Abstract
Epithelial junctions depend on intercellular interactions between β1 subunits of the Na+/K+-ATPase molecules of neighboring cells. The interaction between dog and rat subunits is less effective than the interaction between two dog β1 subunits, indicating the importance of species-specific regions for β1–β1 binding. To identify these regions, the species-specific amino acid residues were mapped on a high-resolution structure of the Na+/K+-ATPase β1 subunit to select those exposed towards the β1 subunit of the neighboring cell. These exposed residues were mutated in both dog and rat YFP-linked β1 subunits (YFP–β1) and also in the secreted extracellular domain of the dog β1 subunit. Five rat-like mutations in the amino acid region spanning residues 198–207 of the dog YFP–β1 expressed in Madin–Darby canine kidney (MDCK) cells decreased co-precipitation of the endogenous dog β1 subunit with YFP–β1 to the level observed between dog β1 and rat YFP–β1. In parallel, these mutations impaired the recognition of YFP–β1 by the dog-specific antibody that inhibits cell adhesion between MDCK cells. Accordingly, dog-like mutations in rat YFP–β1 increased both the (YFP–β1)–β1 interaction in MDCK cells and recognition by the antibody. Conversely, rat-like mutations in the secreted extracellular domain of the dog β1 subunit increased its interaction with rat YFP–β1 in vitro. In addition, these mutations resulted in a reduction of intercellular adhesion between rat lung epithelial cells following addition of the secreted extracellular domain of the dog β1 subunit to a cell suspension. Therefore, the amino acid region 198–207 is crucial for both trans-dimerization of the Na+/K+-ATPase β1 subunits and cell–cell adhesion.
Key words: Na+/K+-ATPase β1 subunit, Epithelial junction, Trans-dimerization
Introduction
The Na+/K+-ATPase (also known as the Na,K-ATPase or sodium/potassium-transporting ATPase) plays a crucial role in epithelial functions. Located in the basolateral membrane, it generates ion gradients that maintain the membrane potential and drive vectorial sodium-dependent transepithelial transport of various solutes. An effective function of the Na+/K+-ATPase in driving vectorial transepithelial transport is ensured by the presence of both tight junctions and adherens junctions. Tight junctions restrict paracellular diffusion of solutes and maintain the polar distribution of basolateral and apical membrane transporters (Steed et al., 2010). Adherens junctions are crucial for the initiation and stabilization of intercellular contacts and are required for the formation and maintenance of the tight junctions (Baum and Georgiou, 2011; Gumbiner, 2005; Irie et al., 2004).
Tight and adherens junctions have a common structural organization that comprises cell adhesion molecules and cytosolic anchoring proteins (Hartsock and Nelson, 2008). Cell adhesion molecules embedded in apposed membranes undergo the trans (cell-to-cell) dimerization by means of the interactions between their extracellular domains, whereas anchoring proteins link cytoplasmic domains of cell adhesion molecules to the cytoskeleton and initiate various signaling pathways (Hartsock and Nelson, 2008; Miyoshi and Takai, 2005; Steed et al., 2010).
E-cadherin is a major cell adhesion molecule of adherens junctions in epithelial cells (Shapiro and Weis, 2009). Homotypic trans-dimerization of E-cadherin is crucial for initiation of cell–cell contact, whereas homotypic cis-dimerization is required for stabilization of adherens junctions (Wu et al., 2010). In addition to E-cadherin, another class of transmembrane proteins, nectins, have been recently identified as cell adhesion molecules in adherens junctions (Miyoshi and Takai, 2007; Takai et al., 2003). Nectins are thought to initiate cell–cell contact by forming hetero-trans dimers even before E-cadherin dimerization, which is then elaborated by nectin-facilitated cadherin recruitment. Nectin and E-cadherin are physically associated with each other through their cytoplasmic-domain-associated proteins (Sakisaka et al., 2007).
Recent data have shown that the Na+/K+-ATPase also acts as a cell adhesion molecule in adherens junctions. The cytoplasmic domain of the Na+/K+-ATPase is linked to the cytoplasmic domain of E-cadherin via an ankyrin and spectrin cytoskeleton (Kizhatil et al., 2007; Nelson et al., 1990; Nelson and Veshnock, 1987), whereas the extracellular domain of the Na+/K+-ATPase β1 subunit interacts with the β1 subunit of the apposed cell (Padilla-Benavides et al., 2010; Tokhtaeva et al., 2011). The removal of N-glycans from the Na+/K+-ATPase β1 subunit or alteration of the amino acid sequence of one of the interacting β1 subunits by ‘swapping species’ weakens β1–β1 binding (Tokhtaeva et al., 2011), indicating that both N-glycans and particular amino acids are important for binding. The impairment of β1–β1 binding, in turn, decreases the detergent resistance of junctional proteins and increases paracellular permeability. Conversely, improvement of β1–β1 binding by reduction of N-glycan branching with specific inhibitors improves β1–β1 interaction and decreases both extractability of junctional proteins and paracellular permeability (Tokhtaeva et al., 2011; Vagin et al., 2008). Therefore, interactions between the Na+/K+-ATPase β1 subunits are important for the integrity of intercellular junctions.
Here, by using site-directed mutagenesis of species-specific residues in the extracellular domains of YFP-linked dog and rat β1 subunits (YFP–β1), as well as in the secreted extracellular domain of the dog β1 subunit (Sec-dog β1), we found that the amino acid region 198–207 exposed towards the neighboring cell in cell monolayers is crucial for homotypic intercellular interactions between the Na+/K+-ATPase β1 subunits.
Results
Rat-like mutations in the amino acid region 198–207 of the YFP–dog-β1 impair its interaction with the endogenous dog β1 subunit in MDCK cells
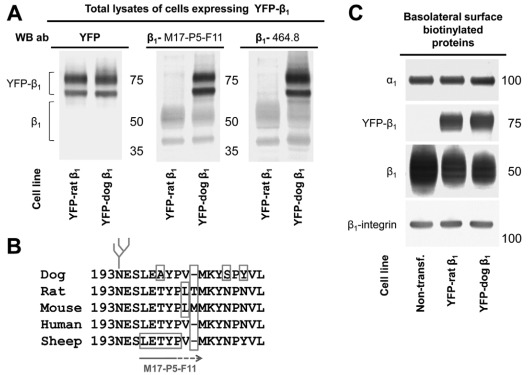
Stable cell lines of MDCK cells expressing either dog or rat YFP–β1 have both YFP-linked and endogenous β1 subunits (Fig. 1A). The endogenous β1 subunits of MDCK cells, which are of dog origin, react with M17-P5-F11 monoclonal antibodies raised against sheep β1 subunits and with 464.8 monoclonal antibodies raised against rabbit β1 subunits (Fig. 1A). Accordingly, YFP-linked dog β1 subunits also react with both M17-P5-F11 and 464.8 antibodies (Fig. 1A). By contrast, YFP–rat-β1 is not recognized by M17-P5-F11 or 464.8 antibodies against β1 (Fig. 1A). As expected, antibodies against YFP react with both dog and rat YFP–β1 but not with endogenous β1 subunits (Fig. 1A). The epitope for the M17-P5-F11 antibody, L196ETYP (Fig. 1B), has been identified by screening a bacteriophage epitope library (Sun and Ball, 1994). However, the L196ETYP regions are present in both rat and sheep β1 subunits, but rat and sheep sequences differ from each other immediately downstream of the L196ETYP region. The lack of reactivity of the M17-P5-F11 antibody with the rat β1 subunit indicates that the epitope for the M17-P5-F11 antibody extends beyond the L196ETYP region, as shown by the arrow below the sequence alignment (Fig. 1B).
Fig. 1.
The monoclonal antibodies raised against the rabbit or sheep β1 subunit react with YFP–dog-β1 but not with YFP–rat-β1. (A) Western blot analysis of total lysates of MDCK cells expressing either YFP–dog-β1 or YFP–rat-β1 using antibodies against YFP and two types of monoclonal antibodies to β1. Both 464.8 and M17-P5-F11 anti-β1 antibodies, raised against rabbit and sheep subunits, respectively, react with the endogenous β1 subunit and YFP–dog-β1 but do not react with YFP–rat-β1. Antibodies to YFP react both with YFP–dog-β1 and with YFP–rat-β1. (B) Multi-species sequence alignment of the region containing the epitope of M17-P5-F11 antibody as deduced from screening a bacteriophage epitope library (Sun and Ball, 1994). The epitope of the 464.1 antibody is unknown. (C) Western blot of proteins isolated by basolateral surface-selective biotinylation showing the presence of both rat and YFP–dog-β1 fusion proteins in the basolateral membranes. Expression of either YFP–dog-β1 or YFP–rat-β1 in MDCK cells resulted in a substantial decrease in the amount of the endogenous Na+/K+-ATPase β1 subunits in the basolateral membranes but did not change the level of the α1 subunits in comparison with that of non-transfected (‘Non-transf.’) cells. β1-integrin was used as a loading control.
Both dog and rat YFP–β1 were detected in the basolateral membrane by surface-specific basolateral biotinylation (Fig. 1C). The amount of the endogenous β1 subunits in the basolateral membrane in both transfected cell lines was less than that in non-transfected cells, whereas the levels of the α1 subunit were similar in all three cell lines (Fig. 1C). These results are consistent with previously published data showing that expressed YFP–β1 competes with the endogenous Na+/K+-ATPase β1 subunit for binding to the α1 subunit in the endoplasmic reticulum (ER) and replaces a fraction of the endogenous α1 subunits in the β1–β1 complexes (Tokhtaeva et al., 2009). As only assembled Na+/K+-ATPase molecules are transported from the ER to the plasma membrane (Tokhtaeva et al., 2009), expression of either YFP–dog-β1 or YFP–rat-β1 results in a decrease of the amount of the endogenous β1 subunit in the basolateral membrane (Fig. 1C).
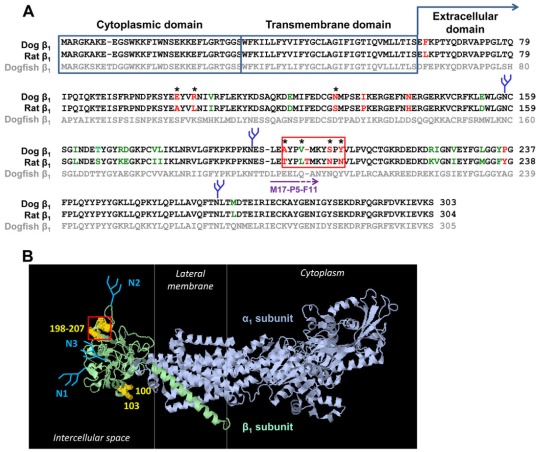
The presence of both β1–(YFP–β1) and β1–β1 in the basolateral membranes enables an assessment of β1–β1 interactions by detecting the amount of the endogenous β1 subunits that co-immunoprecipitate with YFP–β1. Co-immunoprecipitation of the endogenous β1 subunits was considerably more effective with YFP–dog-β1 than with YFP–rat-β1 (Tokhtaeva et al., 2011). As the number and positions of N-glycans are the same in dog and rat β1 subunits, these results indicate that species-specific amino acid regions are important for β1–β1 binding. To predict the putative β1–β1 binding residues, the amino acid sequences of the dog and rat β1 subunits were compared with each other and with the sequence of the chain B of the high-resolution structure of the Na+/K+-ATPase (Shinoda et al., 2009) (dogfish β1 subunit) (Fig. 2). The amino acid sequences of cytoplasmic and transmembrane domains of dog and rat β1 subunits are identical, whereas the extracellular domains contain 26 mismatches (Fig. 2A). The majority of these mismatched residues are conserved in dog and rat subunits (Fig. 2A, green font), but 11 residues are substantially different (Fig. 2A, red font). Mapping the residues that differ in rat and dog subunits on the high-resolution structure of the Na+/K+-ATPase (Shinoda et al., 2009) (Fig. 2B) identified the species-specific residues that are exposed on the surface of the β1 subunit extracellular domain (Fig. 2A, residues marked by asterisks). Some of these residues, such as residues 100, 103 and 106, are located on the lateral surface of the extracellular domain (Fig. 2B) and hence cannot be involved in intercellular interactions between the β1 subunits of neighboring cells, but they could be involved in cis interactions. Other residues are exposed towards the neighboring cell and, therefore, can be considered as candidates for mediators of the trans interactions between the β1 subunits of apposed cells. Particularly, the region highlighted by the red box contains three residues that differ in dog and rat subunits and one residue that shows a conservative change in the dog and rat subunits (Fig. 2A). All these residues are exposed on the surface facing the neighboring cell (Fig. 2B). In addition, this region contains one residue (Thr) that is present in the rat subunit but not in the dog subunit (Fig. 2A).
Fig. 2.
Prediction of putative β1–β1 interacting amino acid residues. (A) A computational alignment of amino acid sequences of the Na+/K+-ATPase dog β1 subunit (NP_001003283.1), rat β1 subunit (NP_037245.2) and chain B, crystal structure of the sodium-potassium pump (CAQ53919.1) was performed by using the Cobalt Multiple Alignment Tool. The amino acid residues that are identical in dog and rat subunits are shown in black font, similar amino acid residues are shown in green font, and different amino acid residues are shown in red font. The most variable region between the dog and rat sequences is highlighted by the red box. (B) The amino acid residues different in dog and rat sequences were mapped on a high-resolution structure of the Na+/K+-ATPase (2ZXE) (Shinoda et al., 2009) by using Jmol, version 1.45, to identify the species-specific residues exposed at the surface of the extracellular domain. These residues are indicated by asterisks in A and by yellow halos in B. N-glycans are shown in blue. The epitope of the M17-P5-F11 antibody is shown by a purple arrow in A.
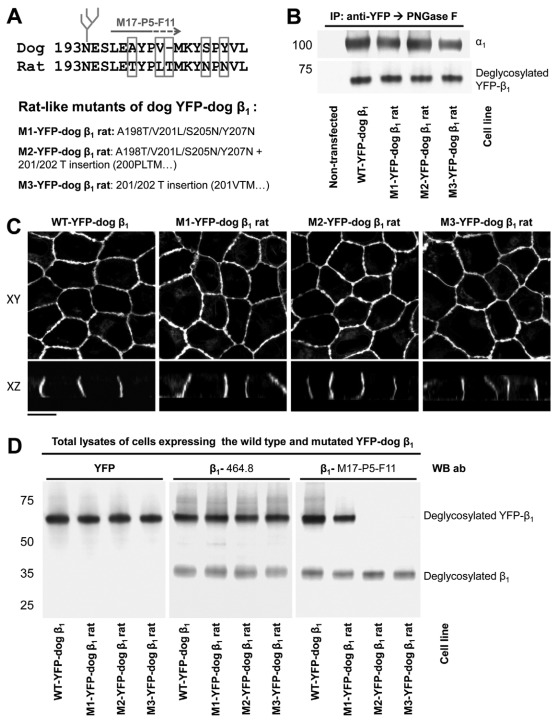
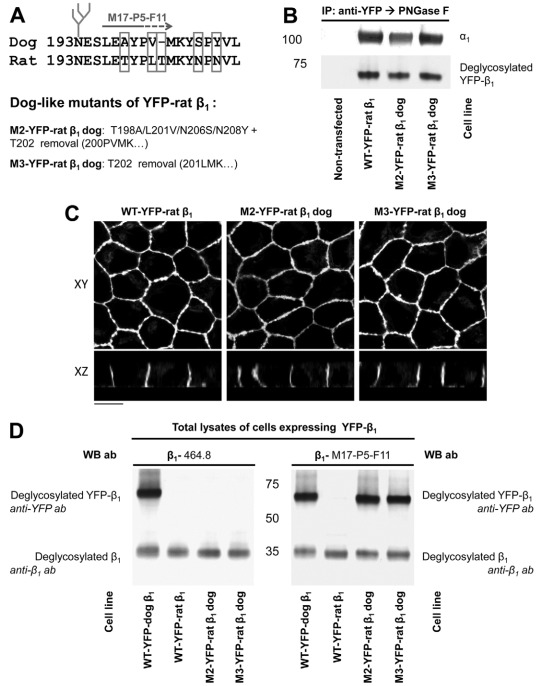
To determine whether this region is important for β1–β1 binding, we replaced four residues in the amino acid region 198–207 of the YFP–dog-β1 with those present in the rat subunit and also inserted the rat-unique Thr residue between Val201 and Met202 of the YFP–dog-β1. Three mutants of YFP–dog-β1, one containing four amino acid replacements, another containing four replacements and one insertion, and the other one containing an insertion only (Fig. 3A), were stably expressed in MDCK cells. Similar to the wild-type YFP–dog-β1, the mutated fusion proteins were associated with the endogenous α1 subunit, as detected by co-immunoprecipitation and were located predominantly in the lateral membranes of MDCK cells, as demonstrated by confocal microscopy (Fig. 3B,C). The levels of expression of the wild-type and mutated YFP–β1 were similar, as detected by antibodies against YFP and by 464.8 antibodies against β1 (Fig. 3D, left and middle panel). In addition to YFP-linked subunits, all three cell lines expressed the endogenous β1 subunit, as detected by 464.8 or M17-P5-F11 antibodies (Fig. 3D, middle and right panels).
Fig. 3.
Rat-like mutations in the amino acid region 198–207 of YFP–dog-β1 impair the recognition of YFP–dog-β1 by an adhesion-blocking M17-P5-F11 antibody. (A) The positions of the amino acid residues mutated in the amino acid region 198–207 of YFP–dog-β1 are shown. Three mutants were constructed, the first with four rat-like amino acid substitutions, the second with four substitutions and one amino acid insertion, and the third with the amino acid insertion only. (B) Western blot analysis of the proteins precipitated by an antibody against YFP shows co-precipitation of the endogenous α1 subunit with the wild-type and mutated YFP–dog-β1 fusion proteins. (C) Confocal microscopy images of stable cell lines expressing the wild-type and mutated YFP–dog-α1 fusion proteins show their predominant localization in the lateral membranes. All images were taken with the same settings. Scale bar: 10 μm. (D) Equal amounts of total cell lysates of MDCK cells expressing either wild-type or mutated YFP–dog-β1 were analyzed by immunoblotting using antibodies to YFP and two types of monoclonal antibodies to β1. Mutations in the amino acid region 198–207 did not have an effect on the reactivity of the 464.1 anti-β1 antibody with YFP–dog-β1. By contrast, replacement of four residues in the amino acid region 198–207 resulted in a substantial decrease in the reactivity of the M17-P5-F11 antibody with YFP–dog-β1. Insertion of a rat-unique Thr residue resulted in a complete loss of reactivity of the M17-P5-F11 antibody with YFP–dog-β1. Here and below, to enable quantitative comparison, immunoprecipitated proteins were deglycosylated by PNGase F prior to SDS-PAGE.
Remarkably, the four rat-like amino acid substitutions in the YFP–dog-β1 resulted in a substantial decrease in its reactivity with the M17-P5-F11 antibody (Fig. 3D, right panel). The Thr insertion between the residues 201 and 202, either alone, or in combination with four rat-like substitutions resulted in a complete loss of the M17-P5-F11 antibody recognition, whereas the 464.8 antibody similarly recognized the wild-type and mutated YFP–dog-β1 (Fig. 3D, middle and right panels). As the M17-P5-F11 antibody inhibits formation of intercellular junctions between MDCK cells (Vagin et al., 2006), the loss of its reactivity with the YFP–dog-β1 due to mutations in the amino acid region 198–207 suggests a contribution of this region to adhesive interactions between dog β1 subunits of neighboring cells.
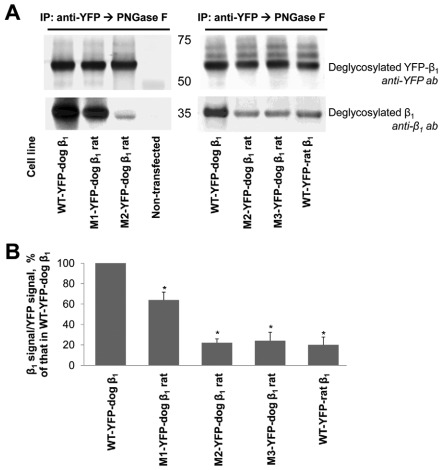
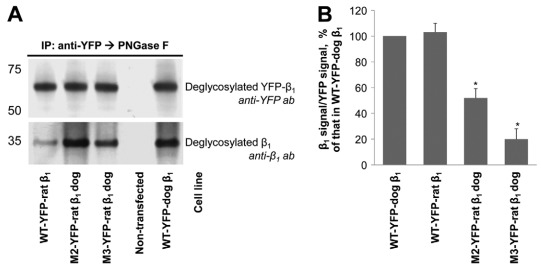
To directly evaluate the effect of mutations on β1–β1 interaction, the wild-type and mutated YFP–β1 fusion proteins were immunoprecipitated using antibodies to YFP, and the amount of co-immunoprecipitated endogenous β1 subunits was determined by immunoblotting. The four rat-like replacements in the amino acid region 198–207 decreased the amount of co-immunoprecipitated β1 subunits by ~40% as detected by either 464.8 or M17-P5-F11 antibodies to β1 (Fig. 4A, left panel; and 4B). The results obtained with the 464.8 antibody are shown. The Thr insertion performed in addition to four rat-like amino acid replacements decreased the amount of co-immunoprecipitated β1 subunits by ~80% in comparison with the wild-type control (Fig. 4A, left panel; and 4B). To evaluate the contribution of a Thr insertion alone in the impairment of β1–β1 binding, a mutant with four rat-like replacements and Thr insertion was compared with the mutant with the Thr insertion alone. The amount of β1 subunit that co-precipitated with these two mutated YFP–dog-β1 fusion proteins was similar. Also, a similar level of co-immunoprecipitation of the endogenous β1 subunits was observed with the YFP–rat-β1 (Fig. 4A, right panel and 4B). These results show the importance of the amino acid region 198–207 for the species-specific binding between two dog β1 subunits.
Fig. 4.
Rat-like mutations in the amino acid region 198–207 of the YFP–dog-β1 impair (YFP–β1)–β1 interactions. (A) Western blot analysis using antibodies against YFP and Na+/K+-ATPase β1 shows that immunoprecipitation of YFP–β1 resulted in co-precipitation of the endogenous Na+/K+-ATPase β1 subunits from cell lysates of MDCK cells expressing the wild-type YFP–dog-β1, or its mutants or the wild-type YFP–rat-β1. (B) Densitometric quantification of the results presented in A was performed by dividing a signal from the antibody to β1 by the corresponding signal from the antibody to YFP. The comparative bar graph shows these ratios as a percentage of the ratio obtained for the wild-type YFP–β1. The results of quantification indicate that amino acid substitutions and insertion in the amino acid region 198–207 decreased the amount of YFP–β1-co-immunoprecipitated endogenous β1 subunits. The positions of the amino acid residues mutated are shown in Fig. 3A. WT, wild-type; error bars, ±s.d. (n=3); *, significant difference from the ‘WT-YFP–dog-β1’, P<0.01, Student's t-test.
Dog-like mutations in the amino acid region 198–208 of the YFP–rat-β1 improve its interaction with the endogenous β1 subunit in MDCK cells
Two dog-like mutants of YFP–rat-β1, one with four amino acid replacements and Thr removal and the other one with just the Thr removed, were assembled with the α1 subunit and were localized predominantly in the lateral membranes of MDCK cells (Fig. 5A–C). In contrast to the wild-type YFP–rat-β1, both dog-like mutants reacted with a cell-adhesion-blocking M17-P5-F11 antibody (Fig. 5D). The removal of T202 resulted in a 2.5-fold increase in the amount of YFP–β1-co-immunoprecipitated endogenous β1 subunit compared with that with the wild-type YFP–rat-β1, as detected by either 464.8 or M17-P5-F11 antibodies against β1 (Fig. 6A,B). The results obtained with 464.8 antibodies are shown. The T202 removal together with four dog-like replacements further increased co-immunoprecipitation of the endogenous β1 subunit up to the level observed with YFP–dog-β1 (Fig. 6). These results confirm the importance of the amino acid region 198–207 for adhesive binding between dog β1 subunits.
Fig. 5.
Dog-like mutations in the amino acid region 198–208 of YFP–rat-β1 increase its reactivity with the M17-P5-F11 antibody. (A) Positions of the amino acid residues mutated in the amino acid region 198–208 of YFP–rat-β1 are shown. Two mutants were constructed, one with four substitutions and one amino acid insertion, and the other with an amino acid insertion only. (B) Western blot analysis of the proteins precipitated by an antibody to YFP shows co-precipitation of the endogenous β1 subunits with the wild-type and mutated YFP–dog-β1 fusion proteins. (C) Confocal microscopy images of stable cell lines expressing the wild-type and mutated YFP–rat-β1 fusion proteins show their predominant localization in the lateral membranes. All images were taken with the same settings. Scale bar: 10 μm. (D) Western blot analysis using antibodies to YFP and Na+/K+-ATPase β1 shows co-precipitation of the endogenous Na+/K+-ATPase β1 subunits with the wild-type YFP–rat-β1, or its mutants or with the wild-type YFP–dog-β1.
Fig. 6.
Dog-like mutations in the amino acid region 198–208 of YFP–rat-β1 increase (YFP–β1)–β1 interactions. (A) Western blot analysis using two anti-β1 antibodies of total lysates of cells expressing the wild-type YFP–dog-β1, or the wild-type YFP–rat-β1, or mutated YFP–rat-β1. Mutations in the amino acid sequence 198–208 of YFP–rat-β1 resulted in recognition of YFP–rat-β1 by the anti-β1 M17-P5-F11, but not by the anti-β1 464.8, antibody. (B) Densitometric quantification of the results presented in C, which was performed as described in the legend to Fig. 4, shows that the dog-like insertion and substitutions in the amino acid region 198–208 increased the amount of YFP–rat-β1-co-immunoprecipitated endogenous β1 subunits. WT, wild-type; error bars, ±s.d. (n=3); *, significant difference from the ‘WT-dog’, P<0.01, Student's t-test.
Rat-like mutations in the amino acid region 198–207 of the secreted extracellular domain of the dog β1 subunit increase its interaction with the rat β1 subunit
The results presented above indicate that the 198–207 region is important for binding between two dog β1 subunits. To determine whether the same region mediates the interaction between two rat β1 subunits, we expressed the secreted extracellular domain of the dog β1 subunit (Sec-dog β1) and studied the effect of rat-like mutations in its region 198–207 on the interaction of Sec-dog β1 with the rat β1 subunit by using two approaches. First, we assessed the in vitro binding between the YFP–rat-β1 and the wild-type or mutated Sec-dog β1. Second, we looked at the effects of the wild-type and mutated Sec-dog β1 on aggregation between rat lung epithelial (RLE-6TN) cells.
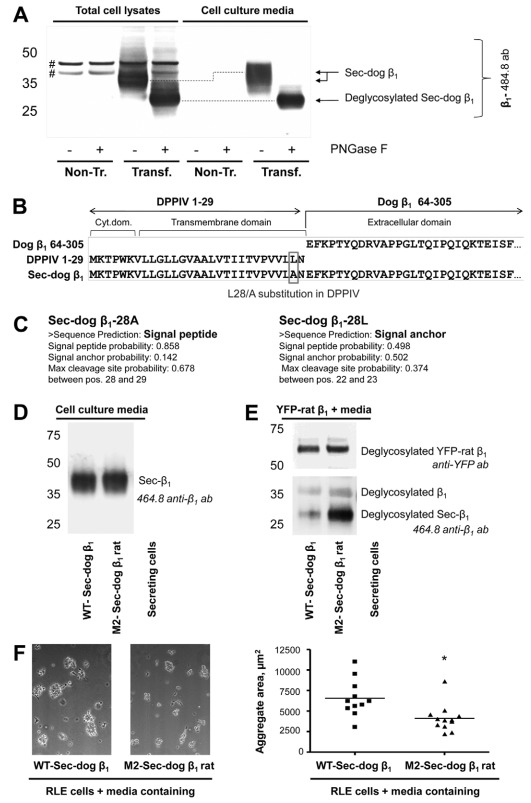
To express Sec-dog β1, human embryonic kidney (HEK-293) cells were transiently transfected with the construct, which was generated in the Fambrough laboratory and was used previously to express the secreted extracellular domain of the dog β1 subunit in CHO cells (Padilla-Benavides et al., 2010). This construct encodes for the chimera containing a cytoplasmic and a transmembrane domain of dipeptidyl peptidase 4 (DPPIV) and an extracellular domain of the dog Na+/K+-ATPase β1 subunit. Western blot analysis of the culture media of transfected cells using the 464.8 antibody against β1 detected a secreted protein of molecular mass ~45 kDa (Fig. 7A), similar to the results reported for CHO cells (Padilla-Benavides et al., 2010). Deglycosylation of this protein by peptide:N-glycosidase F (PNGase F) yielded a core protein of molecular mass ~28 kDa (Fig. 7A), which corresponds to the predicted molecular mass of the deglycosylated extracellular domain of the β1 subunit. The protein detected in the cell lysate moved faster on SDS-PAGE than a secreted protein. However, its deglycosylated product had the same electrophoretic mobility as the deglycosylated protein in the media (Fig. 7A), indicating that the intracellular form is an immature fraction of a secreted extracellular domain of the β1 subunit. Similar results were obtained by using M17-P5-F11 antibodies against β1, which in addition reacted with the endogenous β1 subunit of HEK-293 cells (data not shown). The results indicate that the chimeric protein is inserted in the ER membrane as a type II transmembrane protein (N-terminus in cytoplasm and C-terminus in the ER lumen), allowing co-translational addition of N-glycans to the extracellular domain of the β1 subunit, followed by cleavage of this domain by signal peptidase(s) in the ER lumen and secretion of the β1 extracellular domain into the media.
Fig. 7.
Rat-like mutations in the secreted extracellular domain of the dog β1 subunit improve its binding to the rat Na+/K+-ATPase β1 subunit. (A) Western blot analysis of cell lysates of HEK293 cells, either non-transfected or transiently transfected with a vector encoding a chimera between rat DPPIV and dog Na+/K+-ATPase β1 subunit (Sec-dog β1), as well as corresponding cell culture media. The 464.8 antibody against β1 detected proteins in both the cell lysates and media of transfected, but not of non-transfected, cells. Treatment by PNGase F of both cell lysates and media resulted in the formation of a product of ~28 kDa, which corresponds to the predicted molecular mass of the de-glycosylated extracellular domain of the β1 subunit. #, non-specific bands. (B) Sequence analysis of the construct encoding the chimera showing an L28A amino acid substitution in a DPPIV sequence. (C) The results of signal peptide prediction using SignalP 3.0 (Bendtsen et al., 2004) for Sec-dog β1, containing either Ala or Leu at position 28. (D) Rat-like mutations were introduced into Sec-dog β1 at the positions identical to those introduced in M2-YFP–dog-β1 rat shown in Fig. 3A. Both wild-type and mutated Sec-dog β1 were secreted to the media during transient expression in HEK-293 cells, as detected by immunoblotting. (E) The YFP-linked rat β1 subunit expressed in MDCK cells was immunoprecipitated on anti-YFP antibody bound to protein A in agarose beads. The beads with adherent YFP–rat-β1 were then incubated with the media containing either the wild-type or mutated Sec-dog β1 followed by eluting bead-adherent proteins, SDS-PAGE and immunoblotting. Rat-like mutations in the region 198–207 of Sec-dog β1 considerably increased its co-precipitation with YFP–rat-β1. (F) A suspension of rat lung epithelial (RLE-6TN) cells in the cell culture medium containing either the wild-type or mutated secreted extracellular domain of the dog β1 subunit was placed as drops on the lid of a 24-well dish and cultured for 4 hours after inverting the lid. Representative images of cell aggregates formed in the hanging drops are shown. Quantification was performed by determining the aggregate area in 11 hanging drops, each containing various number of cell aggregates, from four experiments for both wild-type and mutated Sec-dog β1. Each dot represents the mean aggregate area for an individual hanging drop. *P<0.01 significant difference from WT, Student's t-test.
Even though our results on secretion of the extracellular domain of the dog β1 subunit were consistent with previously published data (Padilla-Benavides et al., 2010), it was not clear to us why a chimera between two type II transmembrane proteins, DPPIV and β1 subunit, produced a secreted protein (Fig. 7A). Moreover, a similar chimera between DPPIV and chicken β1 subunit has been reported as a transmembrane protein (Hamrick et al., 1993), which makes the results showing secretion of the dog β1 subunit extracellular domain even more puzzling. DNA sequencing of the construct DPPIV–dog-β1 showed a mutation resulting in a single amino acid substitution, L28A, in the DPPIV sequence (Fig. 7B). Running the program SignalP 3.0 (Bendtsen et al., 2004) predicted the presence of a cleavable signal peptide in the Sec-dog β1 containing 28Ala, but not in the Sec-dog β1 containing 28Leu (Fig. 7C). Thus, secretion of the extracellular domain of the dog β1 subunit appears to be due to the generation of a signal peptidase cleavage site by the single L28A amino acid substitution in a DPPIV–dog-β1 chimera.
As for the wild-type, the mutant Sec-dog β1 with four rat-like amino acid replacements and one amino acid insertion in the region 198–207 was secreted into the cell culture media (Fig. 7D), as detected by the 464.8 antibody against β1. The M17-P5-F11 antibody did not recognize this rat-like mutant of Sec-dog β1. To determine whether these rat-like mutations increase the interaction of Sec-dog β1 with the rat β1 subunit in vitro, YFP–rat-β1 expressed in MDCK cells was immunoprecipitated using antibodies to YFP. After extensive washing, the YFP–rat-β1 attached to protein-A in agarose beads was incubated with the media containing wild-type or mutated Sec-dog β1, followed by collecting the beads, elution and immunoblotting of the bead-adherent proteins. In addition to YFP–rat-β1 and the endogenous dog β1 subunit from MDCK cells, immunoblotting revealed the presence of Sec-dog β1 among proteins eluted from the beads. The amount of the mutated Sec-dog β1 co-precipitated with YFP–rat-β1 was considerably greater than the amount of the YFP–rat-β1-co-precipitated wild-type Sec-dog β1 (Fig. 7E).
To determine whether rat-like mutations in Sec-dog β1 increase its interaction with the rat β1 subunits present in the plasma membrane of live cells, the media containing either wild-type or mutated Sec-dog β1 were added to the suspension of RLE-6TN, cultured epithelial cells of rat origin that endogenously express the β1 subunit (Hao et al., 2003). If the adhesive interaction between rat β1 subunits is mediated by the same amino acid region as binding between dog β1 subunits, the secreted extracellular domain of the dog β1 subunit with rat-like mutations in the region 198–207 is expected to reduce the aggregation of RLE-6TN cells with each other owing to competitive inhibition of intercellular β1–β1 binding.
The ability of RLE-6TN cells to aggregate was assayed by measuring the area of cell aggregates formed in the cell suspension hanging drop. The results show that the area of cell aggregates formed in the presence of the wild-type Sec-dog β1 was significantly greater than the area of cell aggregates formed in the presence of the mutated Sec-dog β1 (Fig. 7F). The results suggest that the presence of a rat-like region on the Sec-dog β1 enabled its interaction with the endogenous rat β1 subunits exposed on the surface of RLE-6TN cells. This interaction, in turn, competitively inhibited the interaction between the β1 subunits of adjacent cells and, hence, cell aggregation. Therefore, as with dog β1 subunits, the interaction between two rat β1 subunits is also mediated by amino acid sequences within the rat 198–208 amino acid region that convey species specificity.
Discussion
Several lines of evidence support the notion of trans-dimerization occurring between the Na+/K+-ATPase β1 subunits of neighboring epithelial cells. First, in mixed cell monolayers, the endogenous β1 subunit of normal rat kidney epithelial cells co-immunoprecipitates with the YFP-linked rat β1 subunit overexpressed in MDCK cells (Padilla-Benavides et al., 2010). Second, the results of fluorescence resonance energy transfer (FRET) show that the β1 subunits of neighboring cells in a cell monolayer have sufficient proximity to permit direct interaction (Padilla-Benavides et al., 2010). Third, removal of Ca2+ from the culture medium followed by disruption of intercellular junctions between MDCK cells expressing both YFP–β1 and the endogenous β1 subunits decreases the amount of YFP–β1-co-precipitated β1 subunits (Tokhtaeva et al., 2011). Finally, increasing exposure of YFP–β1 to endogenous β1 subunits located in neighboring cells by mixing YFP–β1-expressing MDCK cells with non-transfected MDCK cells amplifies the amount of YFP–β1-co-precipitated endogenous β1 subunits (Tokhtaeva et al., 2011).
Experiments in co-cultures of cells of different species showed that the Na+/K+-ATPase β1 subunits were present at homotypic, but not at heterotypic, cell contacts, suggesting that the β1 subunits interact with each other in a species-specific mode (Padilla-Benavides et al., 2010; Shoshani et al., 2005). Direct measurement of β1–β1 interactions by using co-immunoprecipitation showed that the interaction between two dog subunits was much greater than the interaction between dog and rat subunits (Tokhtaeva et al., 2011). As the number and positions of N-glycans are the same, whereas the amino acid sequence is different in dog and rat β1 subunits, these results show the importance of amino-acid-dependent interactions in trans-dimerization of the β1 subunits. The most variable region between dog and rat subunits containing amino acid residues 198–207 and 198–208, respectively, includes the known epitope for the monoclonal antibody against the Na+/K+-ATPase β1 subunit, clone M17-P5-F11 (Sun and Ball, 1994), which reacts with the dog, but not the rat, β1 subunit (Fig. 1). The same antibody inhibits cell–cell adhesion between surface-attached dog MDCK cells (Vagin et al., 2006), which suggests that the region 198–207 contributes to protein–protein interactions between the dog β1 subunits of neighboring cells.
Molecular modeling of dog and rat β1 subunits based on a high-resolution structure of the Na+/K+-ATPase (2ZXE) (Shinoda et al., 2009) showed that all the residues different in dog and rat in the 198–207 (198–208) region are exposed at the surface of the extracellular domain facing the apposed cell (Fig. 2B), further supporting the hypothesis regarding the involvement of this region in adhesive β1–β1 binding. Finally, the results of site-directed mutagenesis of the species-specific residues in this region in both rat and dog subunits confirmed the importance of this region for adhesive β1 dimerization. Particularly, rat-like mutations in the region 198–207 of the YFP–dog-β1 impaired both β1 dimerization and antibody binding (Figs 3, 4). Conversely, dog-like mutations in the region 198–208 of the YFP–rat-β1 improved β1–β1 binding and increased reactivity of the rat β1 with the adhesion-blocking antibody (Figs 5, 6). These results show the importance of this region for binding between dog β1 subunits. The same region is involved in the interaction of two rat β1 subunits as the rat-like mutations in the Sec-dog β1 increased interaction between Sec-dog β1 and rat β1 subunits and also resulted in inhibition of aggregation between rat epithelial cells (Fig. 7). Strikingly, the insertion of the rat-unique Thr residue into YFP–dog-β1 resulted in a complete loss of the M17-P5-F11 antibody recognition and in a dramatic decrease in YFP–β1 binding to the dog subunit down to the level of β1–β1 interaction between dog and rat subunits (Figs 3, 4). Likewise, the removal of the Thr residue from the rat subunit resulted in a dramatic increase in binding to both dog β1 subunit and the dog-reactive antibody (Figs 5, 6). Therefore, the absence of the Thr residue in the regions spanning residues 198–207 is essential for binding between dog β1 subunits. By contrast, two rat β1 subunits effectively interact with each other (Padilla-Benavides et al., 2010), and their regions spanning 198–208 both containing the Thr residue are important for this interaction (Fig. 7). These results indicate that the amino acid residues crucial for β1–β1 binding are located both upstream and downstream of the Thr insertion position. The insertion or removal of the Thr residues in one of the two interacting subunits is likely to misalign these binding residues.
To conclude, the results presented here confirm that the Na+/K+-ATPase acts as a cell adhesion molecule by binding to the Na+/K+-ATPase molecule of a neighboring cell by means of trans-dimerization of their β1 subunits and also identify the amino acid region crucial for this dimerization. Remarkably, the region 198–207 (198–208) is present only in the β1 subunit, which is the major isoform of epithelial cells, but not in the other isoforms of the Na+/K+-ATPase and the homologous H,K-ATPase (Fig. 8). It is possible that, during evolution, this region was conserved in the β1 subunits to maintain a nonconventional role of the Na+/K+-ATPase in the formation and maintenance of epithelial junctions.
Fig. 8.
Multiple alignment of various isoforms of the Na+/K+-ATPase β subunits and the H+/K+-ATPase β subunits. A multiple alignment of various isoforms of the β subunits of these P2-type ATPases shows that the region important for β–β binding is present only in the epithelia-specific β1 isoform.
Materials and Methods
Construction of MDCK stable cell lines
The Na+/K+-ATPase rat β1 and dog β1 subunits linked through their N-termini to YFP were constructed as described previously (Tokhtaeva et al., 2009; Vagin et al., 2006). Mutants of rat and YFP–dog-β1 were constructed by site-directed mutagenesis using the QuikChange mutagenesis kit (Agilent Technologies, Santa Clara, CA). The mutagenic primers were constructed using PrimerSelect software, 5.03 (supplementary material Table S1). Stable MDCK cell lines expressing wild-type and mutated YFP–β1 were obtained as described previously (Vagin et al., 2005).
Production of the wild-type and mutated secreted extracellular domains of the dog β1 subunit
The vector encoding a secreted extracellular domain of the dog β1 subunit (Sec-dog β1) (Padilla-Benavides et al., 2010) constructed in the Fambrough laboratory was a generous gift from Liora Shoshani. This vector encodes a chimera containing 29 N-terminal amino acid residues of DPPIV and amino acid residues 64–303 of the dog Na+/K+-ATPase β1 subunit. The mutant of Sec-dog β1 was constructed by site-directed mutagenesis using the QuikChange mutagenesis kit (Agilent Technologies) with mutagenic primers (for list, see supplementary material Table S1). HEK-293 cells (ATCC, Manassas, VA) were transiently transfected with vectors encoding either the wild-type or mutated Sec-dog β1 using Lipofectamine-2000 transfection reagent (Invitrogen, Carlsbad, CA). The medium was changed 6 hours after transfection, and the media containing Sec-dog β1 were collected 48 hours later.
Cell culture
Cells were grown in DMEM medium (Cellgro Mediatech, Manassas, VA) containing 4.5 g l−1 glucose, 2 mM l-glutamine, 8 mg l−1 phenol red, 100 units ml−1 penicillin, 0.1 mg ml−1 streptomycin and 10% FBS, unless specified otherwise.
Confocal microscopy
Confocal microscopy images were acquired using a Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss MicroImaging GmbH, Germany) and LSM 510 software, version 3.2.
Primary antibodies
A polyclonal antibody against GFP, which recognizes YFP (Clontech, Mountain View, CA), was used for immunoprecipitation. The following monoclonal antibodies were used for western blot analysis: against GFP, clones 7.1 and 13.1, which also recognize YFP (Roche Diagnostics, Indianapolis, IN), against the Na+/K+-ATPase β1 subunit, clone C464.6 (Millipore, MA), against the Na+/K+-ATPase β1 subunit, clone M17-P5-F11 (Affinity Bioreagents, Golden, CO), against the Na+/K+-ATPase β1 subunit, clone 464.8 (Novus Biologicals, Littleton, CO) and against β1-integrin (BD Transduction Laboratories, San Jose, CA).
Immunoprecipitation
Confluent MDCK cell monolayers were rinsed twice with ice-cold PBS and lysed by incubation with 200 μl per well of 150 mM NaCl in 50 mM Tris pH 7.5 containing 1% Nonidet P40, 0.5% sodium deoxycholate and Complete Protease Inhibitor Cocktail, 1 tablet per 50 ml (Roche Diagnostics, Indianapolis, IN) at 4°C for 30 minutes, followed by scraping of the cells. Cell extracts were clarified by centrifugation (15,000 g, 10 minutes) at 4°C. Next, the cell extracts (400 μg protein) were incubated with 30 μl of the suspension of protein A in agarose (Roche Diagnostics, Mountain View, CA) in a total volume of 1 ml of the lysis buffer at 4°C with continuous rotation for at least 3 hours (or overnight) to remove the components that nonspecifically bind to protein A. The pre-cleared cell extract was mixed with 2 μl of polyclonal antibodies against GFP or YFP (Clontech) and incubated with continuous rotation at 4°C for 60 minutes. After addition of 30 μl of the suspension of protein A in agarose, the mixture was incubated at 4°C with continuous rotation overnight. The bead-adherent complexes were washed on the beads first with the lysis buffer and then with 500 mM NaCl in 50 mM Tris pH 7.5 containing 0.1% Nonidet P40 and 0.05% sodium deoxycholate and finally with 10 mM Tris pH 7.5 containing 0.1% Nonidet P40 and 0.05% sodium deoxycholate. The adherent proteins were eluted from the beads by incubation in 45 μl of 2× SDS-PAGE sample buffer (4% SDS, 0.05% Bromophenol Blue, 20% glycerol, 1% β-mercaptoethanol in 0.1 M Tris, pH 6.8) for 5 minutes at 80°C.
To enable quantitative comparison of immunoprecipitated YFP-linked and endogenous β1 subunits, the bead-adherent proteins were deglycosylated by incubation with 30 μl of 50 mM sodium phosphate, pH 7.5 containing 0.16% SDS, 13 mM DTT, 1% Nonidet P40 and 1 μl PNGase F from Flavobacterium meningosepticum (New England BioLabs, Ipswich, MA) for 1 hour at 37°C. After finishing the incubation, the reaction mixture was separated from the beads. The adherent proteins were eluted from the beads by incubation in 30 μl of 2× SDS-PAGE sample buffer for 5 minutes at 80°C. Proteins eluted from the beads were combined with the reaction mixture, separated by SDS-PAGE and analyzed by western blot to detect immunoprecipitated and co-immunoprecipitated proteins by using monoclonal antibodies against GFP (YFP), the Na+/K+-ATPase β1 subunit and the Na+/K+-ATPase β1 subunit.
In vitro binding assay to determine the interaction between Sec-dog β1 and YFP–rat-β1
YFP–rat-β1 stably expressed in MDCK cells was immunoprecipitated and washed from contaminating proteins as described above. Then, the beads with adherent YFP–rat-β1 were incubated with 1 ml of cell culture media produced by HEK-293 cells transiently expressing either the wild-type or mutated Sec-dog β1 at 4°C, with continuous rotation for at least 3 hours (or overnight). The bead-adherent complexes were washed on the beads and eluted from the beads as described above for the immunoprecipitation procedure. The eluted proteins were separated by SDS-PAGE and analyzed by western blot.
Isolation of basolateral plasma membrane proteins of MDCK cells using surface-specific biotinylation
Cells were maintained for 6 days after becoming confluent in transwell inserts. Biotinylation of surface proteins was performed according to previously described procedures (Gottardi et al., 1995; Kroepfl and Gardinier, 2001). Cell monolayers were biotinylated with EZ-Link Sulfo-NHS-SS-biotin (Pierce) that was added into the well only (basolateral surface of the tight cell monolayers). After quenching the biotinylation reaction, cells were washed and then lysed by incubation with 200 μl of 0.15 M NaCl in 15 mM Tris pH 8.0 with 1% Triton X-100 and 4 mM EGTA. Cell extracts were clarified by centrifugation (15,000 g, 10 minutes) at 4°C. To isolate surface-biotinylated proteins, the cell extract was incubated with 100 μl of streptavidin–agarose beads (Sigma-Aldrich) in a total volume of 1 ml of 0.15 M NaCl in 15 mM Tris pH 8.0 with 0.5% Triton X-100 and 4 mM EGTA at 4°C with continuous rotation for 60 minutes. The bead-adherent complexes were washed three times on the beads, and then proteins were eluted from the beads by incubation in 40 μl of SDS-PAGE sample buffer for 5 minutes at 80°C.
Western blot analysis of the total, basolateral and immunoprecipitated proteins of MDCK cells
MDCK cell extracts containing 1–10 μg protein mixed with an equal volume of SDS-PAGE sample buffer or 5–20 μl of proteins eluted from streptavidin–agarose beads or protein-A-conjugated agarose beads were loaded onto 4–12% gradient SDS-PAGE gels (Invitrogen). Where indicated, proteins were treated by PNGase F from Flavobacterium meningosepticum (New England BioLabs) according to the manufacturer's instructions prior to loading for SDS-PAGE. Proteins were separated by SDS-PAGE using MES in SDS running buffer (0.05 M MES, 0.05 M Tris base, 0.1% SDS and 1 mM EDTA, pH 7.3), transferred onto a nitrocellulose membrane (BioRad, Hercules, CA, USA) and detected by western blot analysis using the appropriate primary antibody and anti-mouse or anti-rabbit secondary antibody conjugated to alkaline phosphatase (Promega, Madison, WI) or horseradish peroxidase (American Qualex, San Clemente, CA). Alkaline phosphatase was detected using Nitro Blue Tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate in alkaline phosphatase buffer (150 mM NaCl, 1 mM MgCl2 in 10 mM Tris-HCl, pH 9.0). Horseradish peroxidase was detected by using Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL). Immunoblots were quantified by densitometry using Zeiss LSM 510 software, version 3.2.
Cell aggregation assay
Cell aggregation was assessed by a hanging drop assay performed in a manner similar to a previously described procedure (Qin et al., 2005). RLE-6TN cells (ATCC, Manassas) grown in 10 cm plates were harvested using 0.25% trypsin and 2.21 mM EDTA in HBSS (Cellgro) diluted 1:100 in PBS containing 2 mM EDTA, pH 8, washed twice in PBS and resuspended at 2.5×106 cells ml−1 in normal growth medium. The cell suspension containing 2×104 cells was then diluted five times by the cell culture media containing the wild-type or mutated Sec-dog β1 and placed as 40 μl drops on the lid of a 24-well culture dish. The lid was inverted over cell culture wells, which contained PBS to avoid evaporation of hanging drops, and the cells were allowed to aggregate for 4 hours in a tissue culture incubator. Next, the cell aggregates in each drop were subjected to shear force by passage ten times through a 200-μl wide-bore pipette tip to disperse loosely associated cells and photographed using a Nikon Eclipse TE200 inverted microscope (Nikon Metrology, Brighton, MI) using a ×10 phase-contrast objective. Aggregates were traced, and the aggregate area was measured using MetaMorph software.
Statistical analysis
Statistical analysis was performed by using Student's t tests (GraphPad Prism 4 software and Microsoft Excel). Statistical significance and the number of experiments are specified in the figure legends.
Acknowledgements
We are grateful to Liora Shoshani for providing an expression vector for the secreted extracellular domain of the dog β1 subunit. We thank Glenn Nagami for careful reading of the manuscript and helpful suggestions.
Footnotes
Funding
The work was supported by the National Institutes of Health [grant numbers DK077149 to O.V., DK058333 to G.S. and R37-HL48129 to J.I.S.]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.100149/-/DC1
References
- Baum B., Georgiou M. (2011). Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J. Cell Biol. 192, 907-917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. (2004). Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340, 783-795 [DOI] [PubMed] [Google Scholar]
- Gottardi C. J., Dunbar L. A., Caplan M. J. (1995). Biotinylation and assessment of membrane polarity: caveats and methodological concerns. Am. J. Physiol. 268, F285-F295 [DOI] [PubMed] [Google Scholar]
- Gumbiner B. M. (2005). Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6, 622-634 [DOI] [PubMed] [Google Scholar]
- Hamrick M., Renaud K. J., Fambrough D. M. (1993). Assembly of the extracellular domain of the Na+/K+-ATPase beta subunit with the alpha subunit. Analysis of beta subunit chimeras and carboxyl-terminal deletions. J. Biol. Chem. 268, 24367-24373 [PubMed] [Google Scholar]
- Hao H., Wendt C. H., Sandhu G., Ingbar D. H. (2003). Dexamethasone stimulates transcription of the Na+-K+-ATPase beta1 gene in adult rat lung epithelial cells. Am. J. Physiol. Lung. Cell Mol. Physiol. 285, L593-L601 [DOI] [PubMed] [Google Scholar]
- Hartsock A., Nelson W. J. (2008). Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 1778, 660-669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie K., Shimizu K., Sakisaka T., Ikeda W., Takai Y. (2004). Roles and modes of action of nectins in cell-cell adhesion. Semin. Cell Dev. Biol. 15, 643-656 [DOI] [PubMed] [Google Scholar]
- Kizhatil K., Davis J. Q., Davis L., Hoffman J., Hogan B. L., Bennett V. (2007). Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J. Biol. Chem. 282, 26552-26561 [DOI] [PubMed] [Google Scholar]
- Kroepfl J. F., Gardinier M. V. (2001). Identification of a basolateral membrane targeting signal within the cytoplasmic domain of myelin/oligodendrocyte glycoprotein. J. Neurochem. 77, 1301-1309 [DOI] [PubMed] [Google Scholar]
- Miyoshi J., Takai Y. (2005). Molecular perspective on tight-junction assembly and epithelial polarity. Adv. Drug. Deliv. Rev. 57, 815-855 [DOI] [PubMed] [Google Scholar]
- Miyoshi J., Takai Y. (2007). Nectin and nectin-like molecules: biology and pathology. Am. J. Nephrol. 27, 590-604 [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Veshnock P. J. (1987). Ankyrin binding to (Na++K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature 328, 533-536 [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Shore E. M., Wang A. Z., Hammerton R. W. (1990). Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J. Cell Biol. 110, 349-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Benavides T., Roldan M. L., Larre I., Flores-Benitez D., Villegas-Sepulveda N., Contreras R. G., Cereijido M., Shoshani L. (2010). The polarized distribution of Na+,K+-ATPase: role of the interaction between {beta} subunits. Mol. Biol. Cell 21, 2217-2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Capaldo C., Gumbiner B. M., Macara I. G. (2005). The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J. Cell Biol. 171, 1061-1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakisaka T., Ikeda W., Ogita H., Fujita N., Takai Y. (2007). The roles of nectins in cell adhesions: cooperation with other cell adhesion molecules and growth factor receptors. Curr. Opin. Cell Biol. 19, 593-602 [DOI] [PubMed] [Google Scholar]
- Shapiro L., Weis W. I. (2009). Structure and biochemistry of cadherins and catenins. Cold Spring Harb. Perspect. Biol. 1, a003053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda T., Ogawa H., Cornelius F., Toyoshima C. (2009). Crystal structure of the sodium-potassium pump at 2.4 A resolution. Nature 459, 446-450 [DOI] [PubMed] [Google Scholar]
- Shoshani L., Contreras R. G., Roldan M. L., Moreno J., Lazaro A., Balda M. S., Matter K., Cereijido M. (2005). The polarized expression of Na+,K+-ATPase in epithelia depends on the association between beta-subunits located in neighboring cells. Mol. Biol. Cell 16, 1071-1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed E., Balda M. S., Matter K. (2010). Dynamics and functions of tight junctions. Trends Cell Biol. 20, 142-149 [DOI] [PubMed] [Google Scholar]
- Sun Y., Ball W. J., Jr (1994). Identification of antigenic sites on the Na+/K+-ATPase beta-subunit: their sequences and the effects of thiol reduction upon their structure. Biochim. Biophys. Acta 1207, 236-248 [DOI] [PubMed] [Google Scholar]
- Takai Y., Irie K., Shimizu K., Sakisaka T., Ikeda W. (2003). Nectins and nectin-like molecules: roles in cell adhesion, migration, and polarization. Cancer Sci. 94, 655-667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokhtaeva E., Sachs G., Vagin O. (2009). Assembly with the Na+/K+-ATPase alpha(1) subunit is required for export of beta(1) and beta(2) subunits from the endoplasmic reticulum. Biochemistry 48, 11421-11431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokhtaeva E., Sachs G., Souda P., Bassilian S., Whitelegge J. P., Shoshani L., Vagin O. (2011). Epithelial junctions depend on intercellular trans-interactions between the Na+/K+-ATPase {beta}1 subunits. J. Biol. Chem. 286, 25801-25812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin O., Turdikulova S., Sachs G. (2005). Recombinant addition of N-glycosylation sites to the basolateral Na+/K+-ATPase beta1 subunit results in its clustering in caveolae and apical sorting in HGT-1 cells. J. Biol. Chem. 280, 43159-43167 [DOI] [PubMed] [Google Scholar]
- Vagin O., Tokhtaeva E., Sachs G. (2006). The role of the beta1 subunit of the Na+/K+-ATPase and its glycosylation in cell-cell adhesion. J. Biol. Chem. 281, 39573-39587 [DOI] [PubMed] [Google Scholar]
- Vagin O., Tokhtaeva E., Yakubov I., Shevchenko E., Sachs G. (2008). Inverse correlation between the extent of N-glycan branching and intercellular adhesion in epithelia. Contribution of the Na+/K+-ATPase beta1 subunit. J. Biol. Chem. 283, 2192-2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Jin X., Harrison O., Shapiro L., Honig B. H., Ben-Shaul A. (2010). Cooperativity between trans and cis interactions in cadherin-mediated junction formation. Proc. Natl. Acad. Sci. USA 107, 17592-17597 [DOI] [PMC free article] [PubMed] [Google Scholar]