Abstract
This study examined responses to loud tones before and after cognitive–behavioral treatment for posttraumatic stress disorder (PTSD). Seventy-four women in a PTSD treatment outcome study for rape-related (n = 54) or physical assault-related PTSD (n = 20) were assessed in an auditory loud tone paradigm. Assessments were conducted before and after a 6-week period of cognitive–behavioral therapy. Physiologic responses to loud tones included heart rate (HR), skin conductance (SC), and eye-blink electromyogram (EMG). Groups were formed based upon treatment outcome and included a treatment responder group (no PTSD at posttreatment) and a nonresponder group (PTSD-positive at posttreatment). Treatment was successful for 53 of 74 women (72%) and unsuccessful for 21 women (28%). Responders and nonresponders were not significantly different from each other at pretreatment on the main outcome variables. Treatment responders showed a significant reduction in loud tone-related EMG, HR, and SC responses from pre- to posttreatment (partial η2 = .243, .308, and .365, respectively; all p < .001) and the EMG and HR responses were significantly smaller than nonresponders at posttreatment (partial η22 = .107, p = .004 and .193, p < .001, respectively). Successful cognitive–behavioral treatment of PTSD is associated with a quantifiable reduction in physiological responding to loud tones.
There are now a number of effective treatments for post-traumatic stress disorder (PTSD) including exposure-based therapies (Foa et al., 1999; Rothbaum & Foa, 1992) and cognitive therapies (Resick et al., 2008; Resick, Monson, & Gutner, 2007; Resick, Nishith, Weaver, Astin, & Feuer, 2002; Resick & Schnicke, 1992, 1993). Most of the studies have quantified treatment outcome in terms of a reduction in self-reported symptoms of PTSD. There is, however, very little information about more objective changes in symptoms following treatment of PTSD. One way of generating more objective measures of therapy outcome is to examine changes in physiological responses to treatment.
Psychophysiological measures have demonstrated utility as a means to correctly identify participants who have PTSD from those who do not (Pole, 2007). Orr, Lasko, Shalev, and Pitman (1995) and Orr, Solomon, Peri, Pitman, and Shalev (1997) have calculated the sensitivity and specificity of psychophysiological indices such as heart rate (HR) and skin conductance (SC) responses to traumatic imagery in combat samples to predict PTSD diagnostic status. Findings for diagnostic sensitivity range from 60 to 90% and the specificity from 80 to 100% across multiple studies (Pitman & Orr, 1993). These findings suggest that psychophysiological assessment holds promise to classify individuals with PTSD objectively. There are, however, only a few reports in the literature of this type of methodology being applied to the interpretation of treatment efficacy.
A study of motor-vehicle accident survivors with PTSD showed that after cognitive–behavioral treatment, participants demonstrated significant decreases in HR to audiotaped descriptions of the accident compared to a supportive psychotherapy or a waitlist condition (Blanchard et al., 2002). Most other reports of physiological response to PTSD treatment have relied on case-study designs. Many of these studies report that a positive treatment outcome is associated with a reduction in physiological arousal to reminders of the traumatic event (Boudewyns & Hyer, 1990; Fairbank & Keane, 1982; Keane & Kaloupek, 1982; Nishith, Griffin, & Weaver, 2002; Shalev, Orr, & Pitman, 1992). A number of studies have found that trauma victims with PTSD exhibit heightened startle reactivity and/or a failure to habituate to repeated stimuli when startle is measured in the psychophysiological laboratory. These include studies of male combat veterans (Butler et al., 1990; Morgan, Grillon, Southwick, & Davis, 1995; Orr et al., 1995, 1997), male police officers (Pole, Neylan, Best, Orr, & Marmar, 2003) female survivors of sexual assault (Morgan, Grillon, Lubin, & Southwick, 1997), female survivors of childhood sexual abuse (Metzger et al., 1999), as well as studies of mixed trauma samples (Shalev, Orr, Peri, & Schreiber, 1992). It should be noted that not every study of startle reactivity has produced heightened responses for PTSD-positive participants (e.g., Jovanovic, Norrholm, Sakoman, Esterajher, & Kozaric-Kavacic, 2009). An examination, however, of effect size for eye-blink electromyogram (EMG) and commonly measured autonomic responses to startling auditory stimuli reveals that the average effect sizes fall into the medium to large range (Metzger et al., 1999; Pole, 2007).
In the present study, we investigated whether successful treatment of PTSD was associated with a reduction in response to loud tones. Specifically we compared female trauma survivors who successfully completed treatment as evidenced by no longer having a diagnosis of PTSD with those who completed treatment, but still had PTSD.
Method
Participants
Participants were 74 women who reported either rape (n = 54) or first-degree physical assault (n = 20) for a treatment study for PTSD. The sample consisted of 50 Caucasian participants (67%), 19 African Americans (26%), and five participants of other racial origin (7%). Participants had to be at least 18 years of age (M = 34.7 years, SD = 11.4) and have a current diagnosis of PTSD at pretreatment. They also had to be at least 3 months postcrime (M = 13.4 years postassault; range = 3 months–53.2 years). Exclusionary criteria included current self-mutilation, being actively suicidal, currently psychotic (assessed with the psychotic screen from the Structured Clinical Interview for DSM-IV [SCID]; First, Spitzer, Williams, & Gibbon, 1995), in treatment with another therapist for the index assault, or being drug or alcohol dependent. None of the participants were taking medication that would alter cardiac activity (e.g., beta blockers, calcium channel blockers, etc.). The study was approved by the Institutional Review Board at the University of Missouri–St. Louis and each participant gave written informed consent prior to the start of the study. It was permissible for participants to be on psychotropic medication as long as they were stabilized on that medication for the duration of the study. About 40% of the sample was stabilized on psychotropic medication during the trial. There were no significant differences in the proportion of participants on psychotropic medication in the groups under consideration in this article (treatment responders = 40% and treatment nonresponders = 39% on current psychotropic medications).
Participants were a subset of participants drawn from a large project designed to study cognitive-processing therapy and its active components (cognitive therapy only or written accounts only). Participants in the parent study were given the option of participating in the physiological assessment portion of the study, but could refuse this portion and still remain in the treatment trial. Findings from the parent study and details of the treatment protocol were previously reported (Resick et al., 2008). Therapy sessions were conducted twice weekly for 6 weeks. Participants in the current study were all treatment completers.
Clinical Measures
Posttraumatic stress disorder was assessed using the Clinician-Administered PTSD Scale (CAPS; Blake, Weathers, Nagy, & Kaloupek, 1995). The CAPS provides a continuous score of symptom frequency and intensity as well as the ability to make diagnostic determinations of PTSD status as defined in the Diagnostic and Statistical Manual of Mental Disorders (4th ed., DSM-IV; American Psychiatric Association, 1994). Multiple clinicians with at least a master’s degree who had training and experience working with trauma survivors were used as diagnostic raters. Specific details of the training and methods of assessing interrater reliability are reported in Resick et al. (2008). The evaluation of interrater reliability for the CAPS revealed that the kappa coefficient for the overall PTSD diagnosis was 1.00 with 100% agreement. Kappa values and percent agreement for each of the three PTSD symptom clusters were as follows: reexperiencing (.87; 90% agreement), avoidance (.72; 77% agreement), and arousal (.69; 77% agreement). The Cronbach’s α on the CAPS total score was .91 in this sample.
The SCID (First et al., 1995) mood disorders module was used to assess major depression and the kappa value and percent agreement obtained for major depressive disorder was .80 and 90% agreement. The Peritraumatic Dissociation Experiences Questionnaire (PDEQ; Marmar et al., 1994) coefficient α = .79 and the Beck Depression Inventory (Beck, Steer, & Brown, 1996; coefficient α = .90) were given as self-report instruments.
Procedure
Diagnostic interviews and self-report measures were collected first. Next, a basic auditory screening test was performed using an audiometer to rule out hearing loss. Hearing loss was defined in this study as an inability to hear a pure tone tested at 125, 750, 1000, 3000, and 6000 Hz at a threshold of 35 decibels. No participants were screened out due to hearing loss. Psychophysiological testing took place in a sound-attenuated room adjacent to an observation room containing the physiological equipment. Participants were seated in a comfortable armchair and the recording electrodes were attached. Participants were instructed that following a 4-minute resting baseline period, they would hear a series of loud tones. They were instructed to keep their eyes open and that the entire procedure would not last more than 20 minutes. The same procedure was repeated at posttreatment. The CAPS pretreatment assessment was conducted prior to the start of treatment and we used the standard 1-month version of the CAPS to assess PTSD symptoms and assign diagnosis within one week of the start of therapy. The posttreatment assessment was conducted 2 weeks after the completion of treatment and the CAPS and other symptom measures were conducted using that 2-week assessment period.
The stimuli consisted of ten 95-dB (sound pressure level), 1000 Hz pure tones presented for 500 ms with nearly instantaneous rise and fall times. Intertrial intervals were varied randomly from 32 to 52 s. Tones were generated with a Coulbourn Instruments (Whitehall, PA) programmable digital-to-analog converter amplifier (L65-28) and voltage controlled oscillator (S24-05). Amplification of the tones was accomplished with an audio mixer amplifier (S82-24) and the onset of the tone stimuli were controlled by a shaped rise-fall gate (S84-04) set for a ohms rise time to full intensity. Tones were presented binaurally over Telephonics (Farmingdale, NY) headphones (model TDH-39P).
Physiologic Measures
The left orbicularis oculi electromyogram (EMG) was recorded from two 4-mm Ag/AgCl surface electrodes positioned according to published recommendations (Fridlund & Cacioppo, 1986). The EMG signals were amplified by a Coulbourn high-gain bioamplifier (S75-01) and filtered to retain the portion from 90–250 Hz. The amplified signal was fed into a contour-following integrator (S76-01) that was set to an integration period of 65 ms. For the orbicularis region EMG, a response score was calculated by taking the maximum EMG level derived from the contour-following integrator between 40–200 ms after tone onset minus the average level during the 1 s immediately prior to tone onset (baseline). If an EMG blink artifact was observed in the pretone baseline period, an average EMG level derived from the pretone period of the other nine trials was used to replace the period with artifact. This procedure was used on a small proportion of the total trials (1.8%).
Skin conductance (SC) was measured using Ag/AgCl 9-mm electrodes filled with isotonic paste and attached to the medial phalanges of the first and third finger of the left hand. A constant 0.5 V signal was sent from a Coulbourn isolated SC coupler (V71-23). An SC response was defined as the maximum conductance level between 0.5–6 s after tone onset minus the average level during the 2 s immediately prior to tone onset.
Heart rate was determined using disposable Ag/AgCl leads attached to the left wrist and right ankle and fed to a Coulbourn bioamplifier (S75-01). Peak R-wave signals were detected and interbeat intervals were converted to HR. Heart rate response was calculated as the maximum HR achieved during the period from 0.5–6 s after tone onset minus the average level during the 5 s immediately prior to tone onset.
Data for all physiological signals were continuously sampled at 500 Hz. To reduce the variance associated with extreme scores a square root transformation was performed on the HR, SC, and EMG response scores before statistical analyses were performed. Habituation to the tone stimuli was quantified using a procedure previously used (Griffin, 2008; Orr et al., 1995; Shalev et al., 1992) by calculating a slope coefficient by plotting a linear regression line through the response scores across trials for each measure for each participant.
Statistical Analyses
Main factors included group comparisons (treatment responders vs. nonresponders) and time comparisons (pre- and posttreatment) and group × time interactions (repeated measures ANOVA, ANCOVA). Greenhouse-Geisser corrections were applied to p values for main effects and interactions involving the time factor (pre- and posttreatment). All statistical tests were two-tailed with α level set at .05 for statistical significance. When significant interactions were discovered and follow-up analyses were required, the α level was protected through the use of the false discovery rate (FDR) procedure (Benjamini & Hochberg, 1995), which provides for an adjusted p value when conducting multiple tests. Analyses that were altered by the FDR p value have been noted.
Two participants did not complete the BDI at pretreatment. Because the BDI scores at pretreatment were used as a covariate in a number of analyses, these missing values were replaced with the mean BDI value of the remaining participants. There are different opinions about the appropriateness of the use of analysis of covariance when there are significant group differences (e.g., see Miller & Chapman, 2001, for a discussion). Heart rate data for two participants and SC data for five participants was unusable due to artifact. In addition, the sample size for the SC analyses was reduced because individuals who were deemed to be SC nonresponders were eliminated. Nonresponders (n = 14) were defined as participants who did not have a discernible SC response on 9 of the 10 trials. This issue appeared to influence the SC data for African American participants at a higher rate than Caucasian participants. African American participants have been observed to show less SC than Caucasians (Korol, Bergfeld, & McLaughlin, 1975).
Results
Clinical Data
Pretreatment data revealed that this treatment-seeking sample of 74 women had severe PTSD (CAPS total score: M = 68.8, SD = 7.9). In addition, 31 of the 74 women (42%) were diagnosed with comorbid major depression and the overall mean value for the BDI at pretreatment was M = 26.2, SD = 10.4, indicating moderate-to-severe depression. Additionally, at pretreatment 14 of 74 participants (19%) of the sample met criteria for panic disorder.
At posttreatment, 18 out of the 74 women were still diagnosed with full PTSD (24%). These participants were classified as treatment nonresponders. There were three other participants who were one symptom short of full PTSD and who had total CAPS scores > 45. There is precedent for classifying these highly symptomatic individuals as PTSD-positive based upon previous psychophysiological research (Orr, 1997). These three participants were included in the nonresponder group for all further analyses. The remaining participants who did not have PTSD at posttreatment were designated as treatment responders (N = 53). Assessment of current major depression at pretreatment revealed that 19 of the 53 treatment responders (36%) had comorbid PTSD and depression and 12 of 21 nonresponders (57%) had comorbid depression. At post-treatment only 1 of the 53 treatment responders (2%) still had major depression, but 10 out of 21 (48%) nonresponders still had a comorbid diagnosis indicating a significant difference in the frequency of comorbid major depression across the groups, χ2 (1, N = 74) = 24.9, p < .001. Assessment of panic disorder revealed that at pretreatment there was no significant difference in the frequency of panic in the treatment responders (9 of 53; 17%) compared to the treatment nonresponders (5 of 21; 24%). At posttreatment only one of 53 treatment responders (2%) retained a diagnosis of panic, but 6 out of 21 (29%) of nonresponders had comorbid panic, χ2(1, N = 74) = 11.4, p = .009.
The means and standard deviations for the demographic and clinical data are presented in Table 1. Analyses of the demographic data revealed no significant differences between the responders and the nonresponders on any of the demographic variables. However, findings indicated significant main effects and interactions between the main factors on many of the clinical measures. Analysis of the CAPS data produced significant group × time interactions for the total score and each subscale score, range of F values (1, 71) = 28.7–92.0, all p < .001. The BDI also produced a significant group × time interaction, F (1, 71) = 10.0, p = .002. Follow-up analyses using pretreatment BDI scores as a covariate revealed that for the total CAPS score there was not a significant difference at pretreatment between the responders and nonresponders, F (1, 71) = 3.97, p = .05, FDR = ns. As expected, based upon the definition being used to identify responders and nonresponders there was a large significant difference between the groups at posttreatment, F (1, 71) = 182.05, p <. 001.
Table 1.
Means, Standard Deviations, and Analysis of Variance for Demographic, Trauma Type, and Symptomsa
| Variable | Pretreatment
|
Posttreatment
|
Main effects and interactiona | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Responders
|
Nonresponders
|
Responders
|
Nonresponders
|
||||||
|
n = 53
|
n =21
|
n = 53
|
n = 21
|
||||||
| M | SD | M | SD | M | SD | M | SD | ||
| Age | 34.4 | 11.6 | 35.5 | 11.0 | ns | ||||
| Education | 14.1 | 2.3 | 14.6 | 2.7 | ns | ||||
| Mo. since assault | 157.7 | 171.9 | 167.8 | 168.5 | ns | ||||
| Trauma type | ns | ||||||||
| Rape | 41 | 55.4 | 13 | 17.6 | |||||
| Physical assault | 12 | 16.2 | 8 | 10.8 | |||||
| CAPS total | 64.8 | 17.2 | 78.7 | 16.1 | 17.3 | 11.7 | 71.4 | 20.2 | G***, G × T*** |
| Reexperiencing | 16.5 | 6.7 | 19.7 | 6.8 | 3.7 | 3.8 | 17.9 | 8.4 | G***, G × T*** |
| Avoidance | 27.8 | 9.6 | 33.3 | 7.5 | 5.8 | 6.9 | 30.4 | 11.3 | G***, T**, G × T*** |
| Arousal | 20.6 | 6.8 | 25.6 | 6.6 | 7.8 | 6.3 | 23.1 | 9.2 | G***, G × T*** |
| BDI | 24.1 | 9.9 | 31.1 | 9.9 | 8.0 | 9.3 | 25.1 | 11.1 | G***, T***, G × T** |
| PDI | 15.8 | 6.5 | 15.2 | 7.3 | |||||
| Race | n | % | n | % | |||||
| African American | 14 | 18.9 | 5 | 6.8 | |||||
| Caucasian | 34 | 45.9 | 16 | 21.6 | |||||
| Other | 5 | 6.8 | 0 | 0.0 | |||||
Note. CAPS = Clinician Administered PTSD Scale; BDI = Beck Depression Inventory; PDI = Peritraumatic Dissociation Index; G = Main effect for group; T = main effect for time; G × T = Group by Time interaction; ns = nonsignificant.
If column blank, no statistically significant effects.
p < .05.
p < .01.
p < .001
Examination of the CAPS subscale scores on reexperiencing, avoidance, and arousal between responders and nonresponders revealed that most of the difference in the total CAPS scores at pretreatment could be attributed to differences on the arousal subscale, F (1, 71) = 3.83, p = .053. The other subscales did not approach statistical significance: avoidance: F (1, 71) = 2.09, p = .15; and reexperiencing: F (1, 71) = 0.72, p = .39. At posttreatment, there were large significant differences between the responders and nonresponders on all of the subscales, range of F values (1, 71) = 61.26–109.27, p < .001).
For the BDI, the nonresponders had significantly higher scores than the responder group at pretreatment, F (1, 72) = 8.44, p = .005; and at posttreatment, F (1, 71) = 44.17, p < .001, and there was a significant decrease in depression scores from pre- to posttreatment in the responder group, F (1, 52) = 114.00, p < .001, but not in the nonresponder group, F (1, 19) = 3.23, p = .09. The amount of peritraumatic dissociation during the traumatic event revealed no significant difference between treatment responders and nonresponders, F (1, 71) = 0.11, p = .73.
Physiological Measures
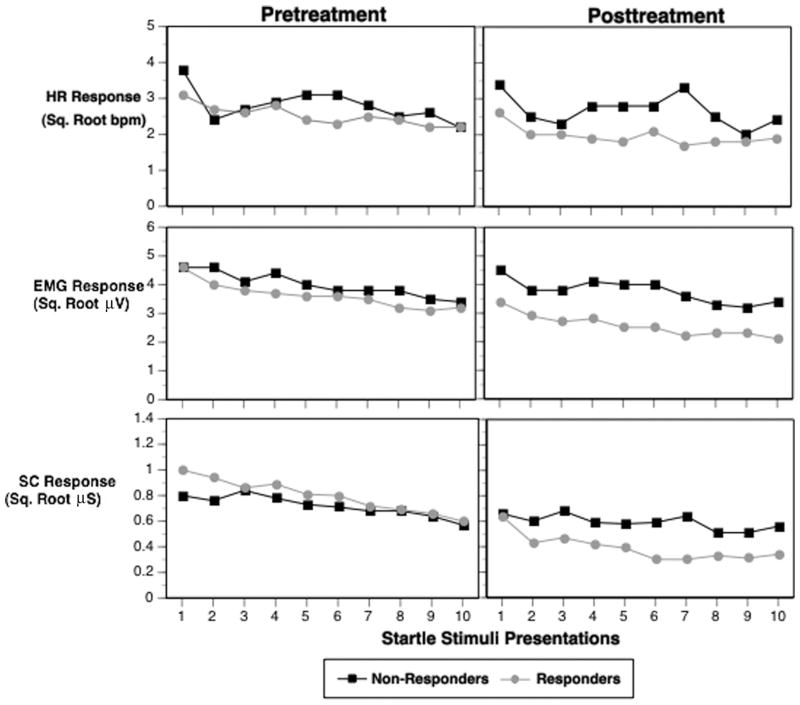
The means, standard deviations and ANOVA findings for the HR, eye-blink EMG, and SC data collapsed over the 10 trials are presented in Table 2. In addition, Figure 1 presents the mean data for the three physiological measures across the 10 tone stimuli presentations grouped by treatment response status. Based upon the clinical findings of statistically significant (or nearly significant) differences at pretreatment between the responders and nonresponders on the BDI and total CAPS scores these measures were included as covariates in the analyses of the physiological variables.
Table 2.
Means, Standard Deviations, and Analysis of Variance for Physiological Responses
| Variable | Pretreatment
|
Posttreatment
|
Main effects and interactiona | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Responders
|
Nonresponders
|
Responders
|
Nonresponders
|
||||||
|
n =53
|
n =21
|
n =53
|
n =21
|
||||||
| M | SD | M | SD | M | SD | M | SD | ||
| Heart rate | |||||||||
| Resting level (bpm) | 74.4 | 8.4 | 77.5 | 10.8 | 72.5 | 10.8 | 76.1 | 8.1 | |
| Pretone level (bpm) | 74.0 | 8.2 | 77.3 | 10.6 | 72.9 | 10.6 | 75.6 | 8.2 | |
| HRR (sq. rt. bpm) | 2.5 | 0.7 | 2.8 | 1.0 | 1.9 | 0.6 | 2.7 | 0.8 | G*, G × T* |
| HRR habituation slope | −0.08 | 0.12 | −0.09 | 0.18 | −0.05 | 0.10 | −0.06 | 0.14 | |
| Eye-blink electromyogram | |||||||||
| Resting level (μV) | 6.2 | 5.4 | 6.3 | 6.3 | 6.2 | 4.6 | 5.1 | 4.3 | |
| Pretone level (μV) | 5.4 | 4.7 | 5.3 | 5.4 | 5.3 | 4.5 | 4.5 | 3.9 | |
| EMGR (sq. rt. μV) | 3.63 | 1.71 | 4.00 | 2.20 | 2.60 | 1.41 | 3.76 | 1.91 | G × T* |
| EMGR habituation slope | −0.14 | 0.15 | −0.14 | 0.10 | −0.12 | 0.13 | −0.11 | 0.19 | |
| Skin conductanceb | |||||||||
| Resting level (μS) | 0.02 | 0.02 | 0.01 | 0.03 | 0.01 | 0.02 | 0.02 | 0.06 | |
| Pretone level (μS) | 0.03 | 0.10 | 0.04 | 0.17 | 0.00 | 0.05 | 0.02 | 0.08 | |
| SCR (sq. rt. μS) | 0.87 | 0.50 | 0.75 | 0.45 | 0.43 | 0.37 | 0.63 | 0.58 | G × T* |
| SCR habituation slope | −0.05 | 0.05 | −0.02 | 0.08 | −0.03 | 0.04 | −0.01 | 0.04 | |
Note. HRR = Heart rate response; bpm = beats per minute; EMGR = electromyogram response; μV = microvolt; SCR = skin conductance response; μS = microSiemens; G = Main effect for group; T = main effect for time; G × T = Group by Time interaction.
If column blank, no statistically significant effects.
For this set of variables, n = 39 and n = 16, respectively.
p < .05.
Figure 1.
Group mean physiologic responses to 10 tone trials at pre- and posttreatment. HRR = Heart rate response (beats per min; bpm); EMG = left orbicularis oculi electromyogram (μV = microvolts); SC = skin conductance (μS = microSiemens).
Examination of the self-reports of drug usage revealed that three participants in the nonresponder group and two participants in the responder group were on a stable regimen of a benzodiazepine from pre- to posttreatment. Exclusion of these participants from the analyses presented below did not change the findings; therefore, they were included in the physiological analyses. In addition, 33% of the nonresponders were on a stable regimen of antidepressant/anxiolytic medication (e.g., bupropion, paroxetine, escitalopram, mirtazapine), which was not significantly different than the proportion of treatment responders (31%) who were currently taking psychotropic medications (e.g., buproprion, venlafaxine, paroxetine, sertraline, escitalopram, fluoxetine).
Analyses of HR, EMG, and SC during the 4-minute resting baseline period and the pretone period prior to stimulus onset revealed no significant treatment response group, measurement time period main effects, or any significant interaction effects.
Analyses of the physiologic response scores revealed that there were significant group × time interactions for all three physiological measures (see Table 2). Follow-up analyses examining the nature of the significant interaction effects revealed that for HR there was not a significant group difference at pretreatment, F (1, 70) = 1.75, p = .19, but that at posttreatment the responder group showed significantly smaller increases in HR to the tone stimuli than the nonresponder group, F (1, 72) = 17.24, p < .001. There was also a significant decrease in HR response from pre- to posttreatment in the responder group, F (1, 50) = 22.22, p < .001, but not in the nonresponders, F (1, 20) = 0.59, p = .45.
For the EMG blink response there was a similar pattern in which there was no significant group difference at pretreatment, F (1, 71) = 0.58, p = .45, but a significantly smaller EMG response at posttreatment for the treatment responders compared to nonresponders, F (1, 72) = 8.63, p = .004. Again, there was a significant decrease in the magnitude of the EMG response from pre- to posttreament in the responder group, F (1, 51) = 16.41, p < .001, but not in the nonresponder group, F (1, 20) = 0.35, p = .56.
Follow-up analyses of the SC data revealed that the significant interaction was driven by a significant decrease in SC magnitude from pre- to posttreatment in the treatment responder group, F (1, 33) = 18.99, p < .001, but there was no significant difference in SC responses in the responder group compared to the nonresponders at posttreatment, F (1, 55) = 2.50, p = .11. Finally, examination of habituation to the loud tones through analysis of response slopes across trials revealed no significant group, time, or interaction effects for any of the physiological measures.
Discussion
The main finding from this study was that successful cognitive–behavioral treatment of PTSD as defined by not retaining a diagnosis of PTSD at post-treatment was associated with a reduction of physiologic responding compared to pretreatment levels and in comparison to treatment nonresponders. Clearly a large proportion of this trauma sample responded very well to cognitive–behavioral treatment (72%). Still, a sizeable portion responded with no or only very modest changes in symptoms following treatment. It is notable that successful treatment resulted not only in a significant reduction in PTSD symptoms, but also in depressive symptoms in this sample. There was a trend for the PTSD pretreatment scores to be higher for the treatment nonresponders and a significantly higher level of pretreatment depression in this group. These differences, however, are not likely to explain our findings because we used these pretreatment variables as covariates in our analyses of the physiological data and still found significant effects on reduction of physiologic responses.
Previous studies have documented that trauma survivors with PTSD have a heightened startle response in comparison to survivors without PTSD across a variety of different traumatic events (e.g., Butler et al., 1990; Metzger et al., 1999; Morgan et al., 1997; Orr et al., 1997; Shalev, Orr, Peri, et al., 1992). In previous studies participants were assessed at a variety of time points posttrauma. This is noteworthy because it suggests that once an elevated startle response has occurred it may become a permanent response lasting decades after trauma exposure. The findings from the present study suggest that the physiological response to loud tones is modifiable with successful treatment. Shalev and colleagues (2000) have found that a time-dependent increase in startle response can occur after exposure to a traumatic event. They observed a significant increase in startle responding that occurred in the acute aftermath of trauma exposure in participants who developed PTSD in a sample composed mostly of motor-vehicle accident survivors. They have argued that this supports a sensitization model of changes that occur after the development of PTSD. More recently Griffin (2008) also found a time-dependent increase in response to loud tones in a prospective study of female rape and physical assault survivors. The present findings support the idea that response to loud tones in PTSD is malleable and can be decreased under appropriate conditions in which the individual can be desensitized with cognitive therapy.
Negatively valenced emotional states previously have been demonstrated to increase startle responding (Lang, Bradley, & Cuthbert, 1990; Vrana & Lang, 1990); it has been suggested that the heightened fear and anxiety acquired by those with PTSD may be a causal defensive mechanism for the exaggerated startle response seen in PTSD (Metzger et al., 1999; Orr et al., 1997). The role of context and emotional state may have played a part in the changes that were observed following treatment. Because the psychophysiological test was conducted at the same location where therapy was conducted, it may be that the participants who no longer had PTSD had come to see the setting as a safe environment, but that those who did not have a reduction in symptoms may have still had an elevated level of anxiety. As depicted in Figure 1, the measures of HR and EMG show significant differences for the treatment responders, yet the initial physiological responses were very much like the nonresponders. For HR, the first trial was comparable for responders and nonresponders and was higher than any other trial. For EMG, the first two trials were elevated for both groups, but Trials 3–10 produced significantly less eye-blink EMG for the treatment responders. Examination of the habituation of the physiological responses over trials, however, revealed no significant effects. This observation indicates that the groups were not different from each other with regard to the rate of changes in responding over repeated presentations.
The exact mechanism by which physiological responding is decreased is still an open question that will require more study. It is unclear if the reduction in response is a nonspecific effect of successful treatment and would be replicated under any successful treatment or if it is a specific effect of some particular element of the treatments used here. The treatment approach that was used here did not have any specific component designed to address physiological responses per se and there was no repeated and extended exposures as would be found in exposure therapies.
It has been suggested that in some studies of combat veterans the stimuli may sound like bursts of gunfire; therefore, physiological reactivity may represent a conditioned response (Prins, Kaloupek, & Keane, 1995). It seems unlikely that the simple loud tone stimuli used here could have been associated with specific elements of the interpersonal assaults that these women experienced. It is also notable that this study used a sample of female trauma survivors compared to the majority of previous studies of startle and loud tone response that have mostly used male trauma survivors.
In addition, it is also important to note that we used a relatively long tone stimulus of 500 ms, which is comparable to some previous research with trauma survivors (e.g., Orr et al, 1995, 1997; Shalev et al., 2000), but that others have used a much shorter stimulus of 40 ms (e.g., Jovanovic et al., 2009; Morgan et al., 1995; Pole et al., 2003). A longer stimulus may elicit defensive responses; thus, any comparison of this study should take that into account. In particular, greater defensive responses have been noted for the cardiac response (Ramirez, Sanchez, Fernandez, Lipp, & Vila, 2005). Attempts at replication of this study should pay attention to the duration of tone stimulus.
To our knowledge this is the first study to show a decrease in physiological response to loud tones following successful treatment of PTSD. The findings suggest that heightened response to loud tones following trauma exposure need not be a life-long consequence of the development of PTSD.
Acknowledgments
This work was supported by NIMH grant MH55542 (Dr. Resick) and NIMH grant MH55688 (Dr. Griffin).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. 2. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1002/jts.2490080106. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Hickling EJ, Veazey CH, Buckley TC, Freidenberg BM, Walsh JD, Keefer L. Treatment-related changes in cardiovascular reactivity to trauma cues in motor vehicle accident-related PTSD. Behavior Therapy. 2002;33:417–426. doi: 10.1016/S0005-7894(02)80036-3. [DOI] [Google Scholar]
- Boudewyns PA, Hyer L. Physiological response to combat memories and preliminary treatment outcome in Vietnam veteran PTSD patients treated with direct therapeutic exposure. Behavior Therapy. 1990;21:63–87. doi: 10.1016/S0005-7894(05)80189-3. [DOI] [Google Scholar]
- Butler RW, Braff DL, Rausch JL, Jenkins MA, Sprock J, Geyer MA. Physiological evidence of exaggerated startle response in a subgroup of Vietnam veterans with combat-related PTSD. American Journal of Psychiatry. 1990;147:1308–1312. doi: 10.1176/ajp.147.10.1308. [DOI] [PubMed] [Google Scholar]
- Chard KM. An evaluation of cognitive processing therapy for the treatment of posttraumatic stress disorder related to childhood sexual abuse. Journal of Consulting and Clinical Psychology. 2005;73:965–971. doi: 10.1037/0022-006X.73.5.965. [DOI] [PubMed] [Google Scholar]
- Fairbank JA, Keane TM. Flooding for combat-related stress disorders: Assessment of anxiety reduction across traumatic memories. Behavior Therapy. 1982;13:499–510. doi: 10.1016/S0005-7894(82)80012-9. [DOI] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV-Patient Edition (SCID-P) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- Foa EB, Dancu CV, Hembree EA, Jaycox LH, Meadows EA, Street GP. A comparison of exposure therapy, stress inoculation training, and their combination for reducing post-traumatic stress disorder in female assault victims. Journal of Consulting & Clinical Psychology. 1999;67:194–200. doi: 10.1037/0022-006X.67.2 .194. [DOI] [PubMed] [Google Scholar]
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Griffin MG. A prospective assessment of auditory startle alterations in rape and physical assault survivors. Journal of Traumatic Stress. 2008;21:91–99. doi: 10.1002/jts.20300. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Sakoman AJ, Esterajher S, Kozaric-Kavacic D. Altered resting psychophysiology and startle response in Croatian combat veterans with PTSD. International Journal of Psychophysiology. 2009;71(3):264–268. doi: 10.1016/j.ijpsycho.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Kaloupek DG. Imaginal flooding in the treatment of a posttraumatic stress disorder. Journal of Consulting and Clinical Psychology. 1982;50:138–140. doi: 10.1037/0022-006X.50.1.138. [DOI] [PubMed] [Google Scholar]
- Korol B, Bergfeld GR, McLaughlin LJ. Skin color and autonomic nervous system measures. Physiology & Behavior. 1975;14:575–578. doi: 10.1016/0031-9384(75)90184-5. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. doi: 10.1037/0033-295X.97.3.377. [DOI] [PubMed] [Google Scholar]
- Marmar CR, Weiss DS, Schlenger WE, Fairbank JA, Jordan KB, Kulka RA, Hough RL. Peritraumatic dissociation and PTSD in male Vietnam theater veterans. American Journal of Psychiatry. 1994;151:902–907. doi: 10.1176/ajp.151.6.902. [DOI] [PubMed] [Google Scholar]
- Metzger LJ, Orr SP, Berry NJ, Ahern CE, Lasko NB, Pitman RK. Physiologic reactivity to startling tones in women with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108:347–352. doi: 10.1037/0021-843X.108.2.347. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037/0021-843X.110.1.40. [DOI] [PubMed] [Google Scholar]
- Monson CM, Schnurr PP, Resick PA, Friedman MJ, Young-Xu Y, Stevens SP. Cognitive processing therapy for veterans with military-related posttraumatic stress disorder. Journal of Consulting and Clinical Psychology. 2006;74:898–907. doi: 10.1037/0022-006X.74.5.898. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Grillon C, Lubin H, Southwick SM. Startle reflex abnormalities in women with sexual assault-related post-traumatic stress disorder. American Journal of Psychiatry. 1997;154:1076–1080. doi: 10.1176/ajp.154.8.1076. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Grillon C, Southwick SM, Davis M. Fear-potentiated startle in posttraumatic stress disorder. Biological Psychiatry. 1995;36:378–385. doi: 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- Nishith P, Griffin MG, Weaver TL. Utility of the heart rate response as an index of emotional processing in a female rape victim with posttraumatic stress disorder. Cognitive & Behavioral Practice. 2002;9:302–307. doi: 10.1016/S1077-7229(02)80024-4. [DOI] [Google Scholar]
- Orr SP. Psychophysiologic reactivity to trauma-related imagery in PTSD: Diagnostic and theoretical implications of recent findings. Annals of the New York Academy of Sciences. 1997;821:114–124. doi: 10.1111/j.1749-6632.1997.tb48273.x. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Shalev AY, Pitman RK. Physiologic responses to loud tones in Vietnam veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1995;104:75–82. doi: 10.1037/0021-843X.104.1.75. [DOI] [PubMed] [Google Scholar]
- Orr SP, Solomon Z, Peri T, Pitman RK, Shalev AY. Physiologic responses to loud tones in Israeli veterans of the 1973 Yom Kippur war. Biological Psychiatry. 1997;41:319–326. doi: 10.1016/S0006-3223(95)00671-0. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP. Psychophysiologic testing for PTSD: Forensic psychiatric application. Bulletin of the American Academy of Psychiatry Law. 1993;21:37–52. [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychological Bulletin. 2007;133:725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Best SR, Orr SP, Marmar CR. Fear-potentiated startle and posttraumatic stress symptoms in urban police officers. Journal of Traumatic Stress. 2003;16:471–479. doi: 10.1023/A:1025758411370. [DOI] [PubMed] [Google Scholar]
- Prins A, Kaloupek DG, Keane TM. Psychophysiological evidence for autonomic arousal and startle in traumatized adult populations. In: Friedman MJ, Charney DS, Deutch AY, editors. Neurobiological and clinical consequences of stress: From normal adaptation to post-traumatic stress disorder. Philadelphia, PA: Lippincott-Raven; 1995. pp. 291–314. [Google Scholar]
- Ramirez I, Sanchez MB, Fernandez MC, Lipp OV, Vila J. Differentiation between protective reflexes: Cardiac defense and startle. Psychophysiology. 2005;42:732–739. doi: 10.1111/j.1469-8986.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- Resick PA, Galovski TE, Uhlmansiek MO, Scher CD, Clum GA, Young-Xu Y. A randomized clinical trial to dismantle components of cognitive processing therapy for posttraumatic stress disorder in female victims of interpersonal violence. Journal of Consulting and Clinical Psychology. 2008;76:243–258. doi: 10.1037/0022-006X.76.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resick PA, Monson CM, Gutner C. Psychosocial treatments for PTSD. In: Friedman MJ, Keane TM, Resick PA, editors. PTSD: Science and practice—A comprehensive handbook. New York, NY: Guilford Press; 2007. pp. 330–358. [Google Scholar]
- Resick PA, Nishith P, Weaver TL, Astin MC, Feuer CA. A comparison of cognitive-processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. Journal of Consulting & Clinical Psychology. 2002;70:867–879. doi: 10.1037/0022-006X.70.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resick PA, Schnicke MK. Cognitive processing therapy for sexual assault victims. Journal of Consulting and Clinical Psychology. 1992;60:748–756. doi: 10.1037/0022-006X.60.5.748. [DOI] [PubMed] [Google Scholar]
- Resick PA, Schnicke MK. Cognitive processing therapy for rape victims: A treatment manual. Newbury Park, CA: Sage; 1993. [Google Scholar]
- Rothbaum BO, Foa EB. Exposure therapy for rape victims with posttraumatic stress disorder. Behavior Therapist. 1992;15:219–222. [Google Scholar]
- Shalev AY, Orr SP, Peri T, Schreiber S. Physiologic responses to loud tones in Israeli patients with posttraumatic stress disorder. Archives of General Psychiatry. 1992;49:870–875. doi: 10.1001/archpsyc.1992.01820110034005. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Orr SP, Pitman RK. Psychophysiologic response during script-driven imagery as an outcome measure in posttraumatic stress disorder. Journal of Clinical Psychiatry. 1992;53:324–326. [PubMed] [Google Scholar]
- Shalev AY, Peri T, Brandes D, Freedman S, Orr SP, Pitman RK. Auditory startle response in trauma survivors with posttraumatic stress disorder: A prospective study. American Journal of Psychiatry. 2000;157:255–261. doi: 10.1176/appi.ajp.157.2.255. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Lang PJ. Fear imagery and startle-probe reflex. Journal of Abnormal Psychology. 1990;99:189–197. doi: 10.1037/0021-843X.99.2.189. [DOI] [PubMed] [Google Scholar]