Abstract
Adaptive immunity, a vertebrate specialization, adds memory and exquisite specificity to the basic innate immune responses present in invertebrates while conserving metabolic resources. In adaptive immunity, antigenic challenge requires extremely rapid proliferation of rare antigen-specific lymphocytes to produce large, clonally expanded effector populations that neutralize pathogens. Rapid proliferation and resulting clonal expansion are dependent on CD98, a protein whose well-conserved orthologs appear restricted to vertebrates. Thus, CD98 supports lymphocyte clonal expansion to enable protective adaptive immunity, an advantage that could account for the presence of CD98 in vertebrates. CD98 supports lymphocyte clonal expansion by amplifying integrin signals that enable proliferation and prevent apoptosis. These integrin-dependent signals can also provoke cancer development and invasion, anchorage-independence and the rapid proliferation of tumor cells. CD98 is highly expressed in many cancers and contributes to formation of tumors in experimental models. Strikingly, vertebrates, which possess highly conserved CD98 proteins, CD98-binding integrins and adaptive immunity, also display propensity towards invasive and metastatic tumors. In this Commentary, we review the roles of CD98 in lymphocyte biology and cancer. We suggest that the CD98 amplification of integrin signaling in adaptive immunity provides survival benefits to vertebrates, which, in turn, bear the price of increased susceptibility to cancer.
Key words: CD98, Cell adhesion, Cell proliferation, Immunity, Integrin
Introduction
The crucial role of clonal expansion in vertebrate adaptive immunity
All multicellular eukaryotic organisms initiate immune responses after recognizing common pathogen-associated molecular patterns (PAMPs) on the surface of invading microbes (Medzhitov and Janeway, 1997). Pattern recognition receptors (PRRs), including toll-like receptors (TLRs), nucleotide oligomerization domain (NOD)-like receptors (NLRs) and retinoic-acid-inducible gene I (RIG-I)-like receptors (RLRs; RIG-I is also known as DDX58), generate signals that activate ‘innate’ immune cells (Kumar et al., 2011) to neutralize or destroy viruses and bacteria either through reactive chemicals [reactive oxygen species (ROS), reactive nitrogen species (RNS) etc.], complement or enzymatic digestion (lysozyme, etc.) (Tosi, 2005; Leclerc and Reichhart, 2004). This process must be repeated every time the organism is exposed to the same pathogen. However, many pathogens that infect higher organisms can overcome innate immunity and thus maintain infections that result in serious damage to the host.
Vertebrates utilize an additional powerful weapon against pathogens, namely, adaptive immunity. Adaptive immune responses provide long-lasting, flexible and specific protection to a wider variety of pathogens than innate immunity alone (Boehm, 2011). Lymphocytes or lymphocyte-like cells are the central adaptive immune cell. They each express unique antigen receptors of a single specificity on their surface. As they develop from lymphoid progenitor cells in the bone marrow, lymphocytes randomly rearrange gene segments that encode three antigen receptor components (V, D and J) through the action of recombination-activating gene (RAG) enzymes (Hsu, 2009). This recombination mechanism generates tremendous diversity (Kuby, 1997) because there are many alleles for each V, D or J gene segment [or variable lymphocyte receptor (VLR)-A and VLR-B gene regions (Saha et al., 2010; Herrin and Cooper, 2010) in the case of agnathans (jawless fish), discussed below], with random nucleotide addition by terminal deoxynucleotidyl transferase (TdT) also adding junctional diversity.
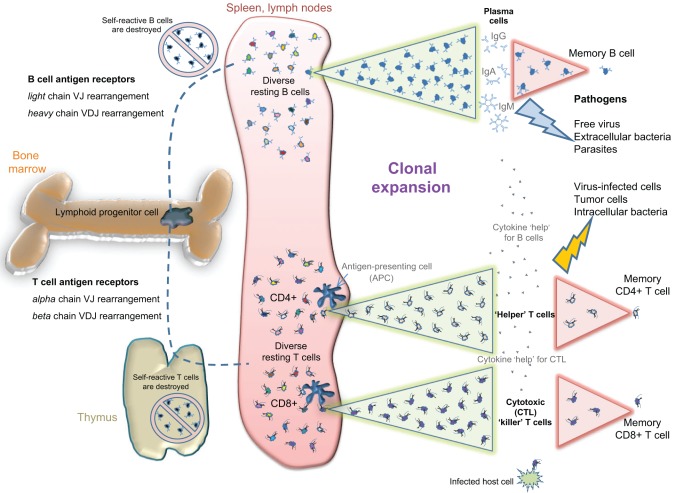
Possessing such a large repertoire of randomly generated antigen specificities has obvious benefits (Murphy et al., 2011). First, the greater diversity of antigen receptors enables the neutralization of a correspondingly larger diversity of pathogens and makes evasion of their neutralizing effects more challenging for pathogens. Second, greater diversity combined with efficient deletion of self-reactive cells (Fig. 1) enables more specific targeting of pathogens rather than host tissues. Third, increased specificity combined with expansion of pathogen-specific lymphocytes allows for formation of specific recall or ‘memory’ responses (Sprent and Surh, 2002). Maintaining a few memory cells that rapidly expand in response to previously experienced antigen conserves resources that would otherwise be expended in maintaining a large population of cells that encompass the entire repertoire of recognition specificities (Murphy et al., 2011). The stimulation of this memory mechanism by vaccines underlies the protection that has saved millions of lives. For these reasons, the vertebrate immune system is usually viewed as more complex and potent than that of invertebrates with respect to diversity, specificity and memory (Boehm, 2011). These benefits depend on the rapid proliferation of lymphocytes that results in clonal expansion, which thus functions as a keystone of adaptive immunity (Fig. 1), allowing vertebrates to take advantage of the striking specificity and diversity of adaptive immunity, while conserving metabolic resources.
Fig. 1.
Vertebrate adaptive immunity. Antigen diversity in jawed vertebrates is generated by somatic recombination of the V, D and J regions (variable, diversity and joining immunoglobulin gene regions) in both immunoglobulin (Ig, B-cell receptor) and TCR (T-cell receptor) chains. After their development in bone marrow (and thymus for T cells), the clones that express self-reactive antigen receptors are deleted. Very few mature resting B and T cells are maintained for each specific antigen (indicated with different colors), thus conserving valuable metabolic resources. Upon antigen exposure in the periphery, the appropriate lymphocyte clone(s) are expanded quickly through rapid proliferation to generate large numbers of antigen-specific effector cells (clonal expansion, illustrated by the green triangles). Effector B cells secrete soluble receptors (antibodies, i.e. IgG, IgA and IgM) that can neutralize or eliminate extracellular pathogens. Cytotoxic (CD8+) T cells scan host cells for the presence of virus or intracellular bacteria, killing any cells that are infected. Helper (CD4+) T cells secrete cytokines that boost both cytotoxic T cells and effector B cells for efficient class-switched antibody responses. Most effector cells die after pathogen clearance, leaving only small numbers of memory B and T cells that are poised for a more rapid clonal expansion upon a secondary exposure (illustrated by the pink triangles). Thus, adaptive immunity utilizes clonal expansion to provide long-lasting specific protection against a wide variety of pathogens while minimizing nutrient cost to the host. IgG, immunoglobulin γ; IgA, immunoglobulin α; IgM, immunoglobulin μ.
Clonal expansion requires resting lymphocytes to enter the cell cycle rapidly upon receiving the appropriate activation signals from antigen receptors, co-receptors, co-stimulatory molecules and cytokines. Extremely rapid cellular proliferation must then follow (with doubling times of less than 5 hours) in order to differentiate and generate sufficient effector cells in time (typically in less 5 days) (Abbas, 2003). For B cells, this rapid proliferation phase can also occur several times, with accompanying somatic hypermutation of receptor genes to increase affinity for antigen, and thus can last up to 3 weeks (Murphy et al., 2011). Both B and T cell clonal expansion rely on the capacity of lymphocytes to accelerate from a resting state to sustained rapid proliferation. Several molecules and mechanisms assist in this process, including growth cytokines or receptors (Nelson and Willerford, 1998; Weaver et al., 2007), adhesion molecules (Mitchell and Williams, 2010), anti-apoptotic proteins (Hildeman et al., 2007) and the vertebrate-specific transmembrane protein CD98 (Cantor et al., 2009; Cantor et al., 2011).
CD98: more than a lymphocyte activation marker
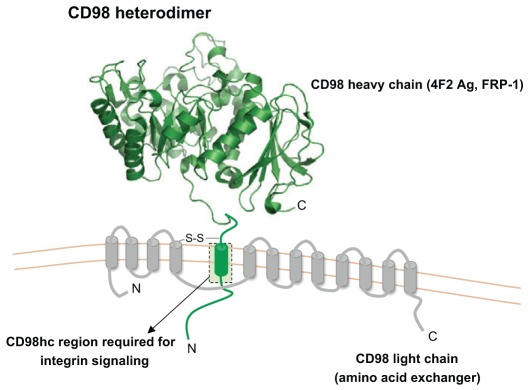
The CD98 heterodimer consists of a type II single-pass transmembrane heavy chain (CD98hc, also known as 4F2 antigen heavy chain or FRP-1; encoded by the genes SLC3A2 and Slc3a2 for human and mouse, respectively) of ~80–85 kDa that is disulfide-linked with a multi-pass light chain of ~40 kDa (Deves and Boyd, 2000) (Fig. 2). Well-conserved orthologues of CD98 are expressed in both jawed and jawless vertebrates but not in invertebrates (Uinuk-Ool et al., 2002). Heterodimeric amino acid transporters containing a single-pass transmembrane heavy chain have been found in Drosophila and in schistosomes, but these do not appear to have all the functions of CD98 described below (Krautz-Peterson et al., 2007; Reynolds et al., 2009). CD98hc was discovered in 1981 when anti-leukocyte monoclonal antibodies were prepared under the theory that “lymphocyte differentiation antigens exist and reflect various immunoregulatory functions” (Haynes et al., 1981b). The 4F2 monoclonal antibody (mAb) bound to all monocytes, weakly to resting lymphocytes, but strongly to activated human B and T cells (Haynes et al., 1981b), and the 4F2 antigen comprises an ~80–85-kDa heavy chain and a ~40-kDa light chain (Hemler and Strominger, 1982). Mouse leukocytes express a similar protein (Bron et al., 1986; Quackenbush et al., 1986), and the human and mouse 4F2 antigens were given the systematic CD designation CD98, with 4F2 mAb recognizing CD98hc (Gottesdiener et al., 1988; Lumadue et al., 1987; Quackenbush et al., 1987). Following its discovery, CD98 was used as an activation marker for both B and T cells during normal and disease states (Hafler et al., 1985; Kehrl et al., 1984; Konttinen et al., 1985; Moretta et al., 1981; Patterson et al., 1984). CD98hc was also designated ‘fusion regulatory protein’ (FRP-1) to reflect its function in cell fusion events that lead to multinucleated giant cells such as osteoclasts or in viral-induced syncitia (Mori et al., 2001; Mori et al., 2004; Ohgimoto et al., 1996; Suga et al., 1997; Tsurudome and Ito, 2000).
Fig. 2.
Schematic illustration of CD98. The CD98 heavy chain (known as CH98hc, 4F2 Ag or FRP-1) is encoded by the Slc3a2 gene in mice and SLC3A2 in humans. CD98hc is a type II transmembrane protein with a large, heavily glycosylated extracellular domain, and a short transmembrane domain and cytoplasmic tail. The extracellular, or ecto-, domain was crystallized, and a ribbon representation of the structure is shown (Fort et al., 2007). The CD98 heterodimer is formed by disulfide bonds between the membrane-proximal section of the CD98hc extracellular domain and any one of at least six possible CD98 light chains (the amino acid transporters LAT-1 or LAT-2, etc.). The integrin signaling function of CD98hc is dependent on the transmembrane and cytoplasmic domains. CD98 is an unusual protein in that it combines adhesive signaling and amino acid transport functions.
CD98 has two known biochemical functions (Fenczik et al., 2001). The heavy chain binds to cytoplasmic tails of integrin-β chains (Miyamoto et al., 2003; Prager et al., 2007; Zent et al., 2000) and mediates adhesive signals that control cell spreading, survival and growth (Fenczik et al., 1997; Feral et al., 2005; Rintoul et al., 2002; Zent et al., 2000). The CD98 light chain can be any one of six permease-type amino acid transporters and is bound to CD98hc by disulfide bond. The light chain functions in amino acid transport (Deves and Boyd, 2000; Verrey, 2003); some of the light chains have broad specificity, but the large neutral amino acid transporters LAT-1 and LAT-2 (encoded by SLC7A6 and SLC7A8), which are the best-studied, have preference for importing certain essential amino acids, particularly leucine, isoleucine and arginine (LAT-1), in exchange for glutamine (Bertran et al., 1992; Palacin, 1994; Pineda et al., 1999; Torrents et al., 1998; Verrey, 2003; Verrey et al., 2000). Indeed, through this nutrient function, CD98 can contribute to the survival and growth of many cell types (Cho et al., 2004; Reynolds et al., 2007). Importantly, surface expression of the light chain is dependent on the presence of the heavy chain, whereas the isolated heavy chain can be expressed without light chains (Cantor et al., 2009; Cantor et al., 2011; Mastroberardino et al., 1998; Teixeira et al., 1987). Thus, CD98hc functions in amplifying integrin signaling and in the transport of amino acids; both of these functions can contribute to cell survival and proliferation. This Commentary will summarize how CD98 enables vertebrate adaptive immunity through support of clonal expansion; however, CD98 also contributes to pathological clonal expansion in the form of cancer.
CD98 in adaptive immunity
Discovery of CD98 in immune cells
Although CD98 was first identified in lymphocytes (Haynes et al., 1981b), its function in immunity has only recently been established. Early studies using antibody blockade and/or CD98 crosslinking suggested that CD98 could function in B and T cell activation, proliferation or effector functions (Diaz et al., 1997; Freidman et al., 1994; Gerrard et al., 1984; Komada et al., 2006; Nakao et al., 1993; Spagnoli et al., 1991; Warren et al., 1996), perhaps by acting as a type of co-stimulatory receptor. However, depending on the conditions used, the effects on proliferation varied (Gerrard et al., 1984). The role of CD98 in integrin signaling, cell survival and proliferation in non-lymphoid cells (Fenczik et al., 1997; Feral et al., 2005; Rintoul et al., 2002; Zent et al., 2000), and in amino acid transport (Bertran et al., 1992; Palacin, 1994; Pineda et al., 1999; Torrents et al., 1998; Verrey, 2003; Verrey et al., 2000), provoked studies that examined the biochemistry and functions of CD98 in lymphocytes upon selective deletion of the Slc3a2 gene followed by reconstitution with mutants that support only one of the biochemical functions of CD98.
CD98 in T lymphocyte function
To examine the function of CD98hc in adaptive immunity, our group generated a conditional knockout for CD98hc by flanking part of the Slc3a2 gene with LoxP sites using homologous recombination in mouse embryonic stem cells (Feral et al., 2007), and bred it with dLck-Cre mice (Zhang et al., 2005), in which Cre recombinase deletes CD98 in the late single-positive stage in both CD4+ and CD8+ T cell lineages (Cantor et al., 2011). CD98hc-null T cells populate secondary lymphoid tissue in normal numbers, form immunological synapses with antigen-presenting cells (APC) normally and are activated by antigen to express activation markers. These cells have moderately impaired homeostatic proliferation in vivo but exhibit markedly defective polyclonal and monoclonal antigen receptor-driven proliferation both in vitro and in vivo (Cantor et al., 2011). Effector functions such as cytokine secretion and cytotoxic target lysis appear to be normal. These data establish a crucial role for CD98hc in the clonal expansion stage of T cell immune responses and show that CD98hc is required for T-cell-dependent adaptive immunity. Given that clonally expanding T cells would have increased metabolic demands, one might expect amino acid transport to be the crucial function of CD98 in T cells. However, reconstitution of CD98hc-null T cells with CD98 mutants shows that the portion of CD98hc required for amino acid transport is not absolutely required, but that the region that is involved in integrin signaling is crucial for T cell proliferation (Cantor et al., 2011). The importance of CD98hc in T cell immune responses is also seen in autoimmune disease. Here, loss of T cell CD98 prevents disease development in two models of T-cell-mediated autoimmunity, type 1 diabetes (T1D) and multiple sclerosis (Cantor et al., 2011). This raises the possibility that CD98hc could serve as a therapeutic target for blocking inappropriate adaptive immune responses.
On the basis of these recent observations, several questions can now be raised regarding CD98hc function in T cells, such as what are the effects an antigen-activated CD98-null T cell might have on the overall immune response? Cytokine secretion and the capacity to differentiate to regulatory-type Foxp3+ T cells are still intact in the absence of CD98hc (Cantor et al., 2011). The ability of a regulatory T cell to suppress inflammatory T cell responses might be less dependent on clonal expansion than the effector functions of an inflammatory subset (Gavin and Rudensky, 2002; Takahashi et al., 1998; Taams et al., 1998). Thus, there is the potential to modulate the quality of an immune response through interfering with CD98hc function. The second question is how partial or transient loss of CD98hc might differ from its complete deletion. Another unresolved issue is whether T cells require CD98hc for secondary responses to the same pathogen. In other words, what is the function of CD98hc in memory CD4 or CD8 T cells? This might have implications in designing intervention strategies that target CD98hc.
CD98 in B lymphocyte function
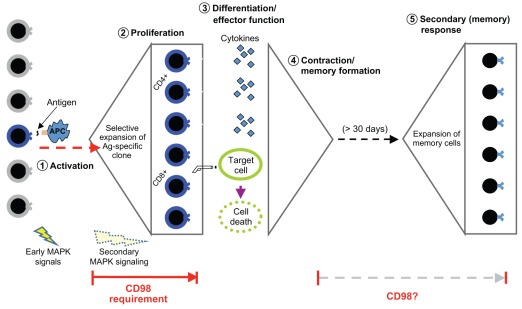
Until recently, only one study had examined CD98 function in B cells using inhibition with the 4F2 antibody, with confusing results that included both inhibition and enhancement of B cell functions (Gerrard et al., 1984). To address this issue, our group genetically deleted CD98hc in B cells using CD19-Cre (Cantor et al., 2009). Similar to the results in T cells, CD98hc is not required for B cell compartmentalization or their initial activation, but is crucial for B cell clonal expansion (Cantor et al., 2009). CD98-null B cells display strikingly reduced proliferation after stimulation with strong mitogens, and thus are unable to differentiate into plasma cells. Consequently, mice lacking CD98hc in B cells have strongly impaired antibody responses following immunization, and class-switching to IgG, a hallmark of mature antibody responses, is dramatically impaired. Because CD98hc-null B cells lack antigen-driven proliferation, the direct role of CD98 in B cell effector function (e.g. antibody secretion) cannot be examined directly. The apparently differing degrees by which T and B cells depend on CD98hc remain puzzling. One possibility is that the intensely competitive nature of B cell clonal expansion in germinal centers (Allen et al., 2007; Pritchard-Briscoe et al., 1977) renders CD98-null B cell unable to compete for available anchorage or nutrients within the environment of a germinal center. Nevertheless, taken together, these studies demonstrate that CD98hc is required for both humoral and cell-mediated adaptive immunity because it is essential for clonal expansion (Fig. 3).
Fig. 3.
The role of CD98hc in cell-mediated adaptive immunity. Upon immune challenge, a rare antigen-specific naive T cell (shown in blue) is activated by peptide antigen (Ag) on an antigen-presenting cell (APC) and rapidly proliferates to expand into a large number of antigen-specific effector T cells. After expansion and differentiation, effector CD4+ ‘helper’ cells secrete cytokines to direct the adaptive immune response, and CD8+ cytotoxic ‘killer’ T cells kill infected target cells. After pathogen clearance, effector T cells die to leave only a few ‘memory’ T cells that are primed for a rapid secondary response. Following secondary exposure to the same antigen, memory T cells re-expand to eliminate the pathogen. CD98hc is required for rapid clonal expansion, but appears dispensable for antigen recognition, activation and effector functions. Whether or not CD98hc is necessary for secondary expansion is an open question.
Mechanism of action for CD98 in lymphocyte proliferation
Although the cellular role of CD98hc in lymphocytes has become fairly clear, the biochemical mechanism by which this protein drives clonal expansion is not fully understood. There are at least three indications that the integrin signaling function of CD98hc is required for rapid lymphocyte proliferation. First, CD98hc mutants that exclusively support integrin signaling can reconstitute proliferation, whereas those that only support amino acid transport do not. Second, CD98-null B cells show defective integrin functions in that they are unable to adhere firmly and spread on anti-αLβ2-integrin-coated solid surfaces. More importantly, mitogen-induced early (<30 min) mitogen-activated protein kinase (MAPK) signaling is intact in CD98-null B cells as indicated by a normal initial wave of extracellular-signal-regulated kinase (ERK1/2) phosphorylation. However, loss of CD98hc abrogates sustained secondary phosphorylation of ERK and downregulation of cyclin-dependent kinase (CDK) inhibitors. This loss of sustained ERK activation and downregulation of CDK inhibitors resembles the effects of the loss of integrin signals that support growth factor stimulation of fibroblasts (Assoian and Schwartz, 2001; Schwartz and Assoian, 2001; Walker and Assoian, 2005). These data raise the question of which integrin ligands support B and T cell proliferation. One possibility is that there is a ligand-independent function of integrins in sustaining ERK signaling, which supports cell cycle progression. Another possibility is that CD98hc-dependent integrins recognize cell-associated ligands during cell–cell interactions (Nguyen et al., 2008) that occur during lymphocyte localization and synapse formation within lymph nodes. Whereas morphological synapse formation appears grossly normal in CD98-null T cells in vitro, it is possible that the quality and duration of contacts is defective in vivo, perhaps preventing T cell polarization (Chang et al., 2007). Additional hints that CD98hc functions in cell–cell contacts comes from its documented roles in cell fusion, and in monocyte or B cell aggregation (Cho et al., 2001; Cho et al., 2004; Mori et al., 2001; Mori et al., 2004; Ohgimoto et al., 1996; Suga et al., 1997; Tsurudome and Ito, 2000). Further study is needed to understand the details of how CD98hc and integrins might cooperate to support lymphocyte proliferation and adaptive immunity.
Given that highly conserved CD98 orthologs are present only in vertebrates (Prager et al., 2007; Uinuk-Ool et al., 2002) and are required for adaptive immunity, they should be found in all adaptive immune systems. Jawless fish (agnathans), which have an alternative adaptive immune system with a unique set of genes that form VLRs (Herrin and Cooper, 2010; Pancer et al., 2005) still rely on clonal expansion of lymphocyte-like cells (Mariuzza et al., 2010) and possess an unambiguous CD98hc orthologue (Uinuk-Ool et al., 2002). This fact adds additional compelling evidence for the absolute requirement of CD98hc for the ability of lymphocytes to mount adaptive immune responses.
CD98 and invasive cancers: the turbocharger gone awry
CD98 expression in tumors
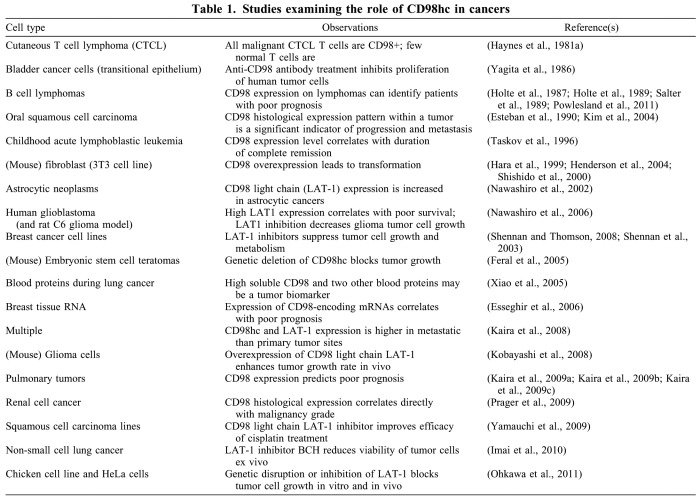
The capacity for rapid proliferation, especially without firm anchorage, can be dangerous in malignant cells, and a large body of evidence implicates CD98 in cancer (Table 1). Many tumors express CD98hc, and its expression correlates with poor prognosis in B cell lymphomas (Holte et al., 1987; Holte et al., 1989; Salter et al., 1989). Furthermore, nearly all studies that have examined the expression of CD98hc or CD98 light chains in solid tumors show that their expression is correlated with progressive or metastatic tumors (Esteban et al., 1990; Kaira et al., 2009a; Kaira et al., 2009b; Kaira et al., 2009c; Kaira et al., 2008; Kaira et al., 2009d; Kobayashi et al., 2008; Nawashiro et al., 2002; Nawashiro et al., 2006; Oleinik et al., 2005; Powlesland et al., 2009; Shennan et al., 2003; Xiao et al., 2005).
Table 1.
Studies examining the role of CD98hc in cancers
CD98 depletion and overexpression studies in cancer
Genetic modulation of CD98 expression in human cell lines and in animal models has established a causal link between CD98 and cancer; CD98 promotes transformation and tumor growth (Feral et al., 2005; Ohkawa et al., 2011). Furthermore, CD98 overexpression drives both anchorage independence and tumorigenesis (Nguyen et al., 2011; Hara et al., 1999; Henderson et al., 2004; Shishido et al., 2000), and the degree of transformation correlates with the level of CD98hc present in the cells (Hara et al., 1999). An early report showed that the transforming ability of CD98hc is lost in a mutant that ablates the ability to form disulfide bonds with the light chain and thus abolishes its amino acid transport function (Shishido et al., 2000). However, this mutation also affects the ability of CD98hc to bind integrins (Kolesnikova et al., 2001), making this result difficult to interpret. Moreover, a later study using CD98hc chimeras, in which its amino acid transport is separated from its integrin signaling function (Fenczik et al., 2001), has shown that the CD98 domain that is involved in integrin signaling is required for transforming Chinese hamster ovary (CHO) cells (Henderson et al., 2004) and for the development of embryonic stem cell teratomas in mice (Feral et al. 2005). Taken together, these genetic studies show that CD98hc expression is important, and that its overexpression is sufficient for cellular transformation.
CD98 blockade in tumors
The third line of evidence for the importance of CD98 in cancer comes from a number of studies that used CD98 inhibitors in a variety of tumor cells; these have resulted in the inhibition of cellular proliferation and tumor growth both in vitro and in vivo (Imai et al., 2010; Nawashiro et al., 2006; Oda et al., 2010; Shennan and Thomson, 2008; Yamauchi et al., 2009). One particular study used anti-CD98 antibodies specific for the heavy chain and showed that the growth of human tumor cells is inhibited in vitro (Yagita et al., 1986). Overall, these studies indicate that CD98 might constitute a target for therapeutic intervention in cancer. In fact, a microRNA that modulates CD98 expression during intestinal epithelial differentiation was recently identified, raising the possibility of an additional approach to alter CD98 expression (Nguyen et al., 2010).
CD98 amino acid transport in cancer
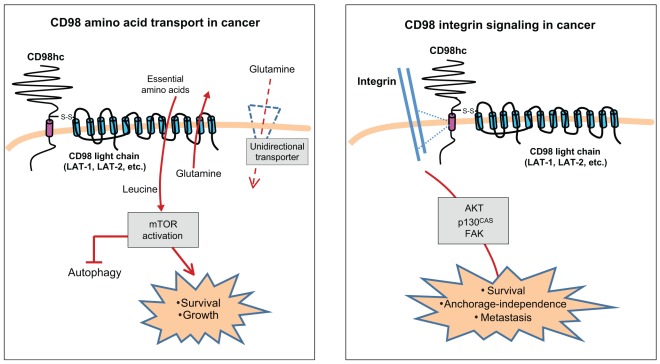
The mechanism by which CD98 promotes tumorigenesis is an important area of current research. Significant questions remain unanswered, such as whether CD98hc allows tumor growth by boosting the transport of nutrients or by augmenting integrin signaling, and if so, how would each of these roles be fulfilled. The rapidly dividing tumor cell has intense metabolic demands, often under conditions of diminishing nutrient availability within a solid tumor. CD98hc could therefore support tumor growth through its association with the amino acid transporter light chain and the subsequent increased supply of amino acids. Under conditions of limited amino acid availability, CD98 light chains such as LAT-1 are upregulated and their expression can be dysregulated in tumor cells (Campbell et al., 2000). LAT-1 and LAT-2 are amino acid exchangers and can import essential amino acids (EAA), such as leucine, isoleucine and arginine in exchange for the export of glutamine that has been imported by other transporters such as through system A transporters (Verrey, 2003). Furthermore, this CD98-mediated exchange of glutamine in the presence of EAAs is a rate-limiting step in the activation of mammalian target of rapamycin (mTOR) (Nicklin et al., 2009), which regulates cell growth and proliferation, providing another pathway through which CD98 could stimulate tumor growth. Cancer cells are heavily dependent on glutamine, not merely as an ingredient for nucleotide and amino acid biosynthesis, but also to promote increased uptake of EAAs through this exchange mechanism (Wise and Thompson, 2010). Interestingly, T lymphocytes are another cell type that is particularly dependent on glutamine (Carr et al., 2010). Thus, it appears probable that high expression levels of CD98hc could allow for rapid proliferation of lymphocytes and tumors by stimulating the exchange of glutamine for limiting EAAs (Fig. 4, left-hand panel).
Fig. 4.
Roles of CD98 in cancer. CD98 has two main biochemical functions, amino acid transport and integrin signaling; both appear important in tumor growth and metastasis. Left panel: CD98 amino acid transport in cancer. LAT-1 and LAT-2, the most commonly studied CD98 light chains, are system L bi-directional transporters of large neutral amino acids. Unidirectional transporters (such as system A or N transporters) import the non-essential amino acid glutamine into a rapidly growing cell. LAT-1 or LAT-2 can then export glutamine in exchange for importing EAAs. EAAs then activate the mTOR pathway, blocking autophagy and promoting cell growth and protein synthesis. Right panel: CD98 integrin signaling in cancer. CD98hc interacts with integrins and mediates integrin-dependent signals that promote tumorigenesis. CD98-dependent integrin signaling through proteins such as AKT, p130CAS (also known as BCAR1) and focal adhesion kinase (FAK) allow tumor cell survival and proliferation without anchorage.
CD98 integrin signaling function in cancer
CD98hc can also contribute to transformation by amplifying integrin signaling that results in reduced anchorage dependence. Integrins themselves have been implicated in invasive cancers because the ‘outside-in’ signals they transduce control cell proliferation and survival (Huck et al., 2010; Lahlou et al., 2007; Desgrosellier and Cheresh, 2010; Harburger and Calderwood, 2009). Integrins act as mechanotransducers that sense the stiffness of the extracellular matrix (ECM). Increased ECM stiffness drives tumor progression through clustering of integrins, which leads to adhesive signaling through focal adhesion kinase (FAK) and phosphatidylinositol 3-kinase (PI3K) (Levental et al., 2009). These signals can also direct the proliferation of micro-metastases in the lungs through β1 integrin (Shibue and Weinberg, 2009). In a softer matrix, such as one with larger amounts of flexible ECM components (elastin, etc.), high expression of CD98hc could permit integrin signaling that would promote cell growth and survival. Thus, the expression level of CD98hc would fine-tune the capacity of the cell to sense the compliance of the matrix. Indeed, CD98hc is important in allowing a cell to exert force on the ECM through integrin-driven RhoA signaling (Feral et al., 2007), which is a key element in the sensing of stiffness (Samuel et al., 2011).
Anchorage independence (i.e. independence of integrin signals provided by adhesion to ECM) is another hallmark of cellular transformation, and CD98hc is important for anchorage independence (Feral et al., 2005). CD98hc co-immunoprecipitates with integrins (Henderson et al., 2004; Rintoul et al., 2002), and binds to the β1 and β3 integrin cytoplasmic domains in a purified system (Prager et al., 2007). Overexpression or crosslinking of CD98hc stimulates FAK and AKT phosphorylation (Cai et al., 2005; Rintoul et al., 2002) (Fenczik et al., 1997), and deletion of CD98hc impairs integrin signaling (Feral et al., 2005). This integrin signaling function of CD98hc is dependent on the transmembrane and cytoplasmic domains (Fenczik et al., 2001), the same region that is crucial for lymphocyte proliferation (Cantor et al., 2009; Cantor et al., 2011) and for cellular transformation caused by overexpression of CD98hc (Henderson et al., 2004). CD98hc can thus regulate integrin signaling, and integrins are crucial for tumor progression through mechanotransduction and control of anchorage-independent growth. Therefore CD98hc might control tumorigenesis by governing integrin signaling (Fig. 4, right-hand panel).
CD98hc might also contribute to other pathologies. For example, in addition to autoimmunity disorders (Cantor et al., 2011), CD98hc overexpression could lead to other adaptive immunopathologies. Arterial restenosis and intestinal villous inflammation are dependent on CD98hc in animal models (Fogelstrand et al., 2009; Nguyen et al., 2011). Vertebrates have stratified squamous epithelium containing rapidly-dividing keratinocytes, which express high levels of CD98hc (Fernandez-Herrera et al., 1989; Lemaitre et al., 2005; Lemaitre et al., 2011; Patterson et al., 1984). The role of CD98hc in pathologic states in these tissues requires further study.
Conclusions
Here, we have summarized the central importance of rapid proliferation for clonal expansion in the vertebrate-specific adaptive immune system. CD98hc enables lymphocyte clonal expansion and adaptive immune responses, probably in part through its effects on integrin signaling. CD98hc is also important in cancer, which is probably mediated through increased transport of amino acids and/or by boosting integrin signals that allow growth in soft ECM or without anchorage. Importantly, clonal expansion is the nexus at which CD98 acts in adaptive immunity, and clonal expansion of tumor cells is also a central feature of many cancers. CD98hc and the integrins that bind to it are vertebrate-specific, as is adaptive immunity, and it is noteworthy that vertebrates appear to be particularly prone to developing invasive and metastatic cancers. This confluence of adaptive immunity, and the expression of CD98hc and CD98-binding integrins (Prager et al., 2007), coincident with increased invasive cancer, in vertebrates, suggests that CD98hc provides vertebrates with the benefit of adaptive immunity, but that this comes with a price: increased susceptibility to invasive cancers. One could speculate that other vertebrate-specific genes that are involved in clonal expansion must exist and are maintained because of the survival benefits they provide through adaptive immunity. Such genes might, like those encoding CD98, be upregulated in activated lymphocytes and could be therapeutic targets for autoimmunity diseases or cancer. Going forwards, it is key for this field to define the specific molecular mechanisms by which CD98 contributes to adaptive immunity and cancer. Second, the dramatic effects of CD98 loss on tumors and on lymphocyte clonal expansion demands that we make efforts to define the therapeutic potential of CD98 inhibition in cancer and autoimmune diseases.
Footnotes
Funding
Work in our laboratories was supported by grants from the National Institutes of Health [grant numbers HL31950, HL 57900, AR 27214, and HL 078784 to M.H.G.]. J.M.C. is supported by the Multiple Sclerosis Society and an NIH K01 grant [grant number K01DK090416]. Deposited in PMC for release after 12 months.
References
- Abbas A. K. (2003). Cellular and molecular immunology. Philadelphia, PA: Saunders; [Google Scholar]
- Allen C. D., Okada T., Tang H. L., Cyster J. G. (2007). Imaging of germinal center selection events during affinity maturation. Science 315, 528-531 [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Schwartz M. A. (2001). Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 11, 48-53 [DOI] [PubMed] [Google Scholar]
- Bertran J., Magagnin S., Werner A., Markovich D., Biber J., Testar X., Zorzano A., Kuhn L. C., Palacin M., Murer H. (1992). Stimulation of system y(+)-like amino acid transport by the heavy chain of human 4F2 surface antigen in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. USA 89, 5606-5610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T. (2011). Design principles of adaptive immune systems. Nat. Rev. Immunol. 11, 307-317 [DOI] [PubMed] [Google Scholar]
- Bron C., Rousseaux M., Spiazzi A. L., MacDonald H. R. (1986). Structural homology between the human 4F2 antigen and a murine cell surface glycoprotein associated with lymphocyte activation. J. Immunol. 137, 397-399 [PubMed] [Google Scholar]
- Cai S., Bulus N., Fonseca-Siesser P. M., Chen D., Hanks S. K., Pozzi A., Zent R. (2005). CD98 modulates integrin beta1 function in polarized epithelial cells. J. Cell Sci. 118, 889-899 [DOI] [PubMed] [Google Scholar]
- Campbell W. A., Sah D. E., Medina M. M., Albina J. E., Coleman W. B., Thompson N. L. (2000). TA1/LAT-1/CD98 light chain and system L activity, but not 4F2/CD98 heavy chain, respond to arginine availability in rat hepatic cells. Loss of response in tumor cells. J. Biol. Chem. 275, 5347-5354 [DOI] [PubMed] [Google Scholar]
- Cantor J., Browne C. D., Ruppert R., Feral C. C., Fassler R., Rickert R. C., Ginsberg M. H. (2009). CD98hc facilitates B cell proliferation and adaptive humoral immunity. Nat. Immunol. 10, 412-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor J., Slepak M., Ege N., Chang J. T., Ginsberg M. H. (2011). Loss of T cell CD98 H chain specifically ablates T cell clonal expansion and protects from autoimmunity. J. Immunol. 187, 851-860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr E. L., Kelman A., Wu G. S., Gopaul R., Senkevitch E., Aghvanyan A., Turay A. M., Frauwirth K. A. (2010). Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 185, 1037-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. T., Palanivel V. R., Kinjyo I., Schambach F., Intlekofer A. M., Banerjee A., Longworth S. A., Vinup K. E., Mrass P., Oliaro J., et al. (2007). Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 315, 1687-1691 [DOI] [PubMed] [Google Scholar]
- Cho J. Y., Fox D. A., Horejsi V., Sagawa K., Skubitz K. M., Katz D. R., Chain B. (2001). The functional interactions between CD98, beta1-integrins, and CD147 in the induction of U937 homotypic aggregation. Blood 98, 374-382 [DOI] [PubMed] [Google Scholar]
- Cho J. Y., Kim A. R., Joo H. G., Kim B. H., Rhee M. H., Yoo E. S., Katz D. R., Chain B. M., Jung J. H. (2004). Cynaropicrin, a sesquiterpene lactone, as a new strong regulator of CD29 and CD98 functions. Biochem. Biophys. Res. Commun. 313, 954-961 [DOI] [PubMed] [Google Scholar]
- Desgrosellier J. S., Cheresh D. A. (2010). Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 10, 9-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deves R., Boyd C. A. (2000). Surface antigen CD98(4F2): not a single membrane protein, but a family of proteins with multiple functions. J. Membr. Biol. 173, 165-177 [DOI] [PubMed] [Google Scholar]
- Diaz L. A., Jr, Friedman A. W., He X., Kuick R. D., Hanash S. M., Fox D. A. (1997). Monocyte-dependent regulation of T lymphocyte activation through CD98. Int. Immunol. 9, 1221-1231 [DOI] [PubMed] [Google Scholar]
- Esseghir S., Reis-Filho J. S., Kennedy A., James M., O'Hare M. J., Jeffery R., Poulsom R., Isacke C. M. (2006). Identification of transmembrane proteins as potential prognostic markers and therapeutic targets in breast cancer by a screen for signal sequence encoding transcripts. J. Pathol. 210, 420-430 [DOI] [PubMed] [Google Scholar]
- Esteban F., Ruiz-Cabello F., Concha A., Perez Ayala M., Delgado M., Garrido F. (1990). Relationship of 4F2 antigen with local growth and metastatic potential of squamous cell carcinoma of the larynx. Cancer 66, 1493-1498 [DOI] [PubMed] [Google Scholar]
- Fenczik C. A., Sethi T., Ramos J. W., Hughes P. E., Ginsberg M. H. (1997). Complementation of dominant suppression implicates CD98 in integrin activation. Nature 390, 81-85 [DOI] [PubMed] [Google Scholar]
- Fenczik C. A., Zent R., Dellos M., Calderwood D. A., Satriano J., Kelly C., Ginsberg M. H. (2001). Distinct domains of CD98hc regulate integrins and amino acid transport. J. Biol. Chem. 276, 8746-8752 [DOI] [PubMed] [Google Scholar]
- Feral C. C., Nishiya N., Fenczik C. A., Stuhlmann H., Slepak M., Ginsberg M. H. (2005). CD98hc (SLC3A2) mediates integrin signaling. Proc. Natl. Acad. Sci. USA 102, 355-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feral C. C., Zijlstra A., Tkachenko E., Prager G., Gardel M. L., Slepak M., Ginsberg M. H. (2007). CD98hc (SLC3A2) participates in fibronectin matrix assembly by mediating integrin signaling. J. Cell Biol. 178, 701-711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Herrera J., Sanchez-Madrid F., Diez A. G. (1989). Differential expression of the 4F2 activation antigen on human follicular epithelium in hair cycle. J. Invest. Dermatol. 92, 247-250 [DOI] [PubMed] [Google Scholar]
- Fogelstrand P., Feral C. C., Zargham R., Ginsberg M. H. (2009). Dependence of proliferative vascular smooth muscle cells on CD98hc (4F2hc, SLC3A2). J. Exp. Med. 206, 2397-2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort J., de la Ballina L. R., Burghardt H. E., Ferrer-Costa C., Turnay J., Ferrer-Orta C., Uson I., Zorzano A., Fernandez-Recio J., Orozco M., et al. (2007). The structure of human 4F2hc ectodomain provides a model for homodimerization and electrostatic interaction with plasma membrane. J. Biol. Chem. 282, 31444-31452 [DOI] [PubMed] [Google Scholar]
- Freidman A. W., Diaz L. A., Jr, Moore S., Schaller J., Fox D. A. (1994). The human 4F2 antigen: evidence for cryptic and noncryptic epitopes and for a role of 4F2 in human T lymphocyte activation. Cell Immunol. 154, 253-263 [DOI] [PubMed] [Google Scholar]
- Gavin M. A., Rudensky A. Y. (2002). Dual TCR T cells: gaining entry into the periphery. Nat. Immunol. 3, 109-110 [DOI] [PubMed] [Google Scholar]
- Gerrard T. L., Jurgensen C. H., Fauci A. S. (1984). Modulation of human B cell responses by a monoclonal antibody to an activation antigen 4F2. Clin. Exp. Immunol. 57, 155-162 [PMC free article] [PubMed] [Google Scholar]
- Gottesdiener K. M., Karpinski B. A., Lindsten T., Strominger J. L., Jones N. H., Thompson C. B., Leiden J. M. (1988). Isolation and structural characterization of the human 4F2 heavy-chain gene, an inducible gene involved in T-lymphocyte activation. Mol. Cell. Biol. 8, 3809-3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler D. A., Hemler M. E., Christenson L., Williams J. M., Shapiro H. M., Strom T. B., Strominger J. L., Weiner H. L. (1985). Investigation of in vivo activated T cells in multiple sclerosis and inflammatory central nervous system diseases. Clin. Immunol. Immunopathol. 37, 163-171 [DOI] [PubMed] [Google Scholar]
- Hara K., Kudoh H., Enomoto T., Hashimoto Y., Masuko T. (1999). Malignant transformation of NIH3T3 cells by overexpression of early lymphocyte activation antigen CD98. Biochem. Biophys. Res. Commun. 262, 720-725 [DOI] [PubMed] [Google Scholar]
- Harburger D. S., Calderwood D. A. (2009). Integrin signalling at a glance. J. Cell Sci. 122, 159-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Bunn P., Mann D., Thomas C., Eisenbarth G. S., Minna J., Fauci A. S. (1981a). Cell surface differentiation antigens of the malignant T cell in Sezary syndrome and mycosis fungoides. J. Clin. Invest. 67, 523-530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L., Fauci A. S. (1981b). Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J. Immunol. 126, 1409-1414 [PubMed] [Google Scholar]
- Hemler M. E., Strominger J. L. (1982). Characterization of antigen recognized by the monoclonal antibody (4F2): different molecular forms on human T and B lymphoblastoid cell lines. J. Immunol. 129, 623-628 [PubMed] [Google Scholar]
- Henderson N. C., Collis E. A., Mackinnon A. C., Simpson K. J., Haslett C., Zent R., Ginsberg M., Sethi T. (2004). CD98hc (SLC3A2) interaction with beta 1 integrins is required for transformation. J. Biol. Chem. 279, 54731-54741 [DOI] [PubMed] [Google Scholar]
- Herrin B. R., Cooper M. D. (2010). Alternative adaptive immunity in jawless vertebrates. J. Immunol. 185, 1367-1374 [DOI] [PubMed] [Google Scholar]
- Hildeman D., Jorgensen T., Kappler J., Marrack P. (2007). Apoptosis and the homeostatic control of immune responses. Curr. Opin. Immunol. 19, 516-521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holte H., Davies C. D., Kvaloy S., Smeland E. B., Foss-Abrahamsen A., Kaalhus O., Marton P. F., Godal T. (1987). The activation-associated antigen 4F2 predicts patient survival in low-grade B-cell lymphomas. Int. J. Cancer 39, 590-594 [DOI] [PubMed] [Google Scholar]
- Holte H., de Lange Davies C., Beiske K., Stokke T., Marton P. F., Smeland E. B., Hoie J., Kvaloy S. (1989). Ki67 and 4F2 antigen expression as well as DNA synthesis predict survival at relapse/tumour progression in low-grade B-cell lymphoma. Int. J. Cancer 44, 975-980 [DOI] [PubMed] [Google Scholar]
- Hsu E. (2009). V(D)J recombination: of mice and sharks. Adv. Exp. Med. Biol. 650, 166-179 [DOI] [PubMed] [Google Scholar]
- Huck L., Pontier S. M., Zuo D. M., Muller W. J. (2010). beta1-integrin is dispensable for the induction of ErbB2 mammary tumors but plays a critical role in the metastatic phase of tumor progression. Proc. Natl. Acad. Sci. USA 107, 15559-15564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H., Kaira K., Oriuchi N., Shimizu K., Tominaga H., Yanagitani N., Sunaga N., Ishizuka T., Nagamori S., Promchan K., et al. (2010). Inhibition of L-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res. 30, 4819-4828 [PubMed] [Google Scholar]
- Kaira K., Oriuchi N., Imai H., Shimizu K., Yanagitani N., Sunaga N., Hisada T., Tanaka S., Ishizuka T., Kanai Y., et al. (2008). l-type amino acid transporter 1 and CD98 expression in primary and metastatic sites of human neoplasms. Cancer Sci. 99, 2380-2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaira K., Ohde Y., Endo M., Nakagawa K., Okumura T., Takahashi T., Murakami H., Tsuya A., Nakamura Y., Naito T., et al. (2009a). Expression of 4F2hc (CD98) in pulmonary neuroendocrine tumors. Oncol. Rep. 26, 931-937 [DOI] [PubMed] [Google Scholar]
- Kaira K., Oriuchi N., Imai H., Shimizu K., Yanagitani N., Sunaga N., Hisada T., Ishizuka T., Kanai Y., Nakajima T., et al. (2009b). Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in stage I pulmonary adenocarcinoma. Lung Cancer 66, 120-126 [DOI] [PubMed] [Google Scholar]
- Kaira K., Oriuchi N., Imai H., Shimizu K., Yanagitani N., Sunaga N., Hisada T., Kawashima O., Kamide Y., Ishizuka T., et al. (2009c). CD98 expression is associated with poor prognosis in resected non-small-cell lung cancer with lymph node metastases. Ann. Surg. Oncol. 16, 3473-3481 [DOI] [PubMed] [Google Scholar]
- Kaira K., Oriuchi N., Shimizu K., Ishikita T., Higuchi T., Imai H., Yanagitani N., Sunaga N., Hisada T., Ishizuka T., et al. (2009d). Evaluation of thoracic tumors with (18)F-FMT and (18)F-FDG PET-CT: a clinicopathological study. Int. J. Cancer 124, 1152-1160 [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Fauci A. S. (1984). Differential expression of cell activation markers after stimulation of resting human B lymphocytes. J. Immunol. 132, 2857-2861 [PubMed] [Google Scholar]
- Kim D. K., Ahn S. G., Park J. C., Kanai Y., Endou H., Yoon J. H. (2004). Expression of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (4F2hc) in oral squamous cell carcinoma and its precusor lesions. Anticancer Res. 24, 1671-1675 [PubMed] [Google Scholar]
- Kobayashi K., Ohnishi A., Promsuk J., Shimizu S., Kanai Y., Shiokawa Y., Nagane M. (2008). Enhanced tumor growth elicited by L-type amino acid transporter 1 in human malignant glioma cells. Neurosurgery 62, 493-504 [DOI] [PubMed] [Google Scholar]
- Kolesnikova T. V., Mannion B. A., Berditchevski F., Hemler M. E. (2001). Beta1 integrins show specific association with CD98 protein in low density membranes. BMC Biochem. 2, 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada H., Imai A., Hattori E., Ito M., Tsumura H., Onoda T., Kuramochi M., Tani M., Yamamoto K., Yamane M., et al. (2006). Possible activation of murine T lymphocyte through CD98 is independent of interleukin 2/interleukin 2 receptor system. Biomed. Res. 27, 61-67 [DOI] [PubMed] [Google Scholar]
- Konttinen Y., Bergroth V., Nykanen P. (1985). Lymphocyte activation in rheumatoid arthritis synovial fluid in vivo. Scand. J. Immunol. 22, 503-507 [DOI] [PubMed] [Google Scholar]
- Krautz-Peterson G., Camargo S., Huggel K., Verrey F., Shoemaker C. B., Skelly P. J. (2007). Amino acid transport in schistosomes: Characterization of the permeaseheavy chain SPRM1hc. J. Biol. Chem. 282, 21767-21775 [DOI] [PubMed] [Google Scholar]
- Kuby J. (1997). Immunology. New York: W. H. Freeman; [Google Scholar]
- Kumar H., Kawai T., Akira S. (2011). Pathogen recognition by the innate immune system. Int. Rev. Immunol. 30, 16-34 [DOI] [PubMed] [Google Scholar]
- Lahlou H., Sanguin-Gendreau V., Zuo D., Cardiff R. D., McLean G. W., Frame M. C., Muller W. J. (2007). Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc. Natl. Acad. Sci. USA 104, 20302-20307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc V., Reichhart J. M. (2004). The immune response of Drosophila melanogaster. Immunol. Rev. 198, 59-71 [DOI] [PubMed] [Google Scholar]
- Lemaitre G., Gonnet F., Vaigot P., Gidrol X., Martin M. T., Tortajada J., Waksman G. (2005). CD98, a novel marker of transient amplifying human keratinocytes. Proteomics 5, 3637-3645 [DOI] [PubMed] [Google Scholar]
- Lemaitre G., Stella A., Feteira J., Baldeschi C., Vaigot P., Martin M. T., Monsarrat B., Waksman G. (2011). CD98hc (SLC3A2) is a key regulator of keratinocyte adhesion. J. Dermatol. Sci. 61, 169-179 [DOI] [PubMed] [Google Scholar]
- Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F., Csiszar K., Giaccia A., Weninger W., et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumadue J. A., Glick A. B., Ruddle F. H. (1987). Cloning, sequence analysis, and expression of the large subunit of the human lymphocyte activation antigen 4F2. Proc. Natl. Acad. Sci. USA 84, 9204-9208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariuzza R. A., Velikovsky C. A., Deng L., Xu G., Pancer Z. (2010). Structural insights into the evolution of the adaptive immune system: the variable lymphocyte receptors of jawless vertebrates. Biol. Chem. 391, 753-760 [DOI] [PubMed] [Google Scholar]
- Mastroberardino L., Spindler B., Pfeiffer R., Skelly P. J., Loffing J., Shoemaker C. B., Verrey F. (1998). Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 395, 288-291 [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C. A., Jr (1997). Innate immunity: the virtues of a nonclonal system of recognition. Cell 91, 295-298 [DOI] [PubMed] [Google Scholar]
- Mitchell D. M., Williams M. A. (2010). An activation marker finds a function. Immunity 32, 9-11 [DOI] [PubMed] [Google Scholar]
- Miyamoto Y. J., Mitchell J. S., McIntyre B. W. (2003). Physical association and functional interaction between beta1 integrin and CD98 on human T lymphocytes. Mol. Immunol. 39, 739-751 [DOI] [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Sekaly P. R., Moretta A., Chapuis B., Cerottini J. C. (1981). Surface markers of cloned human T cells with various cytolytic activities. J. Exp. Med. 154, 569-574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Miyamoto N., Higuchi Y., Nanba K., Ito M., Tsurudome M., Nishio M., Kawano M., Uchida A., Ito Y. (2001). Cross-talk between RANKL and FRP-1/CD98 Systems: RANKL-mediated osteoclastogenesis is suppressed by an inhibitory anti-CD98 heavy chain mAb and CD98-mediated osteoclastogenesis is suppressed by osteoclastogenesis inhibitory factor. Cell Immunol. 207, 118-126 [DOI] [PubMed] [Google Scholar]
- Mori K., Nishimura M., Tsurudome M., Ito M., Nishio M., Kawano M., Kozuka Y., Yamashita Y., Komada H., Uchida A., et al. (2004). The functional interaction between CD98 and CD147 in regulation of virus-induced cell fusion and osteoclast formation. Med. Microbiol. Immunol. 193, 155-162 [DOI] [PubMed] [Google Scholar]
- Murphy K., Travers P., Walport M., Janeway C. (2011). Janeway's immunobiology. New York: Garland Science; [Google Scholar]
- Nakao M., Kubo K., Hara A., Hirohashi N., Futagami E., Shichijo S., Sagawa K., Itoh K. (1993). A monoclonal antibody (H227) recognizing a new epitope of 4F2 molecular complex associated with T cell activation. Cell Immunol. 152, 226-233 [DOI] [PubMed] [Google Scholar]
- Nawashiro H., Otani N., Shinomiya N., Fukui S., Nomura N., Yano A., Shima K., Matsuo H., Kanai Y. (2002). The role of CD98 in astrocytic neoplasms. Hum. Cell 15, 25-31 [DOI] [PubMed] [Google Scholar]
- Nawashiro H., Otani N., Shinomiya N., Fukui S., Ooigawa H., Shima K., Matsuo H., Kanai Y., Endou H. (2006). L-type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. Int. J. Cancer 119, 484-492 [DOI] [PubMed] [Google Scholar]
- Nelson B. H., Willerford D. M. (1998). Biology of the interleukin-2 receptor. Adv. Immunol. 70, 1-81 [DOI] [PubMed] [Google Scholar]
- Nguyen H. T., Dalmasso G., Yan Y., Obertone T. S., Sitaraman S. V., Merlin D. (2008). Ecto-phosphorylation of CD98 regulates cell-cell interactions. PLoS ONE 3, e3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H. T., Dalmasso G., Yan Y., Laroui H., Dahan S., Mayer L., Sitaraman S. V., Merlin D. (2010). MicroRNA-7 modulates CD98 expression during intestinal epithelial cell differentiation. J. Biol. Chem. 285, 1479-1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H. T., Dalmasso G., Torkvist L., Halfvarson J., Yan Y., Laroui H., Shmerling D., Tallone T., D'Amato M., Sitaraman S. V., et al. (2011). CD98 expression modulates intestinal homeostasis, inflammation, and colitis-associated cancer in mice. J. Clin. Invest. 121, 1733-1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin P., Bergman P., Zhang B., Triantafellow E., Wang H., Nyfeler B., Yang H., Hild M., Kung C., Wilson C., et al. (2009). Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136, 521-534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K., Hosoda N., Endo H., Saito K., Tsujihara K., Yamamura M., Sakata T., Anzai N., Wempe M. F., Kanai Y., et al. (2010). L-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci. 101, 173-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgimoto S., Tabata N., Suga S., Tsurudome M., Kawano M., Nishio M., Okamoto K., Komada H., Watanabe N., Ito Y. (1996). Regulation of human immunodeficiency virus gp160-mediated cell fusion by antibodies against fusion regulatory protein 1. J. Gen. Virol. 77, 2747-2756 [DOI] [PubMed] [Google Scholar]
- Ohkawa M., Ohno Y., Masuko K., Takeuchi A., Suda K., Kubo A., Kawahara R., Okazaki S., Tanaka T., Saya H., et al. (2011). Oncogenicity of L-type amino-acid transporter 1 (LAT1) revealed by targeted gene disruption in chicken DT40 cells: LAT1 is a promising molecular target for human cancer therapy. Biochem. Biophys. Res. Commun. 406, 649-655 [DOI] [PubMed] [Google Scholar]
- Oleinik E. K., Shibaev M. I., Oleinik V. M. (2005). [Expression of lymphocyte activation markers in patients with gastrointestinal tumors at different stages]. Vopr. Onkol. 51, 571-574 [PubMed] [Google Scholar]
- Palacin M. (1994). A new family of proteins (rBAT and 4F2hc) involved in cationic and zwitterionic amino acid transport: a tale of two proteins in search of a transport function. J. Exp. Biol. 196, 123-137 [DOI] [PubMed] [Google Scholar]
- Pancer Z., Saha N. R., Kasamatsu J., Suzuki T., Amemiya C. T., Kasahara M., Cooper M. D. (2005). Variable lymphocyte receptors in hagfish. Proc. Natl. Acad. Sci. USA 102, 9224-9229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. A., Eisinger M., Haynes B. F., Berger C. L., Edelson R. L. (1984). Monoclonal antibody 4F2 reactive with basal layer keratinocytes: studies in the normal and a hyperproliferative state. J. Invest. Dermatol. 83, 210-213 [DOI] [PubMed] [Google Scholar]
- Pineda M., Fernandez E., Torrents D., Estevez R., Lopez C., Camps M., Lloberas J., Zorzano A., Palacin M. (1999). Identification of a membrane protein, LAT-2, that Co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J. Biol. Chem. 274, 19738-19744 [DOI] [PubMed] [Google Scholar]
- Powlesland A. S., Hitchen P. G., Parry S., Graham S. A., Barrio M. M., Elola M. T., Mordoh J., Dell A., Drickamer K., Taylor M. E. (2009). Targeted glycoproteomic identification of cancer cell glycosylation. Glycobiology 19, 899-909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powlesland A. S., Barrio M. M., Mordoh J., Hitchen P. G., Dell A., Drickamer K., Taylor M. E. (2011). Glycoproteomic characterization of carriers of the CD15/Lewisx epitope on Hodgkin's Reed-Sternberg cells. BMC Biochem. 12, 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager G. W., Feral C. C., Kim C., Han J., Ginsberg M. H. (2007). CD98hc (SLC3A2) interaction with the integrin beta subunit cytoplasmic domain mediates adhesive signaling. J. Biol. Chem. 282, 24477-24484 [DOI] [PubMed] [Google Scholar]
- Prager G. W., Poettler M., Schmidinger M., Mazal P. R., Susani M., Zielinski C. C., Haitel A. (2009). CD98hc (SLC3A2), a novel marker in renal cell cancer. Eur. J. Clin. Invest. 39, 304-310 [DOI] [PubMed] [Google Scholar]
- Pritchard-Briscoe H., McDougall C., Inchley C. J. (1977). Influence of antigenic competition on the development of antibody-forming cell clones. Clin. Exp. Immunol. 27, 328-334 [PMC free article] [PubMed] [Google Scholar]
- Quackenbush E. J., Linsley P., Letarte M. (1986). Mouse L cells express a molecular complex carrying the human epitopes recognized by monoclonal antibodies 44D7 and 44H7 after DNA-mediated gene transfer. J. Immunol. 137, 234-239 [PubMed] [Google Scholar]
- Quackenbush E., Clabby M., Gottesdiener K. M., Barbosa J., Jones N. H., Strominger J. L., Speck S., Leiden J. M. (1987). Molecular cloning of complementary DNAs encoding the heavy chain of the human 4F2 cell-surface antigen: a type II membrane glycoprotein involved in normal and neoplastic cell growth. Proc. Natl. Acad. Sci. USA 84, 6526-6530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B., Laynes R., Ogmundsdottir M. H., Boyd C. A., Goberdhan D. C. (2007). Amino acid transporters and nutrient-sensing mechanisms: new targets for treating insulin-linked disorders? Biochem. Soc. Trans. 35, 1215-1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B., Roversi P., Laynes R., Kazi S., Boyd C. A., Goberdhan D. C. (2009). Drosophila expresses a CD98 transporter with an evolutionarily conserved structure and amino acid-transport properties. Biochem. J. 420, 363-372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintoul R. C., Buttery R. C., Mackinnon A. C., Wong W. S., Mosher D., Haslett C., Sethi T. (2002). Cross-linking CD98 promotes integrin-like signaling and anchorage-independent growth. Mol. Biol. Cell 13, 2841-2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha N. R., Smith J., Amemiya C. T. (2010). Evolution of adaptive immune recognition in jawless vertebrates. Semin. Immunol. 22, 25-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter D. M., Krajewski A. S., Sheehan T., Turner G., Cuthbert R. J., McLean A. (1989). Prognostic significance of activation and differentiation antigen expression in B-cell non-Hodgkin's lymphoma. J. Pathol. 159, 211-220 [DOI] [PubMed] [Google Scholar]
- Samuel M. S., Lopez J. I., McGhee E. J., Croft D. R., Strachan D., Timpson P., Munro J., Schroder E., Zhou J., Brunton V. G., et al. (2011). Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell 19, 776-791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A., Assoian R. K. (2001). Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 114, 2553-2560 [DOI] [PubMed] [Google Scholar]
- Shennan D. B., Thomson J. (2008). Inhibition of system L (LAT1/CD98hc) reduces the growth of cultured human breast cancer cells. Oncol. Rep. 20, 885-889 [PubMed] [Google Scholar]
- Shennan D. B., Thomson J., Barber M. C., Travers M. T. (2003). Functional and molecular characteristics of system L in human breast cancer cells. Biochim. Biophys. Acta. 1611, 81-90 [DOI] [PubMed] [Google Scholar]
- Shibue T., Weinberg R. A. (2009). Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc. Natl. Acad. Sci. USA 106, 10290-10295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishido T., Uno S., Kamohara M., Tsuneoka-Suzuki T., Hashimoto Y., Enomoto T., Masuko T. (2000). Transformation of BALB3T3 cells caused by over-expression of rat CD98 heavy chain (HC) requires its association with light chain: mis-sense mutation in a cysteine residue of CD98HC eliminates its transforming activity. Int. J. Cancer 87, 311-316 [DOI] [PubMed] [Google Scholar]
- Spagnoli G. C., Ausiello C., Palma C., Bellone G., Ippoliti G., Letarte M., Malavasi F. (1991). Functional effects of a monoclonal antibody directed against a distinct epitope on 4F2 molecular complex in human peripheral blood mononuclear cell activation. Cell Immunol. 136, 208-218 [DOI] [PubMed] [Google Scholar]
- Sprent J., Surh C. D. (2002). T cell memory. Annu. Rev. Immunol. 20, 551-579 [DOI] [PubMed] [Google Scholar]
- Suga S., Tsurudome M., Ito M., Ohgimoto S., Tabata N., Nishio M., Kawano M., Komada H., Ito M., Sakurai M., et al. (1997). Human immunodeficiency virus type-1 envelope glycoprotein gp120 induces expression of fusion regulatory protein (FRP)-1/CD98 on CD4+ T cells: a possible regulatory mechanism of HIV-induced syncytium formation. Med. Microbiol. Immunol. 185, 237-243 [DOI] [PubMed] [Google Scholar]
- Taams L. S., van Rensen A. J., Poelen M. C., van Els C. A., Besseling A. C., Wagenaar J. P., van Eden W., Wauben M. H. (1998). Anergic T cells actively suppress T cell responses via the antigen-presenting cell. Eur. J. Immunol. 28, 2902-2912 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Kuniyasu Y., Toda M., Sakaguchi N., Itoh M., Iwata M., Shimizu J., Sakaguchi S. (1998). Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10, 1969-1980 [DOI] [PubMed] [Google Scholar]
- Taskov H., Pashov A., ffmitrova E., Yordanova M., Serbinova M. (1996). Levels of CAF7 (CD98) expression correlate with the complete remission duration in childhood acute leukemia. Leuk. Res. 20, 75-79 [DOI] [PubMed] [Google Scholar]
- Teixeira S., Di Grandi S., Kuhn L. C. (1987). Primary structure of the human 4F2 antigen heavy chain predicts a transmembrane protein with a cytoplasmic NH2 terminus. J. Biol. Chem. 262, 9574-9580 [PubMed] [Google Scholar]
- Torrents D., Estevez R., Pineda M., Fernandez E., Lloberas J., Shi Y. B., Zorzano A., Palacin M. (1998). Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y+L. A candidate gene for lysinuric protein intolerance. J. Biol. Chem. 273, 32437-32445 [DOI] [PubMed] [Google Scholar]
- Tosi M. F. (2005). Innate immune responses to infection. J. Allergy Clin. Immunol. 116, 241-250 [DOI] [PubMed] [Google Scholar]
- Tsurudome M., Ito Y. (2000). Function of fusion regulatory proteins (FRPs) in immune cells and virus-infected cells. Crit. Rev. Immunol. 20, 167-196 [PubMed] [Google Scholar]
- Uinuk-Ool T., Mayer W. E., Sato A., Dongak R., Cooper M. D., Klein J. (2002). Lamprey lymphocyte-like cells express homologs of genes involved in immunologically relevant activities of mammalian lymphocytes. Proc. Natl. Acad. Sci. USA 99, 14356-14361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrey F. (2003). System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 445, 529-533 [DOI] [PubMed] [Google Scholar]
- Verrey F., Meier C., Rossier G., Kuhn L. C. (2000). Glycoprotein-associated amino acid exchangers: broadening the range of transport specificity. Pflugers Arch. 440, 503-512 [DOI] [PubMed] [Google Scholar]
- Walker J. L., Assoian R. K. (2005). Integrin-dependent signal transduction regulating cyclin D1 expression and G1 phase cell cycle progression. Cancer Metastasis Rev. 24, 383-393 [DOI] [PubMed] [Google Scholar]
- Warren A. P., Patel K., McConkey D. J., Palacios R. (1996). CD98: a type II transmembrane glycoprotein expressed from the beginning of primitive and definitive hematopoiesis may play a critical role in the development of hematopoietic cells. Blood 87, 3676-3687 [PubMed] [Google Scholar]
- Weaver C. T., Hatton R. D., Mangan P. R., Harrington L. E. (2007). IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25, 821-852 [DOI] [PubMed] [Google Scholar]
- Wise D. R., Thompson C. B. (2010). Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 35, 427-433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T., Ying W., Li L., Hu Z., Ma Y., Jiao L., Ma J., Cai Y., Lin D., Guo S., et al. (2005). An approach to studying lung cancer-related proteins in human blood. Mol. Cell. Proteomics 4, 1480-1486 [DOI] [PubMed] [Google Scholar]
- Yagita H., Masuko T., Hashimoto Y. (1986). Inhibition of tumor cell growth in vitro by murine monoclonal antibodies that recognize a proliferation-associated cell surface antigen system in rats and humans. Cancer Res. 46, 1478-1484 [PubMed] [Google Scholar]
- Yamauchi K., Sakurai H., Kimura T., Wiriyasermkul P., Nagamori S., Kanai Y., Kohno N. (2009). System L amino acid transporter inhibitor enhances anti-tumor activity of cisplatin in a head and neck squamous cell carcinoma cell line. Cancer Lett. 276, 95-101 [DOI] [PubMed] [Google Scholar]
- Zent R., Fenczik C. A., Calderwood D. A., Liu S., Dellos M., Ginsberg M. H. (2000). Class- and splice variant-specific association of CD98 with integrin beta cytoplasmic domains. J. Biol. Chem. 275, 5059-5064 [DOI] [PubMed] [Google Scholar]
- Zhang D. J., Wang Q., Wei J., Baimukanova G., Buchholz F., Stewart A. F., Mao X., Killeen N. (2005). Selective expression of the Cre recombinase in late-stage thymocytes using the distal promoter of the Lck gene. J. Immunol. 174, 6725-6731 [DOI] [PubMed] [Google Scholar]