Ecrg4 is a sentinel peptide tethered on the surface of neutrophils and monocytes that is shed upon cell activation with fMLF or LPS, and activates macrophages.
Abstract
We identified fresh human leukocytes as an abundant source of the candidate epithelial tumor suppressor gene, Ecrg4, an epigenetically regulated gene, which unlike other tumor suppressor genes, encodes an orphan-secreted, ligand-like protein. In human cell lines, Ecrg4 gene expression was low, Ecrg4 protein undetectable, and Ecrg4 promoter hypermethylation high (45–90%) and reversible by the methylation inhibitor 5-AzaC. In contrast, Ecrg4 gene expression in fresh, normal human PBMCs and PMNs was 600–800 times higher than in cultured cell lines, methylation of the Ecrg4 promoter was low (<3%), and protein levels were readily detectable in lysates and on the cell surface. Flow cytometry, immunofluorescent staining, and cell surface biotinylation established that full-length, 14-kDa Ecrg4 was localized on PMN and monocyte cell surfaces, establishing that Ecrg4 is a membrane-anchored protein. LPS treatment induced processing and release of Ecrg4, as detected by flow and immunoblotting, whereas an effect of fMLF treatment on Ecrg4 on the PMN cell surface was detected on the polarized R2 subpopulation of cells. This loss of cell surface Ecrg4 was associated with the detection of intact and processed Ecrg4 in the conditioned media of fresh leukocytes and was shown to be associated with the inflammatory response that follows severe, cutaneous burn injury. Furthermore, incubation of macrophages with a soluble Ecrg4-derived peptide increased the P-p65, suggesting that processing of an intact sentinel Ecrg4 on quiescent circulating leukocytes leads to processing from the cell surface following injury and macrophage activation.
Introduction
The normal host response depends on an inflammation cascade, which is sufficient to address the insult but also, equally measured to permit injury resolution and thereby, avoid irreversible tissue damage and even death. To this end, investigators have hypothesized that blood-borne cells intercommunicate and that soluble factors gauge the initial and late responses to injury [1, 2]. In one such paradigm, constitutively expressed, sentinel factors that normally monitor homeostasis in quiescent cells, are down-regulated during the proliferative-reactive phases of injury, and re-emerge to normal levels, coincident with injury resolution.
One candidate sentinel gene is the orphan and epigenetically regulated human C2orf40 gene, also called Ecrg4. Its physiologic function is largely unknown, but its overexpression and/or gene knockdown are associated with changes in cell reactivity, senescence, epithelial cell growth, migration, and differentiation and progenitor cell survival, depending on the experimental model evaluated [3–13]. Ecrg4 gene expression in tissues also changes dynamically during embryonic development, in aging or after injury [3, 5, 6, 13], and its expression is down-regulated in numerous epithelial cancers by DNA hypermethylation of its promoter [4, 12].
Although it is presumed to be a tumor-suppressor gene, the C2orf40/Ecrg4 gene resembles a neuropeptide pro-hormone [11, 13], rather than an intracellular transcription factor or a signal transduction molecule, such as other tumor suppressors [14]. Instead, Ecrg4 processing can produce several candidate orphan ligand-like peptides that are presumed released after trafficking through the secretory pathway [15, 16]. Specifically, cleavage of an unusually long, 30-aa hydrophobic leader sequence generates a mature, 118-aa (14 kDa) protein that is a candidate for further processing by furin, PC1 and PC2, and/or thrombin-like enzymes [16, 17]. Like many neuro-hormone precursors [18, 19], when cells express the Ecrg4 gene, different peptides may be produced, and each may have distinct functions at different times and on different cells. Accordingly, whereas some investigators have suggested that Ecrg4 encodes a growth and migration inhibitor [8, 10], its mechanism of action and even the peptide(s) responsible for Ecrg4 activity remain enigmatic [17]. Ecrg4 gene expression has also been implicated in senescence, apoptosis, and progenitor cell survival in bone marrow and homeostasis in the CNS [3–12, 16, 17, 20, 21].
In this study, we show that leukocytes are a major source of Ecrg4 expression, its expression epigenetically regulated by DNA methylation, and that one of the products of Ecrg4 gene expression localizes to the leukocyte cell surface, where it is dynamically regulated. The findings suggest that the constitutive Ecrg4 expression in leukocytes, gauged in part by DNA methylation, may participate in the shared biology of cancer, immunity, inflammation, and injury.
MATERIALS AND METHODS
Cell and cell culture
All cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in the recommended growth media in a 37°C incubator supplemented with 5% CO2. To determine the effects of demethylation on Ecrg4 gene expression, Jurkat cells were treated daily with 0.5 μM 5-AzaC (Cat. #A3656; Sigma-Aldrich, St. Louis, MO, USA) for 16–48 h. Venous blood was obtained from healthy volunteers by collection into heparinized tubes, and blood was processed and used immediately. Leukocytes were prepared by RBC lysis in ammonium chloride (see below), and PMNs and PBMCs were prepared by further purification over Ficoll after and Dextran fractionation. The UCSD Institutional Review Board approved study participants, protocols, and consent forms, and informed consent was obtained from all participants.
Antibodies
Immunofluorescent studies used commercial anti-MD2 (Cat. #sc-20668; Santa Cruz Biotechnology, Santa Cruz, CA, USA), CD14 (Cat. #sc-6998; Santa Cruz Biotechnology), TLR4 (Cat. #sc-10741; Santa Cruz Biotechnology), and rabbit anti-Ecrg4 antibodies from Sigma-Aldrich (IgG1; Cat. #HPA008546), Phoenix Pharmaceuticals (Burlingame, CA, USA; IgG2; Cat. #G-012-24), or an affinity-purified chicken anti-Ecrg4 IgY antibody prepared by contract to Genway (San Diego, CA, USA). Isotype rabbit and chicken control antibodies were from Jackson ImmunoResearch (West Grove, PA, USA) and Genway, respectively.
PCR and real-time quantitative PCR to detect Ecrg4 gene expression
For gene expression analyses, total RNA was extracted using the RNeasy mini kit (Qiagen, Valencia, CA, USA) and quantified by spectrophotometry. Total RNA (1 μg) was reverse-transcribed using the iScript cDNA synthesis kit (BioRad, Hercules, CA, USA) at the conditions recommended by the vendor in a 20-μl volume. cDNA (1 μl) was used to quantify the gene expression by real-time PCR (BioRad; iQ5 cycler) in a 25-μl reaction containing 200 μM each of sense and antisense primers and iQ SYBR Green Supermix (BioRad). The primers for human ECRG4 were 5′-ACTAAGACTAAAGTGGCCGTTG-3′ (sense) and 5′-AGCCCATGTAGAGAAACTGCT-3′ (anti-sense). GAPDH, 5′-CATGAGAAGTATGACAACAGCCT-3′ (sense) and 5′-AGTCCTTCCACGATACCAAAGT-3′ (anti-sense), was used as the reference gene. Amplification efficiency was >90% for both pairs of primers, and the relative gene expression was calculated by the ΔΔ comparative threshold method, as described on BioRad's Real-Time PCR Applications Guide.
Methylation status of CpG Islands in the Ecrg4 promoter
DNA was prepared using Qiagen blood and tissue kit (Cat. #69,506), and purity as well as concentration were determined by absorbance at 260 and 280 nm. This DNA was digested using the SABiosciences (Qiagen, Valencia, CA, USA) Methylprofiler enzyme kit (MeA-03). Briefly, 125 ng DNA was digested in four equivalent reactions with no enzyme, methylation-sensitive enzyme, methylation-dependent enzyme, or both enzymes at 37°C overnight. The products of these reactions were amplified using the SABiosciences EpiTect Methyl qPCR primer assay for human C2orf40 (CpG Island 03,483; Cat. #MePHO3483–1A) and analyzed using the SABiosciences Methyl-Profiler PCR array template version 1.3 (12/18/2009). The methylation status at CpG Island 03,483 is reported as the sum percentage of hyper plus intermediate methylated DNA.
Cell surface biotinylation
Cell surface biotinylation was performed as we have described elsewhere [22] using the Pierce Biotechnology (Rockford, IL, USA) cell surface protein isolation kit (Cat. #89,881) with some modifications. Cells were washed twice with ice-cold PBS and then incubated with 4 ml ice-cold EZ-Link Sulfo-NHS-SS-Biotin on ice for 30 min. Cells were scraped, centrifuged, and lysed with RIPA buffer (50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 0.1% SDS, 1.0 mM EDTA, 0.5% Na-deoxycholate, and 1.0% Triton X-100) containing protease inhibitors and then sonicated and incubated on ice for 30 min. The cell lysates were cleared by centrifugation at 12,000 rpm for 5 min at 4°C, and 1/12 of the cleared supernatant was used to analyze the cell lysate fraction, and the remaining supernatant was used for Neutravidin bead precipitation, as outlined by the manufacturer's instructions. The conditioned media, cell lysate fraction, and cognate neutravidin precipitates of cell surface proteins were resolved on 4–12% NuPAGE (BioRad) and transferred to nitrocellulose membranes for immunoblotting as described below.
Immunoblotting
Cultured cells and leukocytes were extracted in 4% SDS containing Halt protease inhibitors (100:5) and the supernatants resolved on 4–12% NuPAGE. After transfer to nitrocellulose membranes, nonspecific binding was blocked by incubation with 5% BSA (Sigma-Aldrich) in TBS/Tween for 1 h at room temperature. Antibodies were incubated with the membranes overnight at 4°C and then washed before incubating the membranes with the appropriate HRP-linked secondary antibody (1:10,000 in 1% BSA; Sigma-Aldrich) for 1 h at room temperature. Supersignal West Pico chemiluminescent substrate (Pierce Biotechnology) was used for HRP detection. Images were produced with a Xenogen IVIS Lumina imaging machine, and band densities were determined using the program Living Image 3.1 (Caliper LifeSciences, Hopkinton, MA, USA).
Immunostaining of cells for confocal microscopy
Nonpermeabilized human leukocytes, PBMCs, and PMNs were subjected to immunofluorescence staining following fixation in 2% paraformaldehyde in 2% glucose, 0.1 M sodium phosphate buffer, pH 7.2, for 20 min at room temperature. Cells were blocked with 5% normal donkey serum in 1% BSA for 30 min at room temperature. The affinity-purified chicken anti-Ecrg4 IgY antibody (Genway; 1:2000) and rabbit polyclonal anti-TLR4 (sc-10741; Santa Cruz Biotechnology; 1:250), goat polyclonal anti-CD14 (sc-6998; Santa Cruz Biotechnology; 1:250), or rabbit polyclonal anti-MD2 (sc-20668; Santa Cruz Biotechnology; 1:250) were diluted in 1% BSA/PBS and incubated for 1 h at room temperature. Following further PBS wash, the appropriate Alexa-fluor-conjugated secondary antibodies (Molecular Probes, Eugene, OR, USA; 1:700) were incubated for 1 h at room temperature, washed in PBS, mounted in Slow fade for imaging, and imaged with an Olympus (Melville, NY, USA) Fluoview 1000 (ASW 1.7b) laser-scanning confocal microscope.
Treatment of human blood with fMLF and LPS
Fresh heparinized blood was obtained as described above and treated immediately for 1 min and 5 min with 100 nM/ml fMLF (Sigma-Aldrich) or LPS (0111:B4; Sigma-Aldrich), 100 ng/mL for 30 min at 37°C, before processing for flow cytometry or immunoblotting of supernatant conditioned media.
Flow cytometry
Leukocytes were prepared for FACs from heparinized blood by lysis with ammonium chloride (Cat. #555899; lysing buffer; Becton Dickinson, San Jose, CA, USA) for 15 min at room temperature and washing and fixed in Cytofix (Cat. #554655; Becton Dickinson) for 10 min on ice. Cells were incubated with primary anti-ECRG4 antibody (1:1000) in FACS buffer (1% BSA in PBS, 0.005% sodium azide) and wash in FACS buffer, followed by incubation with anti-chicken Alexa 488-conjugated fluorescent secondary antibodies. Flow cytometry was performed with a Becton Dickinson FACSCalibur and data analysis performed with CellQuestPro software from Becton Dickinson.
Preparation of peritoneal macrophages from mice
The UCSD Institutional Animal Care and Use Committee approved all mouse studies. For the isolation of peritoneal macrophages, cold, 4°C PBS, supplemented with 3% FBS in a volume of 5 mL, was used to flush the peritoneum of each BALB/c mouse. Macrophages from three pooled mice were incubated with synthetic Ecrg4 peptides corresponding to aa 37–62 or 132–148 at the doses indicated (New England Peptide, Gardner, MA, USA). Viable cells were gated and analyzed by flow cytometry for changes in the P-p65 using a PE-conjugated anti-P-p65 antibody (#5733; Cell Signaling Technology, Beverly, MA, USA), as described above, with the exception that cells were incubated with permeabilization buffer prior to antibody incubation, as described by the manufacturer (#554722, CytoFix/CytoPerm; #554723, Perm/Wash buffer; Becton Dickinson).
Statistical analysis
A Student's t test with a P value of <0.05 was used to determine statistical significance. Data are presented as mean value with error bars representing sds from replicate studies, as indicated in the legends for each of the figures. A modified false-discovery rate is used to report Ecrg4 expression levels obtained from microarray data, as described previously [23].
RESULTS
Ecrg4 is expressed in leukocytes but not detectable in blood
While evaluating the distribution of Ecrg4 gene expression in human, rat, and mouse tissues, we unexpectedly observed that in addition to the previously reported high levels of Ecrg4 gene expression in CNS neuroepithelia [3], Ecrg4 mRNA was particularly elevated in human peripheral blood cells (Fig. 1). Indeed, quantitative RT-PCR showed that Ecrg4 expression in leukocytes was 50- to 800-fold higher than other cells and cell lines in culture. Of the many cell lines surveyed, Ecrg4 mRNA was highest in cell lines of leukocyte lineage (i.e., Jurkat, THP1, and HL60 cells), but even then, the expression levels were 50-fold lower than that found in fresh PMNs and PBMCs.
Figure 1. Human peripheral blood cells express the Ecrg4 gene.
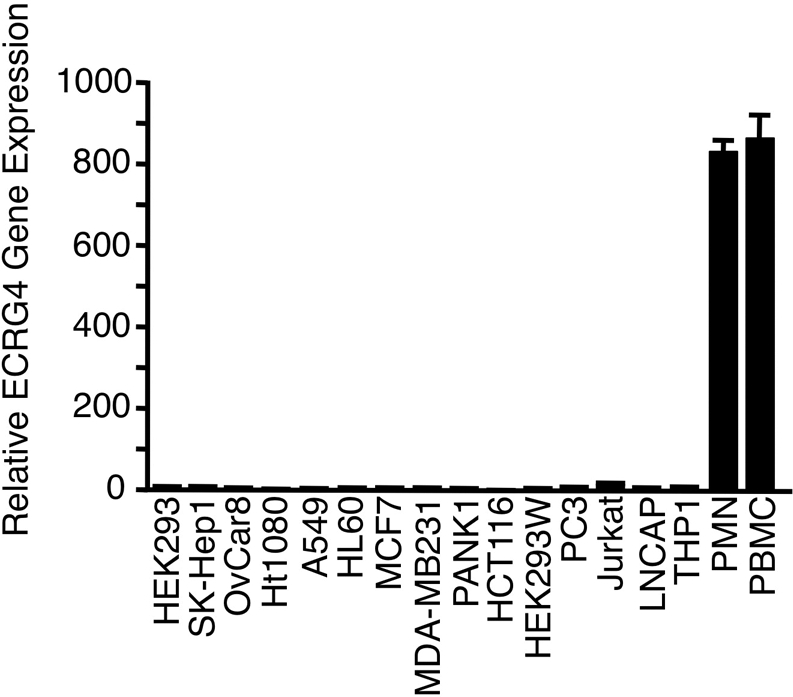
Ecrg4 gene expression in lysates prepared from a panel of cultured human epithelial, monocyte, and T cell cell lines and freshly isolated PMNs and PBMCs was measured by real-time quantitative PCR using GAPDH as a reference gene. Gene expression was normalized to levels in OvCar8 cells (n=3).
Ecrg4 gene expression is regulated by hypermethylation
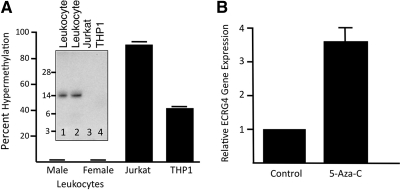
As Ecrg4 is a prototypic example of an epigenetic gene regulated by DNA methylation [4–6, 9, 12], we evaluated whether the differences in gene expression described in Fig. 1 might be attributed, at least in part, to methylation of the Ecrg4 promoter in cancer cell lines. To this end, we measured the methylation status of the Ecrg4 promoter locus in fresh normal human leukocytes prepared from healthy volunteer donors (n=17; Fig. 2A). In these normal leukocytes, we found <3% Ecrg4 promoter methylation. In contrast, there was 40–90% hypermethylation of cognate target CpG Islands in the Ecrg4 promoter of Jurkat and THP1 tumor cell lines (Fig. 2A). Whereas the Ecrg4 precursor peptide was present in leukocyte lysates purified from human blood (Fig. 2A, inset lanes 1 and 2), there was no detectable protein observed in lysates prepared from Jurkat and THP1 cells (Fig. 2A, inset lanes 3 and 4). Using the methylation inhibitor 5-AzaC, we observed an increase in Ecrg4 expression in Jurkat cells, three- to fivefold above control background levels (Fig. 2B). Together, these data support a model in which Ecrg4 gene expression in leukocytes can be regulated by DNA methylation.
Figure 2. Ecrg4 gene expression in human leukocytes.
(A) DNA methylation of the Ecrg4 promoter was evaluated in leukocytes from fresh peripheral blood of 17 healthy volunteers (male, n=8; female, n=9) and compared with DNA prepared from Jurkat and THP1 cells. (Inset) Anti-Ecrg4 antibodies used in immunoblotting detect the 14 kDa Ecrg4 protein in lysates of fresh human leukocytes, but no protein was detected in similarly prepared lysates of cultured Jurkat and THP1 cells. (B) Ecrg4 is hypermethylated in Jurkat cells. The DNA methylation inhibitor 5-AzaC increased Ecrg4 gene expression in Jurkat cells. In four different experiments (n=16), Jurkat cells incubated for 24 h with 0.5 μM 5-AzaC showed an increase of 3.5-fold in Ecrg4 gene expression.
Ecrg4 is a cell membrane protein that localizes to the leukocyte cell surface
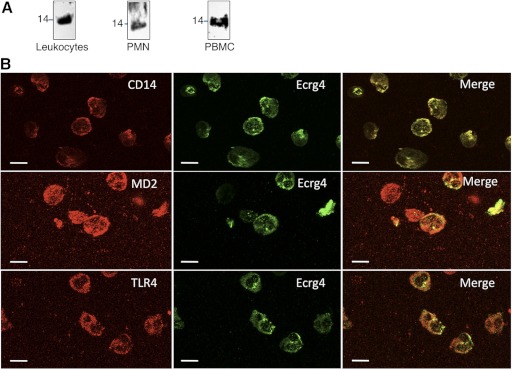
Sequence analyses of the Ecrg4 gene product predict a noncanonical hydrophobic peptide with the dual characteristics of a leader sequence for secretion and a transmembrane domain for cell surface tethering. This was confirmed experimentally by analysis of Ecrg4 on leukocytes by immunoblotting and immunostaining. The leukocyte cell surface was biotinylated and these cell surface proteins recovered by neutravidin precipitation and immunoblotted with an anti-Ecrg4 antibody to reveal the presence of the 14 kDa Ecrg4 (Fig. 3A). Ecrg4 protein was also detected on the surface of human PMNs and PBMCs (Fig. 3A). During these experiments, however, we also noted that the recovery of cell surface Ecrg4 from PMNs and PBMCs from gradients was highly variable, suggesting that its presence on the surface was dependent on cell activation. Therefore, we focused on confocal image analysis (Fig. 3B) and flow cytometry (Figs. 4 and 5) to further analyze Ecrg4-expressing cells. By avoiding cell fractionation and instead, immediately fixing and immunostaining freshly isolated leukocytes after RBC lysis, we were able to identify colocalization of Ecrg4 with TLR4, CD14, and MD2 at the cell surface (Fig. 3B), supporting a cell surface localization of Ecrg4 on leukocytes.
Figure 3. Ecrg4 is a cell membrane protein.
(A) Cell surface biotinylation of human leukocytes or fractionated PMNs and PBMCs revealed the presence of a 14 kDa Ecrg4 protein on the cell surface by precipitated cell surface proteins with neutravidin beads, SDS-PAGE, and immunoblotting with anti-Ecrg4 antibodies (n=4). (B) Confocal microscopy of permeabilized and freshly prepared human leukocytes immunostained for protein components of the innate immunity receptor protein, including CD14, MD2, or TLR4 (red, left panels) and Ecrg4 (green, middle panels). Colocalization was established by examining the overlap of fluorescent signals to reveal the distribution of Ecrg4 with CD14, MD2, or TLR4 (merged image, right panels). Original bars, 10 μm (n=3).
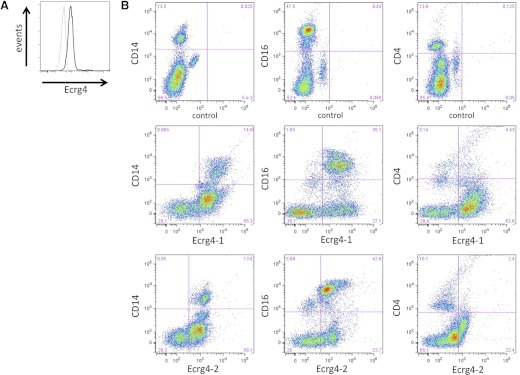
Figure 4. Cell surface expression of Ecrg4 on normal human PMNs and monocytes.
(A) Analysis of cell surface Ecrg4-positive leukocytes using an anti-Ecrg4 antibody (black line) and isotype control (gray line; n=4). (B) Representative analysis of normal human CD16+Ecrg4+, CD14+Ecrg4+, and CD4+Ecrg4+ leukocytes, as described in Materials and Methods (Ecrg4–1, Phoenix Pharmaceuticals 132–148 antibody; Ecrg4–2, Genway 71–148 antibody; n=4).
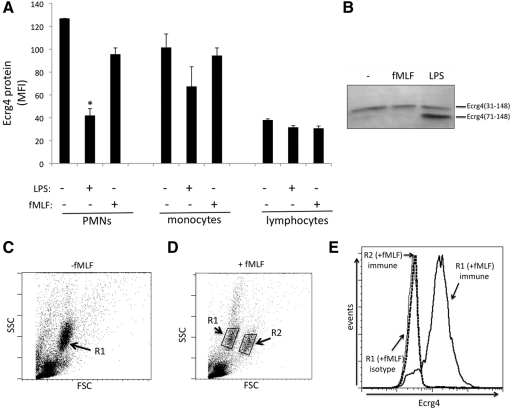
Figure 5. Sensitivity of cell surface Ecrg4 on fresh leukocytes to fMLF or LPS.
(A) Expression levels of Ecrg4 on the surface of PMN, monocyte, and lymphocyte subpopulations in the presence or absence of fMLF (100 nM) or LPS (10 ng/mL) (sem; *P<0.05; n=4). Ecrg4 levels are based on the median fluorescent intensity (MFI) of each population, as described in Materials and Methods. (B) Representative immunoblot with a C-terminal-specific anti-Ecrg4 antibody recognizing aa 132–148 to detect released Ecrg4 from the conditioned media of fresh human leukocytes incubated in buffer, fMLF (100 nM), or LPS (10 ng/mL) at 37°C for 30 min. (C) Representative forward- versus side-scatter (FSC/SSC) plot of normal, fresh human leukocytes with the R1 PMN population highlighted (n=3). (D) Representative forward- versus side-scatter plot of fresh human leukocytes treated with 100 nM fMLF, with R1 regions labeled in contrast to the increased forward-scatter of the polarized R2 side population of PMNs (n=3). (E) Comparison of the cell surface Ecrg4 protein levels in the R1 (solid, thick line) versus R2 regions (dotted line) of the fMLF-treated cells and isotype control (solid, thin line), showing the loss of cell surface protein in PMNs in the R2 population, which distinguishes them from the control R1 population of PMNs having significant cell surface Ecrg4 protein in representative samples (n=3).
To further analyze the expression of Ecrg4 on leukocyte subpopulations, we used flow cytometry to validate the cell surface expression of Ecrg4 on leukocytes (Fig. 4A) and Ecrg4 expression on CD16+ and CD14+ leukocytes versus low levels on CD4+ lymphocytes (Fig. 4B). We observed that Ecrg4 was detected primarily on PMN and monocyte subpopulations of leukocytes based on CD16 and CD14 immunostaining, respectively. We confirmed the cell type distribution of expression with two different anti-Ecrg4 antibodies, indicating that Ecrg4 is on the surface of monocytes and PMNs.
Release of Ecrg4 from the PMN and monocytes surface
To assess the effect of cell stimulation on cell surface levels of Ecrg4, we incubated leukocytes with LPS or fMLF and examined Ecrg4 levels on gated PMNs, monocytes, and lymphocytes (Fig. 5). We initially observed that the most significant decrease in cell surface Ecrg4 was observed on PMNs incubated with LPS (P<0.05; n=3; Fig. 5A), a minor decrease on monocytes, and no significant change in Ecrg4 expression on lymphocytes. Immunoblotting of conditioned media of leukocytes incubated with fMLF, LPS, or buffer, with a C-terminal anti-Ecrg4 antibody recognizing aa 132–148, revealed that a 14-kDa Ecrg4 was present in the media under all three conditions, suggesting that there is a constitutive release of Ecrg4, at least during the incubation of cells ex vivo (Fig. 5B). Furthermore, in the conditioned media of LPS-treated leukocytes, an Ecrg4 peptide was detected at ∼8 kDa, which corresponded to Ecrg4(71–148). Interestingly, the most significant reduction in cell surface Ecrg4 was observed in LPS-treated PMNs and monocytes, which paralleled the presence of a nascent, 8 kDa band in the conditioned media of LPS-treated leukocytes. Whereas fMLF treatment had a modest reduction of Ecrg4 levels on PMNs (termed “R1” population in Fig. 5C), we observed a more striking reduction of surface Ecrg4 on a polarized PMN subpopulation (Fig. 5C–E). In a flow analyses of fMLF-treated PMNs, we noted the presence of a previously characterized [24–26], fMLF-induced side population of PMNs, termed “R2” (Fig. 5C), which denotes PMN polarization, actin polymerization, and adhesion [26, 27]. These fMLF-induced R2 PMNs were distinct from R1 PMNs (Fig. 5D) and were devoid of cell surface Ecrg4 (Fig. 5E, dotted line vs. solid line).
Ecrg4 is a leukocyte injury response gene
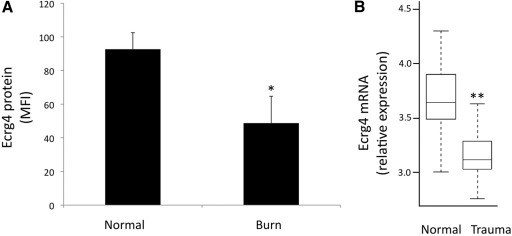
The sensitivity of surface Ecrg4 to LPS or fMLF exposure suggested that Ecrg4 expression levels on leukocytes could be associated with injury. Therefore, we examined the expression of cell surface Ecrg4 on PMNs from patients following a >30% TBSA burn, a systemic inflammatory response syndrome [28–30]. We used these samples to determine the effect of a clinically relevant inflammation state to compare levels of Ecrg4 with leukocytes from healthy volunteers. We focused on PMNs and observed a significant decrease in Ecrg4 cell surface expression on 30% TBSA patients (n=4) versus gender-matched male controls (n=3; Fig. 6A; P<0.05). Whereas severe burn patients are a complex patient group in terms of complications, interventions, and length of stay, the analyses of Ecrg4 on the PMN surface suggested that burn injury releases cell surface Ecrg4. We extended these studies to the analysis of Ecrg4 mRNA levels in healthy volunteers (n=35) compared with blunt trauma patients (n=167) by mining a microarray database for analysis of Ecrg4 expression (Fig. 6B) [23, 31]. We observed a statistically significant reduction in Ecrg4 expression from severely injured blunt trauma patients (Q value<0.001) with a 1.5-fold reduction between control and trauma samples.
Figure 6. Burn injury or trauma decreases Ecrg4 levels in human leukocytes.
(A) Analysis of Ecrg4 protein levels on the surface of PMNs from healthy volunteers (n=3) compared with the systemic inflammation that follows a >30% TBSA cutaneous burn injury (n=4 patients from the UCSD Burn Intensive Care Unit) using flow cytometry as described in Materials and Methods. *P < 0.05. (B) Analysis of Ecrg4 mRNA expression in a microarray analysis of leukocytes from normal donors (n=35) and trauma patients (n=167) from a multicenter study of leukocyte gene expression, as described recently [23] and available online (http://web.mgh.harvard.edu/TRT/). **Q < 0.001 (modified P for false-discovery rate).
C-terminal Ecrg4 peptide induces P-p65
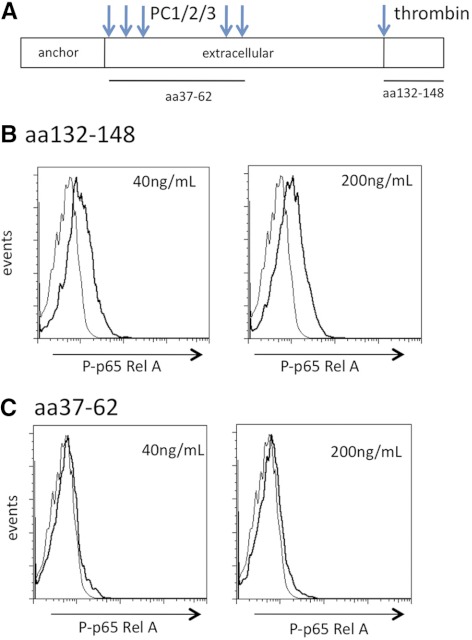
Sequencing of the Ecrg4-derived peptides, which are released from the cell surface, was performed at the UCSD Core Protein Sequencing Laboratories and identified the CΔ16 aa fragment of Ecrg4, which includes aa Ser 132–Tyr 148 of Ecrg4 1–148. This CΔ16 peptide (SPYGFRHGASVNYDDY) was released by processing the Ecrg4 precursor at a thrombin-like consensus sequence and cleavage at Arg 131. Therefore, we used mouse peritoneal macrophages to measure changes in the P-p65 as an indicator of the activation of the NF-κB pathway in macrophages. We observed a significant increase in P-p65 in macrophages treated with CΔ16 at 20 nM or 100 nM, whereas a control peptide corresponding to Ecrg4 37–62 did not affect macrophage P-p65 levels (Fig. 7; P<0.05). Our studies indicate that whereas membrane-bound Ecrg4 is associated with quiescent PMNs and monocytes, a C-terminal and soluble Ecrg4-derived peptide activates the NF-κB pathway in macrophages.
Figure 7. Ecrg4 CΔ16 peptide induced the P-p65.
(A) Diagram of Ecrg4 peptides tested for macrophage activation. (B) Effect of the Ecrg4 CΔ16 peptide (SPYGFRHGASVNYDDY) on the P-p65 in primary peritoneal mouse macrophages incubated at 40 ng/mL and 200 ng/mL (20 nM and 100 nM, respectively) for 15 min at 37°C. Representative plots are shown (n=3). (C) Effect of Ecrg4 37–62 (MLQKREAPVPTKTKVAVDENKAKEFL) on P-p65 in macrophages incubated with 40 ng/mL and 200 ng/mL (15 nM and 70 nM, respectively; n=3).
DISCUSSION
The findings reported here describe a series of new observations regarding the candidate tumor suppressor gene, Ecrg4. First, they identified PMNs and monocytes as major sources of Ecrg4 gene expression. Second, they show that the cell biology of Ecrg4 parallels TNF-α, CX3CL1, and several other inflammatory cytokines, in that it is tethered to the cell membrane, where upon stimulation, it can be released and processed to smaller peptides [32–34]. We have shown that the membrane-bound Ecrg4 protein colocalized with the TLR4/CD14 innate receptor complex on the leukocyte surface and that it is sensitive to LPS or fMLF treatment of cells. Stimulation with LPS, in particular, led to the release and processing of a nascent, cleaved 8 kDa C-terminal Ecrg4 peptide. The pathophysiological relevance of cell surface release of Ecrg4 is supported by the reduction of Ecrg4 following burn injury. The biological activity of a C-terminal Ecrg4 132–148 peptide, leading to increased P-p65, suggests that Ecrg4 released from leukocytes can activate macrophages. These studies support a model in which the membrane-anchored Ecrg4 is present on quiescent cells, but when processed and released, it activates macrophages. Statistically significant changes in Ecrg4 mRNA expression were also detected when mining a public global gene expression database of human leukocytes after severe trauma, suggesting a clinical relevance for Ecrg4 in the biology of injury.
Markov modeling by Mirabeau et al. [16] initially predicted that Ecrg4 was a constitutively secreted protein. Instead our data support the bioinformatic analyses predicting a non-cannonical hydrophobic leader sequence and transmembrane domain at the amino terminus of Ecrg4 that tethers the protein to the cell surface. Accordingly, the Ecrg4 protein(s) joins other cell-tethered ligands and receptors that are present and released from the cell surface after stimulation [35, 36]. As epigenetic mechanisms regulate Ecrg4 gene expression [4, 12, 37], it is interesting to speculate that Ecrg4 promoter methylation regulates the reservoir of Ecrg4 expression and the levels of cell surface protein. If Ecrg4 expression is important for PMN/monocyte quiescence and its reappearance associated with injury recovery [13], then the findings here predict that DNA hypermethylation of the Ecrg4 promoter would interfere with Ecrg4 re-expression on the surface. We initially examined the expression levels of Ecrg4 in various cell lines and tissues but observed high Ecrg4 mRNA and protein in peripheral blood cells, where there was low methylation of the Ecrg4 promoter. Conversely, cultured T cells and monocytes had low levels of Ecrg4 protein, which was associated with high promoter methylation. Treatment with a 5-AzaC to block DNA methylation increased Ecrg4 gene expression, demonstrating that the epigenetic status of the Ecrg4 promoter affected gene expression. This suggested that if the re-expression of Ecrg4 were important to re-establish quiescence, then the epigenetic status of Ecrg4 would be important to evaluate further.
The first suggestion that Ecrg4 might be involved in the inflammatory response was based on its colocalization of CD14, TLR4, and MD2 on the leukocyte cell surface. Confirmation of the cell surface expression of Ecrg4 was established by biotinylation of the cell surface, followed by neutravidin bead pull-down and anti-Ecrg4 immunoblotting of unfractionated leukocyte populations, PMN fractions, and PBMC fractions. Characterization of Ecrg4 immunoreactive leukocytes by flow cytometry led to the identification of Ecrg4+ cells, which were CD14+CD6+CD4−, indicating that Ecrg4 was expressed primarily on the surface of PMNs and monocytes. Further analyses of subpopulations of normal, fresh leukocytes may reveal additional insights into the cell-type distribution of Ecrg4 among PMNs and monocytes.
The release of cell surface Ecrg4 following LPS or fMLF challenge revealed distinct sensitivities to inflammatory stimuli as described for other genes [38–40]. For example, whereas monocyte Ecrg4 was sensitive to LPS, and PMN Ecrg4 was sensitive to fMLF, PMN Ecrg4 was particularly sensitive to LPS. Based on the effects of LPS and fMLF ex vivo on the expression of Ecrg4, we also examined human patient samples to determine if there is pathophysiological relevance of Ecrg4 to injury in human leukocytes. The trauma-induced decrease in Ecrg4 expression detected in leukocytes, along with the loss of Ecrg4 expression in PMNs of burn patients, suggests that Ecrg4 release is an injury response.
To address the biological function of soluble Ecrg4 released following inflammatory insult, we examined the activity of CΔ16, a fragment of the C-terminal Ecrg4 peptide that we detected in conditioned media of transfected cells. Interestingly, this same peptide fragment was identified from the Human Plasma Proteome initiative [41], and we demonstrated here that this Ecrg4 132–148 peptide could induce P-p65, a marker of NF-κB pathway activation. The results, therefore, provide in vivo and in vitro evidence that PMNs and monocytes could modulate the inflammatory phenotype through the release of a soluble Ecrg4. In this model, a membrane-tethered Ecrg4 is associated with quiescence, but the processing and release of a soluble Ecrg4 peptide are associated with activation, and at least one of the soluble peptides that is generated (i.e., Ecrg4 132–148) activates macrophages. In light of these findings, further studies will be necessary to determine whether there is a heretofore unrecognized role for Ecrg4 as a sentinel molecule that gauges inflammation and immunity by its decreased expression in cancer and after injury and its increase at resolution [4, 7, 9, 10, 12, 20, 42]. If so, these data infer that the growth advantage provided by the down-regulation of Ecrg4 by promoter hypermethylation is indirect and that the loss of its sentinel-like capacity to regulate the inflammatory response is ultimately linked to the ability of transformed cells to escape inflammation and immunosurveillance.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health through a P20 Exploratory Center grant for Wound Healing Research from the NIGMS (P20-GM078421) to A.B., B.E., and R.C. and from grants EY018479 (A.B.), DK085871 (A.B.), and HL73396 (B.P.E.) and supplemental funding by the National Eye Institute (NEI) and NIGMS through the American Recovery Act (ARRA). The authors are indebted to David Cauvi, Emelie Amburn, Hyun Bae-Chun, Alexandra Borboa, Anne Marie Hageny, James Putnam, and Lei Xu for their expert laboratory support.
Footnotes
- 5-AzaC
- 5-Aza-2′-deoxycytidine
- Ecrg4
- esophageal cancer-related gene 4
- MD2
- myeloid differentiation protein 2
- NIGMS
- National Institute of General Medical Sciences
- P-p65
- phosphorylation of p65 Rel A
- PC1/2
- protein convertases 1/2
- TBSA
- total body surface area
- UCSD
- University of California San Diego
AUTHORSHIP
A.B., B.P.E., and R.C. designed the experiments and wrote the manuscript. X.D., N.L., J.L., and M.K. performed the experiments. R.W. and B.P. aided A.B. and B.P.E. in the clinical data analysis.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1. Han J., Ulevitch R. J. (2005) Limiting inflammatory responses during activation of innate immunity. Nat. Immunol. 6, 1198–1205 [DOI] [PubMed] [Google Scholar]
- 2. Liew F. Y., Xu D., Brint E. K., O'Neill L. A. (2005) Negative regulation of Toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 5, 446–458 [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez A., Podvin S., Lin S-Y., Miller M., Botfield H., Leadbeater W., Roberton A., Dang X., Knowling S., Cardenas-Galindo E., Donahue J. E., Stopa E. G., Johanson C. E., Coimbra R., Eliceiri B. P., Baird A. (2011) Ecrg4 expression and its product augurin in the choroid plexus: impact on fetal brain development, cerebrospinal fluid homeostasis and neuroprogenitor cell response to CNS injury. Fluids Barriers CNS 8, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gotze S., Feldhaus V., Traska T., Wolter M., Reifenberger G., Tannapfel A., Kuhnen C., Martin D., Muller O., Sievers S. (2009) ECRG4 is a candidate tumor suppressor gene frequently hypermethylated in colorectal carcinoma and glioma. BMC Cancer 9, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huh Y. H., Ryu J. H., Shin S., Lee D. U., Yang S., Oh K. S., Chun C. H., Choi J. K., Song W. K., Chun J. S. (2009) Esophageal cancer related gene 4 (ECRG4) is a marker of articular chondrocyte differentiation and cartilage destruction. Gene 448, 7–15 [DOI] [PubMed] [Google Scholar]
- 6. Kujuro Y., Suzuki N., Kondo T. (2010) Esophageal cancer-related gene 4 is a secreted inducer of cell senescence expressed by aged CNS precursor cells. Proc. Natl. Acad. Sci. USA 107, 8259–8264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li L., Li Y., Li X., Zhang C., Zhou Y., Lu S. (2011) A novel tumor suppressor gene ECRG4 interacts directly with TMPRSS11A (ECRG1) to inhibit cancer cell growth in esophageal carcinoma. BMC Cancer 11, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li L., Yang Y., Li X., Zhou Y., Lu S. (2011) Cloning and expression of soluble recombinant human esophageal cancer related gene 4 protein and its inhibitory effect on tumor growth in vitro and in vivo in esophageal carcinoma. Cancer Sci. 102, 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li L. W., Yu X. Y., Yang Y., Zhang C. P., Guo L. P., Lu S. H. (2009) Expression of esophageal cancer related gene 4 (ECRG4), a novel tumor suppressor gene, in esophageal cancer and its inhibitory effect on the tumor growth in vitro and in vivo. Int. J. Cancer 125, 1505–1513 [DOI] [PubMed] [Google Scholar]
- 10. Li W., Liu X., Zhang B., Qi D., Zhang L., Jin Y., Yang H. (2010) Overexpression of candidate tumor suppressor ECRG4 inhibits glioma proliferation and invasion. J. Exp. Clin. Cancer Res. 29, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tadross J. A., Patterson M., Suzuki K., Beale K. E., Boughton C. K., Smith K. L., Moore S., Ghatei M. A., Bloom S. R. (2010) Augurin stimulates the hypothalamo-pituitary-adrenal axis via the release of corticotrophin-releasing factor in rats. Br. J. Pharmacol. 159, 1663–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yue C. M., Deng D. J., Bi M. X., Guo L. P., Lu S. H. (2003) Expression of ECRG4, a novel esophageal cancer-related gene, downregulated by CpG Island hypermethylation in human esophageal squamous cell carcinoma. World J. Gastroenterol. 9, 1174–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Podvin S., Gonzalez A. M., Miller M. C., Dang X., Botfield H., Donahue J. E., Kurabi A., Boissaud-Cooke M., Rossi R., Leadbeater W. E., Johanson C. E., Coimbra R., Stopa E. G., Eliceiri B. P., Baird A. (2011) Esophageal cancer related gene-4 is a choroid plexus-derived injury response gene: evidence for a biphasic response in early and late brain injury. PLoS One 6, e24609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee E. Y., Muller W. J. (2010) Oncogenes and tumor suppressor genes. Cold Spring Harb. Perspect. Biol. 2, a003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clark H. F., Gurney A. L., Abaya E., Baker K., Baldwin D., Brush J., Chen J., Chow B., Chui C., Crowley C., et al. (2003) The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: a bioinformatics assessment. Genome Res. 13, 2265–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mirabeau O., Perlas E., Severini C., Audero E., Gascuel O., Possenti R., Birney E., Rosenthal N., Gross C. (2007) Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res. 17, 320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ozawa A., Lick A. N., Lindberg I. (2011) Processing of proaugurin is required to suppress proliferation of tumor cell lines. Mol. Endocrinol. 25, 776–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lazure C., Seidah N. G., Pelaprat D., Chretien M. (1983) Proteases and posttranslational processing of prohormones: a review. Can. J. Biochem. Cell Biol. 61, 501–515 [DOI] [PubMed] [Google Scholar]
- 19. Lowry P. J. (1984) Pro-opiocortin: the multiple adrenal hormone precursor. Review. Biosci. Rep. 4, 467–482 [DOI] [PubMed] [Google Scholar]
- 20. Mori Y., Ishiguro H., Kuwabara Y., Kimura M., Mitsui A., Kurehara H., Mori R., Tomoda K., Ogawa R., Katada T., Harata K., Fujii Y. (2007) Expression of ECRG4 is an independent prognostic factor for poor survival in patients with esophageal squamous cell carcinoma. Oncol. Rep. 18, 981–985 [PubMed] [Google Scholar]
- 21. Nishikawa M., Drmanac R. T., Lobal I., Tang Y. T., Lee J., Stache-Crain B. (2008) Polypeptide having an activity to support proliferation or survival of hematopoietic stem cell and hematopoietic progenitor cell, and DNA coding for the same. In United States Patent Trade Office (USPTO, ed.), Nuvelo, San Carlos, CA, USA [Google Scholar]
- 22. Trudel C., Faure-Desire V., Florkiewicz R. Z., Baird A. (2000) Translocation of FGF2 to the cell surface without release into conditioned media. J. Cell. Physiol. 185, 260–268 [DOI] [PubMed] [Google Scholar]
- 23. Xiao W., Mindrinos M. N., Seok J., Cuschieri J., Cuenca A. G., Gao H., Hayden D. L., Hennessy L., Moore E. E., Minei J. P., et al. (2011) A genomic storm in critically injured humans. J. Exp. Med. 208, 2581–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen L. W., Huang H. L., Lee I. T., Hsu C. M., Lu P. J. (2006) Thermal injury-induced priming effect of neutrophil is TNF-α and P38 dependent. Shock 26, 69–76 [DOI] [PubMed] [Google Scholar]
- 25. Howard T. H., Meyer W. H. (1984) Chemotactic peptide modulation of actin assembly and locomotion in neutrophils. J. Cell Biol. 98, 1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kutsuna H., Suzuki K., Kamata N., Kato T., Hato F., Mizuno K., Kobayashi H., Ishii M., Kitagawa S. (2004) Actin reorganization and morphological changes in human neutrophils stimulated by TNF, GM-CSF, and G-CSF: the role of MAP kinases. Am. J. Physiol. Cell. Physiol. 286, C55–C64 [DOI] [PubMed] [Google Scholar]
- 27. Fernandez-Segura E., Garcia J. M., Santos J. L., Campos A. (1995) Shape, F-actin, and surface morphology changes during chemotactic peptide-induced polarity in human neutrophils. Anat. Rec. 241, 519–528 [DOI] [PubMed] [Google Scholar]
- 28. Cavaillon J. M., Adrie C., Fitting C., Adib-Conquy M. (2005) Reprogramming of circulatory cells in sepsis and SIRS. J. Endotoxin. Res. 11, 311–320 [DOI] [PubMed] [Google Scholar]
- 29. Cavaillon J. M., Annane D. (2006) Compartmentalization of the inflammatory response in sepsis and SIRS. J. Endotoxin. Res. 12, 151–170 [DOI] [PubMed] [Google Scholar]
- 30. Dahiya P. (2009) Burns as a model of SIRS. Front. Biosci. 14, 4962–4967 [DOI] [PubMed] [Google Scholar]
- 31. Desai K. H., Tan C. S., Leek J. T., Maier R. V., Tompkins R. G., Storey J. D. (2011) Dissecting inflammatory complications in critically injured patients by within-patient gene expression changes: a longitudinal clinical genomics study. PLoS Med. 8, e1001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bazan J. F., Bacon K. B., Hardiman G., Wang W., Soo K., Rossi D., Greaves D. R., Zlotnik A., Schall T. J. (1997) A new class of membrane-bound chemokine with a CX3C motif. Nature 385, 640–644 [DOI] [PubMed] [Google Scholar]
- 33. Decoster E., Vanhaesebroeck B., Vandenabeele P., Grooten J., Fiers W. (1995) Generation and biological characterization of membrane-bound, uncleavable murine tumor necrosis factor. J. Biol. Chem. 270, 18473–18478 [DOI] [PubMed] [Google Scholar]
- 34. Ludwig A., Weber C. (2007) Transmembrane chemokines: versatile “special agents” in vascular inflammation. Thromb. Haemost. 97, 694–703 [PubMed] [Google Scholar]
- 35. Santos D. O., Lorré K., de Boer M., Van Heuverswyn H. (1999) Shedding of soluble receptor for tumor necrosis factor α induced by M. leprae or LPS from human mononuclear cells. Nihon Hansenbyo Gakkai Zasshi 68, 185–193 [DOI] [PubMed] [Google Scholar]
- 36. Douvdevani A., Einbinder T., Yulzari R., Rogachov B., Chaimovitz C. (1996) TNF-receptors on human peritoneal mesothelial cells: regulation of receptor levels and shedding by IL-1 α and TNF α. Kidney Int. 50, 219–228 [DOI] [PubMed] [Google Scholar]
- 37. Vanaja D. K., Ehrich M., Van den Boom D., Cheville J. C., Karnes R. J., Tindall D. J., Cantor C. R., Young C. Y. (2009) Hypermethylation of genes for diagnosis and risk stratification of prostate cancer. Cancer Invest. 27, 549–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pillay J., Ramakers B. P., Kamp V. M., Loi A. L., Lam S. W., Hietbrink F., Leenen L. P., Tool A. T., Pickkers P., Koenderman L. (2010) Functional heterogeneity and differential priming of circulating neutrophils in human experimental endotoxemia. J. Leukoc. Biol. 88, 211–220 [DOI] [PubMed] [Google Scholar]
- 39. Kopydlowski K. M., Salkowski C. A., Cody M. J., van Rooijen N., Major J., Hamilton T. A., Vogel S. N. (1999) Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J. Immunol. 163, 1537–1544 [PubMed] [Google Scholar]
- 40. Lederer J. A., Brownstein B. H., Lopez M. C., Macmillan S., Delisle A. J., Macconmara M. P., Choudhry M. A., Xiao W., Lekousi S., Cobb J. P., Baker H. V., Mannick J. A., Chaudry I. H., Inflammation and the Host Response to Injury Collaborative Research Program Participants (2008) Comparison of longitudinal leukocyte gene expression after burn injury or trauma-hemorrhage in mice. Physiol. Genomics 32, 299–310 [DOI] [PubMed] [Google Scholar]
- 41. Farrah T., Deutsch E. W., Omenn G. S., Campbell D. S., Sun Z., Bletz J. A., Mallick P., Katz J. E., Malmstrom J., Ossola R., Watts J. D., Lin B., Zhang H., Moritz R. L., Aebersold R. (2011) A high-confidence human plasma proteome reference set with estimated concentrations in PeptideAtlas. Mol. Cell. Proteomics 10, M110.006353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Steck E., Breit S., Breusch S. J., Axt M., Richter W. (2002) Enhanced expression of the human chitinase 3-like 2 gene (YKL-39) but not chitinase 3-like 1 gene (YKL-40) in osteoarthritic cartilage. Biochem. Biophys. Res. Commun. 299, 109–115 [DOI] [PubMed] [Google Scholar]