Abstract
Oxidative stress and impaired antioxidant system have been implicated in the pathophysiology of various diseases. The objective of the study was to determine the quantitative phytochemicals and invitro antioxidant activity of fresh leaves of Syzygium malaccense. The result showed that the methanolic extract exhibited strong antioxidant activity and contains a higher amount of phenolics and flavonoids when compared to aqueous extract.
Keywords: Syzygium malaccense, Flavonoids and phenolics, DPPH
Natural antioxidants have been studied extensively for decades in order to find compounds protecting against a number of diseases related to oxidative stress and free radical induced damage. The plant kingdom offers a wide range of compounds exhibiting antioxidant activities. Polyphenols have been considered as an excellent natural antioxidant. They are widely distributed and can be considered as the most abundant plant secondary metabolites with highly diversified structures (Preiera 2009: Dai 2010). Syzygium malaccense (L.) Merr.& Perry (synonym- Eugenia malaccensis L.), popularly known as ‘Malay apple’ or ‘Mountain apple’belonging to Myrtaceae family and is originated in India and Malaysia , cultivated in Bengal and South India. Different parts of this plant, such as seeds, bark, fruit , and leaves have been used in traditional medicine . Extracts of seeds, fruits, bark, stem and leaves shows varying degrees of antibiotic activity against Micrococcus pyogens Var. aureus, an extract of fruit (without seeds) is moderately effective against Escherichia Coli and those of bark and leaves against Shiegella paradys. The root is used as remedy for itching, diuretic and is given to alleviate edema. The powdered dried leaves are useful against cracked tongue and juice of the crushed leaves are applied as a skin lotion and is added to bathes (Pullaiah ,2006 ) . Leaves of S. malaccense have been used for a wide variety of inflammatory conditions in Western Samoa (Dunstan,1997).The plant is used in Polynesian traditional medicine for the treatment of infectious diseases and has been found to elicit antiviral,antifungal and antibacterial activities (Locher et al.,1995).The Study of hydrodistilled essential oil from the fresh leaves of S.malaccense grown in Nigeria showed the oil to be largely composed of monoterpenes (61.1%) characterized mainly by a-pinene, b-pinene, p-cymene and a-terpineol. The sesquiterpenes constituted 30.8% of the oil with b-caryophyllene as the major component (Karioti et al.,2007).
In search of new resources and potent antioxidant the present study aimed to evaluate the antioxidant potential of methanolic and aqueous leaf extracts of Syzygium malaccense and to measure the impact of polyphenolic constituents on their effectiveness.
Materials and Methods
Plant Material
The leaves of Syzygium malaccense were collected from Palakkad and identified by a plant taxonomist.The collected leaves were washed with clean water and shade dried at room temperature for one week.
Preparation of the Plant Extracts
Plant crude leaf extract was prepared by Soxhlet extraction method (Kokateetal., 2001).
Fifty gram of dried plant powder was extracted thrice with methanol and aqueous (250 ml) using soxhlet apparatus. All the above extracts were concentrated to a small volume by rotary evaporator. The solvent fraction containing the bioactive compounds were used for antioxidant studies.
Estimation of Total Phenolic Content
The total phenolic content of the extract was estimated according to a modified procedure of Singleton et al., (1999).10 mg/ml was oxidized with 1.0 ml of 10% Folin Ciocalteu′s reagent (v/v) and neutralized by adding 0.8 ml of 7.5% sodium carbonate. The absorbance of the resulting mixture was measured at 765 nm after incubating at 30°C for 1.5 hrs. Results were expressed as mg/g of tannic acid equivalent.
Estimation of Total Flavonoid Content
Total flavonoids in methanol and water extract were estimated by Sakanaka et al., (2005). Briefly, 4.2mg of the extract was dissolved in 5ml of 50% methanol, followed by addition of 1ml of a 5% (w/v) sodium nitrite solution. After 6min 1ml of a10% (w/v) aluminium chloride solution was added and the mixture was allowed to stand for a further 5min before 10 ml of a 10% (w/v) NaOH solution was added. The mixture was made up to 50 ml with distilled water and mixed well. The absorbance was measured at 500 nm with a spectrometer after 15 min. The total flavonoid content was expressed as mg/g dry plant material.
Invitro Antioxidant Studies
DPPH Radical Scavenging Activity
A methanol and water solution of the sample extracts at various concentrations (20 - 100μg) was added to 5ml of a 0.1 mM methanolic solution of DPPH and allowed to stand for 20 min at 27°C. The absorbance of the sample was measured at 517 nm. Radical scavenging activity was expressed as the inhibition percentage of free radical by the sample and was calculated using the formula % DPPH radical scavenging activity = (Control OD-sample /Control OD) × 100.
Hydroxyl Radical Scavenging Activity
The effect of methanol and water leaf extract of S. malaccense on hydroxyl radical was assessed by using the deoxyribose method ( Aruoma and Halliwell, 1987). The reaction mixture contained 450μl of 0.2M sodium phosphate buffer (pH 7.0), 150μl of 10mM 2-deoxyribose, 150μl of 10mM FeSO4-EDTA, 150μl of 10mM H2O2, 500μl of H2O, and 100μl of sample solution (0.02-5.00 mg/ml). The reaction was started by the addition of H2O2. After incubation at 37°C for 4 h, the reaction was stopped by adding 750μl of 2.8% trichloroacetic acid and 750μl of 1% thiobarbituric acid in 50mM NaOH, the solution was boiled for 10 min, and then cooled in ice water. The absorbance of the solution was measured at 520 nm. All measurements were made in triplicate and averaged.
3.4.3. Reducing Power Assay
The reducing power of the sample was determined by the method of Oyaizu (1986). An aliquot of the sample (1.0 mL) at various concentrations (20-100 μg/mL) was mixed with phosphate buffer (0.2 M, pH 6.6, 2.5 mL) and 1% potassium ferricyanide (2.5 mL). The mixture was incubated at 50°C for 20 min. After adding 10% trichloroacetic acid (2.5 mL), the mixture was centrifuged at 650 rpm for10 min. The supernatant (2.5 mL) was mixed with distilled water (2.5 mL) and 0.1% iron (III) chloride (0.5 mL), and the absorbance was measured at 700 nm using an appropriate blank. Assays were carried out in triplicate. BHA was used as a reference.
Result and Discussion
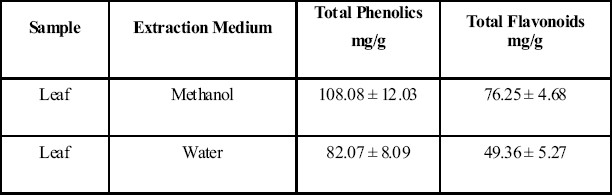
Quantitative estimation of the polyphenolics of S. malaccense studied is presented in table 1. Plant derived antioxidants are regarded as effective in controlling the effects of oxidative damage, and hence had influence in what people eat and drink (Viana et al., 1996; Sun et al., 2002; Pinder and Sandler, 2004). The antioxidative effect is mainly due to phenolic components, such as flavonoids (Pietta, 1998), phenolic acids, and phenolic diterpenes (Shahidi et al., 1992). The total phenolic content in the methanolic extract was 108.08 ± 12.03 mg/g while total flavonoid content was 76.25 ± 4.68 mg/g., the phenolic and flavonoid contents in the aqueous extract showed 82.07 ± 8.09 mg/g and 49.36 ± 5.27 mg/g. The compounds such as flavonoid contain hydroxyls are responsible for the radicals scavenging effects in plants.
Table 1.
Estimation of total phenols and flavonoids
Antioxidant Activities of Extracts
Polyphenolic compounds such as flavonoids, phenolic acids and tannins are considered to be the major contributors to the antioxidant activity of medicinal plants, fruits and vegetables. The antioxidant activities of polyphenols were attributed to their redox properties, which allow them to act as reducing agents, hydrogen donators and singlet oxygen quenchers, as well as their metal chelating abilities (Pereira 2009, Rice Evan 1996, Sanda et al., 2011). Therefore, in the present study four different assays were employed in order to determine and compare the antioxidant properties of selected Syzygium malaccense as well as elucidate their mode of action.
The DPPH assay has been widely used to evaluate the free radical scavenging effectiveness of various antioxidant substances. Antioxidants upon interaction with DPPH either transfer an electron or hydrogen atom to DPPH, thus neutralizing its free radical character. The colour of the reaction mixture changes from purple to yellow with a decrease of the 517 nm absorbance. The degree of discolouration indicates the scavenging potential of the antioxidants [Villano et al., 2007, Foti et al]., 2004]. Figure-1 shows the percent inhibition of DPPH with tested S.malaccense leaf methanolic and aqueous extracts at different concentrations (20-100μg /ml). The higher inhibitory effect was noticed in the methanolic leaf extracts. (78.73% at100μg /ml).The IC50 value of the methanolic extract was 25.74 μg /ml.
Fig 1.
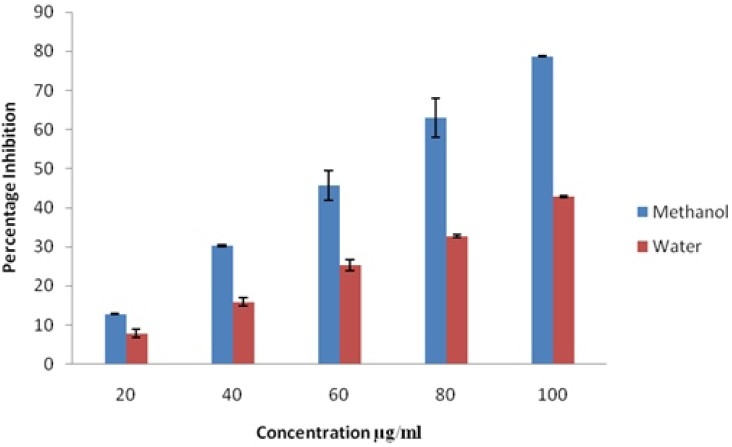
DPPH Scavenging Activity of Methanol and Water Leaf Extracts of Syzygium malaccense
The hydroxyl radical scavenging ability of S.malaccense methanolic and aqueous extracts and a reference antioxidant are presented in the figure- 2. The hydroxyl radical is an extremely reactive oxygen species, capable of modifying several biologically important molecules in the living cells. This radical is able to cause DNA damages leading to carcinogenesis, mutagenesis and cytotoxicity. (Sanda et al., 2011).
Fig 2.
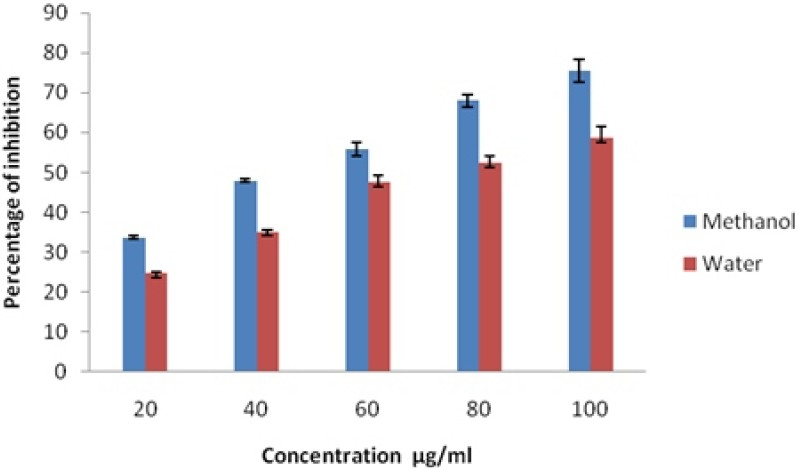
Hydroxyl Radical Scavenging Assay of Methanol and Water Leaf Extracts of Syzygium malaccense
When S. malaccense leaf extract was added to the reaction mixture, it removed the hydroxyl radicals from the sugar and prevented the reaction. The leaf methanolic extracts showed higher inhibitory activities on the hydroxyl radical formation in a concentration dependent manner when compared to the aqueous extract. The IC50 values of the methanolic extract and standard in this assay were 16.25 μg /ml and 7.94 μg /ml respectively. The IC50 value of the aqueous extract is 21.25 μg /ml and the gallic acid is 2.70 μg /ml.
The reducing power assay measures the electron donating ability of antioxidants using the potassium ferricyanide reduction method. Antioxidants cause the reduction of the Fe3+/ferricyanide complex to the ferrous form and activity is measured as the increase in the absorbance at 700 nm. In this assay, the yellow colour of the test solution changes to various shades of green and blue depending on the reducing power of antioxidant samples [Gulcin 2006, 2008 and Prasad et al., 2009].
Figure- 3 shows the plot of reducing power of S.malaccense leaf methanolic and aqueous extracts in comparison with BHA and Gallic acid as a reference antioxidant. At tested concentrations (20-100 μg/mL) all samples possessed the ability to reduce iron(III) ions. The reducing power of the plant extracts increased with increasing concentrations in a strongly linear manner. In this assay, methanolic leaf extract exhibited once again the most powerful effect but lowest effect at 100 μg/mL when compared to Gallic acid.
Fig 3.

Reducing Power of Methanol and Water Leaf Extracts of Syzygium malaccense
Conclusion
Based on the estimation of total polyphenols and antioxidant scavenging assays it could be concluded that methanolic extract of Syzygium malaccense showed most potent antioxidant activity when compared to aqueous leaf extract. Further studies are required to identify the actual chemical constituents and the standardization for further therapeutic effects.24
References
- 1.Aruoma O.I, Halliwell B. Action of hypochlorous acid on the antioxidant protective enzymes superoxide dismutase, catalase and glutathione peroxidase. Biochemical Journal. 1987;248:973–976. doi: 10.1042/bj2480973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunstan A, Noreen Y, Serrano G, Cox PA, Perera P, Bohlin L. Evaluation of some Samoan and Peruvian medicinal plants by prostaglandin biosynthesis and rat ear oedema assays. Journal of Ethnopharmacology. 1997;57(1):35–56. doi: 10.1016/s0378-8741(97)00043-3. [DOI] [PubMed] [Google Scholar]
- 3.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 4.Dai J., Mumper R.J. Plant Phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foti M.C., Daquino C., Geraci C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH radical in alcoholic solutions. J Org Chem. 2004;69:2309–2314. doi: 10.1021/jo035758q. [DOI] [PubMed] [Google Scholar]
- 6.Gülçin İ. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid) Toxicology. 2006;217:213–220. doi: 10.1016/j.tox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Gülçin İ. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Karioti A., Skaltsa H., Gbolade A.A. Analysis of the leaf oil of Syzygium malaccence Merret Perry from Nigeria. Journal of Essential Oil Research. 2007;19(4) [Google Scholar]
- 9.Kokate CK. Practical Pharmacognosy. Vallabh Prakashan; 1999. [Google Scholar]
- 10.Locher O.P., Burch M.T., Mower H.F., Berestecky J., Davis H., VanPoel B., Lasure A., Vanden Berghe D.A., Vlletlnck A.J. Antimicrobial activity and anti-compliment activity of extracts obtained from selected Hawaiian medicinal plants. J. Ethanopharmacol. 1995;49:23–32. doi: 10.1016/0378-8741(95)01299-0. [DOI] [PubMed] [Google Scholar]
- 11.Oyaizu M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986;44:307–315. [Google Scholar]
- 12.Pereira D.M., Valentão P., Pereira J.A., Andrade P.B. Phenolics, From chemistry to biology. Molecules. 2009;14:2202–2211. [Google Scholar]
- 13.Pietta P.G.C., Rice-Evans A, Packer L., editors. Flavonoids in Health and Disease. Dekke: New York; 1998. pp. 61–110. [Google Scholar]
- 14.Pinder R. M., Sandler M. Alcohol, wine and mental health: focus on dementia and stroke. J.Psychopharm. 2004;18:449–456. doi: 10.1177/0269881104047272. [DOI] [PubMed] [Google Scholar]
- 15.Prasad K.N, Yang B., Dong X., Jiang G., Zhang H., Xie H., Jiang Y. Flavonoid contents and antioxidant activities from Cinnamomum species. Innov Food Sci. Emerg. Technol. 2009;10:627–632. [Google Scholar]
- 16.Pullaiah T. Encyclopedia of world medicinal plants. 2006;4 [Google Scholar]
- 17.Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 18.Vladimir-Knežević Sanda, Blažeković Biljana, Štefan Maja Bival, Alegro Antun, Kőszegi Tamás, Petrik József. Antioxidant Activities and Polyphenolic Contents of Three Selected Micromeria Species from Croatia. Molecules. 2011;16:1454–1470. doi: 10.3390/molecules16021454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakanaka S., Tachibana Y., Okada Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinohacha) Food Chemistry. 2005;89:569–575. [Google Scholar]
- 20.Shahidi F., Janitha P.K., Wanasundara P.D. Phenolic antioxidants. Critical Rev. Food Sci. Nutr. 1992;32(1):67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- 21.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin -Ciocalteu Reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 22.Sun A.Y., Simonyi A., Sun G.Y. The “French Paradox” and beyond: neuroprotective effects of polyphenols. Free Rad. Biol. Med. 2002;32:314–318. doi: 10.1016/s0891-5849(01)00803-6. [DOI] [PubMed] [Google Scholar]
- 23.Viana M., Barbas C., Banet B., Bonet M.V., Castro M., Fraile M.U., Herrela L. In vitro effect of a flavonoid-rich extract on LDL oxidation. Athelosclerosis. 1996;123:83–91. doi: 10.1016/0021-9150(95)05763-3. [DOI] [PubMed] [Google Scholar]
- 24.Villaño D., Fernández-Pachón M.S., Moyá M.L., Troncoso A.M., García-Parrilla M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta. 2007;71:230–235. doi: 10.1016/j.talanta.2006.03.050. [DOI] [PubMed] [Google Scholar]


