Graphical abstract
Highlights
► We generated two panels of epitope-matched mouse IgGs anti-PfMSP119 and PyMSP119. ► All IgGs were confirmed to be of the correct subclass and bound to the antigens. ► IgG1 triggers PfMSP119-specific NADPH oxidase activation through FcγR cross-linking. ► Passive transfer of IgG1 anti-PfMSP119 did not protect mice from infection.
Keywords: Mouse IgG, IgG subclasses, Epitope matched antibodies, Malaria
Abstract
Murine immunoglobulin G (IgG) plays an important role in mediating protective immune responses to malaria. We still know relatively little about which IgG subclasses protect against this disease in mouse models, although IgG2a and IgG2b are considered to be the most potent and dominate in successful passive transfer experiments in rodent malarias. To explore the mechanism(s) by which the different mouse IgG subclasses may mediate a protective effect, we generated mouse IgG1, IgG2a, IgG2b and IgG3 specific for the C-terminal 19-kDa region of Plasmodium falciparum merozoite surface protein 1 (PfMSP119), and to the homologous antigen from Plasmodium yoelii (P. yoelii), both major targets of protective immune responses. This panel of eight IgGs bound antigen with an affinity comparable to that seen for their epitope-matched parental monoclonal antibodies (mAbs) from which they were derived, although for reasons of yield, we were only able to explore the function of mouse IgG1 recognizing PfMSP119 in detail, both in vitro and in vivo. Murine IgG1 was as effective as the parental human IgG from which it was derived at inducing NADPH-mediated oxidative bursts and degranulation from neutrophils. Despite showing efficacy in in vitro functional assays with neutrophils, the mouse IgG1 failed to protect against parasite challenge in vivo. The lack of protection afforded by MSP119-specific IgG1 against parasite challenge in wild type mice suggests that this Ab class does not play a major role in the control of infection with mouse malaria in the Plasmodium berghei transgenic model.
1. Introduction
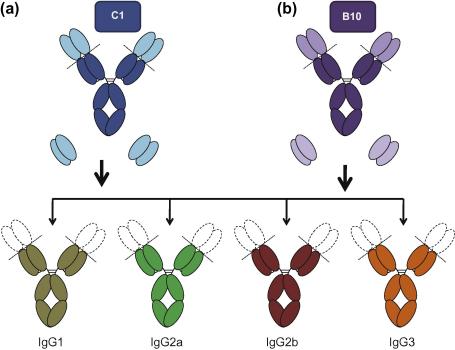
Amongst mouse IgG subclasses, IgG2a and IgG2b are considered to be the most potent activators, and dominate in successful passive transfer experiments in both murine infection (including malaria), and murine tumour models (Clynes et al., 2000; Nimmerjahn and Ravetch, 2005; Spencer Valero et al., 1998). Such functional distinctions have been attributed to differences in their capacity to fix complement and/or recruitment of relevant Fc-receptors for IgG (FcγRs) (Nimmerjahn and Ravetch, 2005). However, very few studies have compared epitope-matched IgG subclasses in the context of protection from both communicable and non-communicable diseases (Chu et al., 2010; Giorgini et al., 2008; Ishizaka et al., 1995; McLean et al., 2002; Reitan and Hannestad, 1995, 2002; Rodrigo et al., 2009; Torres et al., 2007), and in these studies, disease-specific effects were observed between IgG subclasses. For example, mouse IgG1 has been shown to be poor at killing tumours, yet plays an important role in controlling gastrointestinal parasites (McCoy et al., 2008; Wojciechowski et al., 2009). Mouse IgG1 is believed not to be a subclass associated with protective properties as it is not a potent complement activator and it possesses an extremely low activation to inhibition (A:I) ratio (0.1 compared to 69 and 7 for IgG2a and IgG2b respectively), as a consequence of its preference for binding the inhibitory FcγRIIB receptor (Woof, 2005). Work with non-epitope matched mouse monoclonal antibodies (mAbs) targeting Plasmodium yoelii MSP119 has shown conflicting data, with some IgG1 mAbs protecting from malaria (e.g. mAb G3), and others (e.g. mAb B4), having little or no effect on the course of disease (Spencer Valero et al., 1998). However, in the absence of epitope-matched reagents, it is difficult to directly compare the efficacy of IgG1 against the other subclasses in the protection from blood-stage malaria. Due to structural and functional differences between the murine IgG subclasses (in particular with respect to FcγRs they bind), and to attempt to resolve existing controversies as to whether FcRs are indeed even required for protection from malaria in the mouse, we generated two panels of recombinant mouse IgG1, IgG2a, IgG2b and IgG3 targeting identical epitopes on Plasmodium falciparum and P. yoelii MSP119 (Fig. 1). These were then used to investigate the anti-malarial properties of the mouse subclasses in vivo.
Fig. 1.
Generating two panels of epitope-matched murine IgG Abs specific for MSP119 epitopes C1 (P. falciparum-PfMSP119) or B10 (P. yoelii-PyMSP119). Diagram represents a general overview to the construction of (a) C1 and (b) B10 mouse IgGs. Variable genes from parental mAbs C1 or B10 were sub-cloned into redesigned expression vectors for each of the mouse IgG subclasses.
2. Materials and methods
2.1. Generation of PfMSP119-specific antibodies
2.1.1. Construction of pVL-C1-mouse kappa light chain
For all primer sequences, nucleotides in bold refer to restriction enzyme sites whereas an underline highlights stop codons. The plasmid pVL-C1-mouse kappa was generated by a three step PCR. Primer pairs VL-C1for (5′ GGC GTG CAC TCC GAT ATT GTG ATG ACC CAG 3′) and k-joint-rev (5′ ggg aag atg gat aca gtt ggt gca gca tca gtt c 3′) were used to amplify the VL by PCR from pVKExpress C1, whilst introducing an ApaL1 restriction site on the 5′ end. A second PCR with primer pairs k-joint-for (5′ gaa ctg atg ctg cac caa ctg tat cca tct tcc c 3′) and MK-rev (5′ gac tag tcc ctc taa cac tca ttc ctg ttg a 3′) containing a SpeI were used to amplify the mouse kappa light chain constant region from BALB/c genomic DNA. Overlap PCR with primer pairs VL-C1for and MK-rev were then used to fuse as a single PCR product the VL of C1 and the mouse kappa constant region, which was then cloned in pCR2.1-Topo (parking vector) prior to sub-cloning into the parental pVKExpress C1 (McIntosh et al., 2007; Persic et al., 1997) as an ApaLI/SpeI fragment.
2.1.2. Construction of pVH-C1-mIgG1
pVH-C1-IgA1 (Shi et al., 2011) was digested with SalI to remove the IgA constant region sequence and re-ligated to produce pVH-C1-delIgA1. This plasmid was then digested with BssHII/XbaI to release the VH of C1 which was then sub-cloned into pcDNA3.1-C1-hIgG1 from which the C1 and hIgG1 sequences were previously deleted (pcDNA3.1-delC1delIgG1) using the same restriction sites to create pcDNA3.1-C1-delhIgG1. The murine IgG1 constant region was amplified from BALB/c genomic DNA using primer pairs Mg1-for (containing an XbaI site) (5′ GCA AAA GAG CGG CCT TCT AGA AGG TTT G 3′) and Mg1-rev (5′ CAC TGG GAT CAT TTA CCA GGA GAG TG 3′), and cloned into the parking vector, prior to sub-cloning as an XbaI fragment into pcDNA3.1-C1-delhIgG1 to generate pVH-C1-mIgG1.
2.1.3. Construction of pVH-C1-mIgG2a
Primer pairs Mg2a-for (5′ G GTC ACC ATC AAG AGG AGG AAG 3′) and Mg2a-rev (5′ TCT AGA GCT CAT TTA CCC GGA GT 3′) were used to amplify the murine IgG2a from genomic DNA whilst introducing a BstEII site 5′ of the CH1 and an XbaI site 3′ of the stop codon respectively and cloned into the parking vector. pVH-C1-hIgG1 was digested with SalI and blunted with T4 DNA polymerase (New England Biolabs) prior to recovery from gels, and digestion with XbaI, into which a BstEII blunted/XbaI fragment from the parking vector was ligated to create pVH-C1-mIgG2a.
2.1.4. Construction of pVH-C1-mIgG2b and IgG3
Primer pairs Mg2b-for (5′ GAT ATC GGT CAC AGT GCA AGC TCT 3′) and Mg2b-rev (5′TCT AGA GCT CAT TTA CCC GGA GA 3′) were used to amplify the murine IgG2b from genomic DNA whilst introducing an EcoRV site 5′ of the CH1 and an XbaI site 3′ of the stop codon respectively and cloned into the parking vector. pVH-C1-hIgG1 was digested with BamHI and blunted with T4 DNA polymerase (New England Biolabs) prior to recovery from gels, and digestion with XbaI, into which an EcoRV blunted/XbaI fragment from the parking vector was ligated to create pVH-C1-mIgG2b. Primer pairs Mg3-for (5′ GAT ATC GTT CAG GAT AGA GCT GGG 3′) and Mg3-rev (5′ TCT AGA TCT CAT TTA CCA GGG GA 3′) were used in the same way to generate pVH-C1-mIgG3.
2.2. Generation of PyMSP119-specific antibodies
2.2.1. Construction of pVL-B10-mouse kappa light chain
pVL-C1-mouse kappa light chain was digested with ApaLI/XhoI to release the VL of C1. The VL of B10 was subcloned as an ApaLI/XhoI fragment derived from the parental expression vector pB10-VL/10 (Pleass et al., 2003).
2.2.2. Construction of pVH-B10-mIgG2a
pVH-C1-mIgG2a and parental plasmid pB10-VH/5 (Pleass et al., 2003) were digested with BssHII/BstEII to release the VH of C1 (pVH-delC1-mIgG2a) and the VH of B10 respectively. The VH of B10 was sub-cloned into pVH-delC1-mIgG2a using the same restriction sites and renamed pVH-B10-mIgG2a.
2.2.3. Construction of pVH-B10-mIgG1
The plasmid pVH-B10-mIgG1 was generated in two steps. pVH-C1-mIgG1 and pVH-B10-mIgG2a were digested with BssHII/BstEII to release the VH of C1 and VH of B10 respectively. The VH of B10 was sub-cloned into pVH-delC1-mIgG1. Due to the presence of a second BstEII site in exon 1 (CH1 domain) 160 bp after the VH of B10 3′ BstEII site, both plasmids were digested with BstEII and the released fragment sub-cloned into pVH-B10delC1-mIgG1 with the same restriction sites to generate pVH-B10-mIgG1.
2.2.4. Construction of pVH-B10-mIgG2b and IgG3
pVH-B10-mIgG2a was partially digested with MfeI/XbaI to release the IgG2a (pVH-B10-delmIgG2a) sequence after the VH of B10. IgG2b and IgG3 were released from their respective parking vectors with EcoRI/XbaI and sub-cloned into pVH-B10-delmIgG2a compatible cohesive restriction site ends (MfeI–EcoRI) and XbaI. The newly generated expression vectors were renamed as pVH-B10-mIgG2b and pVH-B10-mIgG3. All plasmids were sequenced in both directions to confirm that no errors were introduced into either the variable or constant region genes during PCR/cloning. Amino acid translation of the VH and VL genes for both C1 and B10 expressed in the mouse IgGs confirmed that they are indeed epitope-matched and are shown in Fig. 2.
Fig. 2.
Sequencing of C1 and B10 VH and VL genes. Amino acid sequences alignment of PfMSP119 and PyMSP119-binding epitope-matched mouse IgGs with the respective parental expression plasmids. (a) pVL-C1-mouse kappa compared with pVKExpress C1. (b) IgG1, IgG2a, IgG2b, and IgG3-C1 mouse IgG constructs compared with (h)IgG1-C1. (c) pVL-B10-mouse kappa compared with pB10-VL/10. (d) IgG1, IgG2a, IgG2b, and IgG3-B10 mouse IgG constructs compared with pB10-VH/5.
2.3. Protein expression and purification
Chinese hamster ovary (CHO)-K1 or human embryonic kidney (HEK)-293T cells were transfected by electroporation with corresponding heavy- and light-chain plasmids and the Abs were expressed as follows: IgG1 on HEK-293T and IgG2a, IgG2b, and IgG3 on CHO-K1. Positive clones secreting MSP119-specific IgG detected by ELISA, on plates coated with recombinant PfMSP119-GST (Burghaus and Holder, 1994) or PyMSP119-GST (Lewis, 1989), or immuno-blotting using goat anti-mouse IgG-Fc (Pierce), goat anti-mouse IgG1, IgG2a, IgG2b or IgG3 (Southern Biotech), or a goat anti-mouse kappa (Southern Biotech) all conjugated to horse radish peroxidase (HRP) as previously described (Pleass et al., 2003). From large-scale cultures, mouse IgG was purified on HiTrap protein G-Sepharose (GE Healthcare) by FPLC. The integrity and purity of the antibodies were verified on 4–12% SDS–PAGE gradient gels (Invitrogen).
2.4. Immunofluorescence assay (IFA) and surface plasmon resonance (SPR) analysis
For IFAs, washed erythrocytes from mice infected with the transgenic (Tg) parasite Pb-PfM19 described by de Koning-Ward and colleagues (2003), were fixed on slides in methanol–acetone (1:1, vol/vol) for 10 min. After being blocked in phosphate buffered saline (PBS)/5% (vol/vol) goat serum, slides were incubated with Abs at 5 μg ml−1 in blocking buffer for 1 h, washed, then incubated for 1 h with fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 anti-human IgG (Sigma or Caltag) or goat anti-mouse IgG γ-chain specific (Southern Biotech) diluted 1:500 in blocking buffer. After washing, slides were mounted with DAPI anti-fade and Ab binding examined by IF microscopy on a Zeiss Axioscope 40 microscope. For SPR, quantitative association (KA) and dissociation (KD) constants of the mouse IgG subclasses were measured using a BIAcore X Machine (GE Healthcare). Antigen (PfMSP119-GST) at a concentration of 50 μg/ml was amine-coupled onto a dextran matrix CM5 sensor chip using a Pharmacia Biosensor Amine Coupling Kit following the manufacturer’s instructions. Various concentrations of purified IgGs were then injected into a final volume of 50 μL at a rate of 10 μL/min over the chip and binding kinetics analysed using BIAsimulation 3.0 software.
2.5. Luminol chemiluminescence assay of respiratory burst and myeloperoxidase release
Neutrophils were isolated from heparinized blood taken from healthy volunteers by the sedimentation of erythrocytes in 6% (w/v) dextran T70 (GE Healthcare) in 0.9% (w/v) saline at 37 °C for 30 min, followed by leucocyte separation on a discontinuous density gradient of Lymphoprep (ρ = 1.077 g/cm3; Nycomed, Birmingham, UK) over Ficoll-Hypaque (ρ = 1.119 g/cm3), centrifuged at 700g for 20 min at room temperature. Approval for the collection and use of human cells was obtained from the local Queen’s Medical Centre ethics committee. Wells of chemiluminescence microtiter plates (Dynatech Laboratories, Billinghurst, Sussex, UK) were coated with 150 μl of PfMSP119 at 5 μg ml−1 in coating buffer (0.1 M carbonate buffer, pH 9.6) and incubated overnight at 4 °C. After washing three times with PBS, 150 μl of anti-PfMSP119 IgG at 100 or 50 μg ml−1 was added to antigen coated wells. In each case, triplicate wells were prepared and left for 2 h at room temperature. After washing as before, 100 μl of luminol [67 μg ml−1 in Hank’s buffered saline solution (HBSS) containing 20 mM HEPES and 0.1 g/100 ml globulin-free BSA (HBSS/BSA)] was added to each well. After the addition of 50 μl of purified neutrophils (106/ml in HBSS/BSA) to each well, plates were transferred to a Microlumat LB96P luminometer, and the chemiluminescence was measured at 2 min intervals for 120 min at 37 °C. Data were analysed using Excel software.
2.6. Passive immunization and parasite challenge
Pathogen-free BALB/c mice from 6 to 8 weeks of age (caged individually) were used to test in vivo efficacy of mouse IgG1 in passive transfer experiments. IgG1 was administered (0.5 mg/injection) intraperitoneally (i.p.) on day −1, 0, and +1. Mice were challenged with 5000 parasitized red blood cells (prbc) with Pb-PfM19b that were administered i.p. 5 h after Ab injection on day 0. From day +2 mice were screened daily for weight loss and % infected erythrocytes (parasitemia) counted by blood smears stained with Giemsa (Sigma). At the end of the experiment or when mice lost more than 20% of their initial weight, the animals were humanely sacrificed. All animal experiments were approved by the Home Office and performed in accordance with UK guidelines and regulations (PPL 40/2753).
3. Results
3.1. Characterization of PfMSP119-specific mouse IgG1, IgG2a, IgG2b and IgG3
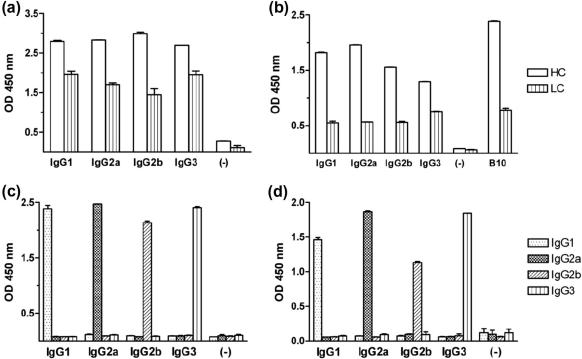
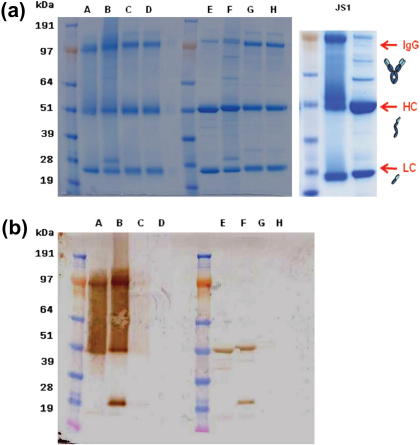
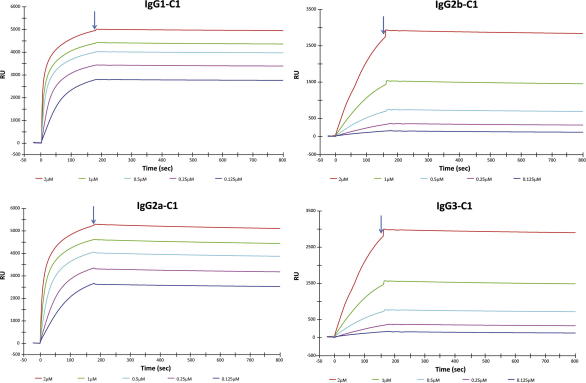
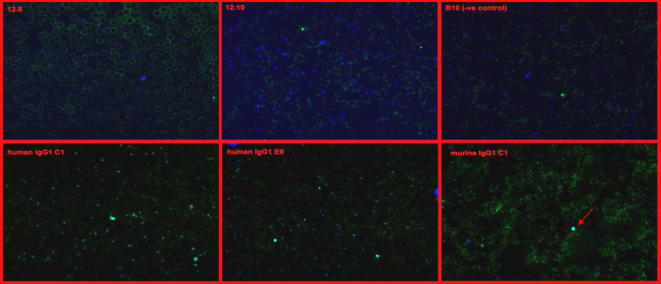
PfMSP119 and PyMSP119-specific mouse IgG1, IgG2a, IgG2b and IgG3 were secreted into culture supernatants from stably transfected CHO-K1 or HEK-293T cells. These recombinant antibodies recognized either recombinant PfMSP119 or PyMSP119 by ELISA with polyclonal anti-mouse IgG Fc- or kappa light chain-specific secondary reagents (Fig. 3a and b). To check that each cell line only secreted the intended antibody class, a second sandwich ELISA was developed to confirm the subclass of the secreted IgG antibodies (Fig. 3c and d). Here we focus on the PfMSP119-specific mouse IgG1, IgG2a, IgG2b and IgG3 that were purified from CHO-K1 or HEK-293T culture supernatants by protein-G affinity-chromatography and appeared pure by SDS–PAGE analysis (Fig. 4a). Total yields of protein after purification, dialysis, and concentration from numerous different batches of culture supernatant over several time points were in the region of 15 mg from 30 l of supernatant for IgG1, and 2.8 mg, 2.1 mg, 1.5 mg from 7 l of supernatant for IgG2a, IgG2b and IgG3 respectively. The poor yields of IgG2a, IgG2b and IgG3 were most likely a consequence of these subclasses being cloned into a different expression plasmid (pVHExpress) compared to the mouse IgG1 (pcDNA3.1), and therefore the majority of work described focuses on IgG1 for which a sufficient quantity of antibody was derived for in vivo studies. The mouse IgG antibodies had an apparent molecular weight (MW) of approximately 150 kDa, although there were minor differences in the MW dependent on subclass. All the preparations contained a significant proportion of free heavy and light chains (arrowed), suggesting that not all the expressed protein folded into intact heterodimeric antibody. Western blotting with the IgG subclass-specific reagents used in the detecting ELISAs were unable to detect any of the mouse IgGs by immunoblotting on both non-reducing and reducing gels, although an alternative polyclonal goat anti-mouse IgG-Fc did recognize mouse IgG1, IgG2a and very faintly IgG2b (Fig. 4b). No significant binding to mouse IgG3 was observed with this reagent despite the presence of an equivalent amount of protein. Importantly, surface plasmon resonance (SPR) analysis for all the IgG subclasses generated revealed no reduction in affinity for PfMSP119 when compared with the parental human IgG1 antibody (Table 1 and Fig. 5), the binding constants remaining essentially the same for each subclass. The recombinant mouse IgG1 by indirect IFA produced a characteristic pattern of MSP1 reactivity from P. falciparum infected erythrocytes (not shown) and Plasmodium berghei parasites Tg for PfMSP119 (Fig. 6, bottom right corner, arrowed).
Fig. 3.
ELISA screening of PfMSP119 and PyMSP119-specific mouse IgGs. HEK-293T or CHO-K1 mammalian cell lines were stably-transfected by electroporation with expression plasmids coding for the panel of mouse IgGs targeting P. falciparum or P. yoelii MSP119. Clones secreting antigen-specific IgGs were detected by enzyme-linked immunosorbent assay (ELISA). Five micrograms of recombinant purified antigen PfMSP119 (a) or PyMSP119 (b) was coated and secreting clones detected with an anti-mouse IgG Fc-specific (HC) or anti-mouse kappa light chain (LC) secondary antibodies. To confirm the secretion of the panel of C1 (c) or B10 (d) epitope-matched mouse IgGs a capture ELISA was performed. Plates were coated with goat anti-mouse F(ab′)2 and supernatant from secreting clones analysed with subclass-specific secondary antibodies. All secondary reagents used were HRP conjugated and all plates were read at 450 nm. Bars represent the mean OD values from duplicate or triplicate wells. Supernatant from untransfected cells was used as negative control. Two micrograms of mAb B10 was used as positive control on panel b. Error bars represent Standard Deviation (SD).
Fig. 4.
Characterization of purified recombinant PfMSP119-specific epitope-matched mouse IgGs. Characterization of the purified PfMSP119-specific mouse IgGs. KEY: IgG1 (A), IgG2a, (B), IgG2b (C), IgG3 (D). (a) Five micrograms of each Ab was run on NuPAGE 4–12% Bis–Tris SDS gels (Invitrogen) under denaturing non-reducing conditions (A–D) or denaturing reducing conditions (E–H) and compared with the control antibody human IgG1 (JS1) from which they were derived (IDEM). An expected MW of ∼150 kDa for the intact antibodies was shown (arrowed, ‘IgG’) although free constitutive heavy chains and light chain were observed (50 and 25 kDa respectively, arrowed). (b) Western Blot analysis of the IgG samples transferred into PVDF membranes. Analysis was made following the same conditions and sample order described on (a). Murine IgG1, IgG2a-C1 antibodies were clearly detectable with polyclonal anti-mouse IgG Fc-specific secondary antibody compared to IgG2b which was detected very faintly, but none of them with the subclass-specific secondary antibodies used in ELISA.
Table 1.
Association (KA) and dissociation (KD) rate constants for the mouse (m) and human (h) IgG antibodies measured by SPR analysis.a
| Antibody | KA (1/M) | KD (M) |
|---|---|---|
| mIgG1-C1 | 4.48 × 107 | 2.3 × 10−8 |
| mIgG2a-C1 | 3.32 × 107 | 3.01 × 10−8 |
| mIgG2b-C1 | 1.2 × 107 | 8.41 × 10−8 |
| mIgG3-C1 | 2.33 × 107 | 3.11 × 10−8 |
| hIgG1-C1 | 3.94 × 107 | 2.3 × 10−8 |
Methodology described in McIntosh et al. (2007) and Pleass et al. (2003).
Fig. 5.
Surface plasmon resonance (SPR)-derived association and dissociation curves of mouse IgGs binding to PfMSP119. SPR association (KA) and dissociation (KD) curves of mouse IgG binding to recombinant purified antigen PfMSP119 amine-coupled and immobilized to a CM5 sensor chip. The antibodies were injected into flow at five different concentrations of 2 μM (red), 1 μM (green), 0.5 μM (cyan), 0.25 μM pink), and 0.125 μM (dark blue) at time 0 and replaced with buffer alone at the times indicated with arrows. No binding was seen from any of the IgGs onto control Ag coated in flow cell 2. Data are summarized in Table 1. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 6.
Characterization of purified mouse IgG1-C1 by immunofluorescence assay (IFA). Mouse IgG1 is reactive with MSP119 on methanol–acetone-fixed smears of merozoites and erythrocytes infected with rodent P. berghei Tg for P. falciparum MSP119 (Pb-PfM19). Top panel: mAbs 12.8 and 12.10 previously described and known not to bind to Pb-PfM19 (Lazarou et al., 2009) and B10 anti-PyMSP119 as negative control (Spencer Valero et al., 1998). Bottom panel: Human IgG1-C1 (JS1) and human IgG1-e9 (JS2) as previously described (McIntosh et al., 2007) and murine IgG1-C1 bind Pb-PfM19 infected erythrocytes. Binding was visualized by staining with a FITC-conjugated secondary reagent with nuclei stained with DAPI and assessed by fluorescent microscopy under 40× magnification.
3.2. IgG1 triggers PfMSP119-specific neutrophil nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation through FcγR cross-linking
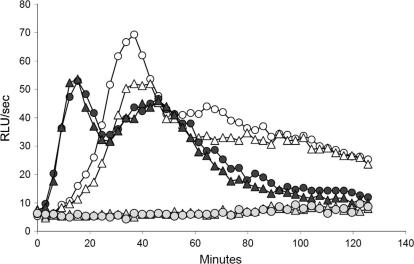
We next assessed the ability of the mouse IgG1 to interact with FcγRs and induce NADPH oxidase activation (respiratory burst) and degranulation in blood neutrophils (Fig. 7). For neutrophils, luminol chemiluminescence provides a read out of NADPH oxidase activation and myeloperoxidase release (Pleass et al., 2007, 2003). When attached to PfMSP119-GST-coated plates, the mouse IgG1 induced comparable respiratory bursts to the parental human IgG1 (Fig. 7), suggesting that binding to PfMSP119 allows mouse IgG1 to be presented to neutrophil FcγRs in an optimal configuration for receptor cross-linking and triggering of functional responses. This finding is similar to that reported previously with a different mouse IgG1 (mAb 12.10) also recognizing PfMSP119 (Lazarou et al., 2009). Since mouse IgG1 is known to bind to human FcγRIIA but not to FcγRIII, the activation of these neutrophils most likely occurs through binding to the former receptor (Pleass et al., 2003).
Fig. 7.
Mouse PfMSP119-specific IgG1 is functional. Neutrophil NADH oxidative bursts were measured by adding antibodies to a chemiluminescence plate coated with recombinant purified antigen PfMSP119. Relative Light Units (RLU, arbitrary units of light produced/second) were calculated from triplicate wells using neutrophils as previously described (McIntosh et al., 2007; Pleass et al., 2007, 2003). Each point in the graph represents the mean value of triplicate wells. RLU were measured for over 120 min after the IgGs were added to neutrophils at two different concentrations of 50 (△) or 100 (○) μg/ml of mIgG1-C1. The overall response was comparable to that derived from human IgG1-C1 at the same concentrations (▴, ● 50 or 100 μg/ml respectively). An IgG2a-Fc fused to antigen (PfMSP119-IgG2a-Fc) was used as an internal negative control ( ,
,  50 or 100 μg/ml respectively).
50 or 100 μg/ml respectively).
3.3. Passive transfer of mouse IgG1 into wild type mice has no effect on the course of a rodent malaria infection
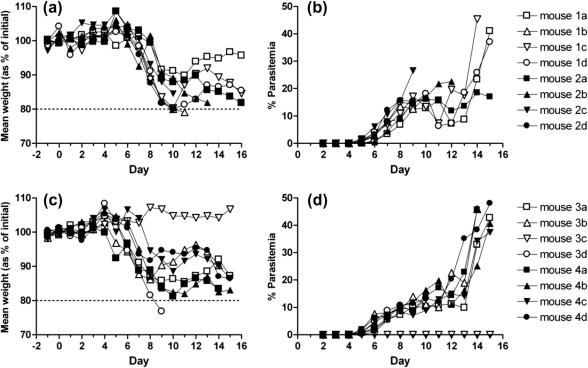
Despite binding PfMSP119 with high affinity, passive transfer of mouse IgG1 into WT BALB/c mice failed to protect seven out of eight animals, in two separate experiments, from a challenge infection with blood-stage P. berghei parasites Tg for PfMSP119 (Fig. 8).
Fig. 8.
Passive transfer of mouse PfMSP119-specific IgG1 does not protect mice from infection. Groups of four BALB/c wild type littermates were injected i.p. with a total dose of 1.5 mg of purified anti-P. falciparum mouse IgG1 (open symbols, groups 1 and 3) or with PBS-vehicle control (closed symbols, groups 2 and 4) and challenged with 5000 parasitized red blood cells (prbc) derived from ‘passage’ animals infected with Pb-PfM19. Similar results were obtained from two independent experiments (a–b and c–d respectively). (a) and (c) Mean weight as % of initial (mean weight from days −1, 0, +1) was assessed daily to determine if an animal had lost more than 20% of its original weight (dotted line) used as a humane end-point in accordance with Home Office regulations. (c) and (d) Seven out of eight animals treated with mIgG1-C1 succumbed to malaria infection and no significant difference was shown between the test and control groups. The percentage of parasitemia between the test and control groups was similar in both experiments.
4. Discussion
Although we have previously shown that mice Tg for human FcRs are very useful for investigating human antibody function in vivo, particularly where there are no suitable animal models for P. falciparum infection in humans (McIntosh et al., 2007; Shi et al., 2011), an argument can be made that they are somewhat contrived, and therefore should be compared in parallel experiments in wild type models of murine malaria infection with murine antibodies. Here we describe the construction of two panels of mouse IgG1, IgG2a, IgG2b and IgG3 recognizing identical epitopes in PfMSP119 or PyMSP119.
Plasmodium spp. merozoites attach to the surface of red blood cells by means of different surface antigens; among these antigens is MSP1 (Holder, 2009). This surface antigen is cleaved twice; the first cleavage takes place at schizogony followed by a second cleavage to reveal PfMSP119 just prior to invasion (Blackman et al., 1994; Morgan et al., 1999). This 19-kDa peptide is relatively conserved with few polymorphic variants and has been described as a major candidate for vaccine development (Pizarro et al., 2003), and continues to be the subject of study for immune therapy (Roussilhon et al., 2007).
These epitope-matched antibodies were generated by cloning variable genes from mAbs previously shown to confer passive protection from rodent malarias (McIntosh et al., 2007; Spencer Valero et al., 1998), into newly generated expression constructs containing mouse IgG1, IgG2a, IgG2b and IgG3 constant region genes respectively. The original plan was to generate the panel of mouse IgGs with the same expression plasmid (pcDNA3.1). However, due to the presence of a BstEII site on the C-terminal site of the VH-C1 in the parental human expression plasmid, we could not find a suitable enzyme site with which releases the human IgG1 sequence from the expression vector and subclones all the mouse IgG sequences in. The approach taken to generate the IgG2a, IgG2b, and IgG3-C1 Abs was to sub-clone in the murine sequences in an expression plasmid (pVHExpress) previously used in the expression of anti-malarial Abs (McIntosh et al., 2007). These plasmids were expressed in CHO-K1 or HEK-293T cells, and all eight secreted antibodies generated were shown to bind their respective MSP119 antigen, and were identified as belonging to the correct subclass by ELISA (Fig. 2).
CHO and HEK-293 mammalian cell lines are widely used in the production of recombinant proteins (Wurm, 2004). Predominantly in industry, CHOs have been used to express a wide range of recombinant proteins including antibodies used in the treatment of numerous diseases, including: multiple sclerosis, arthritis and cancer (Hossler et al., 2009; Kelley, 2009). To a lesser extent, HEK-293s have also been used in the industry to produce recombinant antibodies, but alongside CHOs, they have been the subject of study for increased productivity in the field (Wurm, 2004). HEK-293s have been used to express recombinant proteins for studies in neurology, electrophysiology and to produce mAbs amongst other therapeutic proteins (Chen et al., 2011; Thomas and Smart, 2005). Functional IgGs need to be glycosylated in their Fc-fragment in order to have Fc-mediated effector functions (Jefferis, 2005; Walsh and Jefferis, 2006), although we have not directly assessed the glycosylation status of these reagents, CHO and HEK-293 cell lines have been previously shown to effectively secrete Abs with N-linked carbohydrates (Schlenke et al., 2007; Sethuraman and Stadheim, 2006).
The mouse IgG1, IgG2a, IgG2b and IgG3 all bound PfMSP119 with high affinity as determined by surface plasmon resonance analysis (Fig. 4). The panel of IgG antibodies bound with comparable affinity to recombinant PfMSP119 in line with previous observations with other antibodies (McIntosh et al., 2007), and the mouse IgG1 bound to native protein as determined by immunofluorescence microscopy with infected erythrocytes (Fig. 6). Previous results have shown that the parental human IgG1 from which these mouse antibodies were derived is dependent on FcR-mediated recruitment for function, and that inhibition of erythrocyte invasion and/or inhibition of MSP1 processing are/is not the principal mechanism through which this antibody controls parasites (McIntosh et al., 2007).
Typically in passive transfer experiments with rodent malarias, large concentrations of antibody (1.5 mg total dose per mouse, given as three individual doses) have to be given to provide protection. Although we could produce sufficient quantities of all the antibody classes to allow for preliminary in vitro comparisons to be made, we could only generate sufficient mIgG1 with which to do in vivo work. Intriguingly, passive transfer experiments with this IgG1 antibody failed to protect BALB/c mice from challenge infection with malaria parasites (Fig. 8). Although the mouse IgG1 was fully functional, as determined by its ability to induce NADPH mediated oxidative bursts from human neutrophils, the antibody was ineffective at controlling the development of malaria when passively transferred into BALB/c mice that were challenged with the Tg parasite Pb-PfM19 (de Koning-Ward et al., 2003). In contrast to mouse IgG2a or IgG2b, mouse IgG1 binds preferentially to inhibitory FcγRIIB (CD32B) expressed on murine macrophages, monocytes and B cells (Nimmerjahn and Ravetch, 2006; Pleass and Woof, 2001), but not on neutrophils, offering a possible explanation as to why this subclass was found to be ineffective at controlling the development of a lethal malaria infection when passively transferred into recipient mice.
The similarities of the genomes of the human and rodent Plasmodium spp. with their 14 chromosomes, ranging from 23 to 27 Mb, coding for over 5000 proteins from which around 80% of the +5300 genes found in these genomes are orthologous, highlight the importance of murine malarias as models to understand the human disease (Doolan, 2011; Hall et al., 2005; Janse et al., 2010). These genetic similarities have led to the development of targeted gene knock-outs, mutations, fluorescently-tagged, generation of ‘reporter’ or ‘attenuated’ parasites that contribute to the development of reliable vaccines against the disease (Janse et al., 2010). To date, 561 rodent parasite mutants (predominantly P. berghei) have been reported (Rodent Malaria genetically modified Parasites DataBase (RMgmDB) http://www.pberghei.eu/ accessed on 11th August 2011).
The use of Tg P. berghei rodent parasites expressing vaccine-candidate Ags from orthologous genes of P. falciparum has been described for a wide range of proteins and genes, such as the: sporozoite adhesive protein TRAP (Wengelnik et al., 1999), circumsporozoite protein (CSP) (Persson et al., 2002; Tewari et al., 2002), the chloroquine resistance transporter (CRT) (Ecker et al., 2011), and the UIS4 and HT1 genes that code for a parasitophorous vacuole membrane protein in sporozoites and liver stages (expressed in a Tg P. yoelii) and a hexose transporter, respectively (Blume et al., 2011; Mackellar et al., 2010).
Parasites Tg for MSP119 are no exception. Rodent parasite Plasmodium chabaudi MSP119 has been expressed in P. falciparum and used for in vitro studies (Corran et al., 2004), and two Tg rodent P. berghei parasites expressing PfMSP119 have been described: Pb-PfM19 and PfMSP1–19Pb8.7 (Cao et al., 2009; de Koning-Ward et al., 2003) (respectively). Passive immunization of human IgG1 anti-PfMSP119 in BALB/c mice challenged and infected with Pb-PfM19, protected CD64-Tg mice (but not WT mice) from the course of infection (McIntosh et al., 2007). Similar results were found in mice immunized with purified rabbit anti-PfMSP142 IgG where sterile protection was also observed (Sachdeva et al., 2006). However, alternatively from these experiments, passive transfer of Abs 12.8 and 12.10 into BALB/c mice challenged with Pb-PfM19 confirmed their inability to protect in vivo suggesting that the presence of six amino acids of P. berghei’s MSP119 in the Tg parasite was required for both mAbs to be able to bind to the PfMSP119 epitope (Lazarou et al., 2009). These discrepant results from passive transfer experiments in infections with Pb-PfM19 have encouraged us to assess the efficacy of the alternate panel of IgGs targeting PyMSP119 to elucidate their response in a more natural model of infection.
From 1965 to 2010, more than 1900 immunization studies have been conducted involving all four rodent Plasmodium spp. and human parasites in feasible vaccine development trials. In this vast amount of time and research, many combinations of treatments have been studied such as: (1) immunizations with live, attenuated or dead parasites, (2) purified proteins such as Ags, Abs, DNA subunits, via multiple routes of delivery (Guilbride et al., 2010). Passive transfer experiments in vivo looking at the efficacy of Abs in controlling malaria continue to be of interest as still, the mechanisms of action of the Fc-portion of Abs in controlling the disease is not completely understood. Current improvements in the mass production of recombinant proteins could allow IgG therapies to become the standard choice of treatment against blood-stage malaria in the near future. It is of our interest to continue this project and aim to increase the yield of purified Abs from transfected mammalian cell lines by expressing our panel of IgGs in Sp2/0 myelomas for instance. With greater yields of purified Abs, we would be able to complete the in vitro characterization of the remaining -C1 Abs and compare in vivo both panels of the epitope-matched IgGs constructed.
In summary, two panels of epitope-matched mouse IgGs (IgG1, IgG2a, IgG2b, and IgG3) targeting PfMSP119 or PyMSP119 were constructed and expressed in mammalian cell lines. A novel PfMSP119-specific mouse IgG1 did not show protective capability against parasite challenge in mice but was shown to be fully functional in vitro. Whilst this finding may indicate that IgG1 does not play a major role in protection against malaria, we cannot rule out the possibility that the findings reflect certain shortcomings of the PfMSP119 Tg P. berghei rodent malaria model used, hence future work aims to investigate the role of the other IgG subclasses generated using the anti-P. yoelii panel of antibodies described herein.
Acknowledgments
We thank the Wellcome Trust for provision of funding for this work (082915/B/07/Z) and the Mexican Council of Science and Technology (CONACYT) for provision of a PhD studentship to J.A.-G. We thank Tania de Koning-Ward and Brendan Crabb (WEHI, Melbourne) for provision of the P. berghei transgenic parasites, and Tony Holder (NIMR) for the original B10 hybridomas and provision of recombinant MSP119.
The authors declare that they have no conflict of interest.
Contributor Information
Jaime R. Adame-Gallegos, Email: sbxjra@nottingham.ac.uk.
Richard J. Pleass, Email: richard.pleass@liverpool.ac.uk.
References
- Blackman M.J., Scott-Finnigan T.J., Shai S., Holder A.A. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J. Exp. Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume M., Hliscs M., Rodriguez-Contreras D., Sanchez M., Landfear S., Lucius R., Matuschewski K., Gupta N. A constitutive pan-hexose permease for the Plasmodium life cycle and transgenic models for screening of antimalarial sugar analogs. FASEB J. 2011;25:1218–1229. doi: 10.1096/fj.10-173278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghaus P.A., Holder A.A. Expression of the 19-kilodalton carboxy-terminal fragment of the Plasmodium falciparum merozoite surface protein-1 in Escherichia coli as a correctly folded protein. Mol. Biochem. Parasitol. 1994;64:165–169. doi: 10.1016/0166-6851(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Cao Y., Zhang D., Pan W. Construction of transgenic Plasmodium berghei as a model for evaluation of blood-stage vaccine candidate of Plasmodium falciparum chimeric protein 2.9. PLoS One. 2009;4:e6894. doi: 10.1371/journal.pone.0006894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Chumakov K., Dragunsky E., Kouiavskaia D., Makiya M., Neverov A., Rezapkin G., Sebrell A., Purcell R. Chimpanzee/human monoclonal antibodies for treatment of chronic poliovirus excretors and emergency post-exposure prophylaxis. J. Virol. 2011;85:4354–4362. doi: 10.1128/JVI.02553-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu N., Thomas B.N., Patel S.R., Buxbaum L.U. IgG1 is pathogenic in Leishmania mexicana infection. J. Immunol. 2010;185:6939–6946. doi: 10.4049/jimmunol.1002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes R.A., Towers T.L., Presta L.G., Ravetch J.V. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat. Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- Corran P.H., O’Donnell R.A., Todd J., Uthaipibull C., Holder A.A., Crabb B.S., Riley E.M. The fine specificity, but not the invasion inhibitory activity, of 19-kilodalton merozoite surface protein 1-specific antibodies is associated with resistance to malarial parasitemia in a cross-sectional survey in the Gambia. Infect. Immun. 2004;72:6185–6189. doi: 10.1128/IAI.72.10.6185-6189.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning-Ward T.F., O’Donnell R.A., Drew D.R., Thomson R., Speed T.P., Crabb B.S. A new rodent model to assess blood stage immunity to the Plasmodium falciparum antigen merozoite surface protein 119 reveals a protective role for invasion inhibitory antibodies. J. Exp. Med. 2003;198:869–875. doi: 10.1084/jem.20030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolan D.L. Plasmodium immunomics. Int. J. Parasitol. 2011;41:3–20. doi: 10.1016/j.ijpara.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker A., Lakshmanan V., Sinnis P., Coppens I., Fidock D.A. Evidence that mutant PfCRT facilitates the transmission to mosquitoes of chloroquine-treated Plasmodium gametocytes. J. Infect. Dis. 2011;203:228–236. doi: 10.1093/infdis/jiq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgini A., Brown H.J., Lock H.R., Nimmerjahn F., Ravetch J.V., Verbeek J.S., Sacks S.H., Robson M.G. Fc gamma RIII and Fc gamma RIV are indispensable for acute glomerular inflammation induced by switch variant monoclonal antibodies. J. Immunol. 2008;181:8745–8752. doi: 10.4049/jimmunol.181.12.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbride D.L., Gawlinski P., Guilbride P.D. Why functional pre-erythrocytic and blood stage malaria vaccines fail: a meta-analysis of fully protective immunizations and novel immunological model. PLoS One. 2010;5:e10685. doi: 10.1371/journal.pone.0010685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall N., Karras M., Raine J.D., Carlton J.M., Kooij T.W., Berriman M., Florens L., Janssen C.S., Pain A., Christophides G.K., James K., Rutherford K., Harris B., Harris D., Churcher C., Quail M.A., Ormond D., Doggett J., Trueman H.E., Mendoza J., Bidwell S.L., Rajandream M.A., Carucci D.J., Yates J.R., 3rd, Kafatos F.C., Janse C.J., Barrell B., Turner C.M., Waters A.P., Sinden R.E. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307:82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- Holder A.A. The carboxy-terminus of merozoite surface protein 1: structure, specific antibodies and immunity to malaria. Parasitology. 2009;136:1445–1456. doi: 10.1017/S0031182009990515. [DOI] [PubMed] [Google Scholar]
- Hossler P., Khattak S.F., Li Z.J. Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology. 2009;19:936–949. doi: 10.1093/glycob/cwp079. [DOI] [PubMed] [Google Scholar]
- Ishizaka S.T., Piacente P., Silva J., Mishkin E.M. IgG subtype is correlated with efficiency of passive protection and effector function of anti-herpes simplex virus glycoprotein D monoclonal antibodies. J. Infect. Dis. 1995;172:1108–1111. doi: 10.1093/infdis/172.4.1108. [DOI] [PubMed] [Google Scholar]
- Janse C.J., Kroeze H., van Wigcheren A., Mededovic S., Fonager J., Franke-Fayard B., Waters A.P., Khan S.M. A genotype and phenotype database of genetically modified malaria-parasites. Trends Parasitol. 2010;27:31–39. doi: 10.1016/j.pt.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Jefferis R. Glycosylation of recombinant antibody therapeutics. Biotechnol. Prog. 2005;21:11–16. doi: 10.1021/bp040016j. [DOI] [PubMed] [Google Scholar]
- Kelley B. Industrialization of mAb production technology: the bioprocessing industry at a crossroads. MAbs. 2009;1:443–452. doi: 10.4161/mabs.1.5.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou M., Guevara Patino J.A., Jennings R.M., McIntosh R.S., Shi J., Howell S., Cullen E., Jones T., Adame-Gallegos J.R., Chappel J.A., McBride J.S., Blackman M.J., Holder A.A., Pleass R.J. Inhibition of erythrocyte invasion and Plasmodium falciparum merozoite surface protein 1 processing by human immunoglobulin G1 (IgG1) and IgG3 antibodies. Infect. Immun. 2009;77:5659–5667. doi: 10.1128/IAI.00167-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A.P. Cloning and analysis of the gene encoding the 230-kilodalton merozoite surface antigen of Plasmodium yoelii. Mol. Biochem. Parasitol. 1989;36:271–282. doi: 10.1016/0166-6851(89)90175-8. [DOI] [PubMed] [Google Scholar]
- Mackellar D.C., O’Neill M.T., Aly A.S., Sacci J.B., Jr., Cowman A.F., Kappe S.H. Plasmodium falciparum PF10_0164 (ETRAMP10.3) is an essential parasitophorous vacuole and exported protein in blood stages. Eukaryot. Cell. 2010;9:784–794. doi: 10.1128/EC.00336-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy K.D., Stoel M., Stettler R., Merky P., Fink K., Senn B.M., Schaer C., Massacand J., Odermatt B., Oettgen H.C., Zinkernagel R.M., Bos N.A., Hengartner H., Macpherson A.J., Harris N.L. Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe. 2008;4:362–373. doi: 10.1016/j.chom.2008.08.014. [DOI] [PubMed] [Google Scholar]
- McIntosh R.S., Shi J., Jennings R.M., Chappel J.C., de Koning-Ward T.F., Smith T., Green J., van Egmond M., Leusen J.H., Lazarou M., van de Winkel J., Jones T.S., Crabb B.S., Holder A.A., Pleass R.J. The importance of human FcgammaRI in mediating protection to malaria. PLoS Pathog. 2007;3:e72. doi: 10.1371/journal.ppat.0030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean G.R., Torres M., Elguezabal N., Nakouzi A., Casadevall A. Isotype can affect the fine specificity of an antibody for a polysaccharide antigen. J. Immunol. 2002;169:1379–1386. doi: 10.4049/jimmunol.169.3.1379. [DOI] [PubMed] [Google Scholar]
- Morgan W.D., Birdsall B., Frenkiel T.A., Gradwell M.G., Burghaus P.A., Syed S.E., Uthaipibull C., Holder A.A., Feeney J. Solution structure of an EGF module pair from the Plasmodium falciparum merozoite surface protein 1. J. Mol. Biol. 1999;289:113–122. doi: 10.1006/jmbi.1999.2753. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Ravetch J.V. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Ravetch J.V. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Persic L., Roberts A., Wilton J., Cattaneo A., Bradbury A., Hoogenboom H.R. An integrated vector system for the eukaryotic expression of antibodies or their fragments after selection from phage display libraries. Gene. 1997;187:9–18. doi: 10.1016/s0378-1119(96)00628-2. [DOI] [PubMed] [Google Scholar]
- Persson C., Oliveira G.A., Sultan A.A., Bhanot P., Nussenzweig V., Nardin E. Cutting edge: a new tool to evaluate human pre-erythrocytic malaria vaccines: rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J. Immunol. 2002;169:6681–6685. doi: 10.4049/jimmunol.169.12.6681. [DOI] [PubMed] [Google Scholar]
- Pizarro J.C., Chitarra V., Verger D., Holm I., Petres S., Dartevelle S., Nato F., Longacre S., Bentley G.A. Crystal structure of a Fab complex formed with PfMSP1-19, the C-terminal fragment of merozoite surface protein 1 from Plasmodium falciparum: a malaria vaccine candidate. J. Mol. Biol. 2003;328:1091–1103. doi: 10.1016/s0022-2836(03)00376-0. [DOI] [PubMed] [Google Scholar]
- Pleass R.J., Lang M.L., Kerr M.A., Woof J.M. IgA is a more potent inducer of NADPH oxidase activation and degranulation in blood eosinophils than IgE. Mol. Immunol. 2007;44:1401–1408. doi: 10.1016/j.molimm.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Pleass R.J., Ogun S.A., McGuinness D.H., van de Winkel J.G., Holder A.A., Woof J.M. Novel antimalarial antibodies highlight the importance of the antibody Fc region in mediating protection. Blood. 2003;102:4424–4430. doi: 10.1182/blood-2003-02-0583. [DOI] [PubMed] [Google Scholar]
- Pleass R.J., Woof J.M. Fc receptors and immunity to parasites. Trends Parasitol. 2001;17:545–551. doi: 10.1016/s1471-4922(01)02086-4. [DOI] [PubMed] [Google Scholar]
- Reitan S.K., Hannestad K. A syngeneic idiotype is immunogenic when borne by IgM but tolerogenic when joined to IgG. Eur. J. Immunol. 1995;25:1601–1608. doi: 10.1002/eji.1830250620. [DOI] [PubMed] [Google Scholar]
- Reitan S.K., Hannestad K. Immunoglobulin heavy chain constant regions regulate immunity and tolerance to idiotypes of antibody variable regions. Proc. Natl. Acad. Sci. USA. 2002;99:7588–7593. doi: 10.1073/pnas.052150899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo W.W., Block O.K., Lane C., Sukupolvi-Petty S., Goncalvez A.P., Johnson S., Diamond M.S., Lai C.J., Rose R.C., Jin X., Schlesinger J.J. Dengue virus neutralization is modulated by IgG antibody subclass and Fcgamma receptor subtype. Virology. 2009;394:175–182. doi: 10.1016/j.virol.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussilhon C., Oeuvray C., Muller-Graf C., Tall A., Rogier C., Trape J.F., Theisen M., Balde A., Perignon J.L., Druilhe P. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 2007;4:e320. doi: 10.1371/journal.pmed.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva S., Mohmmed A., Dasaradhi P.V., Crabb B.S., Katyal A., Malhotra P., Chauhan V.S. Immunogenicity and protective efficacy of Escherichia coli expressed Plasmodium falciparum merozoite surface protein-1(42) using human compatible adjuvants. Vaccine. 2006;24:2007–2016. doi: 10.1016/j.vaccine.2005.11.041. [DOI] [PubMed] [Google Scholar]
- Schlenke P. Cell Technology for Cell Products. Springer; 2007. Serum-free transient expression of antibodies using 40 tray cell factories; pp. 527–530. (Chapter VI) [Google Scholar]
- Sethuraman N., Stadheim T.A. Challenges in therapeutic glycoprotein production. Curr. Opin. Biotechnol. 2006;17:341–346. doi: 10.1016/j.copbio.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Shi J., McIntosh R.S., Adame-Gallegos J., Dehal P.K., van Egmond M., van de Winkel J., Draper S.J., Forbes E.K., Corran P.H., Holder A.A., Woof J.M., Pleass R.J. The generation and evaluation of recombinant human IgA specific for Plasmodium falciparum merozoite surface protein 1–19 (PfMSP119) BMC Biotechnol. 2011;11:77. doi: 10.1186/1472-6750-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer Valero L.M., Ogun S.A., Fleck S.L., Ling I.T., Scott-Finnigan T.J., Blackman M.J., Holder A.A. Passive immunization with antibodies against three distinct epitopes on Plasmodium yoelii merozoite surface protein 1 suppresses parasitemia. Infect. Immun. 1998;66:3925–3930. doi: 10.1128/iai.66.8.3925-3930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari R., Spaccapelo R., Bistoni F., Holder A.A., Crisanti A. Function of region I and II adhesive motifs of Plasmodium falciparum circumsporozoite protein in sporozoite motility and infectivity. J. Biol. Chem. 2002;277:47613–47618. doi: 10.1074/jbc.M208453200. [DOI] [PubMed] [Google Scholar]
- Thomas P., Smart T.G. HEK293 cell line: a vehicle for the expression of recombinant proteins. J. Pharmacol. Toxicol. Methods. 2005;51:187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Torres M., Fernandez-Fuentes N., Fiser A., Casadevall A. The immunoglobulin heavy chain constant region affects kinetic and thermodynamic parameters of antibody variable region interactions with antigen. J. Biol. Chem. 2007;282:13917–13927. doi: 10.1074/jbc.M700661200. [DOI] [PubMed] [Google Scholar]
- Walsh G., Jefferis R. Post-translational modifications in the context of therapeutic proteins. Nat. Biotechnol. 2006;24:1241–1252. doi: 10.1038/nbt1252. [DOI] [PubMed] [Google Scholar]
- Wengelnik K., Spaccapelo R., Naitza S., Robson K.J., Janse C.J., Bistoni F., Waters A.P., Crisanti A. The A-domain and the thrombospondin-related motif of Plasmodium falciparum TRAP are implicated in the invasion process of mosquito salivary glands. EMBO J. 1999;18:5195–5204. doi: 10.1093/emboj/18.19.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski W., Harris D.P., Sprague F., Mousseau B., Makris M., Kusser K., Honjo T., Mohrs K., Mohrs M., Randall T., Lund F.E. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30:421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woof J.M. Immunology. Tipping the scales toward more effective antibodies. Science. 2005;310:1442–1443. doi: 10.1126/science.1122009. [DOI] [PubMed] [Google Scholar]
- Wurm F.M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]