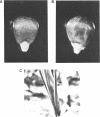
Abstract
The Mu transposons of the Robertsons's Mutator transposable element system in maize are unusual in many respects, when compared to the other known plant transposon systems. The excision of these elements occurs late in somatic tissues and very rarely in the germ line. Unlike the other plant transposons, there is no experimental evidence directly linking Mu element excision and integration. We have analyzed the excision products generated by a Mu1 transposon inserted into the bronze 1 locus of maize. We find that the excision products or 'footprints' left by the Mu1 element resemble those of the other plant transposable elements, rather than those of the animal transposable element systems. We also find some novel types of footprints resembling recombinational events. We suggest that the Mu1 element can promote intrachromosomal crossovers and conversions near its site of insertion, and that this may be another mechanism by which transposons can accelerate the evolution of genomes.
Full text
PDF

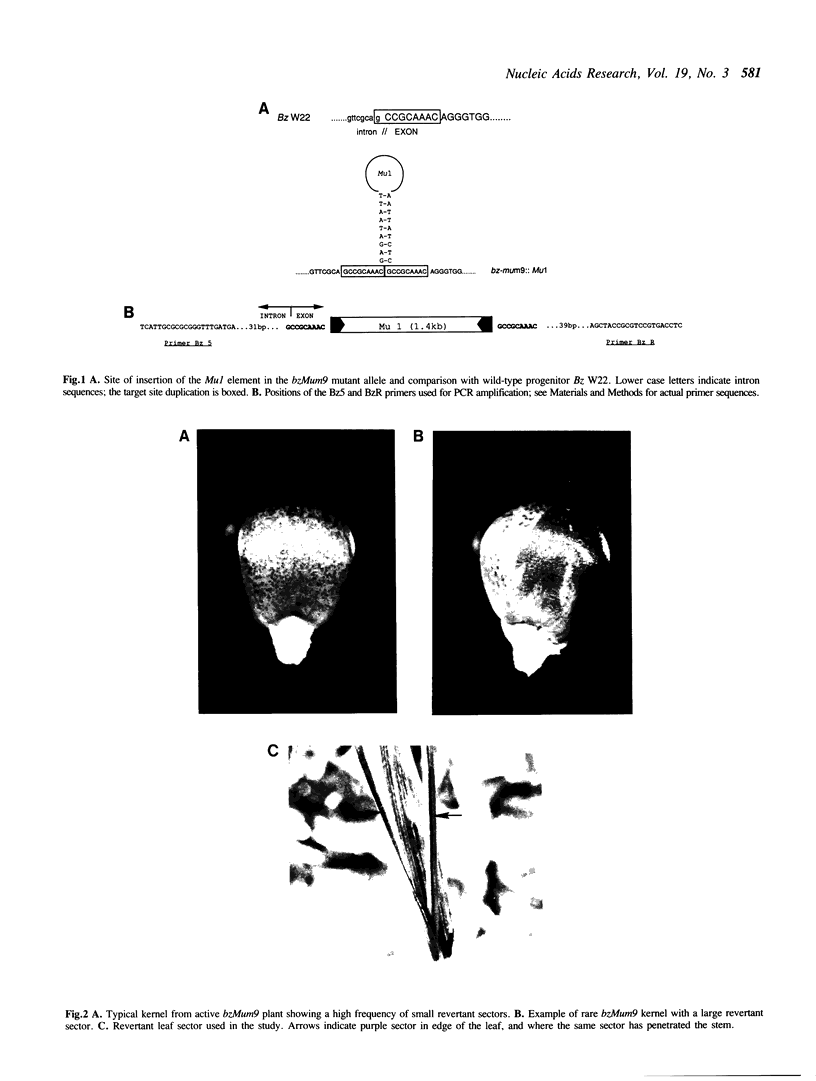



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alleman M., Freeling M. The Mu transposable elements of maize: evidence for transposition and copy number regulation during development. Genetics. 1986 Jan;112(1):107–119. doi: 10.1093/genetics/112.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker R. F., Thompson D. V., Talbot D. R., Swanson J., Bennetzen J. L. Nucleotide sequence of the maize transposable element Mul. Nucleic Acids Res. 1984 Aug 10;12(15):5955–5967. doi: 10.1093/nar/12.15.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen J. L., Swanson J., Taylor W. C., Freeling M. DNA insertion in the first intron of maize Adh1 affects message levels: cloning of progenitor and mutant Adh1 alleles. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4125–4128. doi: 10.1073/pnas.81.13.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. E., Robertson D. S., Bennetzen J. L. Molecular analysis of multiple mutator-derived alleles of the bronze locus of maize. Genetics. 1989 Jun;122(2):439–445. doi: 10.1093/genetics/122.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler V. L., Walbot V. DNA modification of a maize transposable element correlates with loss of activity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1767–1771. doi: 10.1073/pnas.83.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen E. S., Carpenter R., Martin C. Transposable elements generate novel spatial patterns of gene expression in Antirrhinum majus. Cell. 1986 Oct 24;47(2):285–296. doi: 10.1016/0092-8674(86)90451-4. [DOI] [PubMed] [Google Scholar]
- Engels W. R., Johnson-Schlitz D. M., Eggleston W. B., Sved J. High-frequency P element loss in Drosophila is homolog dependent. Cell. 1990 Aug 10;62(3):515–525. doi: 10.1016/0092-8674(90)90016-8. [DOI] [PubMed] [Google Scholar]
- Ralston E. J., English J. J., Dooner H. K. Sequence of three bronze alleles of maize and correlation with the genetic fine structure. Genetics. 1988 May;119(1):185–197. doi: 10.1093/genetics/119.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. S. Genetic studies on the loss of mu mutator activity in maize. Genetics. 1986 Jul;113(3):765–773. doi: 10.1093/genetics/113.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saedler H., Nevers P. Transposition in plants: a molecular model. EMBO J. 1985 Mar;4(3):585–590. doi: 10.1002/j.1460-2075.1985.tb03670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schnable P. S., Peterson P. A., Saedler H. The bz-rcy allele of the Cy transposable element system of Zea mays contains a Mu-like element insertion. Mol Gen Genet. 1989 Jun;217(2-3):459–463. doi: 10.1007/BF02464917. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Gierl A., Cuypers H., Peterson P. A., Saedler H. Plant transposable elements generate the DNA sequence diversity needed in evolution. EMBO J. 1985 Mar;4(3):591–597. doi: 10.1002/j.1460-2075.1985.tb03671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shure M., Wessler S., Fedoroff N. Molecular identification and isolation of the Waxy locus in maize. Cell. 1983 Nov;35(1):225–233. doi: 10.1016/0092-8674(83)90225-8. [DOI] [PubMed] [Google Scholar]
- Sundaresan V., Freeling M. An extrachromosomal form of the Mu transposons of maize. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4924–4928. doi: 10.1073/pnas.84.14.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert L. E., Patterson G. I., Chandler V. L. Mu transposable elements are structurally diverse and distributed throughout the genus Zea. J Mol Evol. 1989 Jul;29(1):28–39. doi: 10.1007/BF02106179. [DOI] [PubMed] [Google Scholar]
- Walbot V. Inheritance of mutator activity in Zea mays as assayed by somatic instability of the bz2-mu1 allele. Genetics. 1986 Dec;114(4):1293–1312. doi: 10.1093/genetics/114.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]