Abstract
Background
High levels of impulsivity have been associated with a number of substance abuse disorders including alcohol abuse. Research has not yet revealed whether these high levels predate the development of alcohol abuse.
Methods
The current study examined impulsivity in 15 inbred strains of mice (A/HeJ, AKR/J, BALB/cJ, C3H/HeJ, C57BL/6J, C57L/J, C58/J, CBA/J, DBA/1J, DBA/2J, NZB/B1NJ, PL/J, SJL/J, SWR/J, and 129P3/J) using a Go/No-go task, which was designed to measure a subject’s ability to inhibit a behavior. Numerous aspects of response to ethanol and other drugs of abuse have been examined in these strains.
Results
There were significant strain differences in the number of responses made during the No-go signal (false alarms) and the extent to which strains responded differentially during the Go and No-go signals (d′). The rate of responding prior to the cue did not differ among strains, although there was a statistically significant correlation between false alarms and precue responding that was not related to basal activity level. Interstrain correlations suggested that false alarms and rate of responding were associated with strain differences in ethanol-related traits from the published literature.
Conclusions
The results of this study do support a link between innate level of impulsivity and response to ethanol and are consistent with a genetic basis for some measures of behavioral inhibition.
Keywords: Impulsivity, Inhibition, Mice, Inbred Strains, Heritability, Go/No-go, Locomotion, Ethanol
High levels of impulsivity have been associated with a number of substance abuse disorders including alcohol abuse (see Perry and Carroll, 2008 for review). Understanding this relationship is made more difficult, because impulsivity is a complex behavioral trait and can be divided into multiple subtypes. One form of impulsivity is poor behavioral inhibition (Evenden, 1999), also known as motor impulsivity (Brunner and Hen, 1997). Poor behavioral inhibition can be defined as a failure to suppress a prepotent motor response and is an established feature of alcohol intoxication (Fillmore and Vogel-Sprott, 1999; Marczinski et al., 2005). Further, several studies have suggested that it may be a risk marker for alcohol and drug use disorders in humans (Nigg et al., 2006), and some data from animal studies suggest that genotypic differences in level of motor impulsivity may predict level of ethanol consumption (Logue et al., 1998).
Behavioral inhibition can be assessed using a variety of tasks. One widely-used method is the 5-choice serial reaction time task (5CSRT: see Robbins, 2002 for review). In this task, the subject pushes a response panel to initiate a trial. After a variable period of time (“the precue period”), a light appears above one of five apertures. The subject earns food rewards by poking its nose in the appropriate aperture. The number of responses that occur before the aperture light appears (“premature responding”) provides a measure of inhibition. Other tasks assess the ability of subjects to respond appropriately to a Go signal and No-go signal. Responses during the No-go signal are counted as false alarms and again provide a measure of inhibition. Appropriately withholding responses during the No-go signal can be reinforced (a symmetric procedure) or not reinforced (an asymmetrical procedure: Hellemans et al., 2005; Mitchell, 2004).
The current study used the symmetrical Go/No-go task described by McDonald and colleagues (1998). In addition to providing data regarding appropriate responding to a Go signal, this task provides a measure of precue responding, when responding is neither reinforced nor punished, like the 5CSRT. Also, we can compare responding during the Go signal (hits), when responses are reinforced, to responding during the No-go signal (false alarms) when responding causes the reinforcer to be omitted (omission training or negative punishment; Rachlin, 1976). Finally, the number of reinforcers earned divided by the total number of responses can be used to calculate efficiency—a measure reminiscent of that used by Logue and colleagues (1998) to compare nose poking in a signaled appetitive task in 13 inbred mouse strains. In that study, Logue and colleagues (1998) assessed the genetic relationship between nose-poking efficiency and subsequent consumption of a 10% ethanol solution in a 2-bottle choice procedure, where the alternative was plain water. This study revealed that those strains that confined their nose poking to periods when it yielded rewards (more efficient behavior) also consumed less ethanol. Logue and colleagues interpreted this as indicating a genetic relationship between impulsivity and ethanol consumption. The Logue and colleagues task included a variable length prestimulus period, followed by a 3-s signaled period during which the first response was reinforced. A tone signaled the end of the Go period and was followed by a fixed duration inter-trial interval. Thus, their measure of efficiency provides an index of the propensity to restrict responding to periods in which it will be reinforced, but does not provide a direct measure of inhibition driven by instrumental omission contingencies under which food is withheld if the response occurs.
The goal of the current study was to identify strain differences in measures of behavioral inhibition and the extent to which these measures are genetically correlated with ethanol-related traits. To accomplish this goal, mice from 15 inbred mouse strains were selected as subjects. The specific strains, or a subset of these strains, have been used in several studies examining ethanol-related traits, e.g., consumption (Belknap et al., 1993), ethanol-induced locomotor activity (Crabbe et al., 1994), measures of acute and chronic withdrawal from ethanol exposure (Metten and Crabbe, 1994, 2005). We anticipated that strains that exhibited lower levels of behavioral inhibition would be found to be those that consumed more ethanol or exhibited higher levels of those traits associated with increased ethanol preference.
Measures of locomotor activity and sucrose preference were obtained for additional animals for all 15 strains. Any strain differences in these activities could be used to better interpret performance on the Go/No-go task, because performance could be influenced by strain differences in basal activity level and the motivational efficacy of sucrose.
METHODS
Subjects
Subjects were male mice (8–11 per strain) obtained from The Jackson Laboratory (Bar Harbor, ME) at 3–5 weeks of age (see Table 1 for numbers and weights of the mice that completed the study from the 15 inbred strains).Mice were 32–46 days old at the time of initiation of operant training, so that animals would be able to complete the experiment quickly to prevent the data being affected by progressive hearing loss in some strains (BALB/cJ, C57BL/6J, C57L/J, CBA/J, DBA/2J; auditory brain response threshold data for mice younger or older than 12 weeks, http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=meas/catlister&req=Cear). The first day of operant training occurred after a minimum of 48 hours on a food-restricted diet (approximately 2 g of mouse chow per day per mouse), and mice were maintained at approximately 90% of age-adjusted free-feeding weight with mouse chow throughout the experiment. This minimal level of food restriction provided motivation for sucrose responding in the Go/No-go task. Mice were housed 2–5 per cage under a 12:12-h light: dark cycle (lights on at 6 am) in a temperature-controlled vivarium (21.7 ± 1°C) and were maintained according to guidelines provided by the Oregon Health & Science University Department of Comparative Medicine. The Institutional Animal Care and Use Committee approved all procedures. Testing was completed before mice reached 3 months of age.
Table 1.
Numbers (n) and Mean Initial Weights of Mice From Each Strain That Completed the Different Experimental Tasks
| Go/No-go task |
Activity and sucrose preference tasks |
|||
|---|---|---|---|---|
| Strain | n | Mean weight (g) | n | Mean weight (g) |
| 129P3/J | 8 | 20.4 | 6 | 19.3 |
| A/HeJ | 10 | 19.7 | 6 | 20.0 |
| AKR/J | 8 | 22.4 | 6 | 25.6 |
| BALB/cJ | 9 | 22.3 | 6 | 21.9 |
| C3H/HeJ | 8 | 20.3 | 6 | 20.6 |
| C57BL/6J | 8 | 19.2 | 6 | 18.6 |
| C57L/J | 8 | 21.0 | 6 | 20.5 |
| C58/J | 8 | 19.2 | 5 | 20.1 |
| CBA/J | 8 | 20.8 | 6 | 21.4 |
| DBA/1J | 8 | 18.3 | 6 | 17.4 |
| DBA/2J | 8 | 20.6 | 6 | 21.4 |
| NZB/B1NJ | 11 | 21.6 | 6 | 22.9 |
| PL/J | 10 | 18.0 | 6 | 18.1 |
| SJL/J | 8 | 20.1 | 6 | 19.6 |
| SWR/J | 8 | 18.0 | 6 | 15.6 |
Go/No-go Task
Go/No-go task performance was assessed in 16 identical Med-Associates (St Albans, VT) chambers housed in sound-attenuating ventilated boxes. Chamber floors consisted of stainless steel rods above a litter pan. A 100 mA house light was mounted outside the chamber above the back wall. The front panel contained three nose poke holes, each mounted 1.27 cm above the floor. Either the left-most or the right-most hole was designated the active hole (counter-balanced between animals), with a 0.50-cm diameter yellow LED light centered 1.91 cm above each. Nose pokes in inactive holes were recorded, but no experimental contingencies were applied to these nose pokes. The active nose poke hole contained a liquid reward cup. Liquid food rewards were delivered to the food cup using a Med-Associates pump (3.33 RPM).
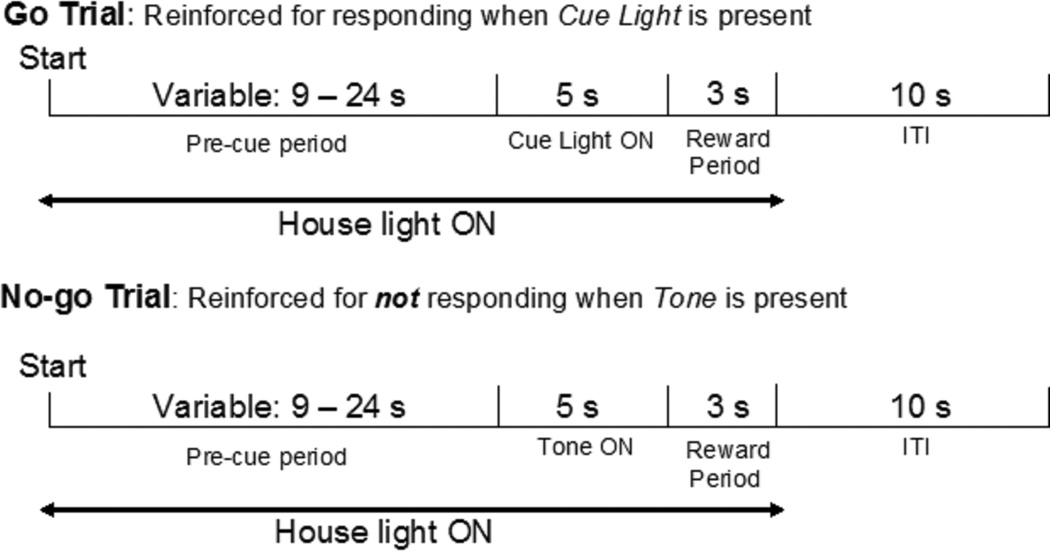
The Go/No-go task was modeled after a task used by McDonald and colleagues (1998). Mice experienced two training phases (Table 2) and an experimental phase lasting 10 sessions. Figure 1 shows a schematic representation of the task as used in the experimental phase. During each of 60 trials, there was a variable duration precue period (9–24 s) during which the house light was illuminated. Responses during the final 3 s of this period reset the trial to prevent premature responding from confounding interpretation of responding during the Go and No-go cues. The precue period was followed by a 5-s cue period, where one of two distinct cues was used to differentiate Go trials from No-go trials. During a Go trial, the light above the left or right nose poke hole was illuminated (counterbalanced between subjects). The first nose poke that occurred during the Go period terminated the Go cue and was reinforced by 19.95 µl of sucrose solution. Mice were permitted 3 s to consume the reinforcer, and then the 10-s inter-trial interval (ITI) began, during which the house light was off. During a No-go trial, a continuous 65-dB 2.9-kHz tone was played. If no nose poke responses occurred during the No-go period, a 19.95-µl reinforcer was delivered at the end of the period, followed by the 3-s consumption period and a 10-s ITI. If a response occurred during the No-go period, the tone was terminated, and the ITI began without a reinforcer being delivered. A response clicker signaled reinforcer delivery.
Table 2.
Mean ± SEM Number of Sessions Required to Complete the Training Phases for the Different Mouse Strains, and the Number of Each Strain That Failed to Advance From Each Phase (see Table 1 for the number of mice that completed all phases and the experimental sessions)
| Strain | Did not complete training |
Phase 1a | Phase 2b |
|---|---|---|---|
| 129P3/J | 1 | 14.63 ± 4.55 | 5.50 ± 2.05 |
| A/HeJ | 1 | 6.40 ± 1.03 | 3.80 ± 0.51 |
| AKR/J | 0 | 8.63 ± 1.76 | 2.50 ± 0.33 |
| BALB/cJ | 0 | 3.89 ± 0.79 | 2.44 ± 0.34 |
| C3/HeJ | 0 | 6.00 ± 1.02 | 4.88 ± 1.92 |
| C57BL/6J | 2 | 7.88 ± 2.77 | 6.38 ± 2.60 |
| C57L/J | 0 | 7.88 ± 2.17 | 3.63 ± 0.71 |
| C58/J | 1 | 3.50 ± 0.63 | 3.50 ± 0.87 |
| CBA/J | 0 | 8.50 ± 1.68 | 3.25 ± 0.56 |
| DBA/1J | 0 | 5.38 ± 1.25 | 5.38 ± 1.02 |
| DBA/2J | 1 | 10.13 ± 2.78 | 5.88 ± 1.98 |
| NZB/B1NJ | 10 | 18.73 ± 1.99 | 5.45 ± 1.57 |
| PL/J | 2 | 7.30 ± 1.22 | 7.50 ± 2.39 |
| SJL/J | 1 | 10.25 ± 2.91 | 10.25 ± 3.71 |
| SWR/J | 2 | 10.00 ± 1.05 | 6.13 ± 2.74 |
Phase 1: There were significant strain differences in sessions required to complete phase 1 of training [F(1, 14) = 4.23, p < 0.001]; Tukey post hoc tests indicated that NZB/B1NJ mice that completed this phase required significantly more sessions than A/HeJ, AKR/J, BALB/cJ, C3/HeJ, C57BL/6J, C57L/J, C58/J, CBA/J, DBA/1J, and PL/J mice, and that 129P3/J mice required significantly more sessions than BALB/cJ and C58/J mice.
Phase 2: There were no strain differences in sessions required to complete phase 2 of training [F(1, 14) = 1.26, p > 0.05].
Correlation between number of training sessions in each phase (Pearson’s product-moment correlation coefficient) = 0.33, N = 15, p = 0.23.
Phase 1 - Go trials only. Nose pokes during a 30-s light cue reinforced with 19.95 µl sucrose. Each session consisted of 60 Go trials. Mice advanced to phase 2 after two consecutive sessions with 30 or more reinforcers earned within 40 minutes. Subjects that did not advance after 35 sessions were dropped from the study.
Phase 2 - Go trials only. Cue period reduced from 30 to 10 s. Mice entered the experimental phase after two consecutive sessions with 30 or more reinforcers earned within 40 minutes. Subjects that did not advance after 30 sessions were dropped from the study.
Fig. 1.
Schematic of contingencies in the Go/No-go task (based on McDonald et al., 1998); see Methods for a complete description.
False alarms and precue response rates were the two main dependent measures of behavioral inhibition for the Go/No-go task. Responses during the No-go period were defined as false alarms. Precue response rate was defined as the total number of responses made during the precue periods divided by the total precue time. The rate measure was used to adjust for potential differences among animals in precue time because responses during the final 3 s of the precue period reset the trial. Additional measures of interest included d′ (d′), a measure of signal discriminability [z-score(hits rate)–z-score (false alarm rate)], the number of hits, i.e., the number of Go trials on which mice responded during the Go period, and efficiency, which is the number of rewards earned divided by the total number of responses.
We conducted preliminary statistical analyses using ANOVAs with strain (genotype) as the between-subjects factor and sessions (6–10) as the within-subjects factor on each of the Go/No-go task measures. There were no statistically significant (all p > 0.05) main effects of session or strain×session interactions for any measure. Based on these findings, the data were collapsed across sessions and analyzed using univariate ANOVAs with strain as a between-subject factor. These early analyses revealed that some strains were not responding differentially to the Go and No-go signals. Data from The Jackson Laboratory (http://jaxmice.jax.org/jaxnotes/archive/485.pdf) indicated that for a subset of strains (C3H/HeJ, CBA/J, PL/J, SJL/J, and SWR/J), this was most probably resulting from a genetically determined blindness. Go/No-go task data from these strains are presented in figures and tables, but have been excluded from the final ANOVAs.
Locomotor Activity
Differences in general activity may affect performance on the Go/No-go task. To assess strain differences in this measure, locomotor activity was measured in experimentally naïve mice using five Accuscan (Columbus, OH) automated activity monitors. These consisted of a 40 × 40 × 30 cm clear acrylic plastic test cage placed inside a monitoring unit that included 8 or 16 evenly spaced photocells. Horizontal beam breaks were assessed, and software converted this into distance traveled (in cm). Each test cage was housed in a black acrylic plastic chamber, lined with foam to attenuate external noise and equipped with a florescent light and ventilated by a fan at the rear. During testing, both the light and fan were on to provide illumination and low-level background noise. Each testing session consisted of 15 minutes, which we divided into 5-minute bins for analysis. Animals were run for 2 consecutive days. Measures of distance traveled on Day 1 provided a measure of response to the novel environment (Piazza et al., 1989), and the difference in distance traveled between Day 2 and Day 1 provided a measure of habituation to the novel environment (Mitchell et al., 2006).
Two-Bottle Sucrose Preference
Following completion of the locomotor activity study, subjects participated in a two-bottle sucrose preference study. Sucrose preference (10%) was measured to assess potential differences in sensitivity to the rewarding effects of sucrose among the inbred mouse strains used in the Go/No-go experiment. Mice were placed individually in cages equipped with a metal wire top and two bottles—one containing de-ionized water and one containing 10%sucrose solution. Placement of the sucrose and water bottles was switched daily to correct for potential side bias. Additional control cages without animals were placed on each mouse rack shelf to estimate daily leakage and evaporation. Leakage amounts were subtracted from the daily drinking values for each subject on that shelf. The total amount of each solution consumed was determined by weighing the bottle at the start of the experiment and every 24 hours for 4 consecutive days. Mice were also weighed daily. Sucrose consumption was calculated as grams of sucrose per kg body weight, averaged across days. Sucrose preference was calculated as average ratio of sucrose consumed divided by the total amount of fluid (water + sucrose) consumed across days. We excluded 10 daily drinking values because bottles leaked (1.4% of values), as shown by wet bedding under the drinking bottle and an almost empty bottle. Additional three daily drinking values were excluded, because negative consumption values indicated that there had been a data collection error (0.4%of values).
Data Analysis
All statistical analyses were carried out using SPSS version 16 (SPSS Inc., Chicago, IL). Huynh-Feldt corrections were performed if there were violations of the sphericity assumption, and in those cases the adjusted degrees of freedom are reported. Main effects of strain were evaluated using univariate ANOVAs, coupled with post hoc Tukey tests to identify the source of statistically significant effects. Heritability was calculated for all measures that included significant strain influences. Only those strains included in the Go/No-go analyses were included. Heritability was calculated as described in Wilhelm and Mitchell (2009) and was based upon the components of variance calculation described in Hegmann and Possidente (1981).
| (1) |
where σ2 is equal to the mean square within strains variation, and σ2s is equal to (σ2 - (mean square between-strains variation))/k. Where k is a function of the unequal replicate numbers for each strain, and the mean square values are derived from a simple, univariate ANOVA with strain as the between-subject factor. Inbred strains are genetically homogenous; however, the calculated heritability may include variation caused by maternal care and/or other early environmental factors in addition to purely genetic influences.
RESULTS
Go/No-go Task Performance
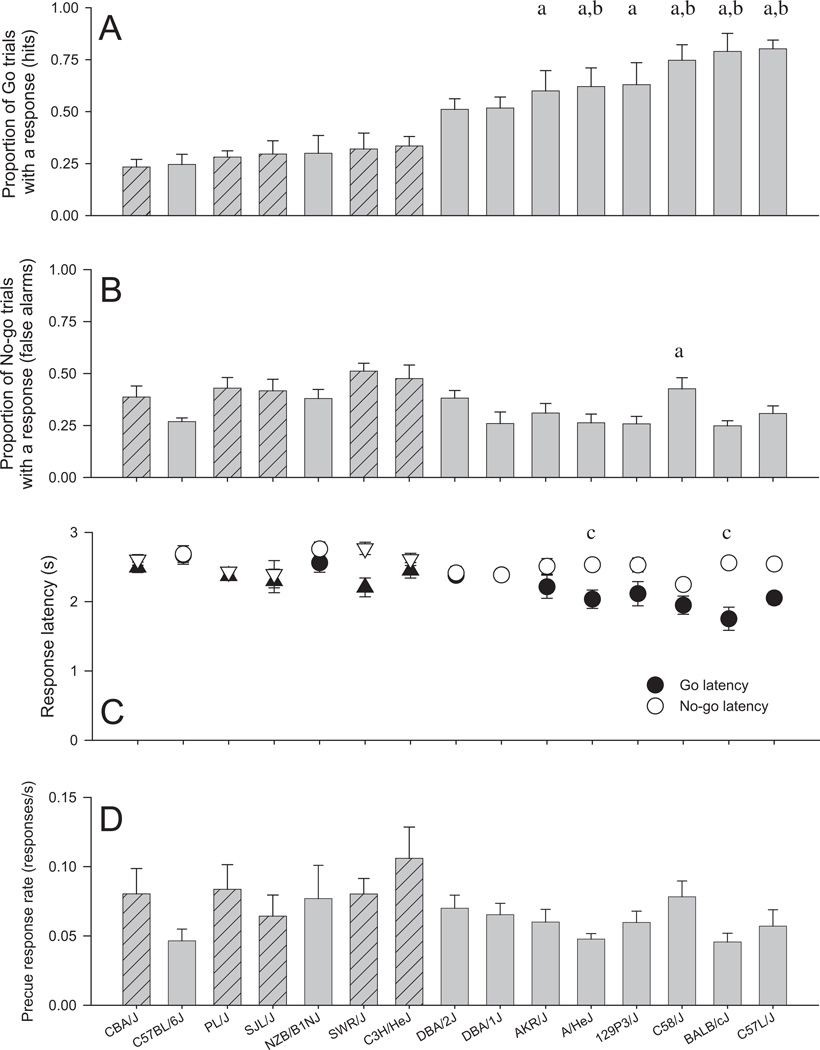
The Go/No-go task assesses the ability of mice to appropriately perform or withhold a nose poke depending on the cues presented. ANOVAs with strain as a between-subject factor were used to analyze the main dependent variables on the Go/No-go task. As shown in Fig. 2, there were strain differences in the number of responses occurring on Go trials [hits: F(9, 76) = 6.74, p < 0.001] and the number of responses occurring on No-go trials [false alarms: F(9, 76) = 2.76, p < 0.01]. Univariate ANOVAs indicated that there were strain differences in the latency to respond when the Go cue occurred [F(9, 76) = 4.73, p < 0.001] and when the No-go cue occurred [F(9, 76) = 2.09, p = 0.04]. As would be expected if subjects were attempting to inhibit responding during the No-go cue, response latency was significantly faster on Go trials than No-go trials [main effect cue type: F(1, 76) = 42.36, p < 0.001], although this was not the case for all strains [cue type × strain: F(9, 76) = 3.29, p = 0.002]. A final measure of impulsive responding, the rate of response during the precue period, did not differ significantly among strains [F(9, 76) = 1.17, p = 0.33]. The interstrain correlation between rate of precue responding and false alarms was 0.84 (p < 0.001, Pearson’s r, N = 10).
Fig. 2.
(A) Mean (±SEM) proportion of Go trials on which mice responded (hits) for the 15 inbred strains of mice: (a) higher responding than C57BL / 6J, (b) higher responding than NZB/B1NJ. (B) Mean (±SEM) proportion of No-go trials on which mice responded (false alarms) for the 15 inbred strains of mice: (a) higher responding than C57BL/6J. (C) Mean (±SEM) response latencies on Go trials and No-go trials for the 15 inbred strains of mice. (c) Longer within strain response latency on No-go trials compared with Go trials (p < 0.01 within subject t-test, uncorrected). (D) Mean (±SEM) rate of responding during the precue period for the 15 inbred strains of mice. Hatched bars and triangle symbols represent the five strains removed from the statistical analyses. All data are averaged over the final 5 sessions.
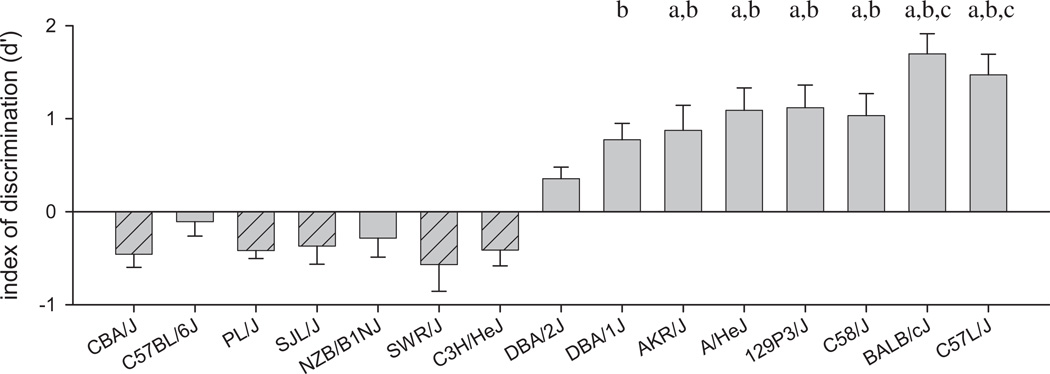
Some strains exhibited large numbers of hits and few false alarms, indicating a high level of discrimination between the Go and No-go signals, while this was not the case for other strains (Fig. 3). These differences resulted in significant strain differences in the index of discrimination, d’ [F(9, 76) = 9.58, p < 0.001]. Eight strains exhibited positive d’ values, and all of these differed significantly from zero, indicating that responding occurred preferentially during the Go signal. The other two strains exhibited negative values, but one-sample t-tests indicated that neither differed significantly from zero, indicating that these strains did not exhibit discrimination between the Go and No-go signals.
Fig. 3.
Mean (±SEM) index of discrimination, d′: (a) more discrimination between Go and No-go trials than C57BL / 6J, (b) more discrimination between Go and No-go trials than NZB/B1NJ, (c) more discrimination between Go and No-go trials than DBA/2J. Hatched bars and triangle symbols represent the five strains removed from the statistical analyses. All data are averaged over the final 5 sessions.
There were no strain differences in efficiency [number of reinforcers earned per response: F(9, 76) = 1.42, p = 0.20]. However, strains differed in the percentage of responses that were made in the rewarded location (the apparatus included two other nose poke receptacles) [F(9, 76) = 6.63, p < 0.001].
Locomotor Activity
There were significant strain differences in distance traveled in the activity chamber on Day 1 [Table 3: F(14, 74) = 12.64, p < 0.001; for strains in Go/No-go analysis only: F(9, 49) = 13.96, p < 0.001]. For all but four strains (BALB/cJ, C3H/HeJ, C58/J, and PL/J), activity levels dropped on Day 2, resulting in significant strain differences in habituation [distance traveled on Day 2 – distance traveled on day 1: F(14, 74) = 6.11, p < 0.001; for strains in the Go/No-go analysis only: F(9, 49) = 5.69, p < 0.001]. Neither measure was significantly correlated with measures of impulsivity from the Go/No-go task for the 10 strains for which these measures were available.
Table 3.
Mean ± SEM Values for Locomotor Activity in the 15 Inbred Strains of Mice
| Locomotor activity (cm) |
||
|---|---|---|
| Strain | Novel environment Day 1a |
Habituation Day 2-Day 1b |
| 129P3/J | 3796 ± 540 | −2486 ± 327 |
| A/HeJ | 2193 ± 296 | −528 ± 159 |
| AKR/J | 4718 ± 349 | −1441 ± 458 |
| BALB/cJ | 2415 ± 203 | 404 ± 249 |
| C3H/HeJ | 2431 ± 63 | 681 ± 305 |
| C57BL/6J | 4437 ± 130 | −1239 ± 518 |
| C57L/J | 3667 ± 515 | −448 ± 252 |
| C58/J | 6754 ± 480 | 796 ± 469 |
| CBA/J | 2544 ± 121 | −9 ± 220 |
| DBA/1J | 3799 ± 155 | −214 ± 236 |
| DBA/2J | 2689 ± 300 | −614 ± 524 |
| NZB/B1NJ | 3422 ± 175 | −620 ± 460 |
| PL/J | 4357 ± 572 | 168 ± 518 |
| SJL/J | 3020 ± 197 | −19 ± 194 |
| SWR/J | 4474 ± 245 | −1711 ± 342 |
Correlation between activity on Day 1 and extent of habituation (Pearson’s product-moment correlation coefficient) = −0.10, N = 15, p = 0.72.
Tukey post hoc tests indicated that locomotor activity in the C58/J strain, which showed the highest levels of activity on Day 1, was significantly higher than all other strains. The AKR/J strain, which showed the second highest levels, differed significantly from the A/HeJ, BALB/cJ, C3H/HeJ, CBA/J, DBA/2J, and SJL/J strains, and the strains showing the third, fourth, and fifth highest levels (SWR/J, C57BL/6J, PL/J) were significantly different from the A/HeJ, BALB/cJ, C3H/HeJ, CBA/J, and DBA/2J.
Negative scores represent a reduced level of activity on Day 2 compared with Day 1. Tukey post hoc tests indicated that the highest degree of habituation, exhibited by 129P3/J, was significantly more than that exhibited by 12 other strains (A/HeJ, BALB/cJ, C3H/HeJ, C57L/J, C58/J, DBA/1J, DBA/2J, NZB/B1NJ, PL/J, SJL/J, and SWR/J). The SWR/J strain exhibited the second highest level of habituation and differed significantly from the BALB/cJ, C3H/HeJ, C58/J, PL/J strains, while the AKR/J mice differed significantly from the BALB/cJ, C3H/HeJ, and C58/J mouse strains. The strain exhibiting the fourth highest rate of habituation, C57BL/6J, differed from the C3H/HeJ and C58/J strains.
Sucrose Preference
ANOVAs with strain as a between subjects factor indicated that there were significant strain differences in sucrose solution consumption (in g sucrose/kg) and preference for the 10% sucrose solution versus water [Table 4: F(14, 74) = 6.63, p < 0.001; F(14, 74) = 2.54, p < 0.01]. A similar pattern of results was obtained when only the strains included in the Go/No-go analyses were examined [F(9, 49) = 6.71, p < 0.001; F(9, 49) = 2.87, p < 0.01]. Sucrose consumption was not significantly correlated with either false alarms or rate of precue responding for these strains (R = −0.49 and −0.31 respectively, N = 10, p > 0.10 for each).
Table 4.
Mean ± SEM Values for 10% Sucrose Drinking Measures in the 15 Inbred Strains of Mice
| Strain | Percent sucrose preferencea |
Consumption (mg sucrose/kg)b |
|---|---|---|
| 129P3/J | 92 ± 4 | 73.86 ± 5.27 |
| A/HeJ | 76 ± 15c | 49.30 ± 12.22 |
| AKR/J | 90 ± 4 | 21.23 ± 2.20 |
| BALB/cJ | 99 ± <1 | 92.24 ± 5.41 |
| C3H/HeJ | 98 ± <1 | 87.88 ± 10.63 |
| C57BL/6J | 99 ± <1 | 93.61 ± 4.78 |
| C57L/J | 94 ± 5 | 86.45 ± 14.64 |
| C58/J | 61 ± 16c | 41.56 ± 20.95 |
| CBA/J | 98 ± 1 | 80.32 ± 2.45 |
| DBA/1J | 98 ± 1 | 41.41 ± 7.23 |
| DBA/2J | 99 ± <1 | 72.62 ± 4.08 |
| NZB/B1NJ | 97 ± 2 | 52.99 ± 8.45 |
| PL/J | 92 ± 4 | 57.97 ± 6.31 |
| SJL/J | 97 ± 1 | 58.28 ± 6.72 |
| SWR/J | 91 ± 9 | 112.07 ± 15.77 |
Tukey post hoc tests indicated that the preference ratio for the C58/J was significantly lower than that of the BALB/cJ, C3H/HeJ, C57BL/6J, C57L/J, CBA/J, DBA/1J, DBA/2J, NZB/B1NJ, and SJL/J strains.
Tukey post hoc tests indicated that AKR/J mice consumed significantly less sucrose than 129P3/J, BALB/cJ, C3H/HeJ, C57BL/6J, C57L/J, CBA/J, DBA/2J, and SWR/J mice strains. In addition to consuming more sucrose than AKR/J mice, the SWR/J mice also consumed significantly more sucrose than A/HeJ, C58/J, DBA/1J, NZB/B1NJ, PL/J, and SJL/J strains. Finally, Tukey post hoc tests indicated that the other two highest consuming strains, C57BL/6J and BALB/cJ, each consumed more sucrose than C58/J and DBA/1J.
Large levels of variability do not reflect the behavior of a single mouse.
Genetic Correlations
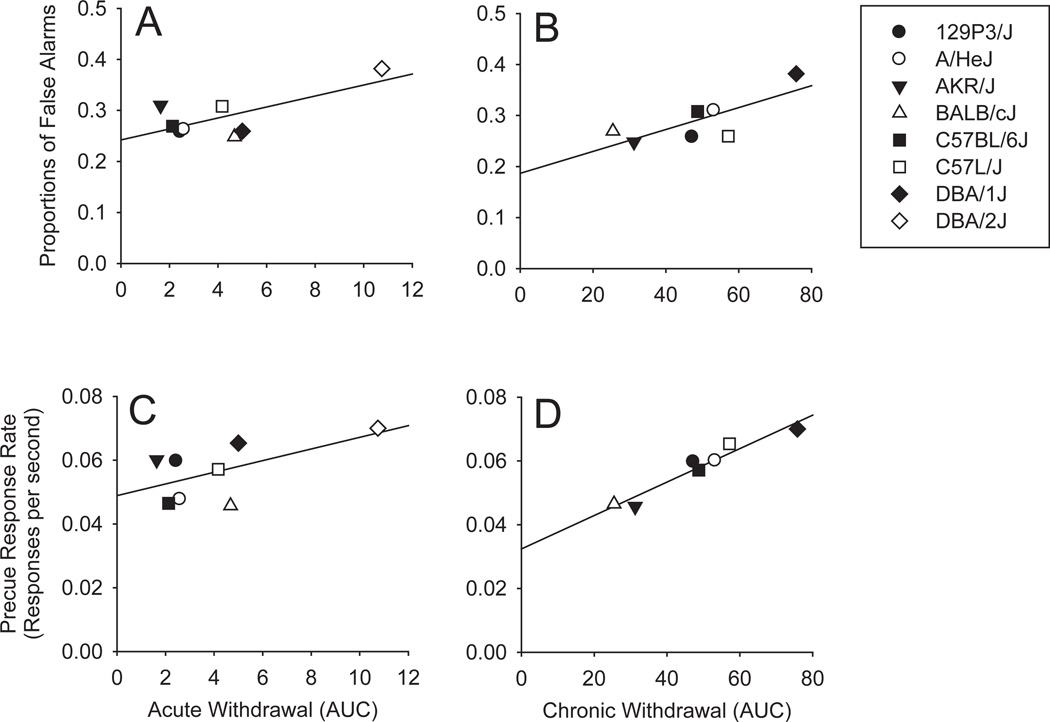
Data from previously published papers that reported strain differences in ethanol responses were examined to determine whether these were significantly associated with strain-related differences in measures of inhibition on the Go/No-go task (rate of precue responses and number of false alarms). As can be seen in Table 5 and Fig. 4, these measures were highly related to measures of ethanol withdrawal sensitivity assessed after chronic exposure. Although the correlation was statistically significant for these measures and acute ethanol exposure, those results were primarily driven by the outlier strain, DBA/2J.
Table 5.
Pearson’s Product-Moment Correlation Coefficients Between Impulsivity Measures (false alarms and precue response rate) for the Inbred Strains on the Go/No-go Task and Ethanol Related Traits From Different Studies in Which at Least 7 Strains Were Shared With the Current Study
| Ethanol-related trait | Na | False alarm rate |
Precue response rate |
|---|---|---|---|
| Sedation/righting reflexb | 8 | 0.07 | 0.05 |
| Ataxia with 2 g/kg ethanolb | 8 | −0.40 | −0.01 |
| Ethanol drinking (3%)c | 8 | −0.28 | −0.52 |
| Ethanol drinking (6%)c | 8 | 0.01 | −0.42 |
| Ethanol drinking (10%)c | 8 | 0.03 | −0.44 |
| Acute withdrawald | 8 | 0.71* | 0.59 |
| Chronic withdrawale | 7 | 0.76* | 0.97** |
p ≤ 0.05.
p ≤ 0.01.
Number of strains in common between the current study and the study examining the specific ethanol related trait.
Fig. 4.
Scatterplots showing the relationships between the proportion of No-go trials on which there were false alarms and the acute and chronic withdrawal scores (A and B) and the relationships between the precue response rate and the acute and chronic withdrawal scores (C and D). Withdrawal scores were the area under the curve produced by scoring handling-induced convulsions (Crabbe et al., 1991) on an hourly basis for 25 hours following the termination of acute or chronic ethanol exposure. Acute and chronic withdrawal data were drawn from Metten and Crabbe (1994, 2005) respectively, also see Table 5 for correlations.
Heritability
Heritability was calculated for all task measures with significant strain effects using Eq. 1. Among behavioral measures from the Go/No-go task, hits and d’ had the largest heritability values (Table 6), while Day 1 locomotor activity had the largest heritability value overall. Strain influences on the two-bottle sucrose preference task were similar to those from the Go/No-go task.
Table 6.
Calculated Heritabilities (Eq. 1) for Measures With Significant Strain Effects
| Behavior | Heritability |
|---|---|
| Hits | 0.40 |
| False alarms | 0.17 |
| d’ | 0.34 |
| Go latency | 0.18 |
| No-go latency | 0.06 |
| Locomotor day 1 activity | 0.69 |
| Locomotor habituation | 0.28 |
| Sucrose consumption | 0.33 |
| Sucrose preference | 0.13 |
DISCUSSION
Strain differences were apparent for most dependent measures of the Go/No-go task including hits, false alarms, and latency to respond on Go and No-go trials, as well as the index of discrimination, d′. However, there were no significant differences among strains for rate of precue responding or efficiency. Those strain differences observed likely indicate the presence of significant genetic influences on this task. Possible influences of early rearing environment, such as differences in maternal care, cannot be ruled out as a source of some variation among strains, but such maternal differences among strains are likely also associated with genetic differences. Future studies using cross-fostering techniques would address the possible impact of early environment on these impulsivity measures, which to the best of our knowledge has not been examined.
Both false alarms (errors of commission) and rate of precue responding have been used in prior research as measures of behavioral inhibition or motor impulsivity (e.g., Mitchell, 2004). However, other interpretations of both measures are possible. One interpretation is that false alarms reflect the ability of the subject to learn the instrumental omission contingency. This suggestion stems from the observation that two strains exhibited no appreciable discrimination between the Go and No-go signals (C57BL/6J and NZB/B1NJ). Although they did learn to respond during the Go signal (Table 2), it could be argued that they did not learn the No-go contingency. However, it could also be argued that ignoring the No-go signal is the essence of a failure of inhibition. This highlights one of the limitations of the current task, that is, its failure to easily differentiate between a failure to learn the No-go signal and complete failures of inhibition. A second interpretation of the false alarms measure is that it reflects the degree of generalization of the approach behavior elicited by the Go signal. This suggestion stems from the observation that Go and No-go response differences could also be partially explained in terms of classical conditioning processes, specifically sign tracking (autoshaping: Brown & Jenkins, 1968). That is, the light above the nose poke hole where food is delivered may elicit approach behavior, because it is a spatially restricted visual cue proximal to the food delivery location. Although it should be noted that, in the archetypal sign tracking procedure, food presentation is independent of responding, while in our procedure food delivery is contingent on nose poking. The tone, being spatially diffuse, is less likely to acquire such a conditioned response, or may create an orienting conditioned response, a behavior incompatible with nose poking (Holland, 1977). Thus, differences in sign tracking for these two stimuli could contribute significantly to the apparent instrumental discrimination, and the number of No-go responses may index the extent of generalization of the sign tracking response from the Go trials. Genetic differences in sign tracking have previously been noted in mice (e.g., O’Connell, 1980) and rats (e.g., Kearns et al., 2006). So it is entirely reasonable that differences in the generalization of a Pavlovian-based conditioned approach response could underlie strain differences in false alarms. However, it can be argued that generalization of approach responding to a situation in which this is inappropriate (No-go trials) is not incompatible with the use of false alarms as a measure of failure to inhibit responding. This explanation would provide a Pavlovian-based mechanism to explain the behavior rather than an instrumentally based one. Future studies could examine the extent to which false alarms are determined by generalization of sign tracking by counterbalancing the stimuli associated with the Go and No-go trials. Additional manipulations to the intensity of the light and tone stimuli could also be used to determine the role of strain differences in responses to stimulus salience on the approach behavior. Finally, alternative interpretations of precue responding are also possible. The nose poking that constitutes the precue responding can be viewed as an anticipatory, classically conditioned response to the onset of the houselight, a conditioned stimulus signaling the possible delivery of food in the food receptacle. Thus, individual differences in precue responding could also reflect an individual’s ability to acquire conditioned responses in general, rather than impairment of behavioral inhibition.
It is somewhat surprising that strain differences were only observed for the false alarm measure, although false alarms and precue responding were highly correlated (r = 0.84). There are several differences between the measures that might account for the lack of effect. First, the contingencies operating on precue responding are different. A response during the No-go signal is followed by the tone and houselights being turned off and a 10-s timeout period, i.e., an omission or negative punishment contingency is in effect. A response during the precue period does not alter the stimulus conditions in the chambers. Thus, the strain effects may reflect a differential sensitivity to the omission contingency, which would not impact precue response rate. This hypothesis could be investigated by examining the first five sessions of the experimental phase to determine whether some strains exhibited decreases in the number of false alarms, suggesting that the omission contingency was affecting behavior. While analyses indicated that there was a significant decline in the number of false alarms over the first five sessions [F(4, 304) = 3.54, p < 0.01], presumably indicating that mice were learning to withhold responses, the strain×session interaction was not statistically significant [F(36, 304) = 1.23, p = 0.18], indicating that differences were not reliable. Future studies specifically targeted to determine whether there are strain differences in sensitivity to omission contingencies might be valuable, given that differences in sensitivity to positive reinforcement contingencies are known to exist and contribute to problems such as drug and alcohol abuse (e.g., Robinson and Berridge, 1993). Further, it should be noted that, while no strain differences were identified for precue rate of responding, this does not negate its value as a measure of impulsivity.
Levels of behavioral inhibition do not appear to be the result of differences in the motivational properties of the reinforce between strains, measured using sucrose consumption, and sucrose preference, as the correlation between these measures and false alarms and rate of precue responding was not significant. However, this does not indicate that performance would be insensitive to changes in motivation, e.g., if levels of food restriction were altered. Further, locomotor activity and level of habituation also did not correlate with precue responding or false alarms, indicating that the Go/No-go task does not simply reflect levels of activity by these strains.
A major goal of the current study was to examine genetic correlations between Go/No-go task performance and previous measures of ethanol responses. Our data did indicate that both false alarms and precue responding were significantly positively correlated with severity of ethanol withdrawal, as measured by scoring handling-induced convulsions (Kosobud & Crabbe, 1986). The chronic measure of withdrawal data were drawn from Metten and Crabbe (2005) and were obtained after mice were exposed to ethanol for 72 hours using the vapor inhalation method (e.g., Crabbe et al., 1983), scores corrected for strain differences in the air/pyrazole control condition. The acute measure of withdrawal data were drawn from Metten and Crabbe (1994) and were obtained following a single 4 g/kg injection, corrected for average baseline scores. The connection between augmented activity in food-restricted animals in the face of signals indicating food is unavailable and higher levels of central nervous system excitability during alcohol withdrawal is not entirely clear but may reflect the action of common activating mechanisms that are initiated when homeostasis is disrupted.
It is somewhat surprising that these impulsivity measures are positively correlated with severity of withdrawal, as other data have indicted that withdrawal severity exhibits a negative genetic correlation with ethanol drinking (Metten et al., 1998). Indeed, there is a tendency for false alarms and precue responding to exhibit a negative relationship, i.e., higher number of false alarms and precue responses (lower inhibition/impulsivity) and lower ethanol consumption. Instead it would be anticipated that lower levels of impulsivity should be associated with higher levels of ethanol consumption. Future research to understand this relationship and the genetic networks are that are responsible is required to resolve these apparent contradictions.
Previous work indicated that strain-dependent levels of efficiency on an appetitive nose poke task could predict strain differences in consumption of 10% ethanol (Logue et al., 1998). The measure of efficiency provided by Logue and colleagues has aspects in common with our measure of precue responding, in that both measures are determined by the number of responses performed that are extraneous to earning reinforcers. Contrary to the results reported in the Logue and colleagues study, we observed negative correlations between ethanol consumption, at a number of concentrations, and precue responding. While these correlations were not statistically significant given the low sample size, they did appear to be relatively similar across conditions, suggesting their robustness. Unfortunately, direct comparisons between measures obtained in the Logue and colleagues study and the current study are very difficult for several reasons. First, it is somewhat unclear the extent to which the strains used by the current study and that of Logue and colleagues overlapped, as Logue and colleagues did not provide entire strain designations, e.g., A versus A/HeJ. Second, while some of their strains originated at The Jackson Laboratory, as did all of the strains in the current study, others were bred on site or were obtained from a different vendor. Third, measures in the Logue and colleagues study were not stable for all strains at the time of testing, so there is a possibility that their results were compromised. Fourth, the current study used a procedure in which precue responding was punished by resetting the precue period, while this was not the case for the Logue study. Thus, our study may be picking up a relationship between sensitivity to this form of punishment and ethanol consumption, while Logue and colleagues would not have been able to address this possibility.
Our previous work using lines of mice selectively bred for high and low levels of ethanol consumption did identify a greater level of impulsivity in the high ethanol drinking line, when measured using the Go/No-go task, but not a delay discounting task (Wilhelm et al., 2007; but also see Oberlin and Grahame, 2009). In rats bred for high and low alcohol drinking, impulsivity measured with a delay discounting task was higher in the high drinking line (Wilhelm and Mitchell, 2008); these rats were not examined using the Go/No-go task. Outcomes of genetic correlation analyses measured using inbred strain panels often do not agree with those from selected lines. We have addressed possible reasons for this in a previous publication (Crabbe et al., 1990). Additional research examining whether the relationship between Go/No-go performance in lines selected for withdrawal severity would be an appropriate way to examine whether the significant correlations between impulsivity and that ethanol-related trait would be informative. In addition, it would be useful to conduct studies examining whether the genetic correlation observed in this study can be observed within subjects, i.e., whether individuals exhibiting lower levels of impulsivity exhibit greater severity to withdrawal examined acutely and chronically. Finally, given the data that performance on the Go/No-go was controlled to some degree by genetic factors, additional research would be valuable that identified the gene networks contributing to the relative sensitivity of the different strains to the positive and the negative outcomes that presumably drives performance on this task.
ACKNOWLEDGMENTS
We wish to thank John Crabbe, Pam Metten, and Andy Cameron for help analyzing the genetic correlation data. The authors also thank William Guethlein, Vanessa Wilson, Kirigin Elstad, and Carly Levine for technical assistance. This research was supported by The Portland Alcohol Research Center (PARC), P60AA010760 and the Department of Veterans Affairs.
REFERENCES
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Brown PL, Jenkins HM. Auto-shaping of the pigeon’s key-peck. J Exp Anal Behav. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D, Hen R. Insights into the neurobiology of impulsive behavior from serotonin receptor knockout mice. Ann NY Acad Sci. 1997;836:81–105. doi: 10.1111/j.1749-6632.1997.tb52356.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Gallaher ES, Phillips TJ, Belknap JK. Genetic determinants of sensitivity to ethanol in inbred mice. Behav Neurosci. 1994;108:186–195. doi: 10.1037//0735-7044.108.1.186. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Kosobud A, Young ER, Janowsky J. Polygenic and single-gene determination of response to ethanol in BXD/TY recombinant inbred mouse strains. Neurobehav Toxicol Teratol. 1983;5:181–187. [PubMed] [Google Scholar]
- Crabbe JC, Merrill CD, Belknap JK. Acute dependence on depressant drugs is determined by common genes in mice. J Pharm Exp Ther. 1991;257:663–667. [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcohol Clin Exp Res. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Vogel-Sprott M. An alcohol model of impaired inhibitory control and its treatment in humans. Exp Clin Psychopharmacol. 1999;7:49–55. doi: 10.1037//1064-1297.7.1.49. [DOI] [PubMed] [Google Scholar]
- Hegmann JP, Possidente B. Estimating genetic correlations from inbred strains. Behav Genet. 1981;11:103–114. doi: 10.1007/BF01065621. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Nobrega JN, Olmstead MC. Early environmental experience alters baseline and ethanol-induced cognitive impulsivity: relationship to forebrain 5-HT(1A) receptor binding. Behav Brain Res. 2005;159:207–220. doi: 10.1016/j.bbr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J Exp Psychol: Anim Behav Proc. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Kearns DN, Gomez-Serrano MA, Weiss SJ, Riley AL. A comparison of Lewis and Fischer rat strains on autoshaping (sign tracking), discrimination reversal learning and negative auto-maintenance. Behav Brain Res. 2006;169:193–200. doi: 10.1016/j.bbr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Kosobud A, Crabbe JC. Ethanol withdrawal in mice bred to be genetically prone or resistant to ethanol withdrawal seizures. J Pharmacol Exp Ther. 1986;238:170–177. [PubMed] [Google Scholar]
- Logue SF, Swartz RJ, Wehner JM. Genetic correlation between performance on an appetitive-signaled nosepoke task and voluntary ethanol consumption. Alcohol Clin Exp Res. 1998;22:1912–1920. [PubMed] [Google Scholar]
- Marczinski CA, Abroms BD, Van Selst M, Fillmore MT. Alcohol-induced impairment of behavioral control: differential effects on engaging vs. disengaging responses. Psychopharmacology (Berl) 2005;182:452–459. doi: 10.1007/s00213-005-0116-2. [DOI] [PubMed] [Google Scholar]
- McDonald MP, Wong R, Goldstein G, Weintraub B, Cheng SY, Crawley JN. Hyperactivity and learning deficits in transgenic mice bearing a human mutant thyroid hormone beta1 receptor gene. Learn Mem. 1998;5:289–301. [PMC free article] [PubMed] [Google Scholar]
- Metten P, Belknap JK, Crabbe JC. Drug withdrawal convulsions and susceptibility to convulsants after short-term selective breeding for acute ethanol withdrawal. Behav Brain Res. 1998;95:113–122. doi: 10.1016/s0166-4328(97)00216-7. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Common genetic determinants of severity of acute withdrawal from ethanol, pentobarbital and diazepam in inbred mice. Behav Pharmacol. 1994;5:533–547. doi: 10.1097/00008877-199408000-00014. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci. 2005;119:911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measuring impulsivity and modeling its association with cigarette smoking. Behav Cogn Neurosci Rev. 2004;3:261–275. doi: 10.1177/1534582305276838. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, Reeves JM, Li N, Phillips TJ. Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcohol Clin Exp Res. 2006;30:429–437. doi: 10.1111/j.1530-0277.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol Clin Exp Res. 2009;33:1–10. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell MF. Autoshaping and food acquisition in mice: a genetic analysis. J Comp Physio Psychol. 1980;94:1149–1159. doi: 10.1037/h0077732. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Rachlin H. Behavior and Learning. San Francisco, CA: Freeman; 1976. [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes Brain Behav. 2008;7:705–713. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Strain differences in delay discounting using inbred rats. Genes Brain Behav. 2009;8:426–434. doi: 10.1111/j.1601-183X.2009.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Reeves JM, Phillips TJ, Mitchell SH. Mouse lines selected for alcohol consumption differ on certain measures of impulsivity. Alcohol Clin Exp Res. 2007;31:1839–1845. doi: 10.1111/j.1530-0277.2007.00508.x. [DOI] [PubMed] [Google Scholar]