Abstract
Discussion on the impact of endogenous polyamines on macrophage function after stimulation with IL-4 or LPS.
Keywords: arginase, ornithine decarboxylase, classical activation, alternative activation, IL-4, LPS
Macrophage activation is a critical step in the immune response against pathogens. The innate immune response of macrophages may determine the outcome of infections and modulate wound healing and inflammatory processes. Alterations in macrophage function have also been implicated in development of neoplastic transformation and in control of transformed cells. Macrophages may respond to various signals from the environment and give rise to different populations of activated cells [1]. In response to IFN-γ and TNF-α, macrophages exhibit enhanced microbicidal or tumoricidal activity by producing proinflammatory cytokines and mediators; this population is defined as classically activated macrophages, also called M1 macrophages. These cells are in contrast to IL-4-dependent, alternatively activated macrophages (M2 cells), which release anti-inflammatory mediators, such as IL-10, and are involved in allergic processes and defense against helminthic infections. Deeper investigations of macrophage physiology have recently indicated that at least two other types of macrophages exist, namely, regulatory and hybrid macrophages, which exhibit dramatic differences in their molecular features and biological properties [1].
In this issue of the Journal of Leukocyte Biology, Van den Bossche et al. [2] examined the effect of polyamines on the expression of markers of classical and alternative macrophage activation. The three biogenic polyamines—putrescine, spermidine, and spermine—are ubiquitous, low molecular weight aliphatic cations, implicated in a large number of cellular processes, including DNA replication, apoptosis, transcription, and translation [3]. In eukaryotes, putrescine is derived from ornithine via the action of ODC. Putrescine is subsequently converted to spermidine and then to spermine by constitutively expressed spermidine and spermine synthases, respectively. The first observation made by the authors was that the treatment of IL-4-stimulated murine macrophages with the ODC inhibitor DFMO for 24 h results in a significant reduction of putrescine content but not of spermidine and spermine. This result is consistent with previous observations from our group [4] and others [5], showing that DFMO fails to reduce spermine levels in macrophages; also, a longer exposure (48–72 h) to DFMO is required to decrease the intracellular concentration of putrescine and spermidine, whereas spermine levels are not affected significantly. However, the authors showed that DENSPM completely inhibits the generation of all of the biogenic polyamines, providing a reliable and important model for studying the biological effects of polyamines on innate immune response.
For these studies, macrophages with or without DENSPM treatment were stimulated for 24 h with IL-4. The expression of one-third of the tested genes that encode markers of alternatively activated macrophages, including the gene arg1, which encodes Arg1, were not modulated by DENSPM; however, two-thirds of these genes, including retnla, ym, cldn11, mrc1, or ear11, were down-regulated by DENSPM and/or DFMO, suggesting that IL-4-dependent expression of these genes is mediated by polyamines (Fig. 1). In this context, the literature helped the authors to generate hypotheses regarding the molecular mechanism(s) of this particular interaction between IL-4 and polyamines, and polyamines may influence gene expression by modulating DNA conformation, chromatin condensation, or DNA and RNA stability. Additionally, polyamines can activate the protein kinase casein kinase 2, which phosphorylates the oncoprotein c-Myc. Interestingly, c-Myc has been shown to be involved in the transcription of a cluster of M2-related genes during macrophage polarization [6]. Therefore, this transcription factor might be implicated in the polyamine-dependent differentiation of M2 macrophages, and this concept deserves further investigation. Of note, our group has established that ODC promoter activation and mRNA expression in Helicobacter pylori-activated macrophages, which present features of classical and alternative activation [7], are also mediated by c-Myc [8]. We can therefore suggest that the c-Myc/ODC/polyamine signaling pathway could be implicated in the regulation of macrophage physiology and consequently in the immune dysregulation in various forms of infection and pathology.
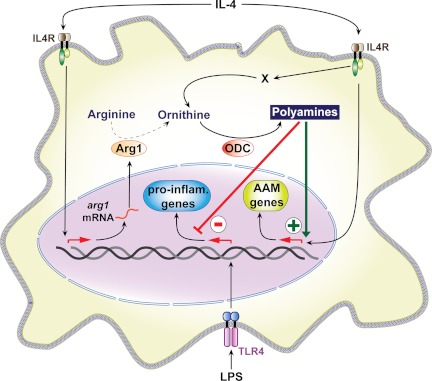
Figure 1. Endogenous polyamines potentiate macrophage polarization.
High levels of polyamines in macrophages treated with IL-4 increase the expression of genes encoding markers of alternatively activated macrophages (AAM), independently of Arg1 activity. Conversely, polyamines inhibit the expression of proinflammatory (pro-inflam.) genes in LPS-stimulated macrophages. How IL-4 increases polyamine synthesis by an ODC-dependent pathway (X), but independently of arginase activity, remains to be determined.
Later in the manuscript, Van den Bossche et al. [2] assessed the role of polyamines on the expression of various proinflammatory genes by LPS-stimulated macrophages. They observed that polyamine depletion was associated with an increase in mRNA expression of nos2, ccl2, ccl5, tnf, il-6, and il-12 p40. Previous work had shown that exogenous spermine down-regulates the proinflammatory cytokine responses of LPS-stimulated macrophages [9] and results in the inhibition of IL-12 p40 production, together with increased generation of IL-10 [10]. This further demonstrates that endogenous polyamines may play a pivotal role in the determination of macrophage activation status, not only by favoring the alternative pathway but also by reducing the inflammatory response (Fig. 1). However, how polyamines influence the activation of regulatory or hybrid macrophages is not addressed in this manuscript.
Arginase is an enzyme that converts arginine to ornithine, which is then metabolized by ODC into putrescine. In macrophages, arginase is thus a rate-limiting enzyme for polyamine synthesis [11]. Two isoforms of arginase exist: the cytoplasmic Arg1, abundant in liver and important for the urea cycle; and Arg2, mainly present in kidneys and localized to mitochondria. Importantly, both isoforms can be induced in cells according to the type of stimulus. As IL-4 induces Arg1 expression in macrophages [12], this enzyme has been considered as a marker for alternatively activated macrophages, although this concept remains controversial. Whereas it has been reported recently that the main immunosuppressive effect of Arg1-expressing macrophages is to compete with CD4+ T cells for arginine rather than regulating the production of IL-10 [13], the prototype cytokine of M2 macrophages, it was of interest to investigate the effect of Arg1 on polyamine production and macrophage activation (Fig. 1). The authors elegantly used the macrophages from Arg1Δ mice, as arg1 genomic deletion is lethal. Surprisingly, the authors found that there was no reduction in polyamine synthesis in IL-4-treated Arg1Δ macrophages that did not express Arg1. Moreover, arginase activity was inhibited completely in these cells, eliminating the likelihood that Arg2 was induced by IL-4. The authors also ruled out the possibility that arginine was metabolized by arginine decarboxylase and agmatinase into putrescine, as the transcripts of the gene encoding agmatinase were not present in the macrophages stimulated with IL-4 or LPS. As the authors discovered that “DFMO treatment of arginase-1-deficient macrophages strongly reduced putrescine levels”, this indicates that polyamine generation is not dependent on Arg1 and that ODC activity was required for putrescine synthesis in these cells. This raises the question as to the arginase-independent source of ornithine substrate for ODC. The authors show in their Fig. 1 that ornithine could derive from proline via the sequential action of proline oxidase and ornithine aminotransferase. However, this pathway remains to be investigated in macrophages under different activating conditions. It will be of interest in future studies to determine if arginine transport into Arg1Δ macrophages is required to allow polyamine synthesis; this could be achieved by blocking the arginine transporter CAT2 (SLC7A2) using small interfering RNA, as reported [14]. Also as CAT2 can transport ornithine into cells, this should be considered as a mechanism for substrate availability for ODC.
Finally, and not surprisingly, the authors show that the polyamine-dependent expression of proinflammatory genes and of markers of M2 macrophages after LPS and IL-4 stimulation, respectively, were identical in WT and Arg1Δ macrophages. Therefore, the authors conclude that “arginase-1-independent polyamine production stimulates the expression of alternatively activated macrophage markers”. This point puts into question the use of Arg1 as a marker of alternatively activated macrophages. The authors imply that there is a mechanism whereby polyamine synthesis occurs through a metabolic pathway that does not involve arginase activity, but this theoretical pathway remains to be elucidated. Regardless of which pathway(s) may be used by macrophages for polyamine biosynthesis, the data in this manuscript demonstrate that endogenous polyamines play a major function in regulation of macrophage activation.
ACKNOWLEDGMENTS
This work was supported by NIH grants R01DK053620, R01AT004821, P01CA116087, and P01CA028842; by the Vanderbilt Digestive Disease Research Center grant P30DK058404; and by a Merit Review grant from the Office of Medical Research, Department of Veterans Affairs (all to K.T.W.). A.P.G. is supported in part by the Philippe Foundation.
SEE CORRESPONDING ARTICLE ON PAGE 685
- Arg1/2
- arginase 1/2
- Arg1Δ
- Arg1F/F/Tie2-Cre+/− mice
- CAT2
- cationic amino acid transporter 2
- DENSPM
- N1,N11-diethylnorspermine
- DFMO
- difluoromethylornithine
- ODC
- ornithine decarboxylase
REFERENCES
- 1. Mosser D. M., Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van den Bossche J., Lamers W. H., Koehler E. S., Geuns J. M. C., Alhonen L., Uimari A., Pirnes-Kahru S., Van Overmeire E., Morias Y., Brys L., Vereecke L., De Baetselier P., Van Ginderachter J. A. (2012) Arginase-1-independent polyamine production stimulates the expression of IL-4-induced alternatively activated macrophage markers while inhibiting LPS-induced expression of inflammatory genes. J. Leukoc. Biol. 91, 685–699 [DOI] [PubMed] [Google Scholar]
- 3. Ruan H., Hill J. R., Fatemie-Nainie S., Morris D. R. (1994) Cell-specific translational regulation of S-adenosylmethionine decarboxylase mRNA. Influence of the structure of the 5′ transcript leader on regulation by the upstream open reading frame. J. Biol. Chem. 269, 17905–17910 [PubMed] [Google Scholar]
- 4. Bussiere F. I., Chaturvedi R., Cheng Y., Gobert A. P., Asim M., Blumberg D. R., Xu H., Kim P. Y., Hacker A., Casero R. A., Jr., Wilson K. T. (2005) Spermine causes loss of innate immune response to Helicobacter pylori by inhibition of inducible nitric-oxide synthase translation. J. Biol. Chem. 280, 2409–2412 [DOI] [PubMed] [Google Scholar]
- 5. Flamigni F., Stanic I., Facchini A., Cetrullo S., Tantini B., Borzi R. M., Guarnieri C., Caldarera C. M. (2007) Polyamine biosynthesis as a target to inhibit apoptosis of non-tumoral cells. Amino Acids 33, 197–202 [DOI] [PubMed] [Google Scholar]
- 6. Pello O. M., De Pizzol M., Mirolo M., Soucek L., Zammataro L., Amabile A., Doni A., Nebuloni M., Swigart L. B., Evan G. I., Mantovani A., Locati M. (2012) Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood 119, 411–421 [DOI] [PubMed] [Google Scholar]
- 7. Lewis N. D., Asim M., Barry D. P., de Sablet T., Singh K., Piazuelo M. B., Gobert A. P., Chaturvedi R., Wilson K. T. (2011) Immune evasion by Helicobacter pylori is mediated by induction of macrophage arginase II. J. Immunol. 186, 3632–3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng Y., Chaturvedi R., Asim M., Bussiere F. I., Scholz A., Xu H., Casero R. A., Jr., Wilson K. T. (2005) Helicobacter pylori-induced macrophage apoptosis requires activation of ornithine decarboxylase by c-Myc. J. Biol. Chem. 280, 22492–22496 [DOI] [PubMed] [Google Scholar]
- 9. Zhang M., Caragine T., Wang H., Cohen P. S., Botchkina G., Soda K., Bianchi M., Ulrich P., Cerami A., Sherry B., Tracey K. J. (1997) Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J. Exp. Med. 185, 1759–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hasko G., Kuhel D. G., Marton A., Nemeth Z. H., Deitch E. A., Szabo C. (2000) Spermine differentially regulates the production of interleukin-12 p40 and interleukin-10 and suppresses the release of the T helper 1 cytokine interferon-γ. Shock 14, 144–149 [DOI] [PubMed] [Google Scholar]
- 11. Kepka-Lenhart D., Mistry S. K., Wu G., Morris S. M., Jr. (2000) Arginase I: a limiting factor for nitric oxide and polyamine synthesis by activated macrophages? Am. J. Physiol. 279, R2237–R2242 [DOI] [PubMed] [Google Scholar]
- 12. Louis C. A., Mody V., Henry W. L., Jr., Reichner J. S., Albina J. E. (1999) Regulation of arginase isoforms I and II by IL-4 in cultured murine peritoneal macrophages. Am. J. Physiol. 276, R237–R242 [DOI] [PubMed] [Google Scholar]
- 13. Pesce J. T., Ramalingam T. R., Mentink-Kane M. M., Wilson M. S., El Kasmi K. C., Smith A. M., Thompson R. W., Cheever A. W., Murray P. J., Wynn T. A. (2009) Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 5, e1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaturvedi R., Asim M., Hoge S., Lewis N. D., Singh K., Barry D. P., de Sablet T., Piazuelo M. B., Sarvaria A. R., Cheng Y., Closs E. I., Casero R. A., Jr., Gobert A. P., Wilson K. T. (2010) Polyamines impair immunity to Helicobacter pylori by inhibiting L-arginine uptake required for nitric oxide production. Gastroenterology 139, 1686–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]