Abstract
Objective
To explore the clinical impact and economic burden of hospital-acquired infections (HAIs) in trauma patients using a nationally representative database.
Design
Retrospective study.
Setting
The Healthcare Cost and Utilization Project Nationwide Inpatient Sample.
Patients
Trauma patients.
Main Outcome Measures
We examined the association between HAIs (sepsis, pneumonia, Staphylococcus infections, and Clostridium difficile–associated disease) and in-hospital mortality, length of stay, and inpatient costs using logistic regression and generalized linear models.
Results
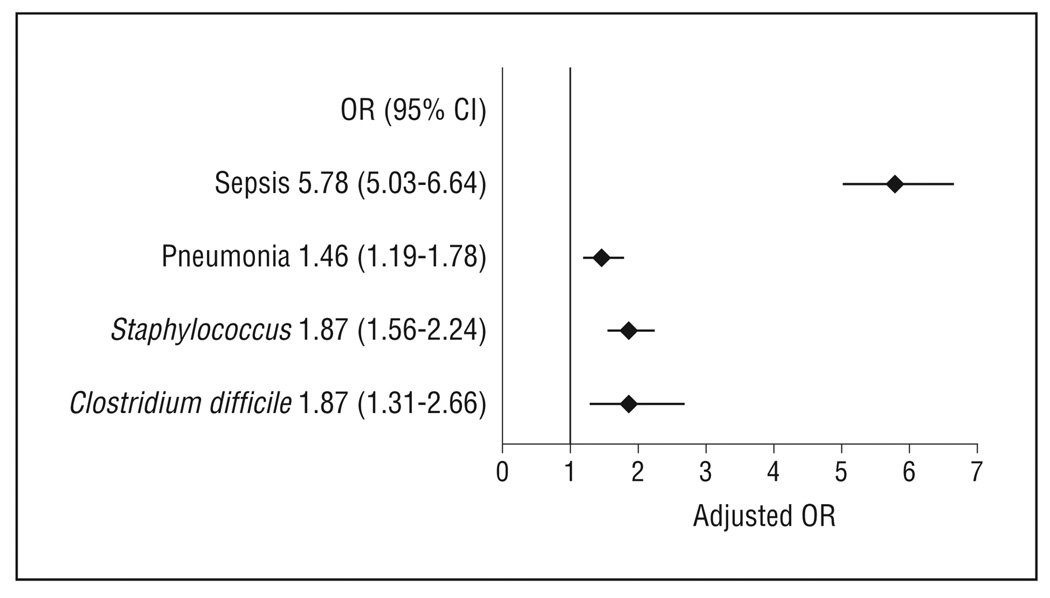
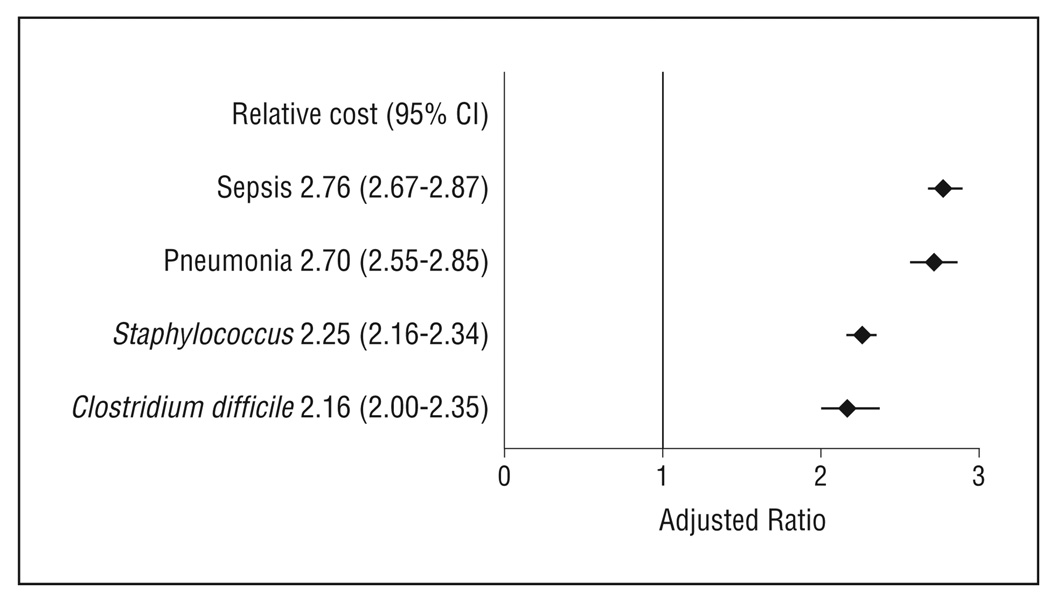
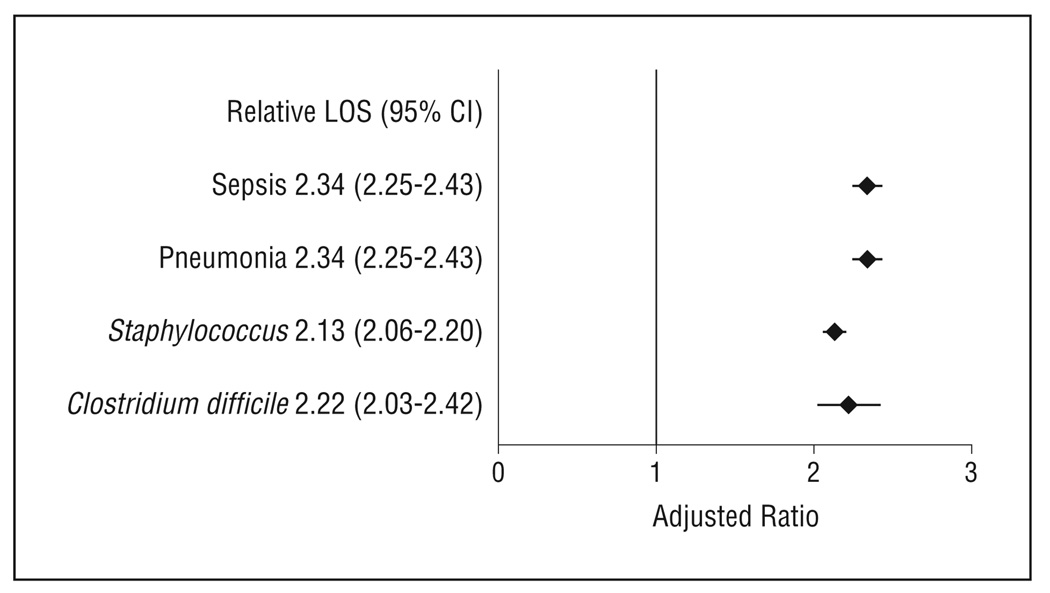
After controlling for patient demographics, mechanism of injury, injury type, injury severity, and comorbidities, we found that mortality, cost, and length of stay were significantly higher in patients with HAIs compared with patients without HAIs. Patients with sepsis had a nearly 6-fold higher odds of death compared with patients without an HAI (odds ratio, 5.78; 95% confidence interval, 5.03–6.64; P < .001). Patients with other HAIs had a 1.5- to1.9-fold higher odds of mortality compared with controls (P < .005). Patients with HAIs had costs that were approximately 2- to 2.5-fold higher compared with patients without HAIs (P < .001). The median length of stay was approximately 2-fold higher in patients with HAIs compared with patients without HAIs (P < .001).
Conclusions
Trauma patients with HAIs are at increased risk for mortality, have longer lengths of stay, and incur higher inpatient costs. In light of the preventability of many HAIs and the magnitude of the clinical and economic burden associated with HAIs, policies aiming to decrease the incidence of HAIs may have a potentially large impact on outcomes in injured patients.
The Institute of Medicine has focused attention on preventing medical errors and improving patient safety.1 Hospital-acquired infections (HAIs) are the most common complication in hospitalized patients,2 with an estimated incidence of 4.5 HAIs per 100 hospital admissions and an annual cost between $35 billion and $45 billion.3 Hospital-acquired infections result in more than 90 000 deaths each year,4,5 ranking death due to HAIs among the top 5 leading causes of death in the United States.6
Trauma patients are at especially high risk for the development of infections7 because of disruptions in tissue integrity and impaired host defense mechanisms.8,9 Trauma remains one of the main causes of mortality worldwide10 and is responsible for nearly one-third of “all lost years of productive life before age 65, exceeding losses from heart disease, cancer, and stroke combined.”11 Infections are a leading cause of death in trauma patients.8 However, to our knowledge, the clinical and economic outcomes of HAIs in trauma patients have not been previously reported using a large nationally representative patient sample.
In light of the preventability of many HAIs, obtaining a better understanding of the clinical impact of HAI on outcomes in trauma patients may provide the impetus for the implementation of best practices for infection control in injured patients. Recent evidence suggests significant variability in outcomes across trauma centers: trauma patients admitted to the highest-mortality hospitals had a 70% higher odds of dying compared with patients admitted to average hospitals.12 Some of these differences in outcomes across hospitals may result from differences in hospital HAI rates. A better understanding of the economic cost of HAI in injured patients may also create a strong business case for reducing the incidence of HAIs in this patient population.
The goal of this study was to explore the clinical impact and economic burden of HAIs in trauma patients using a nationally representative database. The analysis will focus on in-hospital mortality, hospital length of stay (LOS), and hospital cost. We selected HAIs associated with sepsis, pneumonia, Staphylococcus infections, and Clostridium infections because these conditions can be identified using administrative data.13–16 We assumed that infections in patients admitted with traumatic injuries were likely to be HAIs because infection, or illness related to infection, was not the reason for admission of injured patients.
METHODS
DATA SOURCE
This study was based on data from the 2005 and 2006 Nationwide Inpatient Sample developed by the Healthcare Cost and Utilization Project. The Nationwide Inpatient Sample is the largest all-payer hospital inpatient database in the United States and includes data from a 20% stratified sample of US hospitals. The discharge data contain information on patient demographics, admission source, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic and injury codes, Agency for Healthcare Research and Quality comorbidity measures,17 charges, LOS, in-hospital mortality, hospital characteristics, and hospital identifiers. Hospital trauma center status was obtained from the American Hospital Association Annual Survey. Hospital costs were calculated by multiplying the total hospital charges by the group average cost to charges ratios (defined as a weighed average for the hospitals in a group based on state, urban/rural, hospital ownership, and hospital size).18
STUDY POPULATION
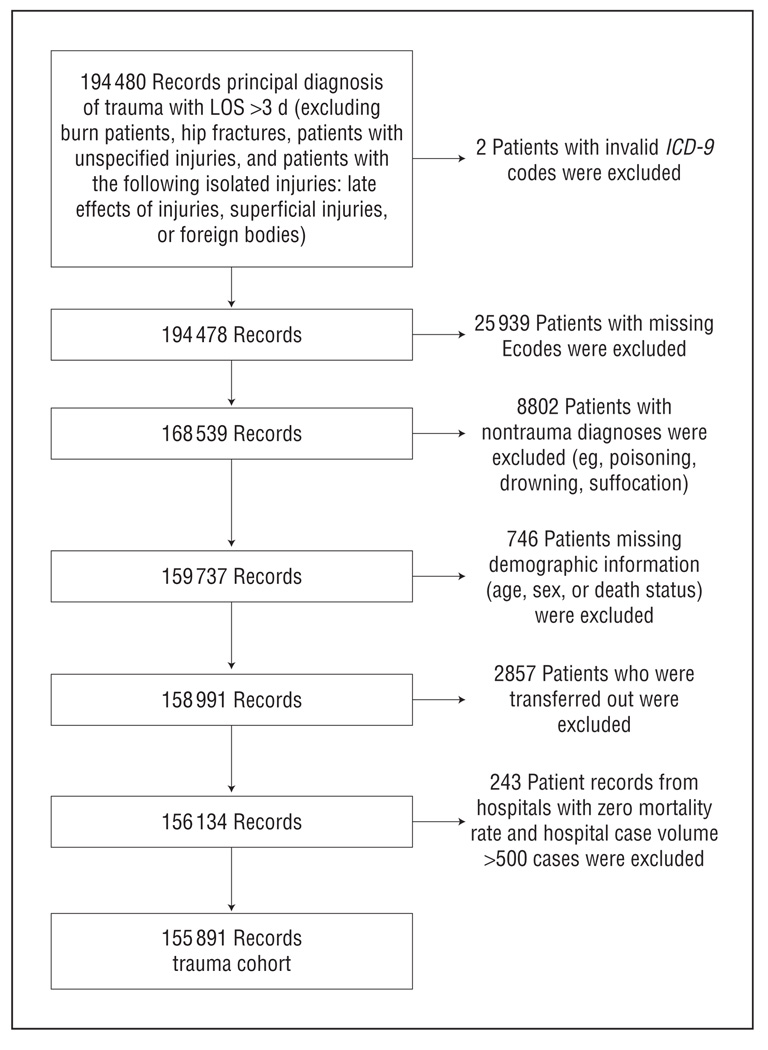
The study population consisted of patients admitted with a principal ICD-9-CM diagnosis of trauma (codes 800–959.9) and LOS more than 3 days, after excluding patients with burns (ICD-9-CM codes 940–949); unspecified injuries (ICD-9-CM codes 959–959.9); and hip fractures (ICD-9-CM codes 820–820.9) and patients with the following isolated injuries: late effects of injury (ICD-9-CM codes 905–909.9), superficial injuries (ICD-9-CM codes 910–924.9), or foreign bodies (ICD-9-CM codes 930–939.0). Patients with LOS less than 3 days were excluded because we assumed that patients who were either discharged or died within 3 days would not have time to have developed HAIs. From this initial cohort of 190 480 patient records, we excluded 25 939 patients with missing External Cause-of-Injury Coding (Ecodes), 8802 patients with nontraumatic mechanisms (eg, poisoning, drowning, suffocation), 746 patients missing demographic information (age, sex, or death status), 2857 patients who were transferred out, and 243 patient records from hospitals with a hospital case volume greater than 500 cases with zero mortality rates. The final study cohort consisted of 155 891 patient records (Figure 1).
Figure 1.
Study population. Ecodes indicates External Cause-of-Injury Coding; ICD-9, International Classification of Diseases, Ninth Revision; LOS, length of stay.
DEFINITIONS
For the purpose of this analysis, 4 different HAI groups were defined using ICD-9-CM codes: (1) sepsis, (2) pneumonia, (3) Staphylococcus infections, and (4) Clostridium difficile–associated disease (CDAD). The criteria used for identifying HAIs are shown in Table 1. Individual patients could have more than 1 HAI. The criteria used to identify cases with sepsis and pneumonia have been previously validated13,19 and used by other investigators.14 Criteria used to identify Staphylococcus infections and CDAD are based on previously published algorithms.15,16 We assumed that all cases identified using these algorithms represented HAIs since it is unlikely that patients admitted with traumatic injuries would have preexisting infections.
Table 1.
Criteria for Identifying Health Care–Associated Infection
| Infection Type | ICD-9-CM Discharge Diagnosis Codes |
|---|---|
| Sepsis | 038–038.9, 112.5, 112.81, 785.52, 995.91, and 995.92 |
| Pneumonia | 482.0–482.2 and 482.4–482.9 |
| Staphylococcus | 730.0–730.09, 711–711.09, 038.11, 041.11, 482.41, V09.0, V09.8, 008.41, 038.1, 790.7, 996.62, 421.0, 996.61, 998.3, and 998.5 |
| Clostridium difficile | 008.45 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
STATISTICAL ANALYSIS
The outcome variables of interest were mortality, LOS, and hospital cost. Separate patient-level analyses were conducted to examine the association between each of the outcome variables and the presence of either sepsis, pneumonia, Staphylococcus infection, or CDAD.
In the first set of analyses, we explored the association between mortality and sepsis, after controlling for patient demographics (age and sex), mechanisms of injury, injury severity, and comorbidities using logistic regression. Patient comorbidities were coded using the Agency for Healthcare Research and Quality Comorbidity Algorithm.17 Injury severity was coded using empirically derived estimates of injury severity based on the previously validated Trauma Mortality Probability Model.12,20 Backward stepwise selection and clinical judgment were used to select comorbidity variables for inclusion in the regression model. Robust variance estimators were used to account for the nonindependence of observations within hospitals. The effect of sepsis on mortality was assessed using the adjusted odds ratio (AOR). We repeated these analyses using either pneumonia, Staphylococcus infection, or CDAD as the exposure variable. In each of the analyses, the reference category only included patients without any of the HAIs (as defined earlier).
In the second set of analyses, we explored the association between cost and sepsis, after controlling for patient demographics (age and sex), mechanisms of injury, injury, comorbidities, and hospital factors (teaching status, rurality, geographic region). The ICD-9-CM injury diagnoses were coded as binary indicator variables. We used a generalized linear model21 with a log link function and a gamma variance function. The variance function was selected using an approach previously described by Manning and Mullahy.22 Robust variance estimators were used because patient outcomes in the same hospital may be correlated.23 The adjusted ratio of hospital cost for patients with sepsis compared with patients without an HAI was assessed by exponentiating the estimated model parameter for cost (eAppendix, http://www.archsurg.com). We repeated these analyses using either pneumonia, Staphylococcus infection, or CDAD as the exposure variable. In the third set of analyses, we separately explored the association between LOS and each of the HAI categories. The models were specified using the same functional form as the cost models.
In the final analysis, we estimated a prediction model for composite measure of HAI (sepsis, pneumonia, Staphylococcus, or CDAD) as a function of patient demographics (age and sex), mechanisms of injury, injury severity, body region, and comorbidities. Backward stepwise selection was used to select comorbidity variables for inclusion in the regression model. Robust variance estimators were used to account for the nonindependence of observations within hospitals.
All statistical analyses were performed using Stata SE/MP version 11.0 (StataCorp, College Station, Texas). The performance of the logistic regression models (for mortality) was assessed using measures of discrimination (C-statistic) and calibration (the Hosmer-Lemeshow statistic).
RESULTS
Table 2 displays information on hospital demographics. Sixty-two percent of the hospitals were located in urban areas and 80% of the hospitals were nonteaching institutions. The hospitals were distributed across all 4 major geographic regions.
Table 2.
Hospital Demographicsa
| Demographic | No. (%) |
|---|---|
| Location | |
| Rural | 586 (38.1) |
| Urban | 951 (61.8) |
| Hospital size | |
| Small | 612 (39.8) |
| Medium | 417 (27.1) |
| Large | 508 (33.0) |
| Teaching status | |
| Teaching | 301 (19.6) |
| Nonteaching | 1236 (80.3) |
| Trauma center accreditationb | |
| Level I | 95 (6.2) |
| Level II | 113 (7.3) |
| Level III | 150 (9.8) |
| None | 650 (42.2) |
| Missing | 530 (34.4) |
| Geographic region | |
| Northeast | 215 (14.0) |
| South | 604 (39.3) |
| Midwest | 424 (20.7) |
| West | 296 (19.2) |
Hospital demographics for study sample. The bed size corresponding to small, medium, and large varies depending on the geographic region, rural vs urban, and teaching status. The number of hospitals within each category does not add up to 1539 because of missing data on hospital demographics.
Thirty-four percent of the hospitals in the Nationwide Inpatient Sample were missing American Hospital Association AHAID identifiers and could not be linked to the American Hospital Association database to determine trauma center designation status.
Table 3 summarizes patient demographics and comorbidities. Patients with pneumonia and Staphylococcus infections were more likely to be younger than control patients, whereas patients with CDAD were older than control patients. Compared with controls, patients with sepsis, pneumonia, and Staphylococcus infections were less likely to be female. Across all patient groups, the most common mechanism of injury was blunt trauma. However, patients with pneumonia were more likely to be in a motor vehicle accident compared with controls (35.2% vs 18.4%) and also more likely to sustain pedestrian trauma (15.6% vs 7.4%). The incidence of congestive heart failure was higher in patients with sepsis and CDAD compared with patients without HAIs. Patients with sepsis and CDAD were also more likely to have renal failure compared with controls. A higher percentage of patients with CDAD had chronic pulmonary disease compared with controls.
Table 3.
Characteristics of Patients With HAI
| % | ||||||
|---|---|---|---|---|---|---|
| Control | HAI | Sepsis | Pneumonia | Staphylococcus |
Clostridium difficile |
|
| No. (%) of patientsa | 148 880 | 7011 (4.50) | 2955 (1.90) | 2479 (1.59) | 3803 (2.44) | 768 (0.49) |
| Age, y, median (IQR) | 57 (34–78) | 53 (32–74) | 58 (38–77) | 47 (27–66) | 49 (31–71) | 69 (45–82) |
| Female | 48.0 | 33.4 | 33.8 | 24.6 | 32.3 | 47.7 |
| Injury mechanism | ||||||
| Blunt trauma | 48.5 | 43.5 | 44.8 | 36.0 | 43.9 | 51.2 |
| Motor vehicle accident | 18.4 | 24.0 | 22.2 | 35.2 | 22.7 | 17.4 |
| Gunshot wound | 3.6 | 4.8 | 5.6 | 5.0 | 4.6 | 2.5 |
| Stab wound | 3.1 | 4.1 | 1.7 | 1.3 | 6.0 | 1.8 |
| Pedestrian trauma | 7.4 | 11.1 | 11.3 | 15.6 | 11.0 | 6.10 |
| Low fall | 19.0 | 14.5 | 14.5 | 7.1 | 11.8 | 21.0 |
| Body regionb | ||||||
| Head | 27.1 | 51.1 | 50.7 | 72.9 | 49.0 | 40.9 |
| Face | 7.1 | 9.3 | 7.9 | 14.0 | 9.1 | 6.4 |
| Chest | 23.7 | 36.1 | 36.7 | 50.9 | 33.2 | 27.8 |
| Abdomen | 18.7 | 23.0 | 26.3 | 24.8 | 19.9 | 20.8 |
| Extremity | 58.4 | 39.0 | 38.1 | 30.6 | 39.2 | 43.3 |
| Superficial | 14.2 | 14.6 | 8.6 | 14.0 | 18.5 | 9.6 |
| Comorbidities | ||||||
| Congestive heart failure | 7.6 | 11.7 | 15.6 | 8.0 | 9.4 | 18.2 |
| Valvular disease | 4.0 | 3.6 | 4.7 | 2.4 | 2.8 | 5.7 |
| Pulmonary circulation disorders | 0.67 | 0.68 | 0.78 | 0.48 | 0.50 | 1.43 |
| Peripheral vascular disease | 2.6 | 3.1 | 2.9 | 1.9 | 3.0 | 5.1 |
| Hypertension | 36.2 | 27.6 | 27.6 | 20.7 | 27.2 | 39.0 |
| Renal failure | 4.1 | 7.1 | 9.6 | 3.6 | 5.9 | 11.8 |
| Liver disease | 1.4 | 2.8 | 3.6 | 2.2 | 3.0 | 1.4 |
| Paralysis | 2.5 | 4.9 | 4.4 | 6.0 | 5.3 | 5.1 |
| Other neurologic disorder | 6.3 | 8.5 | 8.7 | 9.8 | 8.3 | 9.5 |
| Chronic pulmonary disease | 12.5 | 14.0 | 13.8 | 12.7 | 13.0 | 19.1 |
| Diabetes | 12.4 | 9.6 | 10.1 | 7.4 | 9.8 | 11.6 |
| Diabetes, chronic complications | 2.5 | 3.0 | 3.0 | 1.1 | 3.8 | 5.8 |
| Hypothyroidism | 7.7 | 4.1 | 3.8 | 2.5 | 3.8 | 7.4 |
| Lymphoma | 0.33 | 0.43 | 0.64 | 0.20 | 0.42 | 0.52 |
| Metastatic cancer | 0.55 | 0.56 | 0.78 | 0.28 | 0.60 | 0.91 |
| Solid tumor without metastasis | 0.82 | 0.94 | 0.91 | 0.32 | 0.74 | 2.60 |
| Rheumatoid arthritis | 1.9 | 1.2 | 1.1 | 0.89 | 1.2 | 1.3 |
| Coagulation deficiency | 2.7 | 8.3 | 11.1 | 8.6 | 6.8 | 6.8 |
| Obesity | 3.8 | 2.7 | 2.3 | 1.7 | 2.8 | 3.6 |
| Weight loss | 1.8 | 10.3 | 11.7 | 14.2 | 8.5 | 11.2 |
| Fluid and electrolyte disorder | 14.5 | 33.3 | 38.3 | 37.6 | 29.8 | 37.3 |
| Blood loss anemia | 1.5 | 2.6 | 2.5 | 3.2 | 2.3 | 3.5 |
| Deficiency anemia | 10.9 | 12.9 | 12.8 | 11.9 | 13.1 | 18.1 |
| Peptic ulcer disease | 0.03 | 0.01 | 0 | 0.04 | 0 | 0 |
| Alcohol abuse | 9.1 | 13.1 | 12.7 | 16.8 | 13.6 | 9.9 |
| Drug abuse | 4.3 | 5.6 | 5.2 | 6.0 | 6.4 | 3.3 |
| Psychoses | 3.4 | 3.2 | 2.9 | 2.5 | 3.6 | 3.6 |
| Depression | 7.1 | 4.9 | 4.3 | 3.3 | 5.4 | 8.3 |
Abbreviations: HAI, hospital-acquired infection; IQR, interquartile range.
Groups are not mutually exclusive (eg, patients in the sepsis group could also have a Staphylococcus infection).
Groups are not mutually exclusive. Patients could have injuries in more than 1 body region.
Patients with HAIs were less likely to be female (AOR, 0.70; 95% CI, 0.66–0.75). Patients whose mechanism of injury was either a motor vehicle accident (AOR, 1.25; 95% confidence interval [CI], 1.15–1.36; P value < .001), gunshot wound (AOR, 1.28; 95% CI, 1.12–1.45; P value < .001), stab wound (AOR, 1.74; 95% CI, 1.48–2.06; P value < .001), or pedestrian trauma (AOR, 1.49; 95% CI, 1.34–1.64; P value < .001) were more likely to develop an HAI compared with blunt trauma patients (Table 4). Patients with injuries to the head (AOR, 1.32; 95% CI, 1.21–1.43; P value < .001) or chest region (AOR, 1.22; 95% CI, 1.14–1.30; P value < .001) had a higher risk of HAI, whereas injuries to the extremities (AOR, 0.80; 95% CI, 0.74–0.87; P value < .001) were associated with a lower risk of HAI, compared with abdominal injuries.
Table 4.
Results of Multivariate Model for Development of Hospital-Acquired Infection
| AOR (95% CI) | P Value | |
|---|---|---|
| Agea | 1.02 (1.01–1.04) | .001 |
| Female | 0.70 (0.66–0.75) | <.001 |
| Injury mechanism | ||
| Blunt trauma | 1 [Reference] | |
| Motor vehicle accident | 1.25 (1.15–1.36) | <.001 |
| Gunshot wound | 1.28 (1.12–1.45) | <.001 |
| Stab wound | 1.74 (1.48–2.06) | <.001 |
| Pedestrian trauma | 1.49 (1.34–1.64) | <.001 |
| Low fall | 0.90 (0.82–0.98) | .01 |
| Body region | ||
| Head | 1.32 (1.21–1.43) | <.001 |
| Face | 1.07 (0.99–1.16) | .10 |
| Chest | 1.22 (1.14–1.30) | <.001 |
| Abdomen | 1 [Reference] | |
| Extremity | 0.80 (0.74–0.87) | <.001 |
| Superficial | 1.00 (0.91–1.09) | .94 |
| Comorbidities | ||
| Congestive heart failure | 1.63 (1.48–1.79) | <.001 |
| Hypertension | 0.74 (0.69–0.80) | <.001 |
| Renal failure | 1.70 (1.50–1.92) | <.001 |
| Liver disease | 1.43 (1.21–1.68) | <.001 |
| Paralysis | 1.46 (1.27–1.67) | <.001 |
| Other neurologic disorder | 1.55 (1.41–1.71) | <.001 |
| Chronic pulmonary disease | 1.28 (1.18–1.38) | <.001 |
| Diabetes, chronic complications | 1.57 (1.35–1.82) | <.001 |
| Hypothyroidism | 0.70 (0.61–0.81) | <.001 |
| Coagulation deficiency | 1.73 (1.54–1.93) | <.001 |
| Weight loss | 3.09 (2.66–3.59) | <.001 |
| Fluid and electrolyte disorder | 2.22 (2.05–2.39) | <.001 |
| Blood loss anemia | 1.37 (1.08–1.74) | .009 |
| Deficiency anemia | 1.16 (1.06–1.27) | .002 |
| Depression | 0.89 (0.80–0.99) | .03 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval.
Age is in increments of 10 years.
Crude mortality rates, costs, and LOS were all much higher in patients with HAIs compared with patients without HAIs (Table 5). The overall mortality rate for control patients was 1.99%, compared with 21.2% for patients with sepsis, 10.6% for patients with pneumonia, 7.91% for patients with Staphylococcus infections, and 7.27% for patients with CDAD. Compared with other injury mechanisms, low-fall patients with HAIs experienced the highest mortality.
Table 5.
Mortality, Cost, and Length of Stay of Patients With Hospital-Acquired Infections
| Control | Sepsis | Pneumonia | Staphylococcus |
Clostridium difficile |
|
|---|---|---|---|---|---|
| No. (%) of patients | 148 888 | 2955 (1.59) | 2479 (1.59) | 3803 (2.44) | 768 (0.49) |
| Mortality, % | |||||
| Overall | 1.99 | 21.2 | 10.6 | 7.91 | 7.27 |
| Blunt trauma | 2.09 | 23.0 | 12.4 | 9.10 | 8.38 |
| Motor vehicle accident | 2.03 | 17.1 | 7.34 | 6.36 | 0.75 |
| Gunshot wound | 1.99 | 15.7 | 10.6 | 6.86 | 5.26 |
| Stab wound | 0.50 | 8.0 | 3.23 | 0.44 | 7.14 |
| Pedestrian trauma | 1.82 | 18.3 | 9.84 | 6.71 | 6.38 |
| Low fall | 1.99 | 30.3 | 19.9 | 11.8 | 10.5 |
| Cost, $, median | |||||
| Overall | 12 849 | 60 398 | 77 393 | 47 908 | 33 294 |
| Blunt trauma | 11 515 | 45 046 | 70 551 | 33 759 | 26 225 |
| Motor vehicle accident | 17 729 | 86 290 | 82 997 | 74 830 | 65 754 |
| Gunshot wound | 18 213 | 93 770 | 87 045 | 73 677 | 47 489 |
| Stab wound | 12 111 | 49 767 | 61 641 | 10 196 | 27 984 |
| Pedestrian trauma | 19 384 | 99 037 | 86 025 | 79 597 | 90 691 |
| Low fall | 10 270 | 36 229 | 55 945 | 24 670 | 21 276 |
| Length of stay, d, median | |||||
| Overall | 6 | 20 | 24 | 17 | 16 |
| Blunt trauma | 6 | 17 | 23 | 15 | 14 |
| Motor vehicle accident | 6 | 25 | 25 | 23 | 22 |
| Gunshot wound | 7 | 29 | 26 | 24 | 17 |
| Stab wound | 5 | 20 | 22 | 7 | 12 |
| Pedestrian trauma | 7 | 29 | 27 | 25 | 30 |
| Low fall | 5 | 15 | 30 | 13 | 13 |
Total inpatient costs were between 2.6 to 6 times higher in patients with HAI compared with patients without HAIs (Table 5). Patients with sepsis ($60 398) and pneumonia ($77 393) had the highest median costs compared with control patients ($12 849). Inpatients with HAI had nearly a 3- to 4-fold higher LOS compared with patients without HAIs.
After controlling for patient demographics, mechanism of injury, injury type, and comorbidities, we found that mortality, cost, and LOS were significantly higher in patients with HAIs compared with patients without HAIs (Figures 2, 3, and 4). Patients with sepsis had a nearly 6-fold higher odds of death compared with patients without an HAI (OR, 5.78; 95% CI, 5.03–6.64; P < .001). Patients with other HAIs had a 1.5- to 1.9-fold higher odds of mortality compared with controls (P < .005). Patients with HAIs had costs that were 2- to 2.5-fold higher compared with patients without HAIs (P < .001). The median LOS was approximately 2-fold higher in patients with HAIs compared with patients without HAIs (P < .001).
Figure 2.
Impact on mortality of hospital-acquired infections controlling for patient demographics, mechanism of injury, injury severity, and comorbidities. CI indicates confidence interval; OR, odds ratio.
Figure 3.
Impact on inpatient costs of hospital-acquired infections controlling for patient demographics, mechanism of injury, injury, comorbidities, and hospital factors (teaching status, rurality, geographic region). CI indicates confidence interval.
Figure 4.
Impact on length of stay (LOS) of hospital-acquired infections controlling for patient demographics, mechanism of injury, injury, comorbidities, and hospital factors (teaching status, rurality, geographic region). CI indicates confidence interval.
The logistic regression models exhibited excellent discrimination. The C-statistics for the mortality models ranged between 0.88 and 0.90; the C-statistic for the model predicting HAIs was 0.76. Model calibration, assessed using the Hosmer-Lemeshow statistic, ranged between 31 and 186 and is acceptable given the Hosmer-Lemeshow statistic’s well-known sensitivity to sample size and the very large size of our patient cohort.24
COMMENT
In this study based on the Healthcare Cost and Utilization Project Nationwide Inpatient Sample, we found that trauma patients with HAIs are at increased risk for mortality, have longer LOS, and incur higher inpatient costs. In particular, trauma patients with sepsis had a 6-fold higher risk of mortality, whereas patients with other HAIs had a nearly 1.5- to 2-fold higher mortality compared with patients without an HAI. Furthermore, patients with HAIs had LOS and inpatient costs that were approximately 2-fold higher than patients without HAIs.
Reducing HAIs is one of the top priorities in the efforts by the federal government and nongovernmental entities to improve patient safety and health care outcomes in the United States. In particular, the US Department of Health and Human Services has established a national agenda for HAI prevention in an Action Plan that outlines a strategy to reduce the incidence of HAIs by 75% over a 5-year period.25 Furthermore, in this action plan, methicillin-resistant Staphylococcus aureus and CDAD acquired in the acute hospital setting have been identified as priority areas. The National Quality Forum has identified the prevention of health care–associated infections as a key area for improving patient safety in its list of Safe Practices for Better Healthcare.26 Three of the 6 recommended practices in the Institute for Healthcare Improvement’s 100 000 Lives Campaign are focused on the prevention of HAIs.27 Mandatory public reporting of hospital HAI rates is becoming more widespread as part of the effort to increase transparency and accountability to achieve reductions in HAIs.28–30 Finally, the Centers for Medicare and Medicaid Services is no longer reimbursing hospitals for some HAIs as part of the legislatively mandated initiative to penalize hospitals for hospital-acquired conditions.31–33
To our knowledge, our study is the first population-based epidemiologic study of HAIs in trauma patients using a large nationally representative database. Many of the previous studies on trauma patients with HAIs have focused on identifying risk factors for the development of HAIs.9,34–42 Other studies have described the epidemiologic features of HAI in the trauma patients7,43–48 Our findings confirm the findings of previous studies that HAIs in trauma patients are associated with increased mortality,36 LOS,8,36,47 and cost.8,47 In 2 of these previous studies, researchers did not find an independent association between HAI and mortality.45,47 All of these prior studies were relatively small and all were single-center studies, limiting the generalizability of their findings.
There are several important limitations to our study. First, administrative data are not as accurate as clinical records and do not capture all instances of HAIs. However, the accuracy of administrative data for identifying cases of sepsis has been validated in a previous epidemiologic study.13 For pneumonias, ICD-9-CM codes demonstrate high specificity for the detection of pneumonias, but the sensitivity is approximately 50%. The accuracy of ICD-9-CM codes for detecting cases of Staphylococcus infections and CDAD is largely unknown.15,16 The undercoding of other hospital-acquired complications using administrative data has been confirmed in validation studies of the Agency for Healthcare Research and Quality Patient Safety Indicators.49 The undercoding of HAIs may be a source of bias in our analysis and may lead us to underestimate the impact of HAIs on outcomes if a significant number of patients with HAIs are included in the reference population of patients without HAIs.
Second, the use of the Trauma Mortality Probability Model may not have completely adjusted for disease severity because of the lack of information on patient physiology in administrative data.20 The Trauma Mortality Probability Model–ICD-9 is based on ICD-9-CM injury codes but does not include important information on patient physiology such as Glasgow Coma Scale scores and vital signs on hospital admission. However, the statistical performance of the Trauma Mortality Probability Model–ICD-9 is excellent, minimizing the potential for omitted variable bias.20 Third, we were unable to explore the impact of HAIs on other important quality domains such as functional outcomes because these outcome data are not included in administrative data. Future work exploring the impact of HAIs on other quality domains will be necessary once these additional outcomes data become available.
Third, the Nationwide Inpatient Sample does not allow us to determine whether infections identified as HAIs were present on admission or developed as a complication of the hospital stay. Therefore, it is possible that some of the infections represent community-acquired infections as opposed to HAIs. However, it is likely that most infections in trauma patients are hospital acquired. Although this is a reasonable assumption for trauma patients, we were not able to verify this assumption because of the absence of a present-on-admission indicator in the Nationwide Inpatient Sample. Despite these limitations, to our knowledge, this study is the first analysis of the impact of HAIs in trauma patients using a large nationally representative database. Although it shares many of the same limitations of other epidemiological investigations conducted using large administrative data sets, it has the advantage of a large sample size not possible in studies based on prospectively collected clinical data.
Finally, our estimate of the association between HAIs and LOS may overestimate the effect of HAI on LOS. A priori, patients who develop HAIs would be expected to have longer LOS. However, patients who stay longer in the hospital would also be expected to be at higher risk of developing HAIs. As a result, the estimated correlation may overstate the influence of HAI on LOS. Statistical techniques to deal with this problem of endogeneity between LOS and HAIs, ie, the use of instrumental variables, would not be feasible here. This limitation is partially offset because the LOS model includes many of the important determinants of LOS. This same issue applies to the association between HAIs and cost because the LOS is an important element of cost. The practical impact of this bias from a policy perspective is lessened by the fact that policies designed to reduce the likelihood of HAIs could also include efforts to reduce LOS.
In summary, HAIs are associated with increased mortality, LOS, and inpatient costs in patients admitted with traumatic injuries. In light of the preventability of many hospital-acquired conditions50,51 and the magnitude of the clinical and economic burden of HAIs, the current emphasis on implementing interventions aiming to decrease the incidence of HAIs may have a potentially large impact. The current shift in payment policies away from “output-based funding” toward “outcomes-based funding” may act as a catalyst for patient safety initiatives designed to reduce HAIs and improve patient outcomes.32 Future studies will be necessary to assess the impact of recent changes in Centers for Medicare and Medicaid Services payment policies on the incidence of HAIs.
Acknowledgments
Funding/Support: This project was supported by grant RO1 HS 16737 from the Agency for Healthcare and Quality Research and grant R01 NR01 0107 from the National Institutes of Health (Prevention of Nosocomial Infections and Cost-Effectiveness).
Footnotes
Author Contributions: Dr Glance had full access to the data and takes responsibility for the accuracy of the data analysis. Study concept and design: Glance, Mukamel, and Dick. Acquisition of data: Glance. Analysis and interpretation of data: Glance, Stone, and Dick. Drafting of the manuscript: Glance, Stone, and Dick. Critical revision of the manuscript for important intellectual content: Glance, Stone, Mukamel, and Dick. Statistical analysis: Glance, Mukamel, and Dick. Obtained funding: Glance and Dick. Administrative, technical, and material support: Glance. Study supervision: Glance.
Financial Disclosure: None reported.
Disclaimer: The views presented in this article are those of the authors and may not reflect those of the Agency for Healthcare and Quality Research and the National Institutes of Health.
Online-Only Material: The eAppendix is available at http://www.archsurg.com.
REFERENCES
- 1.Institute of Medicine. To Err is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000. [Google Scholar]
- 2.Burke JP. Infection control: a problem for patient safety. N Engl J Med. 2003;348(7):651–656. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 3.Scott RD. The direct medical costs of healthcare-associated infections in US hospitals and the benefits of prevention. [Accessed May 5, 2010]; www.cdc.gov/ncidod/dhqp/pdf/Scott_CostPaper.pdf. Published 2009.
- 4.Leape LL, Berwick DM. Five years after To Err Is Human: what have we learned? JAMA. 2005;293(19):2384–2390. doi: 10.1001/jama.293.19.2384. [DOI] [PubMed] [Google Scholar]
- 5.Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating health care-associated infections and deaths in US hospitals, 2002. Public Health Rep. 2007;122(2):160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson RN. Deaths: leading causes for 1999. Natl Vital Stat Rep. 2001;49(11):1–87. [PubMed] [Google Scholar]
- 7.Wallace WC, Cinat M, Gornick WB, Lekawa ME, Wilson SE. Nosocomial infections in the surgical intensive care unit: a difference between trauma and surgical patients. Am Surg. 1999;65(10):987–990. [PubMed] [Google Scholar]
- 8.Pories SE, Gamelli RL, Mead PB, Goodwin G, Harris F, Vacek P. The epidemiologic features of nosocomial infections in patients with trauma. Arch Surg. 1991;126(1):97–99. doi: 10.1001/archsurg.1991.01410250105017. [DOI] [PubMed] [Google Scholar]
- 9.Jamulitrat S, Narong MN, Thongpiyapoom S. Trauma severity scoring systems as predictors of nosocomial infection. Infect Control Hosp Epidemiol. 2002;23(5):268–273. doi: 10.1086/502047. [DOI] [PubMed] [Google Scholar]
- 10.Krug EG, Sharma GK, Lozano R. The global burden of injuries. Am J Public Health. 2000;90(4):523–526. doi: 10.2105/ajph.90.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute of Medicine. Reducing the Burden of Injury: Advancing Prevention and Treatment. Washington, DC: National Academy Press; 1999. [PubMed] [Google Scholar]
- 12.Glance LG, Dick AW, Mukamel DB, Meredith W, Osler TM. The effect of preexisting conditions on hospital quality measurement for injured patients. Ann Surg. 2010;251(4):728–734. doi: 10.1097/SLA.0b013e3181d56770. [DOI] [PubMed] [Google Scholar]
- 13.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 14.Eber MR, Laxminarayan R, Perencevich EN, Malani A. Clinical and economic outcomes attributable to health care-associated sepsis and pneumonia. Arch Intern Med. 2010;170(4):347–353. doi: 10.1001/archinternmed.2009.509. [DOI] [PubMed] [Google Scholar]
- 15.Noskin GA, Rubin RJ, Schentag JJ, et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med. 2005;165(15):1756–1761. doi: 10.1001/archinte.165.15.1756. [DOI] [PubMed] [Google Scholar]
- 16.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis. 2006;12(3):409–415. doi: 10.3201/eid1203.051064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Cost-to-charge ratio files. [Accessed April 8, 2010];Healthcare Cost and Utilization Project Web site. http://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp.
- 19.Aronsky D, Haug PJ, Lagor C, Dean NC. Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328. doi: 10.1177/1062860605280358. [DOI] [PubMed] [Google Scholar]
- 20.Glance LG, Osler TM, Mukamel DB, Meredith W, Wagner J, Dick AW. TMPM-ICD9: a trauma mortality prediction model based on ICD-9-CM codes. Ann Surg. 2009;249(6):1032–1039. doi: 10.1097/SLA.0b013e3181a38f28. [DOI] [PubMed] [Google Scholar]
- 21.McCullagh P, Nelder JA. Generalized Linear Models. 2nd ed. New York, NY: Chapman & Hall/CRC; 1989. [Google Scholar]
- 22.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 23.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48(4):817–830. [Google Scholar]
- 24.Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: the Hosmer-Lemeshow test revisited. Crit Care Med. 2007;35(9):2052–2056. doi: 10.1097/01.CCM.0000275267.64078.B0. [DOI] [PubMed] [Google Scholar]
- 25.HHS Action Plan to Prevent Healthcare-Associated Infections. US Department of Health and Human Services Web site. [Accessed May 10, 2010]; http://www.hhs.gov/ash/initiatives/hai/actionplan/index.html.
- 26.National Quality Forum. Safe Practices for Better Healthcare—2009 Update: A Consensus Report. Washington, DC: National Quality Forum; 2009. [Google Scholar]
- 27.Wachter RM, Pronovost PJ. The 100,000 Lives Campaign: a scientific and policy review. Jt Comm J Qual Patient Saf. 2006;32(11):621–627. doi: 10.1016/s1553-7250(06)32080-6. [DOI] [PubMed] [Google Scholar]
- 28.McKibben L, Fowler G, Horan T, Brennan PJ. Ensuring rational public reporting systems for health care-associated infections: systematic literature review and evaluation recommendations. Am J Infect Control. 2006;34(3):142–149. doi: 10.1016/j.ajic.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Stone PW, Horan TC, Shih HC, Mooney-Kane C, Larson E. Comparisons of health care-associated infections identification using two mechanisms for public reporting. Am J Infect Control. 2007;35(3):145–149. doi: 10.1016/j.ajic.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Jhung MA, Banerjee SN. Administrative coding data and health care-associated infections. Clin Infect Dis. 2009;49(6):949–955. doi: 10.1086/605086. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Medicare and Medicaid Services. Roadmap for implementing value driven healthcare in the traditional Medicare fee-for-service program. [Accessed April 16, 2010]; http://www.cms.gov/QualityInitiativesGenInfo/downloads/VBPRoadmap_OEA_1-16_508.pdf. Published 2010.
- 32.Stone PW, Glied SA, McNair PD, et al. CMS changes in reimbursement for HAIs: setting a research agenda. Med Care. 2010;48(5):433–439. doi: 10.1097/MLR.0b013e3181d5fb3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNair PD, Luft HS, Bindman AB. Medicare’s policy not to pay for treating hospital-acquired conditions: the impact. Health Aff (Millwood) 2009;28(5):1485–1493. doi: 10.1377/hlthaff.28.5.1485. [DOI] [PubMed] [Google Scholar]
- 34.Hurr H, Hawley HB, Czachor JS, Markert RJ, McCarthy MC. APACHE II and ISS scores as predictors of nosocomial infections in trauma patients. Am J Infect Control. 1999;27(2):79–83. doi: 10.1016/s0196-6553(99)70085-4. [DOI] [PubMed] [Google Scholar]
- 35.Tejada Artigas A, Bello Dronda S, Chacón Vallés E, et al. Risk factors for nosocomial pneumonia in critically ill trauma patients. Crit Care Med. 2001;29(2):304–309. doi: 10.1097/00003246-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Bochicchio GV, Joshi M, Knorr KM, Scalea TM. Impact of nosocomial infections in trauma: does age make a difference? J Trauma. 2001;50(4):612–617. doi: 10.1097/00005373-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Flores JM, Jiménez PI, Rincón MD, et al. Early risk factors for sepsis in patients with severe blunt trauma. Injury. 2001;32(1):5–12. doi: 10.1016/s0020-1383(00)00103-0. [DOI] [PubMed] [Google Scholar]
- 38.Bochicchio GV, Napolitano LM, Joshi M, et al. Persistent systemic inflammatory response syndrome is predictive of nosocomial infection in trauma. J Trauma. 2002;53(2):245–250. doi: 10.1097/00005373-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 39.El-Masri MM, Hammad TA, McLeskey SW, Joshi M, Korniewicz DM. Predictors of nosocomial bloodstream infections among critically ill adult trauma patients. Infect Control Hosp Epidemiol. 2004;25(8):656–663. doi: 10.1086/502457. [DOI] [PubMed] [Google Scholar]
- 40.Cavalcanti M, Ferrer M, Ferrer R, Morforte R, Garnacho A, Torres A. Risk and prognostic factors of ventilator-associated pneumonia in trauma patients. Crit Care Med. 2006;34(4):1067–1072. doi: 10.1097/01.CCM.0000206471.44161.A0. [DOI] [PubMed] [Google Scholar]
- 41.Hoover L, Bochicchio GV, Napolitano LM, et al. Systemic inflammatory response syndrome and nosocomial infection in trauma. J Trauma. 2006;61(2):310–316. doi: 10.1097/01.ta.0000229052.75460.c2. [DOI] [PubMed] [Google Scholar]
- 42.Rangel EL, Butler KL, Johannigman JA, Tsuei BJ, Solomkin JS. Risk factors for relapse of ventilator-associated pneumonia in trauma patients. J Trauma. 2009;67(1):91–95. doi: 10.1097/TA.0b013e3181a8b2b2. [DOI] [PubMed] [Google Scholar]
- 43.Schimpff SC, Miller RM, Polkavetz S, Hornick RB. Infection in the severely traumatized patient. Ann Surg. 1974;179(3):352–357. doi: 10.1097/00000658-197403000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papia G, McLellan BA, El-Helou P, et al. Infection in hospitalized trauma patients: incidence, risk factors, and complications. J Trauma. 1999;47(5):923–927. doi: 10.1097/00005373-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 45.Laupland K, Gregson DB, Kirkpatrick AW, et al. Bloodstream infection complicating trauma. Clin Invest Med. 2004;27(5):253–258. [PubMed] [Google Scholar]
- 46.Lazarus HM, Fox J, Lloyd JF, et al. A six-year descriptive study of hospital-associated infection in trauma patients: demographics, injury features, and infection patterns. Surg Infect (Larchmt) 2007;8(4):463–473. doi: 10.1089/sur.2005.43. [DOI] [PubMed] [Google Scholar]
- 47.Lazarus HM, Fox J, Burke JP, et al. Trauma patient hospital-associated infections: risks and outcomes. J Trauma. 2005;59(1):188–194. doi: 10.1097/01.ta.0000171535.75484.df. [DOI] [PubMed] [Google Scholar]
- 48.Osborn TM, Tracy JK, Dunne JR, Pasquale M, Napolitano LM. Epidemiology of sepsis in patients with traumatic injury. Crit Care Med. 2004;32(11):2234–2240. doi: 10.1097/01.ccm.0000145586.23276.0f. [DOI] [PubMed] [Google Scholar]
- 49.Romano PS, Mull HJ, Rivard PE, et al. Validity of selected AHRQ patient safety indicators based on VA National Surgical Quality Improvement Program data. Health Serv Res. 2009;44(1):182–204. doi: 10.1111/j.1475-6773.2008.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 51.Miller RS, Norris PR, Jenkins JM, et al. Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma. 2010;68(1):23–31. doi: 10.1097/TA.0b013e3181c82678. [DOI] [PubMed] [Google Scholar]