 2005; Hayashi et al., 2007; Oishi et al., 1998). Clock mRNA levels typically oscillate in phase with Bmal1 (Arjona and Sarkar, 2005; Young et al., 2001), however, we did not detect significant fluctuations in expression levels of Clock (Table 1; Fig 2A), which is consistent with what was previously shown in peritoneal macrophages under LD conditions (Hayashi et al., 2007). The clock controlled transcription factors Rev-erbα and Dbp undergo similar robust oscillations that are consistent with what was observed in splenic macrophages (Table 1; Fig. 1A) but again, peak expression levels in DCs, ZT15 and ZT17 for Rev-erbα and Dbp, respectively, (Table 1; Fig. 2A) were approximately four hours after the maximum levels observed in peritoneal macrophages, NK cells and murine liver (Arjona and Sarkar, 2005; Boorman et al., 2005; Hayashi et al., 2007). Under constant conditions (DD) Per1 and Per2 expression levels gradually increase through the subjective day and reach maximum levels late in the subjective night, CT17 and CT21, respectively (Table 1; Fig. 2B; Table S2), which is later than what is observed in peritoneal macrophages (Keller et al., 2009). Per1 transcript levels also have a longer acrophase than what was observed in splenic (Fig. 1B) and peritoneal macrophages (Keller et al., 2009). Per2 mRNA levels (Fig. 2B) are similar to what we observed in splenic macrophages (Fig. 1B; Table 1). Bmal1 and Clock do not undergo significant circadian oscillations (Table 1). Rev-erbα and Dbp experience robust oscillations, similar to what we observed in splenic macrophages (Table 1; Fig. 1B; Table S1) but maximum expression levels occur approximately 5 hours later in the subjective night, at CT17, than what was seen in peritoneal macrophages (Keller et al., 2009).
Finally, we examined clock gene expression under LD conditions in splenic enriched B cells. Per1 expression levels have been shown to peak at the light-dark transition in murine tissues and cells (Arjona and Sarkar, 2005, 2006; Oishi et al., 1998; Young et al., 2001), including splenic macrophages and DCs (Fig. 1A and 2A), however in splenic B cells, expression peaks approximately four hours after the light to dark transition (Table 1; Fig. 3A; Table S3). Consistent with what others have previously reported (Arjona and Sarkar, 2005; Young et al., 2001) and we have observed here in splenic macrophages and DCs (Fig. 1A and 2A), Per2 levels peak slightly after Per1 and have a longer acrophase (Table 1; Fig. 3A; Table S3). Bmal1 does not oscillate in antiphase to the Per genes as seen in other murine tissues and cells (Arjona and Sarkar, 2005; Young et al., 2001) and Clock transcripts do not undergo significant oscillations over the daily cycle (Table 1). We observed robust oscillations of mRNA levels in the clock controlled transcription factors Rev-erbα and Dbp (Table 1; Fig. 3A; Table S3). Their peak corresponds to 3 hours after the light-dark transition, which is consistent to what we observed in splenic macrophages and DCs, except Dbp mRNA levels peak five hours after the transition in DCs (Table 1; Fig. 1A and 2A). The rhythm of Dbp is similar to that of Per1 (Fig. 3A), as seen in other peripheral tissues and cells (Arjona and Sarkar, 2005; Damiola et al., 2000; Hayashi et al., 2007; Hughes et al., 2009; Kennaway et al., 2003; Yagita and Okamura, 2000; Young et al., 2001). We then examined clock gene expression under DD conditions. According to JTK_CYCLE, Per1 and Per2 did not experience circadian oscillations (Table 1) and their expression rhythms (Fig. 3B) differed from what has previously been shown in murine peritoneal macrophages (Keller et al., 2009) and what we observed in this study with splenic macrophages and DCs (Fig. 1B and 2B) as transcript levels were lowest at the transition from subjective day to subjective night. Bmal1 did not undergo significant circadian oscillations (Table 1; Fig. 3B; Table S3), however, its expression level was highest at the beginning of the subjective day, which is consistent with expression in peritoneal macrophages under DD conditions (Keller et al., 2009). Clock levels appear to oscillate in antiphase with the Per genes as it has a significant expression peak at the same circadian time (CT11) as the nadir in both Per genes and Clock mRNA levels decrease through the subjective night while mRNA levels remain high in both Per genes (Fig. 3B; Table S3) but as stated earlier it was determined neither of the Per genes oscillate under DD conditions (Table 1). Rev-erbα transcript levels underwent robust oscillations, peaking at the transition from subjective day to subjective night (Table 1; Fig. 3B; Table S3), which is similar to what was previously shown in peritoneal macrophages (Keller et al., 2009) but slightly earlier than what we observed in splenic macrophages and DCs (Table 1; Fig. 1B and 2B). Similar to Rev-erbα, Dbp mRNA levels have a significant expression peak at the end of the subjective day (Fig. 3B; Table S3).
Our data demonstrate that macrophages, DCs and B cells enriched from mouse spleen possess functional molecular clocks as demonstrated by the daily oscillations in clock gene expression. Moreover, the circadian oscillations of the clock controlled transcription factors, Rev-erbα and Dbp, demonstrate these clocks are able to generate functional outputs, which further reveals these splenic immune cells possess working molecular clocks. Functional clocks have now been described in the spleen (Arjona and Sarkar, 2006; Keller et al., 2009), splenic NK cells (Arjona and Sarkar, 2005) and from this study, splenic macrophages, DCs and B cells. Interestingly, the rhythms of the clock genes in the spleen possess many of the same characteristics as in the immune cells but they are not identical, nor are the rhythms in the cells identical to each other. Adding another layer of complexity to the circadian rhythm of the spleen, it is a heterogeneous immunological tissue composed of several different types of cells (e.g. DCs, macrophages, B cells and NK cells), some of which have been shown to fluctuate in relative and/or absolute numbers throughout the day (Keller et al., 2009).
There is an ever-increasing body of literature that describes the influence of daily rhythms on immune function (e.g. lymphocyte proliferation, antigen presentation, and cytokine gene expression) (Arjona and Sarkar, 2005, 2006; Levi et al., 1991; Maestroni and Conti, 1996). It is intriguing to speculate that aspects of the host innate immune system are under circadian regulation in order to anticipate the time of day when a pathogenic encounter is most likely to occur. The recognition of microbial infection by innate immune cells triggers the production of TNF-α among other cytokines. Interestingly, it has been shown that TNF-α downregulates expression of several clock genes and induces increased rest periods in mice (Cavadini et al., 2007) and elevated TNF-α levels have also been linked to fatigue in patients suffering from the autoimmune disease rheumatoid arthritis (Pollard et al., 2006). It is plausible that the cross talk between the immune system and circadian system induces an altered sleep state in order to maximize the host defense during infection. Disruption of circadian rhythms, in the form of jet lag or night shift work, has been shown to result in an increased risk for gastrointestinal disease, cardiovascular disease, and cancer, which is further evidence that the circadian and immune systems are intertwined (Bovbjerg, 2003; Majde and Krueger, 2005). The presence of functional molecular clocks in splenic macrophages, dendritic cells, and B cells strengthens the belief that the circadian and immune systems are tightly connected.
Supplementary Material
Acknowledgments
We thank Manchuan Chen for technical assistance with the qPCR. This work was supported by National Institutes of Health Training Grant, T32 AI07019 (to AS) and in part by N01 HHSN272201100019C. EF is an Investigator with the Howard Hughes Medical Institute.
Footnotes
Conflict of Interest Statement
All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arjona A, Sarkar DK. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J Immunol. 2005;174:7618–7624. doi: 10.4049/jimmunol.174.12.7618. [DOI] [PubMed] [Google Scholar]
- Arjona A, Sarkar DK. The circadian gene mPer2 regulates the daily rhythm of IFN-gamma. J Interferon Cytokine Res. 2006;26:645–649. doi: 10.1089/jir.2006.26.645. [DOI] [PubMed] [Google Scholar]
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflugers Arch. 2007;454:849–855. doi: 10.1007/s00424-007-0242-2. [DOI] [PubMed] [Google Scholar]
- Boorman GA, Blackshear PE, Parker JS, Lobenhofer EK, Malarkey DE, Vallant MK, Gerken DK, Irwin RD. Hepatic gene expression changes throughout the day in the Fischer rat: implications for toxicogenomic experiments. Toxicol Sci. 2005;86:185–193. doi: 10.1093/toxsci/kfi166. [DOI] [PubMed] [Google Scholar]
- Bovbjerg DH. Circadian disruption and cancer: sleep and immune regulation. Brain, behavior, and immunity. 2003;17(Suppl 1):S48–50. doi: 10.1016/s0889-1591(02)00066-1. [DOI] [PubMed] [Google Scholar]
- Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. The biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system. The Journal of endocrinology. 2003;177:17–26. doi: 10.1677/joe.0.1770017. [DOI] [PubMed] [Google Scholar]
- Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A. TNF-alpha suppresses the expression of clock genes by interfering with E-box- mediated transcription. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12843–12848. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YG, Mantalaris A, Bourne P, Keng P, Wu JH. Expression of mPer1 and mPer2, two mammalian clock genes, in murine bone marrow. Biochemical and biophysical research communications. 2000;276:724–728. doi: 10.1006/bbrc.2000.3536. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes & development. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Garabette M, King VM, Chahad-Ehlers S, O’Brien J, Maywood ES. Expression of clock gene products in the suprachiasmatic nucleus in relation to circadian behaviour. Novartis Foundation symposium. 2003;253:203–217. doi: 10.1002/0470090839.ch15. discussion 102–209, 218–222, 281-204. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Shimba S, Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biological & pharmaceutical bulletin. 2007;30:621– 626. doi: 10.1248/bpb.30.621. [DOI] [PubMed] [Google Scholar]
- Hughes ME, DiTacchio L, Hayes KR, Vollmers C, Pulivarthy S, Baggs JE, Panda S, Hogenesch JB. Harmonics of circadian gene transcription in mammals. PLoS genetics. 2009;5:e1000442. doi: 10.1371/journal.pgen.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ME, Hogenesch JB, Kornacker K. JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. Journal of biological rhythms. 2010;25:372–380. doi: 10.1177/0748730410379711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennaway DJ, Varcoe TJ, Mau VJ. Rhythmic expression of clock and clock- controlled genes in the rat oviduct. Molecular human reproduction. 2003;9:503–507. doi: 10.1093/molehr/gag067. [DOI] [PubMed] [Google Scholar]
- Levi F, Canon C, Dipalma M, Florentin I, Misset JL. When should the immune clock be reset? From circadian pharmacodynamics to temporally optimized drug delivery. Ann N Y Acad Sci. 1991;618:312–329. doi: 10.1111/j.1749-6632.1991.tb27251.x. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ, Conti A. Melatonin and the immune-hematopoietic system therapeutic and adverse pharmacological correlates. Neuroimmunomodulation. 1996;3:325–332. doi: 10.1159/000097292. [DOI] [PubMed] [Google Scholar]
- Majde JA, Krueger JM. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005;116:1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Oishi K, Sakamoto K, Okada T, Nagase T, Ishida N. Antiphase circadian expression between BMAL1 and period homologue mRNA in the suprachiasmatic nucleus and peripheral tissues of rats. Biochemical and biophysical research communications. 1998;253:199–203. doi: 10.1006/bbrc.1998.9779. [DOI] [PubMed] [Google Scholar]
- Pollard LC, Choy EH, Gonzalez J, Khoshaba B, Scott DL. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology (Oxford, England) 2006;45:885–889. doi: 10.1093/rheumatology/kel021. [DOI] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science (New York, NY. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Ueda HR, Hayashi S, Chen W, Sano M, Machida M, Shigeyoshi Y, Iino M, Hashimoto S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nature genetics. 2005;37:187–192. doi: 10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- Wang X, Reece SP, Van Scott MR, Brown JM. A circadian clock in murine bone marrow-derived mast cells modulates IgE-dependent activation in vitro. Brain, behavior, and immunity. 25:127–134. doi: 10.1016/j.bbi.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Yagita K, Okamura H. Forskolin induces circadian gene expression of rPer1, rPer2 and dbp in mammalian rat-1 fibroblasts. FEBS letters. 2000;465:79–82. doi: 10.1016/s0014-5793(99)01724-x. [DOI] [PubMed] [Google Scholar]
- Young ME, Razeghi P, Taegtmeyer H. Clock genes in the heart: characterization and attenuation with hypertrophy. Circulation research. 2001;88:1142– 1150. doi: 10.1161/hh1101.091190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.