Abstract
The dysfunction of TAR DNA-binding protein-43 (TDP-43) is implicated in neurodegenerative diseases. However, the function of TDP-43 is not fully elucidated. Here we show that the protein level of endogenous TDP-43 in the nucleus is increased in mouse cortical neurons in the early stages, but return to basal level in the later stages after glutamate accumulation-induced injury. The elevation of TDP-43 results from a downregulation of phosphatase and tensin homolog (PTEN). We further demonstrate that activation of NR2A-containing NMDA receptors (NR2ARs) leads to PTEN downregulation and subsequent reduction of PTEN import from the cytoplasm to the nucleus after glutamate accumulation. The decrease of PTEN in the nucleus contributes to its reduced association with TDP-43, and thereby mediates the elevation of nuclear TDP-43. We provide evidence that the elevation of nuclear TDP-43, mediated by NR2AR activation and PTEN downregulation, confers protection against cortical neuronal death in the late stages after glutamate accumulation. Thus, this study reveals a NR2AR–PTEN–TDP-43 signaling pathway by which nuclear TDP-43 promotes neuronal survival. These results suggest that upregulation of nuclear TDP-43 represents a self-protection mechanism to delay neurodegeneration in the early stages after glutamate accumulation and that prolonging the upregulation process of nuclear TDP-43 might have therapeutic significance.
Key words: TAR DNA-binding protein-43, NR2A-containing NMDA receptor, PTEN, Glutamate neurotoxicity, Neuroprotection, Neurodegeneration
Introduction
The TAR DNA-binding protein-43 (TDP-43) is a highly conserved, heterogeneous ribonucleoprotein. TDP-43 has both nuclear export and import signals, but its distribution is primarily nuclear (Ayala et al., 2008; Winton et al., 2008). It has been shown that TDP-43 regulates gene transcription, exon splicing and exon inclusion (Sreedharan et al., 2008). However, the function of TDP-43 and it underlying mechanisms are not fully understood. Evidence from in vivo models of murine TDP-43 deletion indicates that TDP-43 is required for embryogenesis and is essential for viability (Kraemer et al., 2010; Sephton et al., 2010; Wu et al., 2010). Recently, the major proteins of the pathological inclusions in amyotrophic lateral sclerosis (ALS) have been identified as TDP-43 and its C-terminal fragments of ~20–25 kDa (Arai et al., 2006; Igaz et al., 2008; Mackenzie et al., 2007; Neumann et al., 2006). TDP-43 has also been identified as a component in the inclusions of frontotemporal lobar degeneration (FTLD) and other neurodegenerative diseases (Arai et al., 2009; Hasegawa et al., 2007; Neumann et al., 2006).
Glutamate accumulation-mediated neurotoxicity is known to play a crucial role in traumatic and ischemic brain injuries, as well as in neurodegenerative diseases including ALS (Culcasi et al., 1994; Fiszman et al., 2010; Grosskreutz et al., 2010; Lafon-Cazal et al., 1993; Perry et al., 1987; Plaitakis and Caroscio, 1987). The elevation of glutamate concentration causes neurotoxicity through overactivation of ionotropic glutamate receptors (Arundine and Tymianski, 2004; Hanson et al., 2010; Hardingham et al., 2002; Lee et al., 1999; Rothstein et al., 1990; Sarraf-Yazdi et al., 1998). NMDA receptors are the major subtypes of ionotropic glutamate receptors to mediate glutamate neurotoxicity-induced neuronal death or neurodegeneration (Annis and Vaughn, 1998; Arundine and Tymianski, 2004; Brunet et al., 2009; Hanson et al., 2010; Hardingham et al., 2002; Lee et al., 1999; Sarraf-Yazdi et al., 1998; Sen et al., 2005). However, how NMDA receptors induce neuronal death or neurodegeneration remains unclear. The NMDA receptors containing NR2A and NR2B subunits (NR2ARs and NR2BRs) are the major subtypes of NMDA receptors expressed in the central nervous system (CNS) (Dingledine et al., 1999). Studies demonstrate that NR2ARs and NR2BRs play opposing role in regulating neuronal survival or death (Chen et al., 2008; DeRidder et al., 2006; Hardingham et al., 2002; Liu et al., 2007; Ning et al., 2004; Vanhoutte and Bading, 2003). This might explain why use of NMDA receptor antagonists as neuroprotective agents has been disappointing in clinical trials (Gredal et al., 1997; Traynor et al., 2006). Thus, investigating the specific effects differentially mediated by NR2AR- and NR2BR-dependent intracellular signaling would provide molecular evidence for the development of selective neuroprotection therapies.
Studies by others and us have revealed that suppression of PTEN (phosphatase and tensin homolog) protects against neuronal death (Chang et al., 2007; Ning et al., 2004). Although it functions in the cytoplasm, PTEN can enter the nucleus to regulate transcription, alternative splicing and mRNA stability (Planchon et al., 2008). Under normal conditions, PTEN shuttles between the cytoplasm and nucleus (Gil et al., 2006; Planchon et al., 2008). In the nucleus, PTEN has been shown to cause downregulation of extracellular signal-regulated kinase (ERK), leading to a decrease in cyclin D1 levels and G0–G1 arrest (Planchon et al., 2008). By interacting with CENP-C, PTEN enhances centromere stability and overall genomic stability (Planchon et al., 2008). Similar to its role in the cytoplasm, nuclear PTEN also induces apoptosis (Planchon et al., 2008).
To understand the cellular and molecular mechanisms that mediate the role of TDP-43 in both physiological and neurodegenerative conditions, we investigated the effect of NMDA receptors and PTEN on TDP-43 expression in an in vitro glutamate accumulation-induced neurodegeneration model using cultured mouse cortical neurons. We show that the protein level of endogenous TDP-43 in the nucleus is increased in the early stages after glutamate accumulation. The increase of TDP-43 is mediated through a reduced association of PTEN with TDP-43 in the nucleus, which results from the activation of NR2ARs and subsequent downregulation of nuclear PTEN. We also provide evidence that the upregulation of nuclear TDP-43 by NR2AR activation and PTEN downregulation in the early stages after glutamate accumulation is neuroprotective. Thus, the NR2AR–PTEN–TDP-43 signaling pathway might represent a general mechanism against neuronal death in the early stages of glutamate neurotoxicity-induced neuronal injury and neurodegeneration.
Results
The protein level of nuclear TDP-43 is increased in cortical neurons in the early stages after endogenous glutamate accumulation
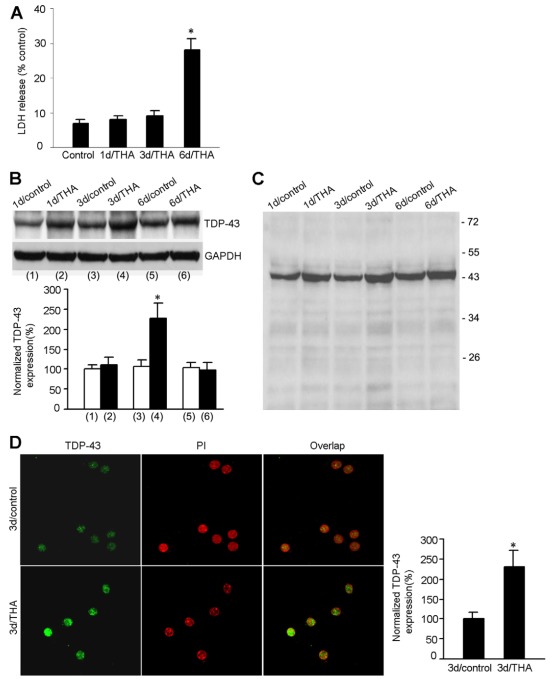
To investigate the functional consequences of TDP-43 in the CNS, we established an in vitro glutamate-induced neurotoxicity model in cultured mouse cortical neurons. DL-threo-beta-hydroxyaspartate (THA), an inhibitor of glutamate transporters, was used to induce neuronal injury. By promoting extracellular glutamate accumulation, THA treatment causes glutamate neurotoxicity and has been used to induce neuronal injury in vitro (Corse et al., 1999; Kidd and Isaac, 2000; Matyja et al., 2006; Nagańska et al., 2010; Tolosa et al., 2008; Van Westerlaak et al., 2001). To characterize this model, we first performed a lactate dehydrogenase (LDH) release assay to measure THA-induced neuronal damage in the cortical cultures. We showed that the rate of neuronal death was not significantly altered during the first 3 days after treatment with 100 μM THA, but increased at 6 days after treatment (Fig. 1A). We then performed western blot assays to measure the protein level of TDP-43 in the specific injury paradigm. We found that the level of TDP-43 was remarkably increased in cortical neurons during the first 3 days after THA treatment (Fig. 1B). However, the increased expression of TDP-43 returned to basal levels at 6 days after THA insult (Fig. 1B). Our data also showed that there were no C-terminal fragments of TDP-43 in THA-treated groups or in the respective controls (Fig. 1C).
Fig. 1.
Protein expression of nuclear TDP-43 is increased in mouse cortical neurons in the early stages after THA treatment. (A) Time course of THA-induced neuronal damage. Summarized data indicate that LDH release is remarkably increased at 6 days after 100 μM THA treatment (mean ± s.e.m.; n=6 animals for each group; *P<0.05 vs control). (B) Representative immunoblots (top panel) and summarized data (bottom panel) show that the protein level of TDP-43 is increased at 3 days after 100 μM THA treatment (n=8 animals for each group; *P<0.05 vs 3d/control; data are normalized to 1d/control). (C) A full blot image of the sample TDP-43 blot in B. (D) Sample images (left) and summarized data (right) show that the protein expression of TDP-43 in the nucleus of cultured cortical motor neurons is increased at 3 days after 100 μM THA treatment (for each group, n=30 cells from three independent experiments; *P<0.05 vs 3d/control; data are normalized to 3d/control).
Because TDP-43 is mainly expressed in the nucleus, we next tested whether the distribution of TDP-43 was altered in the nucleus of cortical neurons after glutamate accumulation. Immunocytochemical staining was performed to determine TDP-43 expression with the use of propidium iodide (PI) to label the nucleus in cultured cortical neurons (Fig. 1D). Although we confirmed that TDP-43 expression was increased at 3 days after THA treatment (Fig. 1D), we found that the increased TDP-43 was confined to the nucleus (Fig. 1D). We did not observe significant expression of TDP-43 in the cytoplasm in either control or THA-treated neurons (Fig. 1D). Taken together, these results indicate that TDP-43 expression is increased only in the nucleus in the early stages after glutamate accumulation.
Downregulation of PTEN contributes to the upregulation of TDP-43 after glutamate accumulation
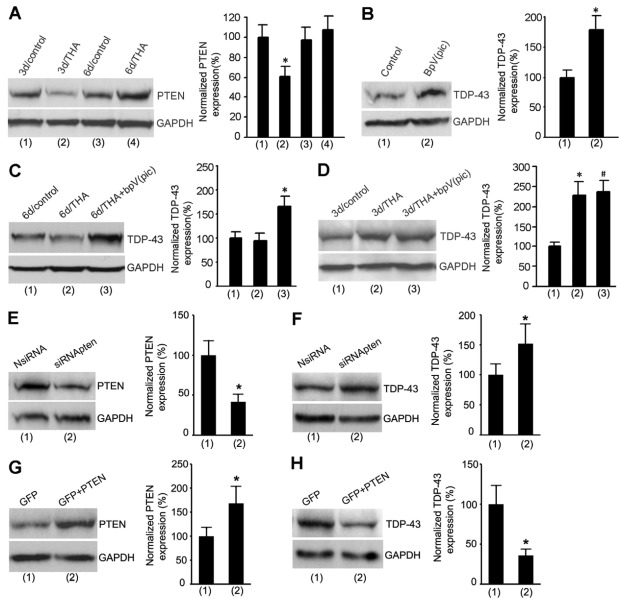
Given that the phosphatase PTEN is involved in regulating neuronal survival and exerts its effects in both cytoplasm and nucleus, we tested the effect of PTEN on TDP-43. We first measured the level of PTEN protein in cortical neurons at 3 days after THA treatment. We showed that, in contrast to the increase of TDP-43, PTEN was decreased at 3 days after THA treatment (Fig. 2A). To determine whether PTEN downregulation contributed to TDP-43 upregulation, we tested the effect of a selective PTEN inhibitor bisperoxovanadium [bpV(pic)] on the protein expression of TDP-43 in the cortical neurons (Schmid et al., 2004). We found that PTEN inhibition by bpV(pic) not only enhanced TDP-43 expression in control cortical neurons (Fig. 2B), but also increased TDP-43 expression at 6 days after THA treatment (Fig. 2C). However, bpV(pic) treatment had no significant effect on TDP-43 expression at 3 days after THA treatment (Fig. 2D). To provide further evidence for the regulation of TDP-43 by PTEN, the cultured neurons were transfected with PTEN siRNAs (siRNApten) or PTEN cDNAS (Fig. 2E–H). Our data showed that suppression or overexpression of PTEN increased and inhibited TDP-43 expression, respectively (Fig. 2E–H). These results suggest that TDP-43 is negatively regulated by endogenous PTEN, and that the upregulation of TDP-43 expression is mediated by PTEN downregulation in the early stages after glutamate accumulation.
Fig. 2.
The increase of TDP-43 is mediated by PTEN downregulation soon after THA treatment. (A) Representative immunoblots (left) and summarized data (right) show that the protein expression of PTEN is decreased at 3 days after 100 μM THA treatment (n=7 for each group; *P<0.05 vs 3d/control; data are normalized to 3d/control). (B) Sample immunoblots (left) and summarized data (right) show that treatment with the PTEN inhibitor bpV(pic) (100 nM) for 24 hours increases the protein expression of TDP-43 in normal cortical neurons (n=8 for each group; *P<0.05 vs control). (C) Representative immunoblots (left) and summarized data (right) show that treatment with bpV(pic) (100 nM) at 5 days after 100 μM THA insult increases the protein expression of TDP-43 in cortical neurons at 6 days after THA insult (n=6 for each group; *P<0.05 vs 6d/THA; data are normalized to 6d/control). (D) Sample immunoblots (left) and summarized data (right) show that treatment withbpV(pic) (100 nM) at 2 days after 100 μM THA insult has no effect on the protein expression of TDP-43 at 3 days after THA insult (n=6 for each group; *P<0.05 vs 3d/control; #P>0.05 vs 3d/THA). (E) Treatment with PTEN siRNA (siRNApten) but not the non-targeting control siRNA (NsiRNA) in cultured cortical neurons suppresses PTEN expression (n=5 for each group; *P<0.05 vs NsiRNA). (F) Treatment with siRNApten but not NsiRNA increases TDP-43 expression (n=5 for each group; *P<0.05 vs NsiRNA). (G) Transfection of PTEN cDNA increases PTEN expression in cultured cortical neurons (n=4 for each group; *P<0.05 vs GFP). (H) PTEN overexpression reduces TDP-43 expression in cultured cortical neurons (n=4 for each group. *P<0.05 vs. GFP). All bar graphs show means ± s.e.m.
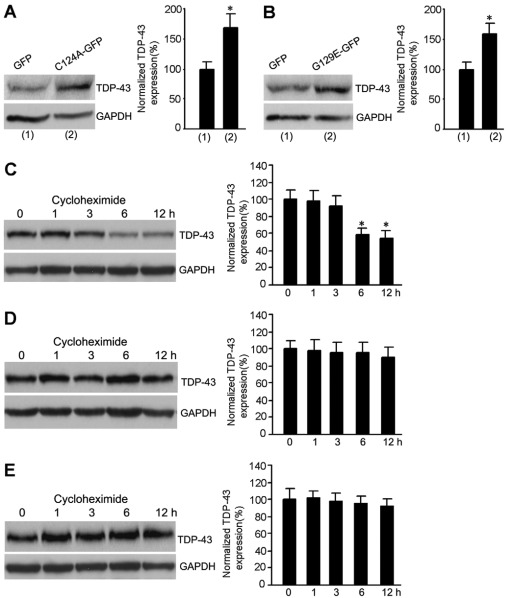
To determine how TDP-43 was regulated by PTEN, two different dominant-negative forms of phosphatase-inactivated PTEN mutants, C124A and G129E, were used to inhibit endogenous lipid and protein phosphatase activities of PTEN (Myers et al., 1998; Tamura et al., 1998; Weng et al., 2001). The C124A mutation causes a loss of both lipid and protein phosphatase activities, and the mutant G129E results only in loss of lipid phosphatase activity with retention of protein phosphatase activity. Our results demonstrated that the level of TDP-43 was increased in neurons transfected with both C124A and G129E (Fig. 3A,B), suggesting that the downregulation of lipid phosphatase activity of PTEN contributes to upregulation of TDP-43.
Fig. 3.
The lipid phosphatase activity of PTEN mediates the regulation of TDP-43 by PTEN. (A) Transfection of C124A induces increased expression of TDP-43 in cultured cortical neurons (n=4 for each group; *P<0.05 vs GFP). (B) Transfection of G129E increases TDP-43 expression in cultured cortical neurons (n=4 for each group; *P<0.05 vs GFP). (C) The level of TDP-43 is decreased in control neurons at 6 hours after cycloheximide treatment in cultured cortical neurons (n=6 for each group; *P<0.05 vs 0 h). (D) The protein level of TDP-43 is not altered following treatment for 12 hours with cycloheximide in THA-treated neurons. (E) The protein level of TDP-43 remains unchanged following treatment with cycloheximide for 12 hours in bpV(pic)-treated neurons. All bar graphs show means ± s.e.m.
We also performed cycloheximide chase experiments to determine the effect of PTEN on TDP-43 stability. The neuronal cultures were first treated with vehicle or THA (100 μM) for 3 days and then the cultures were treated with cycloheximide (20 μg/ml) for 1, 3, 6 and 12 hours (Turgeon et al., 2001). We found that the protein level of TDP-43 was significantly decreased in control neurons at 6 hours after cycloheximide treatment (Fig. 3C). By contrast, in THA-treated neurons, the protein level of TDP-43 remained unchanged following treatment with cycloheximide for 12 hours (Fig. 3D). Moreover, our data showed that bpV(pic) treatment stabilized the level of TDP-43 after 12 hours of treatment with cycloheximide (Fig. 3E). These results indicate that downregulation of PTEN leads to the enhancement of TDP-43 protein stability, which might contribute to THA-induced increase of TDP-43 expression in the early stages after glutamate accumulation.
NR2AR activation leads to PTEN downregulation and subsequent TDP-43 upregulation after glutamate accumulation
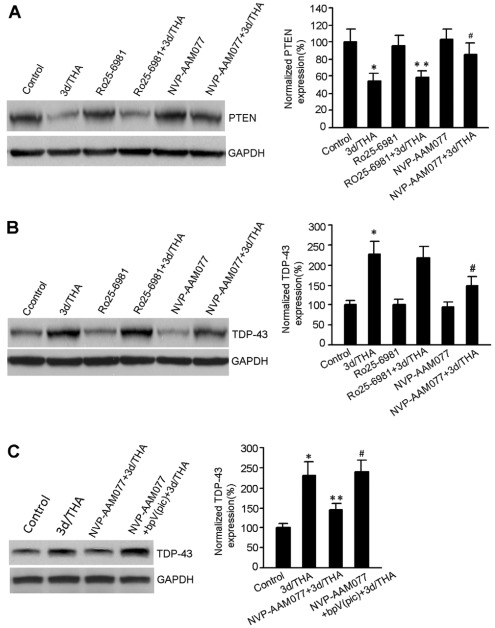
To further reveal the upstream signaling to regulate TDP-43, we tested the effects of NMDA receptors on the protein expression of both PTEN and TDP-43 after glutamate accumulation. We showed that the NR2AR antagonist NVP-AAM077 (0.4 μM) but not NR2BR antagonist Ro25-6981 (0.5 μM) attenuated THA-induced PTEN downregulation after glutamate accumulation (Fig. 4A). Although NVP-AAM077 has small degree of cross-inhibition on NR2BRs (Liu et al., 2007), this cross-effect can be ignored because our data indicated that NR2BR inhibition had no effect on TDP-43 expression (Fig. 4A). Thus, PTEN downregulation is in part mediated by NR2AR activation after glutamate accumulation.
Fig. 4.
NR2AR activation mediates PTEN downregulation and TDP-43 upregulation in early stages after THA treatment. (A) Sample immunoblots (left) and summarized data (right) show that the NR2AR antagonist NVP-AAM077 (0.4 μM) but not the NR2BR antagonist Ro25-6981 (0.5 μM) reduces THA-induced PTEN downregulation at 3 days after 100 μM THA treatment (n=7 for each group; *P<0.05 vs control; **P>0.05 vs 3d/THA; #P<0.05 vs. 3d/THA; data are normalized to control). (B) Representative immunoblots (left) and summarized data (right) show that NVP-AAM077 (0.4 μM) but not Ro25-6981 (0.5 μM) prevented upregulation of TDP-43 expression at 3 days after 100 μM THA treatment (n=6 for each group; *P<0.05 vs control; #P<0.05 vs 3d/THA; data are normalized to control). (C) Sample immunoblots (left) and summarized data (right) show that bpV(pic) (100 nM) prevented NR2AR-inhibition-induced blockade of TDP-43 upregulation in cortical neurons at 3 days after 100 μM THA treatment (n=6 for each group; *P<0.05 vs control; **P<0.05 vs 3d/THA; #P<0.05 vs NVP-AAM077+3d/THA; data are normalized to control). All bar graphs show means ± s.e.m.
As our data indicated that TDP-43 was a downstream effector of PTEN (Fig. 2), we reasoned that NR2AR activation would lead to TDP-43 upregulation through PTEN downregulation after glutamate accumulation. To verify this possibility, the effects of NVP-AAM077 (0.4 μM) and Ro25-6981 (0.5 μM) on TDP-43 expression were tested in cortical neurons treated with THA (100 μM). We showed that the inhibition of NR2ARs but not NR2BRs blocked the upregulation of TDP-43 expression at 3 days after THA treatment (Fig. 4B). We also showed that inhibiting NR2AR activity prevented THA-induced upregulation of TDP-43, and this effect was blocked by treatment of PTEN inhibitor bpV(pic) (Fig. 4C). These data suggest that NR2AR activation leads to PTEN downregulation, which in turn results in TDP-43 upregulation in the early stages after glutamate accumulation.
Decreased association of PTEN with TDP-43 in the nucleus mediates TDP-43 upregulation after glutamate accumulation
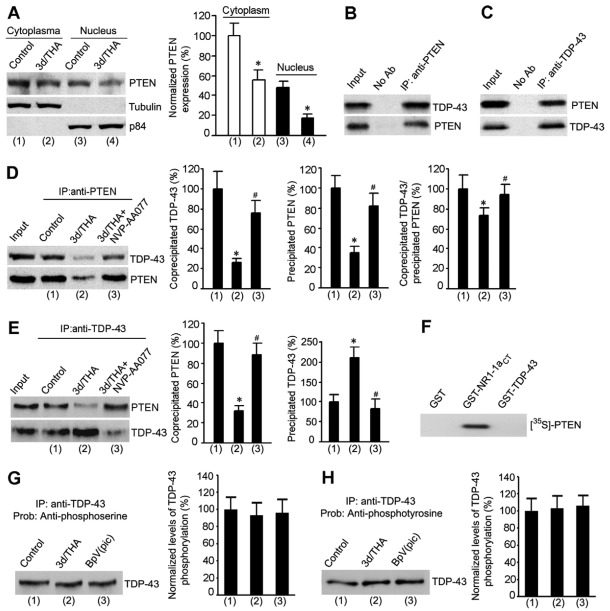
Because the downregulation of PTEN might occur in the nucleus to regulate TDP-43 expression, we performed subcellular fractionation assays to measure the PTEN levels in both the cytoplasm and nucleus. Our data showed that the protein expression of PTEN was decreased not only in the cytosolic fraction but also in the nuclear fraction at 3 days after THA treatment (Fig. 5A), suggesting that a reduced import of PTEN from cytoplasm into the nucleus leads to the decrease of PTEN in the nucleus.
Fig. 5.
The association of PTEN with TDP-43 is reduced at the early stage after glutamate accumulation. (A) Sample immunoblots (left) and summarized data (right) from subcellular fractionation assays show that the protein expression of PTEN is decreased in both cytosolic and nuclear fractions at 3 days after 100 μM THA treatment (n=6 for each group; *P<0.05 vs cytoplasm or nucleus control; data were normalized to cytoplasm control). Tubulin, a marker of cytoplasmic fraction; p85, a marker of nucleus fraction. (B) Representative immunoblots from co-immunoprecipitation assays show that TDP-43 is co-immunoprecipitated by an antibody against PTEN. No Ab, no antibody added to the assay. (C) Sample immunoblots from co-immunoprecipitation assays show that PTEN is co-immunoprecipitated by by an antibody against TDP-43. (D) Representative immunoblots (left) and summarized data (right) from co-immunoprecipitation assays show that the level of co-precipitated TDP-43 by anti-PTEN antibody is significantly reduced at 3 days after 100 μM THA treatment compared with that in control cortical neurons, and that the NR2AR antagonist NVP-AAM077 (0.4 μM) blocks the reduction of coprecipitated TDP-43 induced by THA treatment (n=6 for each group; *P<0.05 vs control; #P<0.05 vs 3d/THA; data are normalized to control). (E) Representative immunoblots (left) and summarized data (right) from co-immunoprecipitation assays show that the level of coprecipitated PTEN by anti-TDP-43 antibody is significantly decreased at 3 days after 100 μM THA treatment compared with that in control cortical neurons, and that the NR2AR antagonist NVP-AAM077 (0.4 μM) blocks the reduction of coprecipitated PTEN induced by THA treatment (n=6 for each group; *P<0.05 vs control; #P<0.05 vs 3d/THA; data are normalized to control). (F) In vitro binding assay showing the direct binding of [35S]PTEN to GST–NR1-1aCT but not GST–TDP-43. (G) Representative immunoblot (left) and summarized data (right) show that THA (treatment for 3 days) and bpV(pic) have no significant effect on the serine phosphorylation of TDP-43 (n=3 for each group). (H) Representative immunoblot (left) and summarized data (right) show that THA (treatment for 3 days) and bpV(pic) have no significant effect on the tyrosine phosphorylation of TDP-43 (n=3 for each group). All bar graphs show means ± s.e.m.
To understand how reduced nuclear PTEN led to the upregulation of TDP-43 in the nucleus, we performed a co-immunoprecipitation assay to test whether PTEN could physically associate with TDP-43 to regulate TDP-43 expression. Indeed, our data indicated that TDP-43 was co-immunoprecipitated with PTEN by an anti-PTEN antibody in the homogenates of cultured cortical neurons (Fig. 5B). Conversely, immunoprecipitation with an anti-TDP-43 antibody co-precipitated PTEN (Fig. 5C). These data indicate that PTEN forms a protein complex with TDP-43 in the nucleus to negatively regulate TDP-43 expression. To further determine whether this was also true in cortical neurons subjected to glutamate insult, we measured the association levels of PTEN with TDP-43 in cortical neurons in both control and THA-treated conditions. Our data showed that both the level of co-precipitated TDP-43 by anti-PTEN antibody and the level of co-precipitated PTEN by anti-TDP-43 antibody were significantly lower at 3 days after THA treatment compared with those in control cortical neurons (Fig. 5D,E), and that the NR2AR antagonist NVP-AAM077 blocked the reduction of co-precipitated TDP-43 or PTEN induced by THA treatment (Fig. 5D,E). Together, these results suggest that NR2AR activation/PTEN downregulation may contribute to the reduced association of PTEN with TDP-43 in the nucleus, which leads to the upregulation of TDP-43 after glutamate accumulation.
To determine whether PTEN regulated TDP-43 through direct binding, we performed an in vitro binding assay (Ning et al., 2004). Our data showed that PTEN was not directly associated with TDP-43 (Fig. 5F). To determine whether PTEN-dependent dephosphorylation was involved in the regulation of TDP-43 expression after glutamate insult, we measured the levels of serine and tyrosine phosphorylation of TDP-43 in neurons treated with THA or PTEN inhibitor (Wan et al., 1997a). TDP-43 was immunoprecipitated by antibody against TDP-43, and then anti-phosphoserine/phosphotyrosine antibodies were used to detect the phosphorylation level of precipitated TDP-43 proteins. We found that both serine and tyrosine phosphorylation of TDP-43 were not altered at 3 days after THA treatment and that PTEN inhibitor bpV(pic) had no significant effects on serine and tyrosine phosphorylation of TDP-43 (Fig. 5G,H). These data indicate that regulation of TDP-43 by PTEN is not mediated through a dephosphorylation process.
Upregulation of nuclear TDP-43 confers protection against glutamate accumulation-induced neuronal death
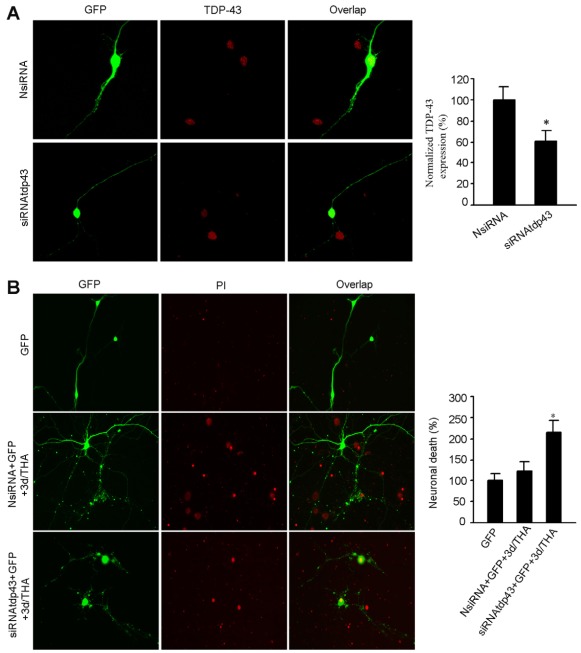
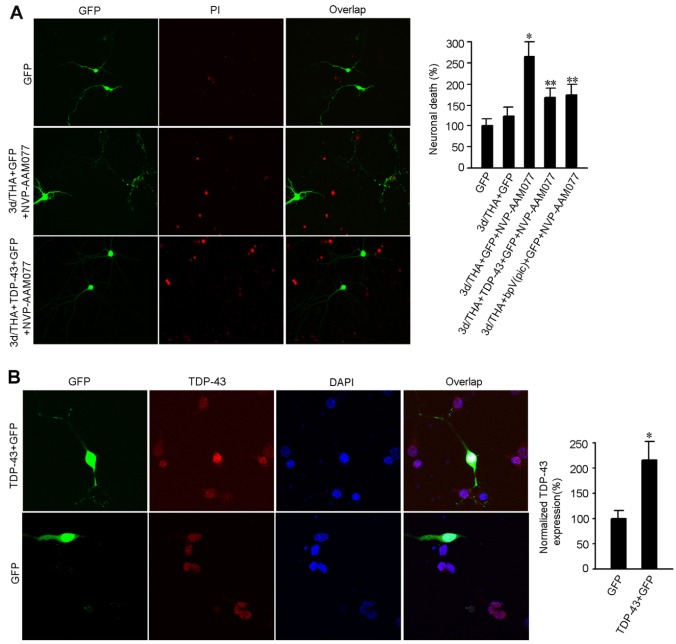
Because NR2AR activation and PTEN downregulation are believed to promote neuronal survival (Chang et al., 2007; Chen et al., 2008; Liu et al., 2007; Ning et al., 2004), it is possible that the effect delivered by nuclear TDP-43 upregulation in the early stages after glutamate accumulation represents a neuroprotective response. To test this possibility, we used siRNA to knock down TDP-43 (siRNAtdp43) in the nucleus of cultured cortical neurons (Fig. 6A). We showed that the cell death in cortical neurons transfected with siRNAtdp43 was enhanced at 3 days after THA treatment compared with the control neurons transfected with non-targeting control siRNA (NsiRNA) (Fig. 6B). These data indicate that the endogenous TDP-43 in the nucleus is a pro-survival signal in nature. To determine whether NR2AR and PTEN were upstream of TDP-43 to regulate neuronal survival, we first measured the effect of the NR2AR antagonist NVP-AAM077 on neuronal death in cortical neurons subjected to glutamate insult. We found that NR2AR inhibition by NVP-AAM077 (0.4 μM) remarkably increased cell death in cortical neurons at 3 days after THA treatment (Fig. 7A). However, the transient overexpression of TDP-43 (Fig. 7B) or inhibition of PTEN by bpV(pic) attenuated the NR2AR inhibition-induced increase of neuronal death after glutamate insult (Fig. 7A). Thus, the upregulation of TDP-43 by NR2AR activation and PTEN downregulation plays a neuroprotective role in the early stages after glutamate neurotoxicity-induced injury.
Fig. 6.
Suppressing nuclear TDP-43 increases the death of cortical neurons at early stages after THA treatment. (A) Sample images (left) and summarized data (right) indicate that TDP-43 siRNA (siRNAtdp43) but not the non-targeting control siRNA (NsiRNA) inhibits TDP-43 expression in the nucleus of normal cortical neurons (means ± s.e.m.; for each group, n=35 cells from three independent experiments; *P<0.05 vs NsiRNAtdp43; data are normalized to NsiRNA). (B) Representative images (left) and summarized data (right) show that knockdown of TDP-43 with siRNAtdp43 promotes cortical neuronal death at 3 days after 100 μM THA treatment compared with the control neurons transfected with NsiRNA (means ± s.e.m.; for each group, n=50 cells from three independent experiments; *P<0.05 vs GFP; data are normalized to GFP). The neurons were transfected with siRNAs at 1 day after THA treatment.
Fig. 7.
Upregulation of nuclear TDP-43 protects against cortical neuronal death. (A) Representative images (left) and summarized data (right) show that overexpression of TDP-43 or treatment with bpV(pic) reduces NR2AR inhibition-mediated increase of neuronal death at 3 days after 100 μM THA treatment (means ± s.e.m.; for each group, n=50 cells from three independent experiments; *P<0.05 vs GFP; **P<0.05 vs 3d/THA+GFP+NVP-AAM077; data are normalized to GFP). The neurons were transfected with cDNA encoding TDP-43 at 1 day after THA treatment. (B) Sample images (left) and summarized data (right) indicate that transient transfection of TDP-43 cDNA increases TDP-43 expression in nucleus in normal cortical neurons (means ± s.e.m.; for each group, n=30 cells from three independent experiments; *P<0.05 vs GFP; data are normalized to GFP).
Discussion
By knockdown or deletion of TDP-43 in vitro and in vivo, recent studies suggest that TDP-43 is a pro-survival protein in the nucleus and that the loss of function of nuclear TDP-43 promotes neurodegeneration (Fiesel et al., 2010; Iguchi et al., 2009). However, there is no direct evidence showing the neuroprotective effect of endogenous TDP-43 in neuronal injury and neurodegeneration. Our study provides the first evidence that nuclear TDP-43 expression is increased at the early stages in an in vitro glutamate accumulation-induced neurodegeneration model (Corse et al., 1999; Kidd and Isaac 2000; Matyja et al., 2006; Nagańska et al., 2010; Tolosa et al., 2008; Van Westerlaak et al., 2001). We demonstrate that glutamate accumulation results in an upregulation of nuclear TDP-43, and that the TDP-43 upregulation confers a neuroprotective effect. These results reveal a self-protection mechanism mediated by TDP-43 upregulation at the early stage of neurodegeneration. It would be interesting in the future to test whether TDP-43 also protects from other forms of neuronal death or cytotoxicity in different cell types.
Aberrant regulation of TDP-43 has been found to play complex roles in both the nucleus and cytoplasm in the pathogenesis of ALS (Barmada et al., 2010; Winton et al., 2008). In ALS patients, TDP-43 expression is remarkably reduced in the nucleus in the affected neurons with cytoplasmic inclusions (Cairns et al., 2007; Hasegawa et al., 2007; Neumann et al., 2006). In transgenic mice with TDP-43 overexpression, TDP-43 accumulates in the nucleus and aggregates in the cytoplasm (Wils et al., 2010). These findings indicate that a translocation of TDP-43 from the nucleus to cytoplasm might contribute to the accumulation of TDP-43 in the cytoplasmic inclusions. In the in vitro neurodegeneration model, we demonstrate that in response to glutamate accumulation, endogenous TDP-43 is only increased in the nucleus and does not translocate to cytoplasm. Thus, although TDP-43 behaves as a pro-survival signaling protein in the nucleus, the upregulation of endogenous TDP-43 in the nucleus would not lead to its translocation to the cytoplasmic compartment.
To understand the cellular and molecular mechanism by which TDP-43 plays a neuroprotective role during the neurodegeneration process, we investigated the effect of NMDA receptors on TDP-43 expression in an in vitro neurodegeneration model. We demonstrate for the first time that the upregulation of TDP-43 is mediated by the activation of NR2AR after glutamate accumulation. Recent evidence shows that NR2AR activation promotes neuronal survival in acute CNS injury such as cerebral ischemia and traumatic spinal injury (Alilain and Goshgarian, 2008; Liu et al., 2007; Terasaki et al., 2010). However, the underlying mechanisms remain largely unknown. In the present study, we identify TDP-43 as a novel downstream signaling protein of NR2ARs in a glutamate neurotoxicity-induced neurodegeneration model. Our results suggest that upregulation of TDP-43 by NR2AR activation counteracts neurodegeneration in the early stages after glutamate insult. By contrast, NR2BR is found to have no effect on TDP-43 expression in our experimental model, although previous studies, including ours, indicate that NR2BR overactivation increases neuronal death by suppression of CREB-, ERK- and PINK1-dependent survival signaling pathways (Hardingham et al., 2002; Shan et al., 2009; Wang et al., 2004). Because blocking NMDA receptors would suppress NR2AR-mediated neuroprotection while inhibiting NR2BR-mediated neuronal death, simply inhibiting NMDA receptors would not be a proper strategy to prevent neurodegeneration. This might explain why the use of glutamate antagonists as neuroprotective agents has been disappointing in clinical trials (Gredal et al., 1997; Traynor et al., 2006). Thus, investigating cellular and molecular mechanism mediating the differential role of NR2AR and NR2BR in neuronal survival or death is crucial for us to develop a selective neuroprotection strategy for the treatment of ischemic or traumatic CNS injury and neurodegenerative diseases.
Our results also show that PTEN downregulation mediates NR2AR-dependent elevation of nuclear TDP-43 following glutamate accumulation. Interestingly, we provide evidence that the downregulation of PTEN leads to a reduced association of PTEN with TDP-43 in the nucleus, which contributes to the increased nuclear expression of TDP-43 after glutamate accumulation. It is unclear how the decreased association of PTEN and TDP-43 affects TDP-43 levels. However, based on our evidence that: (1) TDP-43 expression is negatively regulated by PTEN (Fig. 2); (2) PTEN does not directly interact with TDP-43 (Fig. 5F); (3) PTEN has no effect on serine/tyrosine phosphorylation of TDP-43 (Fig. 5G,H); and (4) PTEN downregulation enhances the stability of TDP-43, we reason that the decrease in the PTEN–TDP-43 association might indirectly lead to a relief of TDP-43 inhibition by PTEN in a phosphorylation-independent manner, which enhances the stability of TDP-43 and thus increases TDP-43 expression. Future studies are required to further address how this process occurs.
In summary, the present study provides the first evidence that upregulation of TDP-43, mediated by NR2AR activation and PTEN downregulation, confers neuroprotection in the early stages after glutamate accumulation. Given that NMDA receptors, PTEN and TDP-43 are involved in various CNS disorders, the NR2AR–PTEN–TDP-43 signaling might represent novel therapeutic targets for the development of a neuroprotective strategy.
Materials and Methods
Culture of mouse cortical motor neurons and THA treatment
The cortical neuronal cultures were prepared from C57BL/6 mice at gestational day 17 using a modified protocol (Shan et al., 2009). After removing meninges, cortices were placed in ice-cold plating medium (Neurobasal medium, 2% B-27 supplement, 0.5% FBS, 0.5 μM L-glutamax and 25 μM glutamic acid). Dissociated motor neurons were prepared from precentral gyrus that was carefully dissected from the cortices. The neurons were suspended in plating medium and plated on Petri dishes coated with poly-D-lysine. After 3 days in culture, half of the plating medium was removed and replaced with maintenance medium (Neurobasal medium, 2% B-27 supplement, and 0.5 μM L-glutamine). Thereafter, maintenance medium was changed in the same manner every 3 days. To induce chronic glutamate neurotoxicity, the cultures were exposed to THA (100 μM) at 8 days after plating. The cultured neurons were collected for experiments at 1, 3 and 6 days after THA treatment.
Western blotting, co-immunoprecipitation and in vitro binding assays
Western blotting, co-immunoprecipitation and in vitro binding assays were performed as reported previously (Ning et al., 2004). For western blotting, total proteins were extracted with lysis buffer. Equal amounts of proteins were separated by 8–10% SDS polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were electronically transferred to polyvinylidene difluoride membrane and incubated with a blocking buffer for 1 hour at room temperature. The membranes were incubated with primary antibodies overnight at 4°C, and then incubated with HRP-conjugated secondary antibodies for 1 hour at room temperature. The anti-TDP-43 antibody was purchased from Santa Cruz Biotech (Santa Cruz, CA) and the anti-PTEN antibody was purchased from Chemicon (Temecula, CA). The protein bands were imaged using ECL (Amersham Pharmacia Biotech). The membranes were re-probed with anti-GAPDH antibody as a loading control. For co-immunoprecipitation experiments, the cell lysates (500 μg proteins in 1 ml) were pre-absorbed with 20 μl protein A/G agarose beads at 4°C for 30 minutes, spun at 10,000 r.p.m. for 10 seconds, and the supernatant was incubated with specific primary antibody at 4°C overnight. After incubation with 20 μl protein A/G agarose beads for 1.5 hours at 4°C, the immunocomplexes were collected by centrifugation and washed three times with ice-cold washing buffer. The final products were boiled for 5 minutes and resolved with SDS-PAGE, and immunoblotted with specific antibodies. Images were analyzed using ImageJ software (Version 1.34). For in vitro binding assay, GST–NR1-1aCT, GST–TDP-43 or GST (10 μg) alone was incubated with [35S]PTEN probe for 4 hours at room temperature. The beads were washed six times with tissue homogenizing buffer and eluted with 10 mM glutathione. Eluates were separated by SDS-PAGE and visualized by autoradiography (Ning et al., 2004).
Immunocytochemical staining
Immunocytochemical staining was performed as described previously (Liu et al., 2006; Wan et al., 1997b). Briefly, the transfected cells were fixed with 4% paraformaldehyde and blocked in 5% normal goat serum. The specimen was incubated with primary antibody overnight at 4°C and then incubated with fluorochrome-conjugated secondary antibody for 1 hour at room temperature. The primary anti-TDP-43 antibody was purchased from Santa Cruz Biotech (Santa Cruz, CA). Secondary antibodies, Alexa Fluor 594 (red fluorescence) and Alexa Fluor 488 (green fluorescence), were purchased from Invitrogen (Carlsbad, CA). DAPI or propidium iodide (PI) was used to label the nuclei. Fluorescent-labeled proteins were imaged using a 63× or 40× objective mounted on a Zeiss LSM 510 META confocal microscope (Oberkochen, Germany) as described previously (Liu et al., 2006; Wan et al., 1997b). Images were acquired using a Zeiss AxioCam digital camera in the linear range with constant settings and were analyzed using ImageJ software. Each image was a z-series of 6–13 images, taken at 0.75-μm-depth intervals. The resultant stack was ‘flattened’ into a single image using a maximum projection. For individual experiments, all images in all experiments were analyzed using identical acquisition parameters. During data acquisition and analysis, the investigator was blind to the treatment group.
Transfection
The small interfering RNAs specific to mouse TDP-43 and PTEN (siRNAtdp43 and siRNApten) and non-targeting control siRNA (NsiRNA) were purchased from Santa Cruz Biotech (Santa Cruz, CA). The cortical neurons were transfected with using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer's protocol (Ning et al., 2004). cDNA encoding green fluorescent protein (GFP) was used as a marker of successful neuronal transfection. The cells were fixed for immunocytochemical labeling at 48 hours after transfection.
Subcellular fractionation assay
The assay was performed as described (Ayala et al., 2008). Briefly, cultured neurons were harvested and washed in PBS by repeated centrifugation. The experiment was carried out at 4°C. The pellet was resuspended in five volumes of buffer N [15 mM Tris-HCl, pH 7.5, 60 mM KCl, 15 mM NaCl, 5 mM MgCl2, 1 mM CaCl2, 1 mM DTT, 2 mM Na3VO4, 1 mM PMSF, 0.25 M sucrose, Complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN)]. Cell lysis was obtained by adding an equal amount of buffer N plus 0.6% NP-40. After incubation for 5 minutes, nuclei were pelleted and gently resuspended in 1 ml of buffer N. The nuclei were again pelleted and lysed using an equal volume of solution 2 (10 mM HEPES, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.1 mM EGTA, 0.1 mM DTT, 0.5 mM PMSF, 5% glycerol, 0.4 M NaCl). The nuclear fraction was cleared by centrifugation following incubation for 30 minutes. Nuclear and cytoplasmic fractions were quantified and visualized by western blotting.
Neuronal death assays
Lactate dehydrogenase (LDH) is a cytoplasmic enzyme retained by viable cells with intact plasma membranes and released from cells with damaged membranes. LDH release was measured using a CytoTox 96 Cytotoxicity kit based on the manufacturer's instructions (Promega, Madison, WI) (Shan et al., 2009). The levels of maximal neuronal death were measured by treating the cultures with 10× lysis solution to yield complete lysis of the cells. Absorbance data were obtained using a 96-well plate reader at 490 nm.
PI labeling was performed to detect neuronal death as described in our previous study (Ning et al., 2004). Briefly, all the experimental cultures were plated with equal number of cells. The culture medium of cells co-transfected with GFP and specific siRNAs or cDNAs was replaced by extracellular solution containing PI at a final concentration of 50 μg/ml. After incubation for 20 minutes in an ambient gas incubator at 37°C, cultures were washed with extracellular solution and then fixed with 4% paraformaldehyde. Neuronal death (percentage) was determined by calculating the number of PI-labeled neurons expressing GFP. The investigator for the cell count was blinded to the experimental treatment.
Statistics
All population data were expressed as means ± s.e.m. The Student's t-test or the ANOVA test was used when appropriate. Statistical significance was placed at P<0.05.
Acknowledgements
We thank James Shen and Virginia Lee for providing us with TDP-43 cDNA constructs. We also thank Y. P. Auberson for the gift of NVP-AAM077.
Footnotes
Funding
This study was supported by University of Nevada Start-up fund and grants from the National Center for Research Resources [grant number RR024210] and the National Institute of General Medical Sciences [grant number GM103554] from the National Institutes of Health. Deposited in PMC for release after 12 months.
References
- Alilain W. J., Goshgarian H. G. (2008). Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp. Neurol. 212, 348-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annis C. M., Vaughn J. E. (1998). Differential vulnerability of autonomic and somatic motor neurons to N-methyl-D-aspartate-induced excitotoxicity. Neuroscience 83, 239-249 [DOI] [PubMed] [Google Scholar]
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., et al. (2006). TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351, 602-611 [DOI] [PubMed] [Google Scholar]
- Arai T., Mackenzie I. R., Hasegawa M., Nonoka T., Niizato K., Tsuchiya K., Iritani S., Onaya M., Akiyama H. (2009). Phosphorylated TDP-43 in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathol. 117, 125-136 [DOI] [PubMed] [Google Scholar]
- Arundine M., Tymianski M. (2004). Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell. Mol. Life Sci. 61, 657-668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala Y. M., Zago P., D'Ambrogio A., Xu Y. F., Petrucelli L., Buratti E., Baralle F. E. (2008). Structural determinants of the cellular localization and shuttling of TDP-43. J. Cell Sci. 121, 3778-3785 [DOI] [PubMed] [Google Scholar]
- Barmada S. J., Skibinski G., Korb E., Rao E. J., Wu J. Y., Finkbeiner S. (2010). Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis. J. Neurosci. 30, 639-649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet N., Tarabal O., Esquerda J. E., Calderó J. (2009). Excitotoxic motoneuron degeneration induced by glutamate receptor agonists and mitochondrial toxins in organotypic cultures of chick embryo spinal cord. J. Comp. Neurol. 516, 277-290 [DOI] [PubMed] [Google Scholar]
- Cairns N. J., Neumann M., Bigio E. H., Holm I. E., Troost D., Hatanpaa K. J., Foong C., White C. L., 3rd, Schneider J. A., Kretzschmar H. A., et al. (2007). TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am. J. Pathol. 171, 227-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N., El-Hayek Y. H., Gomez E., Wan Q. (2007). Phosphatase PTEN in neuronal injury and brain disorders. Trends Neurosci. 30, 581-586 [DOI] [PubMed] [Google Scholar]
- Chen M., Lu T. J., Chen X. J., Zhou Y., Chen Q., Feng X. Y., Xu L., Duan W. H., Xiong Z. Q. (2008). Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke 39, 3042-3048 [DOI] [PubMed] [Google Scholar]
- Corse A. M., Bilak M. M., Bilak S. R., Lehar M., Rothstein J. D., Kuncl R. W. (1999). Preclinical testing of neuroprotective neurotrophic factors in a model of chronic motor neuron degeneration. Neurobiol. Dis. 6, 335-346 [DOI] [PubMed] [Google Scholar]
- Culcasi M., Lafon-Cazal M., Pietri S., Bockaert J. (1994). Glutamate receptors induce a burst of superoxide via activation of nitric oxide synthase in arginine-depleted neurons. J. Biol. Chem. 269, 12589-12593 [PubMed] [Google Scholar]
- DeRidder M. N., Simon M. J., Siman R., Auberson Y. P., Raghupathi R., Meaney D. F. (2006). Traumatic mechanical injury to the hippocampus in vitro causes regional caspase-3 and calpain activation that is influenced by NMDA receptor subunit composition. Neurobiol. Dis. 22, 165-176 [DOI] [PubMed] [Google Scholar]
- Dingledine R., Borges K., Bowie D., Traynelis S. F. (1999). The glutamate receptor ion channels. Pharmacol. Rev. 51, 7-61 [PubMed] [Google Scholar]
- Fiesel F. C., Voigt A., Weber S. S., Van den Haute C., Waldenmaier A., Görner K., Walter M., Anderson M. L., Kern J. V., Rasse T. M., et al. (2010). Knockdown of transactive response DNA-binding protein (TDP-43) downregulates histone deacetylase 6. EMBO J. 29, 209-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszman M. L., Ricart K. C., Latini A., Rodríguez G., Sica R. E. (2010). In vitro neurotoxic properties and excitatory aminoacids concentration in the cerebrospinal fluid of amyotrophic lateral sclerosis patients. Relationship with the degree of certainty of disease diagnoses. Acta Neurol. Scand. 121, 120-126 [DOI] [PubMed] [Google Scholar]
- Gil A., Andrés-Pons A., Fernández E., Valiente M., Torres J., Cervera J., Pulido R. (2006). Nuclear localization of PTEN by a Ran-dependent mechanism enhances apoptosis: Involvement of an N-terminal nuclear localization domain and multiple nuclear exclusion motifs. Mol. Biol. Cell 17, 4002-4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredal O., Werdelin L., Bak S., Christensen P. B., Boysen G., Kristensen M. O., Jespersen J. H., Regeur L., Hinge H. H., Jensen T. S. (1997). A clinical trial of dextromethorphan in amyotrophic lateral sclerosis. Acta Neurol. Scand. 96, 8-13 [DOI] [PubMed] [Google Scholar]
- Grosskreutz J., Van Den Bosch L., Keller B. U. (2010). Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium 47, 165-174 [DOI] [PubMed] [Google Scholar]
- Hanson K. A., Kim S. H., Wassarman D. A., Tibbetts R. S. (2010). Ubiquilin modifies TDP-43 toxicity in a Drosophila model of amyotrophic lateral sclerosis (ALS). J. Biol. Chem. 285, 11068-11072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham G. E., Fukunaga Y., Bading H. (2002). Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 5, 405-414 [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Arai T., Akiyama H., Nonaka T., Mori H., Hashimoto T., Yamazaki M., Oyanagi K. (2007). TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain 130, 1386-1394 [DOI] [PubMed] [Google Scholar]
- Igaz L. M., Kwong L. K., Xu Y., Truax A. C., Uryu K., Neumann M., Clark C. M., Elman L. B., Miller B. L., Grossman M., et al. (2008). Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Am. J. Pathol. 173, 182-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi Y., Katsuno M., Niwa J., Yamada S., Sone J., Waza M., Adachi H., Tanaka F., Nagata K., Arimura N., et al. (2009). TDP-43 depletion induces neuronal cell damage through dysregulation of Rho family GTPases. J. Biol. Chem. 284, 22059-22066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd F. L., Isaac J. T. (2000). Glutamate transport blockade has a differential effect on AMPA and NMDA receptor-mediated synaptic transmission in the developing barrel cortex. Neuropharmacology 39, 725-732 [DOI] [PubMed] [Google Scholar]
- Kraemer B. C., Schuck T., Wheeler J. M., Robinson L. C., Trojanowski J. Q., Lee V. M., Schellenberg G. D. (2010). Loss of murine TDP-43 disrupts motor function and plays an essential role in embryogenesis. Acta Neuropathol. 119, 409-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon-Cazal M., Pietri S., Culcasi M., Bockaert J. (1993). NMDA-dependent superoxide production and neurotoxicity. Nature 364, 535-537 [DOI] [PubMed] [Google Scholar]
- Lee J. M., Zipfel G. J., Choi D. W. (1999). The changing landscape of ischaemic brain injury mechanisms. Nature 399 Suppl. A7-A14 [DOI] [PubMed] [Google Scholar]
- Liu B., Liao M., Mielke J. G., Ning K., Chen Y., Li L., El-Hayek Y. H., Gomez E., Zukin R. S., Fehlings M. G., et al. (2006). Ischemic insults direct glutamate receptor subunit 2-lacking AMPA receptors to synaptic sites. J. Neurosci. 26, 5309-5319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wong T. P., Aarts M., Rooyakkers A., Liu L., Lai T. W., Wu D. C., Lu J., Tymianski M., Craig A. M., et al. (2007). NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 27, 2846-2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie I. R., Bigio E. H., Ince P. G., Geser F., Neumann M., Cairns N. J., Kwong L. K., Forman M. S., Ravits J., Stewart H., et al. (2007). Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann. Neurol. 61, 427-434 [DOI] [PubMed] [Google Scholar]
- Matyja E., Taraszewska A., Nagańska E., Rafałowska J., Gebarowska J. (2006). Astroglial alterations in amyotrophic lateral sclerosis (ALS) model of slow glutamate excitotoxicity in vitro. Folia Neuropathol. 44, 183-190 [PubMed] [Google Scholar]
- Myers M. P., Pass I., Batty I. H., Van der Kaay J., Stolarov J. P., Hemmings B. A., Wigler M. H., Downes C. P., Tonks N. K. (1998). The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc. Natl. Acad. Sci. USA 95, 13513-13518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagańska E., Taraszewska A., Matyja E., Grieb P., Rafałowska J. (2010). Neuroprotective effect of erythropoietin in amyotrophic lateral sclerosis (ALS) model in vitro. Ultrastructural study. Folia Neuropathol. 48, 35-44 [PubMed] [Google Scholar]
- Neumann M., Sampathu D. M., Kwong L. K., Truax A. C., Micsenyi M. C., Chou T. T., Bruce J., Schuck T., Grossman M., Clark C. M., et al. (2006). Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130-133 [DOI] [PubMed] [Google Scholar]
- Ning K., Pei L., Liao M., Liu B., Zhang Y., Jiang W., Mielke J. G., Li L., Chen Y., El-Hayek Y. H., et al. (2004). Dual neuroprotective signaling mediated by downregulating two distinct phosphatase activities of PTEN. J. Neurosci. 24, 4052-4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry T. L., Hansen S., Jones K. (1987). Brain glutamate deficiency in amyotrophic lateral sclerosis. Neurology 37, 1845-1848 [DOI] [PubMed] [Google Scholar]
- Plaitakis A., Caroscio J. T. (1987). Abnormal glutamate metabolism in amyotrophic lateral sclerosis. Ann. Neurol. 22, 575-579 [DOI] [PubMed] [Google Scholar]
- Planchon S. M., Waite K. A., Eng C. (2008). The nuclear affairs of PTEN. J. Cell Sci. 121, 249-253 [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Tsai G., Kuncl R. W., Clawson L., Cornblath D. R., Drachman D. B., Pestronk A., Stauch B. L., Coyle J. T. (1990). Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann. Neurol. 28, 18-25 [DOI] [PubMed] [Google Scholar]
- Sarraf-Yazdi S., Sheng H., Miura Y., McFarlane C., Dexter F., Pearlstein R., Warner D. S. (1998). Relative neuroprotective effects of dizocilpine and isoflurane during focal cerebral ischemia in the rat. Anesth. Analg. 87, 72-78 [DOI] [PubMed] [Google Scholar]
- Schmid A. C., Byrne R. D., Vilar R., Woscholski R. (2004). Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 566, 35-38 [DOI] [PubMed] [Google Scholar]
- Sen I., Nalini A., Joshi N. B., Joshi P. G. (2005). Cerebrospinal fluid from amyotrophic lateral sclerosis patients preferentially elevates intracellular calcium and toxicity in motor neurons via AMPA/kainate receptor. J. Neurol. Sci. 235, 45-54 [DOI] [PubMed] [Google Scholar]
- Sephton C. F., Good S. K., Atkin S., Dewey C. M., Mayer P., 3rd, Herz J., Yu G. (2010). TDP-43 is a developmentally regulated protein essential for early embryonic development. J. Biol. Chem. 285, 6826-6834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y., Liu B., Li L., Chang N., Li L., Wang H., Wang D., Feng H., Cheung C., Liao M., et al. (2009). Regulation of PINK1 by NR2B-containing NMDA receptors in ischemic neuronal injury. J. Neurochem. 111, 1149-1160 [DOI] [PubMed] [Google Scholar]
- Sreedharan J., Blair I. P., Tripathi V. B., Hu X., Vance C., Rogelj B., Ackerley S., Durnall J. C., Williams K. L., Buratti E., et al. (2008). TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319, 1668-1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Gu J., Matsumoto K., Aota S., Parsons R., Yamada K. M. (1998). Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280, 1614-1617 [DOI] [PubMed] [Google Scholar]
- Terasaki Y., Sasaki T., Yagita Y., Okazaki S., Sugiyama Y., Oyama N., Omura-Matsuoka E., Sakoda S., Kitagawa K. (2010). Activation of NR2A receptors induces ischemic tolerance through CREB signaling. J. Cereb. Blood Flow Metab. 30, 1441-1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosa L., Mir M., Asensio V. J., Olmos G., Lladó J. (2008). Vascular endothelial growth factor protects spinal cord motoneurons against glutamate-induced excitotoxicity via phosphatidylinositol 3-kinase. J. Neurochem. 105, 1080-1090 [DOI] [PubMed] [Google Scholar]
- Traynor B. J., Bruijn L., Conwit R., Beal F., O'Neill G., Fagan S. C., Cudkowicz M. E. (2006). Neuroprotective agents for clinical trials in ALS: a systematic assessment. Neurology 67, 20-27 [DOI] [PubMed] [Google Scholar]
- Turgeon D., Carrier J. S., Lévesque E., Hum D. W., Bélanger A. (2001). Relative enzymatic activity, protein stability, and tissue distribution of human steroid-metabolizing UGT2B subfamily members. Endocrinology 142, 778-787 [DOI] [PubMed] [Google Scholar]
- Van Westerlaak M. G., Joosten E. A., Gribnau A. A., Cools A. R., Bär P. R. (2001). Chronic mitochondrial inhibition induces glutamate-mediated corticomotoneuron death in an organotypic culture model. Exp. Neurol. 167, 393-400 [DOI] [PubMed] [Google Scholar]
- Vanhoutte P., Bading H. (2003). Opposing roles of synaptic and extrasynaptic NMDA receptors in neuronal calcium signalling and BDNF gene regulation. Curr. Opin. Neurobiol. 13, 366-371 [DOI] [PubMed] [Google Scholar]
- Wan Q., Man H. Y., Braunton J., Wang W., Salter M. W., Becker L., Wang Y. T. (1997a). Modulation of GABAA receptor function by tyrosine phosphorylation of beta subunits. J. Neurosci. 17, 5062-5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q., Xiong Z. G., Man H. Y., Ackerley C. A., Braunton J., Lu W. Y., Becker L. E., MacDonald J. F., Wang Y. T. (1997b). Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature 388, 686-690 [DOI] [PubMed] [Google Scholar]
- Wang J. Q., Tang Q., Parelkar N. K., Liu Z., Samdani S., Choe E. S., Yang L., Mao L. (2004). Glutamate signaling to Ras-MAPK in striatal neurons: mechanisms for inducible gene expression and plasticity. Mol. Neurobiol. 29, 1-14 [DOI] [PubMed] [Google Scholar]
- Weng L. P., Brown J. L., Eng C. (2001). PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum. Mol. Genet. 10, 599-604 [DOI] [PubMed] [Google Scholar]
- Wils H., Kleinberger G., Janssens J., Pereson S., Joris G., Cuijt I., Smits V., Ceuterick-de Groote C., Van Broeckhoven C., Kumar-Singh S. (2010). TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc. Natl. Acad. Sci. USA 107, 3858-3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winton M. J., Igaz L. M., Wong M. M., Kwong L. K., Trojanowski J. Q., Lee V. M. (2008). Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J. Biol. Chem. 283, 13302-13309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. S., Cheng W. C., Hou S. C., Yan Y. T., Jiang S. T., Shen C. K. (2010). TDP-43, a neuro-pathosignature factor, is essential for early mouse embryogenesis. Genesis 48, 56-62 [DOI] [PubMed] [Google Scholar]