Abstract
A reaction based fluorescence turn-on strategy for hydrogen sulfide (H2S) was developed. This strategy was based on a H2S-specific Michael addition-cyclization sequence. Other biological thiols such as cysteine and glutathione did not pursue the reaction and therefore did not turn on the fluorescence/consume the substrates. The probes showed good selectivity and sensitivity for hydrogen sulfide.
Hydrogen sulfide (H2S) has traditionally been considered a highly toxic gas. However, recent studies have demonstrated that H2S is an endogenously generated gaseous signalling molecule with very potent cytoprotective actions that are on par with two other well-known endogenous gaseotransmitters: nitric oxide (NO) and carbon monoxide (CO). 1 Up to date, hydrogen sulfide's exact mechanisms of action are still under active investigation. Some chemical and biochemical catabolic reactions of H2S have been reported. For example, H2S can react readily with methemoglobin to form sulfhemoglobin, which might act as the metabolic sink for H2S. As a potential reductant, H2S is likely to be consumed by endogenous oxidant species such as hydrogen peroxide, superoxide, peroxynitrite, etc. H2S can also cause protein S-sulfhydration (i.e. to form –S-SH).2 This process is potentially significant because it provides a possible mechanism by which H2S alters the functions of a wide range of cellular proteins and enzymes. It is likely that many more important reactions of H2S are to be discovered. Nevertheless, the production of endogenous H2S and the exogenous administration of H2S elicit a wide range of protective actions including vasodilation, anti-inflammatory, antioxidant, anti-apoptotic, and down regulation of cellular metabolism during stressful states.1 These results strongly suggest that modulation of H2S levels could have potential therapeutic values for a number of diseases. It is important, therefore, to understand the chemistry and properties of H2S and to appreciate the limitation and errors that may be generated when measuring H2S in biological samples.
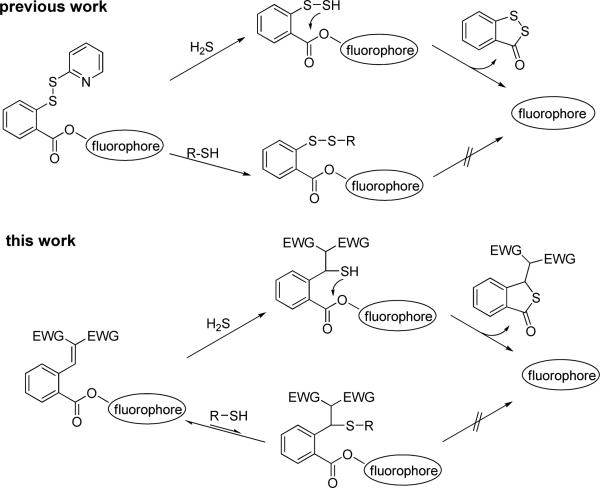
Currently a critical debate in the field is about the biologically relevant concentrations of H2S as reports varying over 105-fold range.3 Traditional H2S detection methods (colorimetric/electrochemical assays, gas chromatography, and sulfide precipitation)4 often require complicated post-mortem processing and/or destruction of tissues or cell lysates. Given the high reactivity of H2S in biological environments, these methods could yield inconsistent results. Fluorescence based assays could be useful in this field due to the high sensitivity and convenience. Fluorescence methods are suitable for nondestructive detection of bio-targets in live cells or tissues with readily available instruments. Since 2011 several fluorescent probes which can potentially be used for H2S detection in living systems have been reported.5 The fluorescence turn-on strategies of these probes were based on several different H2S-specific reactions.5 Among these methods, the strategy developed by our group utlized the unique dual-nucleophilicity of H2S.5b In our first generation probes (Scheme 1), 2-pyridinyl disulfide was employed as the H2S trap to initiate the tandem reaction to release the fluorophore. Such probes showed high selectivity for H2S and potential applications in monitoring H2S in living cells.5b However, the 2-pyridinyl disulfide could also react with biological thiols. Although the reaction won't lead to fluorescence turn-on, relatively high probe loading may be necessary. To solve this problem, here we report a new generation of probes which only react with H2S while do not interfere with other thiols.
Scheme 1.
Reaction-based fluorescence turn-on strategies.
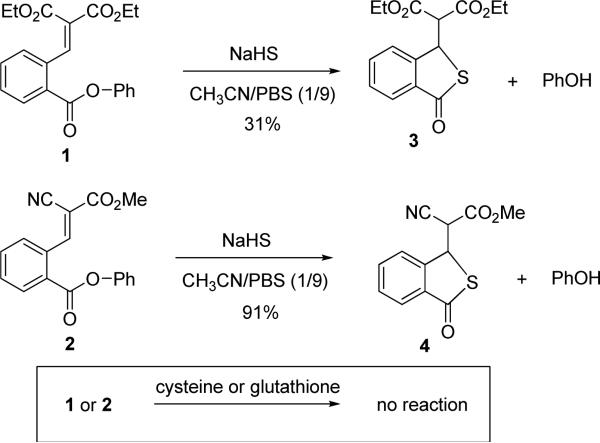
The design of the 2nd generation probes is shown in Scheme 1. Experiments by Holmes et al revealed that simple thiols reacted readily with some Michael acceptors at physiological pH, but the products could not be obtained or identified.6 This is possibly due to that the Michael addition was a rapid-equilibrium process and no stable covalent product was formed. Based on Holmes’ results, we envisioned that certain Michael acceptors might be useful to differentiate H2S from biological thiols. It is also known that H2S in aqueous has a pKa of 7.0 while thiols have higher pKa values ~8.5. So, H2S should be a better nucleophile than thiols in physiological media. If Michael acceptors are employed in the probes, they should be able to react with H2S and promote the intramolecular cyclization to release the fluorophore. The reactions between Michael acceptors and biological thiols such as cysteine and glutathione, however, should be reversible and therefore should not consume the probes.
With this idea in mind, two model compounds 1 and 2 were prepared (see Supporting Information for the synthesis). When compound 1 was treated with H2S (using NaHS as the equivalent) for 1 hour at rt, the desired cyclization product 3 was obtained in 31% yield (Scheme 2). The rest material was un-reacted 1. Compound 2 was proved to be more reactive in this reaction. Under the same conditions, cyclization product 4 was obtained in 91% yield. As expected, when both compounds were treated with cysteine or glutathione, no Michael addition products were isolated. Only the starting materials were recovered. In addition, compounds 1 and 2 showed no reaction towards primary amines and ammonia (see supporting information). These results confirmed our hypothesis that certain Michael acceptors can be used to selectively trap H2S and they are not consumed by thiols.
Scheme 2.
Model reactions
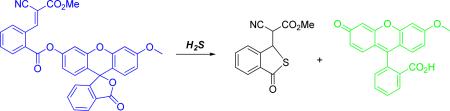
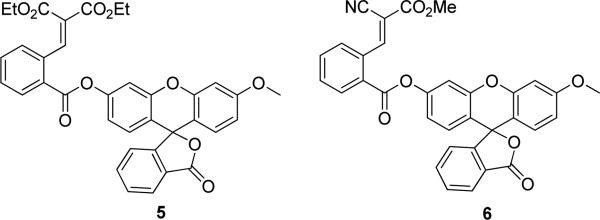
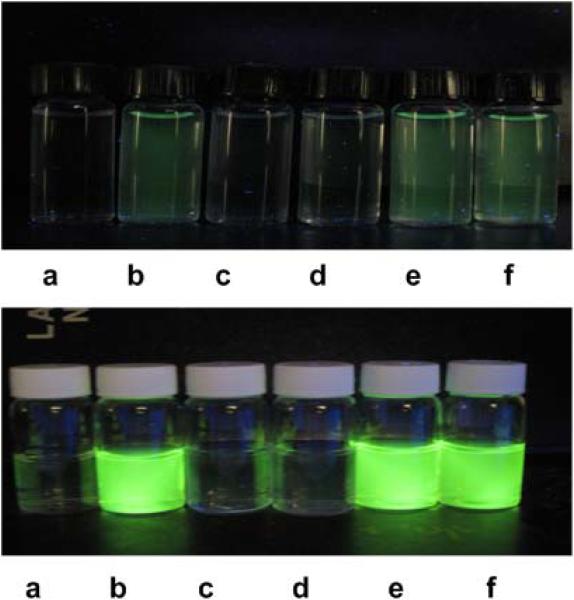
We then prepared two Michael acceptor-based fluorescent probes (5 and 6, Figure 1) and tested their fluorescence properties in aqueous buffers. Both compounds exhibited no absorption features in the visible region (fluorescence quantum yield of 5: Φ = 0.0002; 6: Φ = 0.0008). As shown in the images in Figure 2, the reactions of 5 and 6 with H2S (using NaHS as the equivalent) yielded significant fluorescence signals. Control experiments using cysteine or glutathione did not lead to any fluorescence increase. As expected, when H2S and thiols co-existed, we still observed strong fluorescence change. In addition, we noticed that probe 6 apparently showed much better responses than probe 5. This was consistent with the fact observed in Scheme 2 that the cyanoacrylate derivative had better reactivity with H2S than the benzylidenemalonate derivative.
Figure 1.
Structures of Michael acceptor-based fluorescent probes.
Figure 2.
Fluorescence images of probe 5 (top row) and probe 6 (bottom row) in pH 7.4 phosphate buffer. a) probe only (5 μM); b) probe + NaHS (100 μM); c) probe + cysteine (1 mM); d) probe + glutathione (1 mM), e) probe + cysteine (1 mM) + NaHS (100 μM), f) probe + glutathione (1 mM) + NaHS (100 μM).
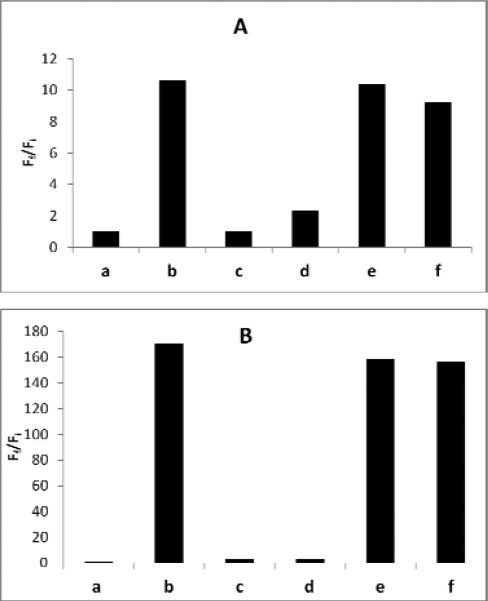
The turn-on responses of the probes were also measured by a spectrofluorometer. As shown in Figure 2, upon treatment of 5 μM probes 5 or 6 with 100 μM NaHS, significant increases in fluorescence intensity were observed. Within 30 min reaction time under these conditions, the benzylidenemalonate probe 5 produced 11-fold turn-on response. Again, the cyanoacrylate probe 6 was proved to be more sensitive to H2S and it led to 160-fold turn-on response (Figure 3). The analysis of reaction products confirmed the production of the corresponding fluorescein dye. When the probes were treated with abundant biologically relevant thiols, including 1 mM cysteine and 1 mM glutathione, no significant fluorescence increase was observed. Moreover, when H2S (100 μM) and thiols (1 mM) co-existed, we still observed strong fluorescence enhancements using both probes. These results demonstrated that the turn-on responses of probes 5 and 6 were selective for H2S and the probes could be used for the detection of H2S in the presence of high concentration of biological thiols.
Figure 3.
Fluorescence responses of (A) 5 μM probe 5 and (B) 5 μM probe 6 to H2S and biologically relevant thiols. : a) probe only, b) probe + NaHS (100 μM); c) probe + cysteine (1 mM); d) probe + glutathione (1 mM), e) probe + cysteine (1 mM) + NaHS (100 μM), f) probe + glutathione (1 mM) + NaHS (100 μM). Data were acquired in phosphate buffer (pH 7.4, 10 mM) with excitation at λex 465 nm, 25 °C.
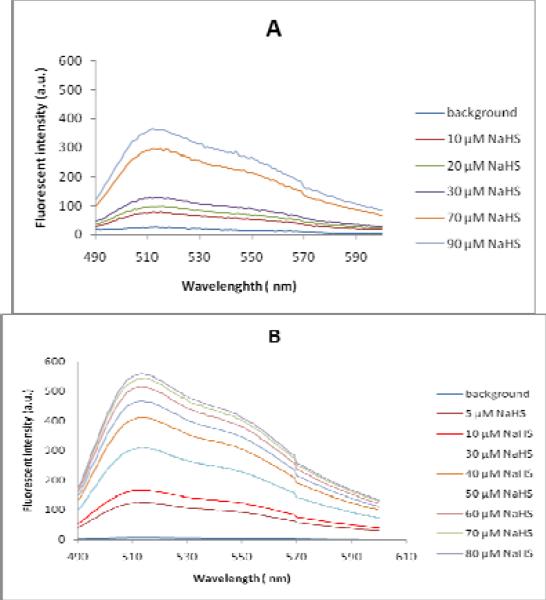
To demonstrate the efficiency of the probes in the measurement of H2S concentration, varying concentrations of NaHS (1-100 μM) were added to the solutions of 5 and 6 (5 μM). The fluorescence intensities were indeed linearly related to the concentrations of NaHS in such concentration ranges (Figure 4 and Figure S2 in Supporting Information). The detection limit for H2S using these probes was found to be around 1 μM.
Figure 4.
Fluorescence spectra of probe 5 (A) and probe 6 (B) in phosphate buffer (pH 7.4, 10 mM). Probe concentration was 5μM. Spectra were recorded after incubation with different concentrations of NaHS for 30 min.
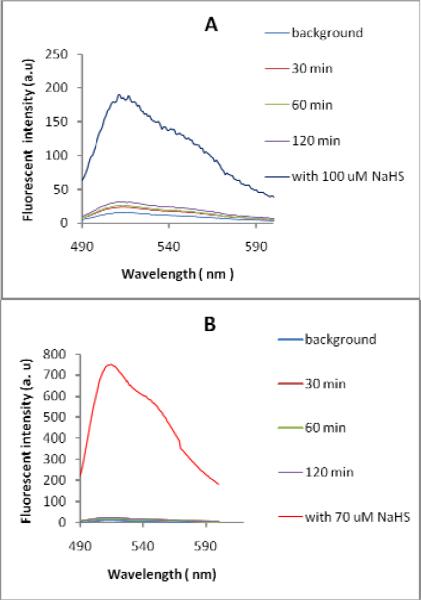
We recognized that probes 5 and 6 contained ester groups which could potentially be hydrolyzed by cellular esterase. We then tested the stability of probes in the presence of esterase. As shown in Figure 5, fluorescene intensity of both probes did not shown significant increase when they were treated with esterase for 2 h. After that, NaHS was added and fluorescence increase was immediately observed. These results suggested that the probes were quite stable towards esterase.
Figure 5.
Fluorescence spectra of probe 5 (A) and probe 6 (B) in the presence of esterase. Probe concentration: 5μM. Esterase concentration: 0.025 g/ mL. Spectra were recorded after incubation for different times.
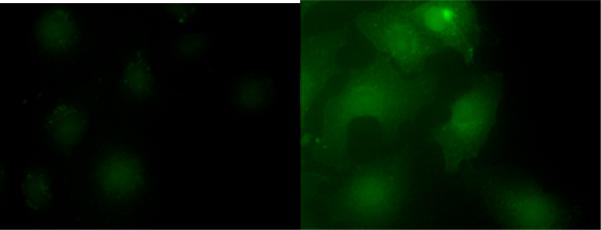
Since probe 6 was identified as a better probe than compound 5, due to higher sensitivity and faster reaction with H2S, this probe was selected for monitoring H2S in living cells. Briefly, COS7 cells were incubated with compound 6 for 10 min and then washed twice with phosphate buffer. We did not observe any significant fluorescent cells. However, strong fluorescence in the cells was observed after treatment with sodium sulfide for 10 min. Thus we conclude that probe 6 can be used for the detection of H2S in cultured cells.
In summary, we reported in this study an improved fluorescent probe for H2S detection. The design was based on a Michael addition of H2S followed by an intramolecular cyclization to release the fluorophore. This process proved to be selective for H2S. Other biological thiols reacted with the Michael acceptors in a reversible way and did not lead to covalent products. Therefore, thiols could not turn-on the fluorescence and did not consume the probe. Compared to other reaction based H2S probes5, our design has some advantages. Our probes contain three structural subunits: H2S trapper, linker, and fluorophore. A lot of chemical modifications can be carried out to improve the efficiency of the probes. We believe this strategy will be useful for the design of more sensitive and selective probes for the detection of H2S in biological systems.
Supplementary Material
Figure 6.
Fluorescence images of H2S detection in COS7 cells using probe 6. COS7 cells on glass coverslips were incubated with 6 (100 μM) for 10 min, washed, and then subjected to different treatments. Left was control (no sodium sulfide was added); right was treated with sodium sulfide (200 μM).
Acknowledgment
This work is supported in part by a CAREER award from NSF (0844931) to MX and NIH (R01HL088559) to ARW.
Footnotes
Supporting Information Available: Spectroscopic and analytical data and selected experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.a Li L, Rose P, Moore PK. Annu. Rev. Pharmacol. Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]; b Szabo C. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]; c Olson KR. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R297–R312. doi: 10.1152/ajpregu.00045.2011. [DOI] [PubMed] [Google Scholar]; d Lavu M, Bhushan S, Lefer DJ. Clin. Sci. 2011;120:219–229. doi: 10.1042/CS20100462. [DOI] [PubMed] [Google Scholar]; e Whiteman M, Moore PK. J. Cell. Mol. Med. 2009;13:488–507. doi: 10.1111/j.1582-4934.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Lynn EG, Austin RC. Expt. Rev. Clin. Pharmacol. 2011;120:97–108. doi: 10.1586/ecp.10.130. [DOI] [PubMed] [Google Scholar]
- 2.a Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. Sci. Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gadalla MM, Snyder SH. J. Neurochem. 2010;113:14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a Kabil O, Banerjee R. J. Biol. Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Olson KR. Biochim. Biophys. Acta. 2009;1787:856–863. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]; c Furne J, Saeed A, Levitt MD. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R1479–R1485. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]; d Han Y, Qin J, Chang X, Yang Z, Du Z. Cell. Mol. Neurobiol. 2006;26:101–107. doi: 10.1007/s10571-006-8848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ubuka T. Analyt. Technol. Biomed. Life Sci. 2002;781:227–249. doi: 10.1016/s1570-0232(02)00623-2. [DOI] [PubMed] [Google Scholar]; e Warenycia MW, Goodwin LR, Benishin CG, Reiffenstein RJ, Francom DM, Taylor JD, Dieken FP. Biochem. Pharmacol. 1989;38:973–981. doi: 10.1016/0006-2952(89)90288-8. [DOI] [PubMed] [Google Scholar]
- 4.a Tangerman A. J. Chromatogr. B. 2009;877:3366–3377. doi: 10.1016/j.jchromb.2009.05.026. [DOI] [PubMed] [Google Scholar]; b Ubuka T. J. Chromatogr. B. 2002;781:227–249. doi: 10.1016/s1570-0232(02)00623-2. [DOI] [PubMed] [Google Scholar]; c Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR., Jr. Anal. Biochem. 2005;341:40–51. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]; d Nagata T, Kage S, Kimura K, Kudo K, Noda M. J. Forensic. Sci. 1990;35:706–712. [PubMed] [Google Scholar]
- 5.a Lippert AR, New EJ, Chang CJ. J. Am. Chem. Soc. 2011;133:10078–10080. doi: 10.1021/ja203661j. [DOI] [PubMed] [Google Scholar]; b Liu C, Pan J, Li S, Zhao Y, Wu LY, Berkman CE, Whorton AR, Xian M. Angew. Chem. Int. Ed. 2011;50:10327–10329. doi: 10.1002/anie.201104305. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Peng H, Cheng Y, Dai C, King AL, Predmore BL, Lefer DJ, Wang BH. Angew. Chem. Int. Ed. 2011;50:9672–9675. doi: 10.1002/anie.201104236. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Qian Y, Karpus J, Kabil O, Zhang S, Zhu H, Banerjee R, Zhao J, He C. Nat. Commun. 2011;2:495–501. doi: 10.1038/ncomms1506. [DOI] [PubMed] [Google Scholar]; e Sasakura K, Hanaoka K, Shibuya N, Mikami Y, Kimura Y, Komatsu T, Ueno T, Terai T, Kimura H, Nagano T. J. Am. Chem. Soc. 2011;133:18003–18005. doi: 10.1021/ja207851s. [DOI] [PubMed] [Google Scholar]
- 6.Pritchard RB, Lough CE, Currie DJ, Holmes HL. Can. J. Chem. 1968;46:775–781. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.