Introduction
It has been widely accepted that adequate calcium intake is critical for development and maintenance of bone health, particularly in children and elderly adults. In addition to a pivotal role in skeletal metabolism, the potential effects of calcium on non-skeletal health outcomes have received growing attention recently,[1] among which the effect of calcium intake on cardiovascular disease (CVD) is a subject of intense interest. In vitro and in vivo experimental studies provide evidence for the involvement of calcium in multiple physiologic processes that may modify the function or structure of the cardiovascular system. Some, but not all, epidemiological studies report associations between inadequate calcium intake and both an adverse CVD risk factor profile and increased risk of CVD events. Moreover, several completed randomized clinical trials have evaluated the risks and benefits of calcium supplementation on bone mineral density and/or fracture incidence. These trials offer valuable, though preliminary, data regarding possible effects of calcium supplements on risk of CVD. Since many generally healthy US adults take supplemental calcium for bone health,[2] it is important to better understand the balance of risks and benefits related to calcium supplement use. This review covers experimental, epidemiological, and clinical evidence regarding the role of calcium intake in the development of CVD among adults.
Metabolism and Physiologic Significance of Calcium
Calcium is quantitatively the most abundant mineral in the human body. An average adult’s body typically contains about 1.0–1.5 kg calcium, 99% of which resides in bones and teeth. Besides the structural role in skeleton, calcium is a vital electrolyte that is required for many critical biological functions, including muscle contraction, vascular tone, nerve transmission, and many enzyme-mediated processes.[3] Intracellular Ca2+ is typically 103–104 fold lower than the extracellular level. When the cell is stimulated, calcium enters the cell from extracellular compartments to activate the proteins that carry out the proper actions. Upon the completion of response, calcium is pumped outside the cell or into intracellular storage to await the next activation cycle. Calcium homeostasis is tightly controlled by the calciotropic hormones: vitamin D, parathyroid hormone and calcitonin. These hormones regulate calcium absorption from intestine, excretion or re-absorption from kidney, and deposition or release from bone. As a result, circulating concentration of calcium is usually maintained constant in the range of 1.0 to 1.2 mmol/L. Unless there is prolonged and severe calcium deficiency, calcium level in blood is rarely compromised. Excessively high levels of calcium in blood - known as hypercalcemia - rarely occurs due to excessive dietary or supplemental calcium intake, but commonly results from primary hyperparathyroidism or malignancy. Hypercalcemia can cause many medical disorders, such as renal insufficiency, vascular and soft tissue calcification, and kidney stone.
Calcium Intake in the US Population
Since calcium cannot be produced in humans and its excretory conservation is weak, sufficient daily intake is required to maintain adequate calcium supply in each individual. The amount of daily calcium need varies by age. The National Institutes of Health (NIH) recommends a daily intake of 1000 mg for men between 25 and 65 years of age. The same amount is also recommended for women between 25 and 50 years, except for pregnant or lactating women or postmenopausal women not on estrogen replacement therapy, for whom 1,500 mg/day is recommended. For all men and women over 65 years old, the NIH recommends daily calcium intake to be 1,500 mg.[4] The US Institute of Medicine (IOM) recently updated the dietary reference intakes for calcium and recommends intake of 1,000 mg/day for all adults aged 19 through 50 and for men until age 70 and of 1,200 mg/day for women starting at age 51 and both men and women aged >70 years.[5] Calcium-rich foods (milk and other dairy products) are the preferred source of calcium intake due to higher absorption efficiency,[6] while calcium supplementation is an alternative means to reach optimal intake for those who cannot obtain adequate calcium through diet alone.
Despite the well-recognized benefits of calcium on bone health, the daily calcium intake in general US population remains below current recommendations, particularly among elderly adults and women. The mean estimated dietary intake of calcium was 952 and 872 mg/d respectively for men aged 51–70 and ≥71 years and 788 and 750 mg/d respectively for women in the same age groups.[5] More than half of American men older than 50 years and American women in all age groups fail to meet the recommended intake of calcium from food sources; less than 25% of women aged >50 years achieved the recommended level.[5] Because a large number of US adults, mostly older women, take high-dose calcium supplements, it is also necessary to consider the health effects of not only dietary calcium but also supplemental calcium intake.
Potential Effect of Calcium on Cardiovascular Disease Risk
Laboratory studies have shown multiple biological mechanisms through which calcium may affect the risk of developing CVD (Table 1). When consumed in large amounts, calcium binds to fatty acids and bile acids in intestine to form insoluble soaps, subsequently decreases fatty acid absorption and lowers blood cholesterol levels.[7, 8] Dietary calcium also down-regulates activity of renin-angiotensin system,[9] improves sodium-potassium balance,[10] and decreases vascular smooth muscle tone,[11] which all contribute beneficially to blood pressure regulation. High calcium intake suppresses the influx of calcium from outside the cells. In adipocytes, reductions in intracellular calcium inhibit fatty acid synthase and activate lipolysis, potentially leading to an anti-obesity effect.[12] In pancreatic β-cells, insulin secretion is a calcium-dependent process that will be compromised when intracellular calcium is either too high or too low.[13] An optimal range of intracellular calcium is also required for insulin-mediated activities in liver, skeletal muscle, and adipose tissues.[14–17] Maintaining relatively low intracellular calcium levels in these target organs has favorable effects on insulin signal transduction[16, 18] and peripheral insulin sensitivity.[17, 18] In addition, low intracellular calcium inhibits platelet aggregation,[19] attenuates cytokine-induced inflammation,[20] and augments vascular relaxation.[21–23] Lastly, the cardiovascular benefits of greater calcium consumption may also be indirectly mediated through induced activities of the calciotropic hormones.[12] However, excessively high calcium intake may also lead to hypercalcemia and vascular calcification, thereby raising CVD risk.[24]
Table 1.
Potential effects and mechanisms of calcium in pathogenesis of cardiovascular disease
| Effect | Biological Mechanisms |
|---|---|
| Favorable cholesterol changes | Binds to fatty acids and bile acids in intestine to form insoluble soaps, increases lipid excretion, and decreases amount of lipids entering enterohepatic circulation. |
| Blood pressure lowering | Down-regulates activity of renin-angiotensin system, improves sodium-potassium balance, and suppresses vascular smooth muscle tone. |
| Anti-obesity effect | Reduces adipocyte intracellular calcium, inhibits fatty acid synthase, and activates lipolysis. |
| Improvement of insulin secretion | Maintains the balance between extracellular and intracellular calcium pools of pancreatic β cell. |
| Enhancement of insulin sensitivity | Improves insulin signal transduction in primary insulin target tissues, and enhances peripheral insulin sensitivity. |
| Improvement in inflammatory profile | Inhibits cytokine-induced apoptosis. |
| Anti-thrombotic property | Reduces platelet intracellular free calcium load and inhibits platelet aggregation. |
| Augmentation of vasorelaxation | Enhances hyperpolarization by opening of calcium-activated potassium channels, increases sensitivity to nitric oxide, and decreases production of superoxide and vasoconstrictor prostanoids. |
| Vascular calcification | Calcium deposition in atherosclerotic lesions. |
Epidemiologic Studies of Dietary Calcium Intake and Cardiovascular Disease Risk
As reviewed before,[3] ecologic studies and cross-sectional studies found both positive and inverse correlations between higher calcium intake and CVD risk factors, including high blood pressure, dyslipidemia, diabetes, and obesity. Reverse causation is a concern in these studies, and thus prospective cohort studies provide more valuable information in assessing the potential effects of long-term dietary and supplemental calcium intake on subsequent development of CVD (Table 2). Among 34,486 US postmenopausal women in the Iowa Women’s Health Study, reduction in coronary heart disease (CHD) mortality was observed with high calcium intake: the relative risk (RR) of CHD mortality in the highest versus the lowest quartile of calcium intake was 0.67 (95% CI: 0.47–0.94) for total calcium and 0.63 (95% CI: 0.40–0.98) for dietary calcium without supplements.[25] Among 23,366 participants in a population-based cohort of Swedish men, higher dietary calcium was associated with a borderline significant lower rate of CVD mortality during 10 years of follow-up (RR in the highest compared with the lowest tertile of intake: 0.77, 95% CI: 0.58–1.01).[26] However, no such associations between dietary calcium intake and CHD mortality was found in cohorts of Dutch civil servants,[27] US male health professionals,[28] and Japanese men and women.[29] There was also no association between dietary calcium intake and subsequent incidence of total CHD (including nonfatal myocardial infarction (MI) and CHD death) in US,[28] Finnish,[30] and Japanese cohorts.[31]
Table 2.
Summary of prospective observational studies that examined the association between dietary calcium intake and risk of cardiovascular disease
| Source | Country | Study Design |
Study Subjects | Dietary Calcium Intake (mg/day) |
Endpoints | Follow -up |
Main Findings |
|---|---|---|---|---|---|---|---|
| Van der Vijver et al., 1992 [27] | Netherlands | Cohort study | Civil servants, 1583 M & 1508 W, 40–65 y | M: ≤585, 585–1245, >1245 F: ≤445, 445–850, >850 |
CVD & CHD mortality | 28 y | OR for CVD mortality in the lowest vs. highest quintile: M: 1.3 (0.8–1.9), W: 1.1 (0.6–2.0) OR for CHD mortality in the lowest vs. highest quintile: M: 0.9 (0.6–1.6), W: 1.1 (0.5–2.5) |
| Abbott et al., 1996 [32] | United States | Cohort study | Honolulu Heart Program, 3,150 M, 55–68 y | <275, 276–406, 407–605, 606–3109 | Incident stroke | 22 y | RR for thromboembolic stroke in the lowest vs. highest quartile: 1.8 (1.1–2.9) |
| Ascherio et al., 1998 [34] | United States | Cohort study | HPFS, 43,738 M, 40–75 y | 500, 700, 800, 1000, 1400 (medians) | Incident stroke | 8 y | RR for total stroke in the highest vs. lowest quintile: 1.05 (0.72–1.53). |
| Bostick et al., 1999 [25] | United States | Cohort study | IWHS, 34,486 W, 55–69 y | <543, 543–742, 743–1110, >1110 | CHD mortality | 8 y | RR for CHD mortality in the highest vs. lowest quartile: 0.63 (0.40–0.98). |
| Iso et al., 1999 [33] | United States | Cohort study | NHS, 85,764 W, 34–59 y | 393, 543, 670, 829, 1128 (medians) | Incident stroke | 14 y | RR for ischemic stroke excluding nonatherogenic embolic infarction in the highest vs. lowest quintile: 0.73 (0.53–1.01); P trend=0.04. |
| Al-Delaimy et al., 2003 [28] | United States | Cohort study | HPFS, 39,800 M, 40–75 y | 497, 625, 738, 880, 1190 (medians) | Incident CHD | 12 y | RR for total CHD in the highest vs. lowest quintile: 0.93 (0.77–1.14); p trend=0.27. RR for nonfatal MI in the highest vs. lowest quintile: 0.82 (0.65–1.05); p trend=0.09. RR for CHD mortality in the highest vs. lowest quintile: 1.21 (0.85–1.71); p trend=0.53. |
| Marniemi et al., 2005 [30] | Finland | Cohort study | 361M & 394W, 65–99 y, | 1420 (overall mean), tertiles | Incident MI & stroke | 10 y | RR for MI in the highest vs. lowest tertile: 1.14 (0.70–1.84). RR for stroke in the highest vs. lowest tertile: 1.34 (0.70–2.55). |
| Umesawa et al., 2006 [29] | Japan | Cohort study | JACC, 21,068 M & 32,319 W, 40–79 y | M: 250, 363, 449, 536, 665 (medians) F: 266, 379, 462, 545, 667 (medians) |
CVD, CHD, and stroke mortality | 9.6 y | RR for CVD mortality in the highest vs. lowest quintile: M: 0.97 (0.64–1.48); p trend=0.95. W: 1.14 (0.74–1.74); p trend=0.14. RR for CHD mortality in the highest vs. lowest quintile: M: 0.92 (0.37–2.29); p trend=0.43. W: 0.87 (0.31–2.45); p trend=0.50. RR for stroke mortality in the highest vs. lowest quintile: M: 0.68 (0.37–1.26); p trend=0.13. W: 0.94 (0.51–1.72); p trend=0.01. |
| Larsson et al., 2008 [35] | Finland | Cohort study | ATBC Study, 26,556 M, 50–69 y | 876, 1178, 1379, 1581, 1916 (medians) | Incident stroke | 13.6 y | RR for cerebral infarction stroke in the highest vs. lowest quintile: 1.10 (0.98–1.26); p trend=0.09. RR for intracerebral hemorrhagic stroke in the highest vs. lowest quintile: 1.20 (0.87–1.64); p trend=0.23. RR for subarachnoid hemorrhagic stroke in the highest vs. lowest quintile: 1.56 (0.98–2.47); p trend=0.10. |
| Umesawa et al., 2008 [31] | Japan | Cohort study | JPHC Study, 19,947 M & 21,579 W, 40–59 y | 233, 344, 439, 603, 753 (medians) | Incident stroke and CHD | 13 y | RR for total stroke in the highest vs. lowest quintile: 0.71 (0.56–0.89). RR for CHD in the highest vs. lowest quintile: 0.93 (0.58–1.50). |
| Kaluza et al., 2010 [26] | Sweden | Cohort study | Cohort of Swedish Men, 23,366 M, 45–79 years | <1,230, 1,230–1,598, ≥1,599 | CVD mortality | 10 y | HR for CVD mortality in the highest vs. lowest tertile: 0.77 (0.58–1.01), p trend=0.064 |
Abbreviation: M: men; W: women; CVD: cardiovascular disease; CHD: coronary heart disease; MI: myocardial infarction; OR: odds ratio; RR: relative risk; HR: hazard ratio; HPFS: Health Professionals Follow-up Study; IWHS: Iowa Women Health Study; NHS: Nurses’ Health Study; JACC: Japan Collaborative Cohort Study; ATBC: Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; JPHC: Japan Public Health Center Study.
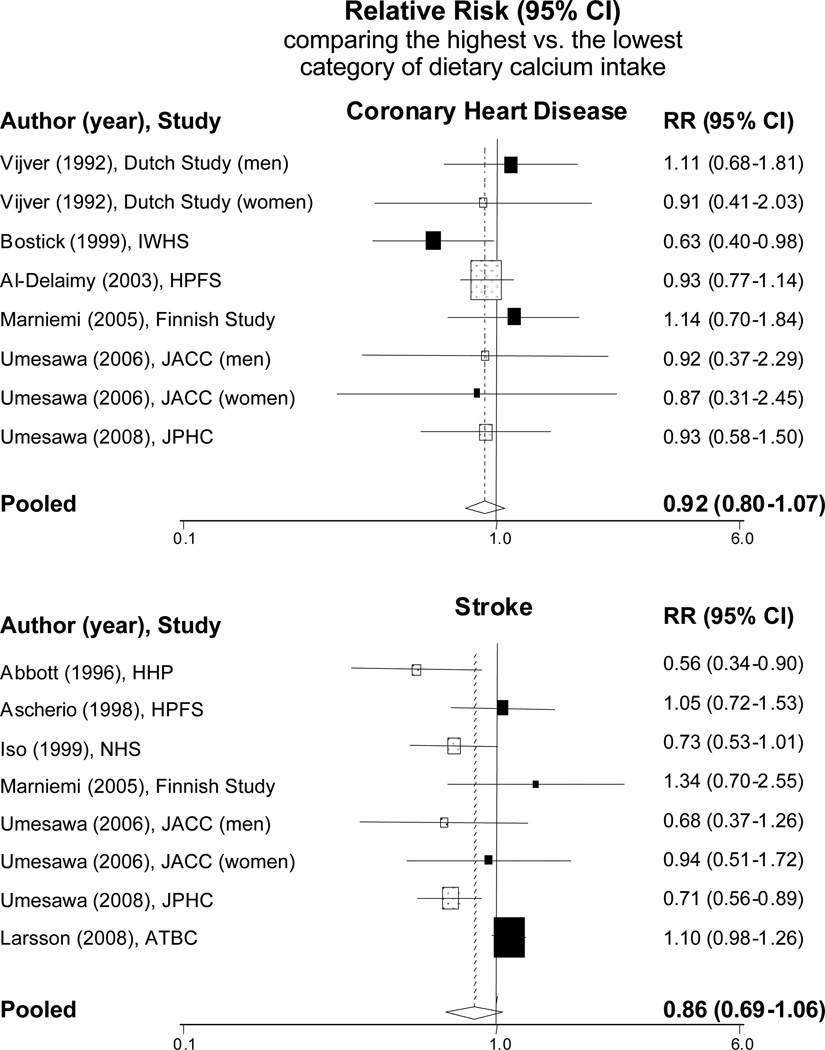
For stroke, in the Honolulu Heart Program, the multivariable RR of thromboembolic stroke in the lowest quartile of dietary calcium intake compared with the highest quartile was 1.8 [95% CI: 1.1–2.9].[32] In the Nurses’ Health Study, women in the highest quintile of dietary calcium had a multivariable RR of incident ischemic stroke of 0.73 [95% CI: 0.53–1.01] compared to those in the lowest quintile (p for trend=0.04).[33] Similar inverse associations between dietary calcium intake and incident stroke were also observed in two Japanese cohorts[29, 31] but not in the Health Professionals Follow-up Study[34] or the Alpha-Tocopherol, Beta-carotene Cancer Prevention Study.[35] When we conducted meta-analyses to combine these data, the pooled RR of CVD comparing the highest to the lowest level of dietary calcium intake was 0.92 [95% CI: 0.80–1.07] for any CHD and 0.86 [95% CI: 0.69–1.06] for any stroke (Figure 1).
Figure 1.
Meta-analysis of prospective observational studies that examined dietary calcium intake in association with risk of cardiovascular disease.
Epidemiologic Studies of Calcium Supplement Use and Cardiovascular Disease Risk
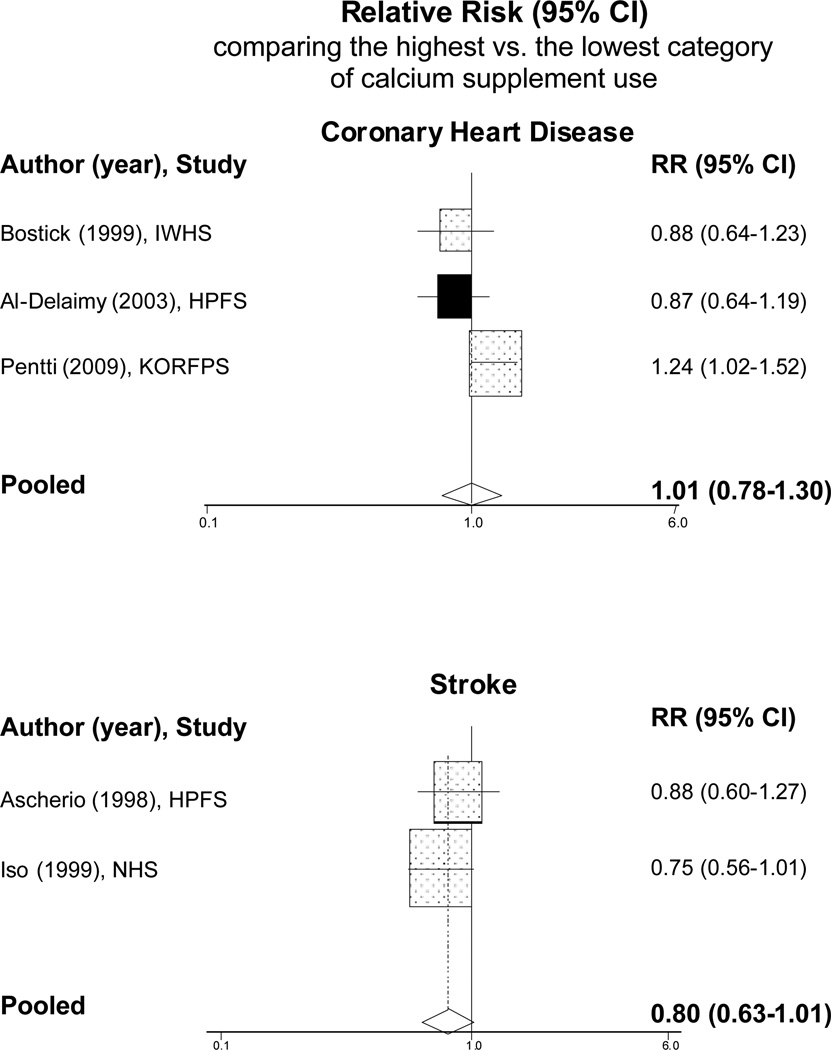
We are aware of five prospective studies that have specifically examined calcium supplement use and risk of CVD, of which four studies found no significant associations (Table 3). In the Health Professionals Follow-up Study, comparing men who took the highest dose of calcium supplement (median of 1000 mg/d) with nonusers, the RRs were 0.87 (95% CI: 0.64–1.19) for total CHD, 1.02 (95% CI: 0.71–1.46) for nonfatal MI, and 0.61 (95% CI: 0.34–1.10) for CHD death;[28] comparing men who took ≥400 mg/d of calcium supplements with nonusers, the RR of stroke was 0.88 [95% CI: 0.60–1.27].[34] In the Iowa Women’s Health Study, the RR of CHD mortality comparing women taking ≥500 mg/d of calcium supplements versus nonusers was 0.88 [95% CI: 0.64–1.23].[25] In the Nurses’ Health Study, the RR of stroke for women taking ≥400 mg/d of calcium supplements versus nonusers was 0.75 [95% CI: 0.56–1.01].[33] One recent study of 10,555 Finnish women, aged 52–62 years old, from the Kuopio Osteoporosis Risk Factor and Prevention Study found an increased risk of CHD with the use of calcium supplements or calcium plus vitamin D supplements. During an average of 6.6 years of follow-up, the multivariable RR of CHD comparing women who used calcium or calcium+vitamin D supplements to nonusers was 1.24 (95% CI: 1.02–1.52).[36] Combining these data, the pooled RR of CVD in the highest versus the lowest dose of calcium supplement use was 1.01 (95% CI: 0.78–1.30) for CHD and 0.80 (95% CI: 0.63–1.01) for stroke (Figure 2). These pooled data show no significant benefits of calcium supplements use in reducing the risk of CHD or stroke.
Table 3.
Summary of prospective observational studies that examined the association between calcium supplement use and risk of cardiovascular disease
| Source | Country | Study Design |
Study Subjects | Calcium Supplement Use |
Endpoints | Follow -up |
Main Findings |
|---|---|---|---|---|---|---|---|
| Ascherio et al., 1998 [34] | United States | Cohort study | HPFS, 43,738 M, 40–75 y | Nonuser, < 400 mg/d, ≥400 mg/d | Incident stroke | 8 y | RR in user ≥400 mg/d. vs. non-users: 0.88 (0.60–1.27) for total stroke 0.83 (0.52–1.34) for ischemic stroke |
| Bostick et al., 1999 [25] | United States | Cohort study | IWHS, 34,486 W, 55–69 y | 0, 1–500, >500 mg/d | CHD mortality | 8 y | RR in users vs. nonusers: 0.76 (0.58–1.00) for 1–500 mg/d, 0.88 (0.64–1.23) for >500 mg/d, P trend=0.46. |
| Iso et al., 1999 [33] | United States | Cohort study | NHS, 85,764 W, 34–59 y | Nonuser, < 400 mg/d, ≥400 mg/d | Incident Stroke | 14 y | RR in user ≥400 mg/d. vs. non-users: 0.75 (0.56–1.01). |
| Al-Delaimy et al., 2003 [28] | United States | Cohort study | HPFS, 39,800 M, 40–75 y | 0, 57, 200, 325, 500, 1000 mg/d (Medians) | Incident CHD | 12 y | RR for total CHD in the highest vs. the lowest quintile of users: 0.87 (0.64–1.19); P trend=0.31. RR for nonfatal MI in the highest vs. the lowest quintile of users: 1.02 (0.71–1.46); P trend=0.84. RR for CHD mortality in the highest vs. the lowest quintile of users: 0.61 (0.34–1.10); P trend=0.05. |
| Pentti et al., 2009 [36] | Finland | Cohort study | Kuopio Osteoporosis Risk Factor and Prevention Study, 10,555 W, 52–62 y | Nonuser, user | Incident CHD | 6.55 y | HR in user vs. non-users: 1.24 (1.02–1.52) in entire cohort 1.26 (1.01–1.57) in postmenopausal women |
Abbreviation: M: men; W: women; CHD: coronary heart disease; MI: myocardial infarction; RR: relative risk; HR: hazard ratio; HPFS: Health Professionals Follow-up Study; IWHS: Iowa Women Health Study; NHS: Nurses’ Health Study.
Figure 2.
Meta-analysis of prospective observational studies that examined calcium supplement use in association with risk of cardiovascular disease.
Epidemiologic Studies of Blood Calcium level and Cardiovascular Disease Risk
Since blood concentration of calcium is controlled by calciotropic hormones, calcium intake only modestly changes blood calcium levels. We found only few studies that examined the association between circulating calcium levels and CVD. In a cohort of 2183 middle-aged Swedish men, serum calcium was an independent risk factor for MI during 18-year follow-up. The estimated risk of MI varied from 0.06 to 0.15 over the range of mean ±2 SDs of serum calcium levels. The odds ratio of MI corresponding to the difference was 2.33 (95% CI: 1.21–4.51).[37] In the ARIC study, by contrast, serum calcium was not associated with risk of CHD but was positively associated with risk of stroke over 12.6 years follow-up. The hazard ratio per 0.4 mg/dL increase in serum calcium was 1.01 (95% CI: 0.96–1.06) for CHD and 1.16 (95% CI: 1.07–1.26) for stroke.[38] These data seem to suggest a possible adverse effect of serum calcium on risk of CVD, but the evidence is limited and weak. In a Finnish cohort study of elderly men and women with follow-up for up to 10 years, higher serum concentrations of calcium was not significantly associated with increased risk of acute MI or stroke.[30]
Effect of Calcium Supplementation on Cardiovascular Disease Outcomes in Randomized Clinical Trials
To our knowledge, no randomized clinical trial has specifically tested the effect of calcium supplementation on CVD as a primary endpoint. However, several trials have considered CVD outcomes in secondary analyses or as adverse events (Table 4). A trial of 930 US men and women documented number of hospitalized events due to CVD during 4 years intervention, and found that similar proportions of participants in the groups of 1200 mg/d calcium supplementation and placebo had cardiac disease (11% vs. 10%) and stroke (3% vs. 2%).[39] A randomized, double-blind, placebo-controlled trial of 1,460 Australian women reported a RR of 1.12 (95%CI: 0.77–1.64) for clinically diagnosed CHD comparing women taking 1200 mg/d of calcium carbonate (given in 2 divided doses) versus those taking placebo.[40] A more recent report from the same trial extended analyses to examine the combined endpoint of mortality or first-hospitalization due to CVD after 5 years of randomized treatment plus 4.5 years of post-trial follow-up. The multivariable RR was 0.94 (95%: 0.69–1.28) at the conclusion of treatment, and 0.92 (95%: 0.74–1.15) after all 9.5 years of follow-up.[41] In another trial in New Zealand, 1,471 healthy postmenopausal women were randomized to take 1g/day of calcium citrate or placebo. After 5 years treatment and follow-up, women in the calcium group experienced more adjudicated MI events and composite CVD endpoints including MI, stroke, and sudden death than women in the placebo group.[42] However, when unreported events identified from the national database of hospital admissions were added, the increased RR in the calcium group was no longer significant (RR=1.49, 95% CI: 0.86–2.57 for MI and RR=1.21, 95% CI: 0.84–1.74 for composite CVD). Finally, a trial of 323 healthy men ≥40 years old from New Zealand randomized participants to take 600 mg/d calcium citrate, 1200 mg/d calcium citrate, or placebo. The composite endpoint of vascular events including angina, MI, sudden death, and coronary revascularization were more common in calcium supplement versus placebo group. Because the vascular event rates were low, however, statistical power to detect any difference was small.[43]
Table 4.
Summary of randomized clinical trials of calcium supplementation (with and without vitamin D) that reported the events of cardiovascular disease.
| Source | Country | Study Subjects | Intervention | Duration | Primary Endpoints |
Main findings on CVD endpoints |
|---|---|---|---|---|---|---|
| Calcium Supplement only | ||||||
| Baron et al, 1999 [39] | U.S. | 672 M&258 W, 61 y (mean) | 1200 mg/d as calcium carbonate Placebo |
4 years | Recurrence of colorectal adenoma | N of hospitalized cardiac events: 50 (11%) in calcium group 46 (10%) in placebo group N of hospitalized stroke vents: 12 (3%) in calcium group 11 (2%) in placebo group |
| Prince et al, 2006 [40] | Australia | 1460 W, >70 y | 1200 mg/d (in 2 divided dose) as calcium carbonate Placebo |
5 years | Clinical fracture, vertebral deformity | RR of diagnosed CHD in intervention vs. placebo: 1.12 (0.77–1.64) |
| Bolland et al, 2008 [42] | New Zealand | 1471 W, postmenopausal, 74 y (mean) | 1000 mg/d as calcium citrate Placebo |
5 years | Fracture incidence, bone density | RR of CVD events in intervention vs. placebo group: 1.49 (0.86–2.57) for MI 1.37 (0.83–2.28) for stroke 0.51 (0.13–2.01) for sudden death 1.21 (0.84–1.74) for composite CVD |
| Reid et al, 2008 [43] | New Zealand | 323 M, ≥40 y | 1200 mg/d and 600 mg/d as calcium citrate Placebo |
2 years | Bone mineral density | Composite vascular events: N=3 in 1200 mg/d calcium group N=2 in 600 mg/d calcium group N=0 in placebo group P=0.24 |
| Calcium + Vitamin D Supplement | ||||||
| Brazier et al, 2005 [44] | France | 192 W, >65 y | 1000 mg/d as calcium carbonate and vitamin D 800 IU/d (in 2 divided dose) Placebo |
1 year | Bone mineral density | N of adverse cardiovascular events: 6 (6.3%) in treatment group 5 (5.2%) in placebo group |
| Hsia et al, 2007 [45] | United States | 36,282 W, postmenopausal, 50–79 y | 1000 mg/d as calcium carbonate and vitamin D3 400 IU/d (in 2 divided dose) Placebo |
7 years | Fracture incidence | RR of CVD events in intervention vs. placebo group: 1.04 (0.92–1.18) for total CHD 1.05 (0.91–1.20) for nonfatal MI 1.01 (0.79–1.29) for CHD death 0.95 (0.82–1.10) for total stroke |
| Lappe et al, 2008 [46] | United States | 1179 healthy W, postmenopausal, >55 y | 1400 mg/d as calcium citrate or 1500 mg/d as calcium carbonate and vitamin D placebo Calcium and 1000 IU vitamin D3/d Double placebos |
4 years | Fracture incidence | Total vascular event rate: 4.76/1000 person-year in calcium group (with and without vitamin D), 6.94 /1000 person-year in placebo group RR=0.69 (not statistically significant) |
Abbreviation: M: men; W: women; CVD: cardiovascular disease; CHD: coronary heart disease; CABG: coronary artery bypass surgery; PCI: percutaneous coronary interventions; MI: myocardial infarction; RR: relative risk.
Amount of calcium provided in the table is elemental calcium dosage.
Some trials also evaluated the cardiovascular effects of combined calcium and vitamin D supplementation. In a multi-center trial conducted in France, 192 elderly women with vitamin D insufficiency (25-dehydroxyvitamin D ≤12 ng/mL) were randomized to receive either a combination tablet containing 1,000 mg calcium carbonate and 800 IU vitamin D3 daily, or placebo tablet. Six patients in active supplement group and five patients in placebo group reported occurrence of cardiovascular event during 1 year of follow-up.[44] Among 36,282 postmenopausal women in the Women’s Health Initiative (WHI), daily supplementation of 1000 mg calcium and 400 IU vitamin D (as 25-dehydroxyvitamin D3) did not alter the risk of CVD during 7 years of follow-up (RR=1.05, 95% CI: 0.91–1.20 for non-fatal MI, RR=1.01, 95% CI: 0.79–1.29 for CHD death, and RR=0.95, 95% CI: 0.82–1.10 for stroke).[45] In another trial of 1,179 community-dwelling healthy postmenopausal women, there was no significant difference in MI or other vascular events after 4 years supplementation with either vitamin D (1000 IU/d) plus calcium (as calcium citrate 1400 mg/d or calcium carbonate 1500 mg/d) or calcium alone compared with placebo.[46] When the two calcium treatment groups were combined, the vascular event rate was 4.76/1000 person-year in supplement group and 6.94/1000 person-year in placebo group.
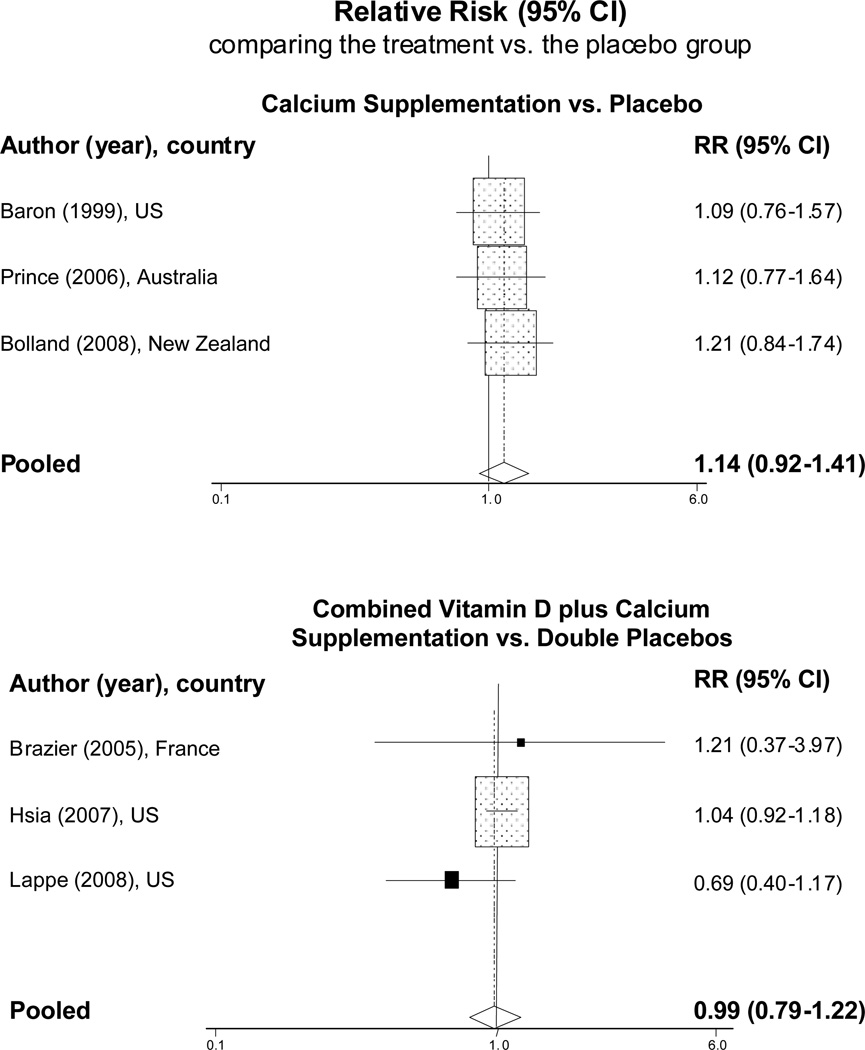
When we combined the data from randomized trials, the pooled RRs of CVD were 1.14 [95% CI: 0.92–1.41] for calcium supplements vs. placebo and 0.99 [95% CI: 0.79–1.22] for combined calcium plus vitamin D supplements vs. double placebos (Figure 3).
Figure 3.
Meta-analysis of randomized controlled trials of calcium supplementation (with and without vitamin D) that reported the cardiovascular events in the treatment group versus respective placebos.
Comments
Experimental studies have demonstrated that calcium is involved in multiple physiologic processes potentially related to development of CVD. There have also been several epidemiologic studies examining dietary and supplemental calcium intake in relation to CVD risk, with considerable heterogeneity in study design, participant characteristics, and potential for confounding. Randomized trials specifically designed to evaluate the cardiovascular effects of short- and long-term calcium supplementation remain lacking; current available data is primarily derived from secondary analyses of existing trials. In assessing the totality of evidence to date, it appears that calcium intake, either from diet or from supplements, has little or no effect on CVD risk.
Pooled analyses combining data from several large-scale, population-based, prospective cohort studies showed a non-significant inverse association of dietary calcium intake with incident CHD, incident stroke, and total CVD. A seemingly stronger association with risk of stroke might be attributed to an established blood pressure-lowering effect of dietary calcium.[47] Fewer studies have examined the association between calcium supplement use and incident CVD. The generally null findings noted to date suggest that calcium supplementation is unlikely to confer a strong effect on CVD risk. The Kuopio Osteoporosis Risk Factor and Prevention Study[36] was the only study that reported a significantly increased risk of CHD among users of calcium supplements versus nonusers.[36] Of note, in this study the mean dietary calcium intake from liquid milk products and cheese was 773.8 mg/day for calcium supplements users and 818.2 mg/day for nonusers. Although the study did not determine the daily dose of calcium supplements, total calcium intake likely exceeded the upper limit of 2000 mg/day by NIH[4] and IOM guidelines[5] for participants with both high habitual dietary and supplemental calcium intake, and these individuals might be at risk for hypercalcemia and its complications. In addition, since this study did not separate calcium and calcium plus vitamin D supplement users, it is not clear whether the increased CHD risk is specifically related to calcium or vitamin D.
There are some discrepancies in our findings on dietary versus supplemental calcium intake with CVD risk. It has been postulated that these discrepancies may be explained in part by their differential impact on circulating calcium.[48] For calcium supplements, usually taken on their own and without food, there would be an immediate and sizable increase in blood calcium concentrations within a couple of hours, and the calciotropic hormone secretion and bone resorption may be affected.[49] In contrast, calcium from foods, typically during a mixed meal, will be absorbed slowly over several hours. This absorption process is less likely to cause detectable change in blood calcium concentration, and the metabolic response will vary according to simultaneous ingestion of other nutrients.[50] Since available studies on blood calcium in association with risk of CVD are very limited and inconclusive, more studies are needed to provide further insight into a complex relation between intake, metabolism, and biologic effect of calcium on pathogenesis of CVD.
Clinical trial data on the effect of calcium supplementation on CVD risk are limited to secondary analyses. Following the first original study that raised concerns about a possible adverse cardiovascular effect of calcium supplementation,[42] a recently published meta-analysis combined data from a total of 15 eligible clinical trials and investigated whether calcium supplements increase the risk of CVD events.[51] In the analysis of 5 trials with patient level data, RR of MI for participants allocated to calcium supplementation compared with those allocated to placebo was 1.31 (95% CI: 1.02–1.67). The corresponding RRs for stroke, composite endpoint of MI, stroke, or sudden death, and all-cause mortality were 1.20 (95% CI: 0.96–1.50), 1.18 (95% CI: 1.00–1.39), and 1.09 (95% CI: 0.96–1.23) respectively. The analysis of trial level data showed similar results, with a pooled RR for MI of 1.27 (95% CI: 1.01–1.59). The authors concluded that calcium supplements (without coadministered vitamin D) are associated with an increased risk of MI and suggested a reassessment of calcium supplement use.
Although the randomized, controlled trial design provides the strongest support for potential causality, post-hoc analyses of secondary endpoints as presented in this meta-analysis should be interpreted with caution. First, none of the included trials was specifically designed to test the effect of calcium supplementation on risk of CVD, and the numbers of CVD events in many trials were too small to draw clinically meaningful conclusions. Second, CVD events were not pre-specified endpoints in most trials and therefore were not systematically ascertained. Only 2 of the 15 trials had CVD events adjudicated by blinded investigators. Third, not a single trial included in this meta-analysis reported a significant difference in CVD events between calcium and placebo groups, but only the pooled RR showed a statistically significant effect. Conclusion of this meta-analysis also heavily depends on unpublished data, which can not be evaluated rigorously. Fourth, the combined trial data seem to suggest that calcium supplements increase the risk of MI, but not the risk of stroke or all-cause mortality. The biological mechanisms explaining this specific effect remain uncertain. Taken these limitations together, currently available evidence from clinical trials does not definitively indicate an adverse effect of calcium supplementation on risk of CVD.
The new report recently released by IOM[5] on dietary reference intakes for calcium and vitamin D cited vast evidence for a role of calcium in promoting skeletal growth and maintenance. However, the evidence for any benefits of calcium beyond bone health remains insufficient. Though the report noted that once intake of calcium surpasses 2000 mg per day for both men and women aged ≥51 years, the risk for harm may increase, it is premature to make definitive statements about the cardiovascular effects associated with high intake of calcium. Our review of epidemiologic studies and clinical trials support the IOM report. Future studies need to include not only more prospective cohorts but also randomized trials specifically designed to evaluate the risks or benefits of calcium supplementation on CVD endpoints as the primary pre-specified outcome.
Acknowledgement
The authors thank the technical assistance from Dr. Yiqing Song for his scientific input throughout the meta-analysis. Dr. Wang was supported by a career grant HL095649 from the National Institutes of Health, Bethesda, MD, USA.
Reference
- 1.Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005 May;35(5):290–304. doi: 10.1111/j.1365-2362.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 2.Subar AF, Block G. Use of vitamin and mineral supplements: demographics and amounts of nutrients consumed. The 1987 Health Interview Survey. Am J Epidemiol. 1990 Dec;132(6):1091–1101. doi: 10.1093/oxfordjournals.aje.a115752. [DOI] [PubMed] [Google Scholar]
- 3.Vaskonen T. Dietary minerals and modification of cardiovascular risk factors. J Nutr Biochem. 2003 Sep;14(9):492–506. doi: 10.1016/s0955-2863(03)00074-3. [DOI] [PubMed] [Google Scholar]
- 4.NIH Consensus conference. Optimal calcium intake. NIH Consensus Development Panel on Optimal Calcium Intake. JAMA. 1994 Dec 28;272(24):1942–1948. [PubMed] [Google Scholar]
- 5.Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press; 2010. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine. [Google Scholar]
- 6.Bennett T, Desmond A, Harrington M, McDonagh D, FitzGerald R, Flynn A, et al. The effect of high intakes of casein and casein phosphopeptide on calcium absorption in the rat. Br J Nutr. 2000 Jun;83(6):673–680. doi: 10.1017/s0007114500000854. [DOI] [PubMed] [Google Scholar]
- 7.Fleischman AI, Yacowitz H, Hayton T, Bierenbaum ML. Effects of dietary calcium upon lipid metabolism in mature male rats fed beef tallow. J Nutr. 1966 Mar;88(3):255–260. doi: 10.1093/jn/88.3.255. [DOI] [PubMed] [Google Scholar]
- 8.Yacowitz H, Fleischman AI, Bierenbaum ML, Kritchevsky D. Calcium and lipid metabolism: effects of increased dietary calcium on atherosclerosis in rabbits. Trans N Y Acad Sci. 1971 Mar;33(3):344–350. doi: 10.1111/j.2164-0947.1971.tb02602.x. [DOI] [PubMed] [Google Scholar]
- 9.Resnick LM, Laragh JH, Sealey JE, Alderman MH. Divalent cations in essential hypertension. Relations between serum ionized calcium, magnesium, and plasma renin activity. N Engl J Med. 1983 Oct 13;309(15):888–891. doi: 10.1056/NEJM198310133091504. [DOI] [PubMed] [Google Scholar]
- 10.Resnick LM. The role of dietary calcium in hypertension: a hierarchical overview. Am J Hypertens. 1999 Jan;12(1 Pt 1):99–112. doi: 10.1016/s0895-7061(98)00275-1. [DOI] [PubMed] [Google Scholar]
- 11.Bohr DF. Vascular Smooth Muscle: Dual Effect of Calcium. Science. 1963 Feb 15;139(3555):597–599. doi: 10.1126/science.139.3555.597. [DOI] [PubMed] [Google Scholar]
- 12.Zemel MB. Calcium modulation of hypertension and obesity: mechanisms and implications. J Am Coll Nutr. 2001 Oct;20(5 Suppl):428S–435S. doi: 10.1080/07315724.2001.10719180. discussion 40S–42S. [DOI] [PubMed] [Google Scholar]
- 13.Draznin B. Intracellular calcium, insulin secretion, and action. Am J Med. 1988 Nov 28;85(5A):44–58. doi: 10.1016/0002-9343(88)90397-x. [DOI] [PubMed] [Google Scholar]
- 14.Ojuka EO. Role of calcium and AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. Proc Nutr Soc. 2004 May;63(2):275–278. doi: 10.1079/PNS2004339. [DOI] [PubMed] [Google Scholar]
- 15.Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes. 2004 Feb;53(2):330–335. doi: 10.2337/diabetes.53.2.330. [DOI] [PubMed] [Google Scholar]
- 16.Williams PF, Caterson ID, Cooney GJ, Zilkens RR, Turtle JR. High affinity insulin binding and insulin receptor-effector coupling: modulation by Ca2+ Cell Calcium. 1990 Sep;11(8):547–556. doi: 10.1016/0143-4160(90)90031-o. [DOI] [PubMed] [Google Scholar]
- 17.Draznin B, Sussman K, Kao M, Lewis D, Sherman N. The existence of an optimal range of cytosolic free calcium for insulin-stimulated glucose transport in rat adipocytes. J Biol Chem. 1987 Oct 25;262(30):14385–14388. [PubMed] [Google Scholar]
- 18.Zemel MB. Nutritional and endocrine modulation of intracellular calcium: implications in obesity, insulin resistance and hypertension. Mol Cell Biochem. 1998 Nov;188(1–2):129–136. [PubMed] [Google Scholar]
- 19.Renaud S, Ciavatti M, Thevenon C, Ripoll JP. Protective effects of dietary calcium and magnesium on platelet function and atherosclerosis in rabbits fed saturated fat. Atherosclerosis. 1983 May;47(2):187–198. doi: 10.1016/0021-9150(83)90154-5. [DOI] [PubMed] [Google Scholar]
- 20.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007 Jun;92(6):2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makynen H, Kahonen M, Wu X, Arvola P, Porsti I. Endothelial function in deoxycorticosterone-NaCl hypertension: effect of calcium supplementation. Circulation. 1996 Mar 1;93(5):1000–1008. doi: 10.1161/01.cir.93.5.1000. [DOI] [PubMed] [Google Scholar]
- 22.Tolvanen JP, Makynen H, Wu X, Hutri-Kahonen N, Ruskoaho H, Karjala K, et al. Effects of calcium and potassium supplements on arterial tone in vitro in spontaneously hypertensive rats. Br J Pharmacol. 1998 May;124(1):119–128. doi: 10.1038/sj.bjp.0701810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jolma P, Kalliovalkama J, Tolvanen JP, Koobi P, Kahonen M, Hutri-Kahonen N, et al. High-calcium diet enhances vasorelaxation in nitric oxide-deficient hypertension. Am J Physiol Heart Circ Physiol. 2000 Sep;279(3):H1036–H1043. doi: 10.1152/ajpheart.2000.279.3.H1036. [DOI] [PubMed] [Google Scholar]
- 24.Seely S. Is calcium excess in western diet a major cause of arterial disease? Int J Cardiol. 1991 Nov;33(2):191–198. doi: 10.1016/0167-5273(91)90346-q. [DOI] [PubMed] [Google Scholar]
- 25.Bostick RM, Kushi LH, Wu Y, Meyer KA, Sellers TA, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to ischemic heart disease mortality among postmenopausal women. Am J Epidemiol. 1999 Jan 15;149(2):151–161. doi: 10.1093/oxfordjournals.aje.a009781. [DOI] [PubMed] [Google Scholar]
- 26.Kaluza J, Orsini N, Levitan EB, Brzozowska A, Roszkowski W, Wolk A. Dietary calcium and magnesium intake and mortality: a prospective study of men. Am J Epidemiol. Apr 1;171(7):801–807. doi: 10.1093/aje/kwp467. [DOI] [PubMed] [Google Scholar]
- 27.Van der Vijver LP, van der Waal MA, Weterings KG, Dekker JM, Schouten EG, Kok FJ. Calcium intake and 28-year cardiovascular and coronary heart disease mortality in Dutch civil servants. Int J Epidemiol. 1992 Feb;21(1):36–39. doi: 10.1093/ije/21.1.36. [DOI] [PubMed] [Google Scholar]
- 28.Al-Delaimy WK, Rimm E, Willett WC, Stampfer MJ, Hu FB. A prospective study of calcium intake from diet and supplements and risk of ischemic heart disease among men. Am J Clin Nutr. 2003 Apr;77(4):814–818. doi: 10.1093/ajcn/77.4.814. [DOI] [PubMed] [Google Scholar]
- 29.Umesawa M, Iso H, Date C, Yamamoto A, Toyoshima H, Watanabe Y, et al. Dietary intake of calcium in relation to mortality from cardiovascular disease: the JACC Study. Stroke. 2006 Jan;37(1):20–26. doi: 10.1161/01.STR.0000195155.21143.38. [DOI] [PubMed] [Google Scholar]
- 30.Marniemi J, Alanen E, Impivaara O, Seppanen R, Hakala P, Rajala T, et al. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis. 2005 Jun;15(3):188–197. doi: 10.1016/j.numecd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Umesawa M, Iso H, Ishihara J, Saito I, Kokubo Y, Inoue M, et al. Dietary calcium intake and risks of stroke, its subtypes, and coronary heart disease in Japanese: the JPHC Study Cohort I. Stroke. 2008 Sep;39(9):2449–2456. doi: 10.1161/STROKEAHA.107.512236. [DOI] [PubMed] [Google Scholar]
- 32.Abbott RD, Curb JD, Rodriguez BL, Sharp DS, Burchfiel CM, Yano K. Effect of dietary calcium and milk consumption on risk of thromboembolic stroke in older middle-aged men. The Honolulu Heart Program. Stroke. 1996 May;27(5):813–818. doi: 10.1161/01.str.27.5.813. [DOI] [PubMed] [Google Scholar]
- 33.Iso H, Stampfer MJ, Manson JE, Rexrode K, Hennekens CH, Colditz GA, et al. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke. 1999 Sep;30(9):1772–1779. doi: 10.1161/01.str.30.9.1772. [DOI] [PubMed] [Google Scholar]
- 34.Ascherio A, Rimm EB, Hernan MA, Giovannucci EL, Kawachi I, Stampfer MJ, et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation. 1998 Sep 22;98(12):1198–1204. doi: 10.1161/01.cir.98.12.1198. [DOI] [PubMed] [Google Scholar]
- 35.Larsson SC, Virtanen MJ, Mars M, Mannisto S, Pietinen P, Albanes D, et al. Magnesium, calcium, potassium, and sodium intakes and risk of stroke in male smokers. Arch Intern Med. 2008 Mar 10;168(5):459–465. doi: 10.1001/archinte.168.5.459. [DOI] [PubMed] [Google Scholar]
- 36.Pentti K, Tuppurainen MT, Honkanen R, Sandini L, Kroger H, Alhava E, et al. Use of calcium supplements and the risk of coronary heart disease in 52–62-year-old women: The Kuopio Osteoporosis Risk Factor and Prevention Study. Maturitas. 2009 May 20;63(1):73–78. doi: 10.1016/j.maturitas.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Lind L, Skarfors E, Berglund L, Lithell H, Ljunghall S. Serum calcium: a new, independent, prospective risk factor for myocardial infarction in middle-aged men followed for 18 years. J Clin Epidemiol. 1997 Aug;50(8):967–973. doi: 10.1016/s0895-4356(97)00104-2. [DOI] [PubMed] [Google Scholar]
- 38.Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008 Sep;156(3):556–563. doi: 10.1016/j.ahj.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999 Jan 14;340(2):101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 40.Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med. 2006 Apr 24;166(8):869–875. doi: 10.1001/archinte.166.8.869. [DOI] [PubMed] [Google Scholar]
- 41.Lewis JR, Calver J, Zhu K, Flicker L, Prince RL. Calcium supplementation and the risks of atherosclerotic vascular disease in older women: results of a 5-year RCT and a 4.5-year follow-up. J Bone Miner Res. Jul 7; doi: 10.1002/jbmr.176. [DOI] [PubMed] [Google Scholar]
- 42.Bolland MJ, Barber PA, Doughty RN, Mason B, Horne A, Ames R, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. Bmj. 2008 Feb 2;336(7638):262–266. doi: 10.1136/bmj.39440.525752.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reid IR, Ames R, Mason B, Reid HE, Bacon CJ, Bolland MJ, et al. Randomized controlled trial of calcium supplementation in healthy, nonosteoporotic, older men. Arch Intern Med. 2008 Nov 10;168(20):2276–2282. doi: 10.1001/archinte.168.20.2276. [DOI] [PubMed] [Google Scholar]
- 44.Brazier M, Grados F, Kamel S, Mathieu M, Morel A, Maamer M, et al. Clinical and laboratory safety of one year's use of a combination calcium + vitamin D tablet in ambulatory elderly women with vitamin D insufficiency: results of a multicenter, randomized, double-blind, placebo-controlled study. Clin Ther. 2005 Dec;27(12):1885–1893. doi: 10.1016/j.clinthera.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 45.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007 Feb 20;115(7):846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 46.Lappe JM, Heaney RP. Calcium supplementation: Results may not be generalisable. Bmj. 2008 Feb 23;336(7641):403. doi: 10.1136/bmj.39493.476667.1F. author reply 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarron DA, Reusser ME. Finding consensus in the dietary calcium-blood pressure debate. J Am Coll Nutr. 1999 Oct;18(5 Suppl):398S–405S. doi: 10.1080/07315724.1999.10718904. [DOI] [PubMed] [Google Scholar]
- 48.Reid IR, Bolland MJ. Calcium supplementation and vascular disease. Climacteric. 2008 Aug;11(4):280–286. doi: 10.1080/13697130802229639. [DOI] [PubMed] [Google Scholar]
- 49.Reid IR, Schooler BA, Hannan SF, Ibbertson HK. The acute biochemical effects of four proprietary calcium preparations. Aust N Z J Med. 1986 Apr;16(2):193–197. doi: 10.1111/j.1445-5994.1986.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 50.Green JH, Booth C, Bunning R. Postprandial metabolic responses to milk enriched with milk calcium are different from responses to milk enriched with calcium carbonate. Asia Pac J Clin Nutr. 2003;12(1):109–119. [PubMed] [Google Scholar]
- 51.Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. Bmj. 341:c3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]