Abstract
Langerhans cells (LCs) are a distinct population of dendritic cells that form a contiguous network in the epidermis of the skin. Although LCs possess many of the properties of highly proficient dendritic cells, recent studies have indicated that they are not necessary to initiate cutaneous immunity. In this study, we used a tractable model of cutaneous GVHD, induced by topical application of a Toll-like receptor agonist, to explore the role of LCs in the development of tissue injury. By adapting this model to permit inducible and selective depletion of host LCs, we found that GVHD was significantly reduced when LCs were absent. However, LCs were not required either for CD8 T-cell activation within the draining lymph node or subsequent homing of effector cells to the epidermis. Instead, we found that LCs were necessary for inducing transcription of IFN-γ and other key effector molecules by donor CD8 cells in the epidermis, indicating that they license CD8 cells to induce epithelial injury. These data demonstrate a novel regulatory role for epidermal LCs during the effector phase of an inflammatory immune response in the skin.
Introduction
The skin is the largest organ in area and contains multiple immune-competent cells. Langerhans cells (LCs) are a population of myeloid cells characterized by their expression of the C-type lectin, Langerin, and their location as a dense, interlacing network within the epidermal layer of the skin.1 Although LCs were the first dendritic cell (DC) population to be characterized,2 their precise role in regulating cutaneous immunity has proven elusive. LCs possess many of the key attributes of highly proficient DCs, including their ability to migrate to draining lymph nodes (LNs) on activation. However, only a few studies have shown a specific requirement for this population in initiating cutaneous immunity.3,4 Moreover, recent experimental innovations permitting isolated depletion of Langerin+ DCs have demonstrated the existence of a novel Langerin+ dermal DC population that is distinct from migratory LCs,5–7 and these DCs are probably responsible for some of the functions attributed to LCs in earlier studies.5,8 Indeed, when viewed purely from the perspective of immune response initiation, the role of LCs appears to be relatively minor. Genetic studies, including selective depletion of epidermal LCs, have suggested alternative roles as negative regulators of cutaneous immunity9 or in innate immunity relating to barrier functions.10 However, a clear function for LCs compared with other skin DCs has not, to our knowledge, been defined.
In the steady state, the LC population is maintained without recruitment of bone marrow (BM) precursors from proliferation of radio-resistant Langerin+ LCs within the epidermis.1,11 This fact has been exploited in elegant BM chimera experiments to demonstrate that LCs are capable of initiating GVHD-like lesions under circumstances where MHC allo- or model self-antigen presentation is restricted to the LC compartment.12,13 However, it remains unclear as to whether this population is necessary to induce injury under circumstances where other DC populations capable of presenting the relevant antigens are also present.14
We have previously demonstrated that induced innate immune activation via topical application of a TLR-7 or TLR-8 agonist (imiquimod) at the time of T-cell transfer to MHC-mismatched allogeneic chimeras induces recruitment of effector T cells to the skin and induction of severe, localized epithelial GVHD.15 By adapting this model to allow inducible and selective host LC depletion, we have examined their role in regulating the development of cutaneous injury.
Methods
Animals
Balb/c.PL-ThyaCy (Thy1.1) and CD11c.DTR/GFP (B6.FVB-Tg(Itgax-DTR/EGFP)57Lan/J) were purchased from The Jackson Laboratory. Langerin.DTREGFP (B6.129S2-Cd207tm3Mal/Orl) mice were kindly provided by Bernard Malissen and Adrien Kissenpfennig (Université de la Mediterrannée, France). BALB/c mice were purchased from Charles River Laboratories, and C57BL/6 (B6) mice were bred in house. Procedures were conducted in accordance with the United Kingdom Home Office Animals (Scientific Procedure) Act of 1986 and were approved by the Ethics and Welfare Committee of the Royal Free and University College London Medical School.
BM transplantation and delayed leukocyte transfer
Procedures for the generation of allogeneic chimeras and transfer of donor splenocytes were performed as described previously.16 Recipient mice were lethally irradiated (11 Gy x-ray irradiation, 0.55 Gy/min split into 2 fractions separated by 48 hours), and T cell–depleted BM cells were injected intravenously 4 hours later. To generate mixed allogeneic chimeras, a total of 2 × 107 T cell–depleted BM cells from B6 (or CD11c-DTR) and BALB/c mice were injected at a 1:1 ratio into B6 or Langerin.DTR mice. Eight weeks after reconstitution, established allogeneic chimeras received 3 × 107 BALB/c Thy1.1 (donor) splenoctyes intravenously.
GVHD model
One-fourth of a 12.5-mg sachet of imiquimod (∼ 3 mg imiquimod; Aldara, Meda Pharmaceuticals) was topically applied to a shaved area of 2.25 cm2 on the back of established chimeras on days 0, 5, and 10 relative to transfer of donor splenocytes. To deplete CD11chigh DCs or LCs, 100 ng or 500 ng of diphtheria toxin (DT; Sigma-Aldrich), respectively, was administered by intraperitoneal injection as described previously.17,18 GVHD scoring of histologic sections was performed as described previously.16
Antibodies and flow cytometry
The following antibodies were used for cell surface staining: anti-CD4–fluorescein isothiocyanate, phycoerythrin (PE), or allophycocyanin (L3T4), anti-CD8α–PE or allophycocyanin (Ly-2), anti-CD11b–allophycocyanin (M1/70), anti-CD90.1–FITC or biotin (HIS51), anti-B220–PE-Cy5 (RA3-6B2), anti-CD62L–PE (MEL-14), and anti-CD86–PE (GL1), all purchased from eBioscience. Anti-CD11c–PE (N418) was purchased from Miltenyi Biotec. Anti-I-Ab–PE or biotin antibodies (AF6-120.1) were purchased from BD Biosciences. Detection of biotinylated antibodies was performed using fluorescein isothiocyanate, peridinin chlorophyll protein, or allophycocyanin conjugated to streptavidin (BD Biosciences). Flow cytometry was performed on a FACSCalibur (BD Biosciences).
Isolation of epidermal cells
Excised skin was incubated in 0.25% (weight/volume) trypsin solution (Sigma-Aldrich) for 2 hours at 37°C. Epithelial sheets were peeled carefully from the dermis and agitated in PBS/10% FBS to generate single-cell suspensions. Thy1.1+CD8+ epidermal cells were sorted on a FACSAria II (BD Biosciences).
Quantitative real-time PCR
Methods for quantitative real-time PCR have been described previously.19,20 For tumor necrosis factor-related apoptosis-inducing ligand mRNA, the forward primer was 5′-ATGGTGATTTGCATAGTGCTCC-3′ and reverse primer was 5′-GCAAGCAGGGTCTGTTCAAGA-3′.
Cytotoxicity assays
Cytotoxicity assays were performed in vivo as described previously.20 B cells were isolated from B6 (host-type) and BALB/c (donor-type) splenocytes by immunomagnetic separation. The B cells were then differentially labeled with either low-dose (0.5μM) or high-dose (5μM) carboxyfluorescein succinimidyl ester, mixed at a ratio of 1:1, and injected intravenously at a total of 1 × 107 cells per recipient. Sixteen hours later, cells recovered from the spleen were analyzed by flow cytometry for the presence of carboxyfluorescein succinimidyl ester-labeled cells. Specific cyotoxicity was calculated from the ratio of target (host) and control (donor) cells as previously described.20
Statistics
Analysis was performed using the Student t test (2-tailed), except for evaluation of GVHD scores where the Mann Whitney test (2-tailed) was used. A P value ≤ .05 was considered significant.
Histology
Cutaneous GVHD was scored blind on skin sections stained with H&E. Images were acquired with a Zeiss Axioskop 2 upright microscope (objective lens 20×/0.50) fitted with an AxioCam camera (Zeiss), using Axiovision 4 software (Zeiss).
Results
LCs are required for the development of cutaneous GVHD
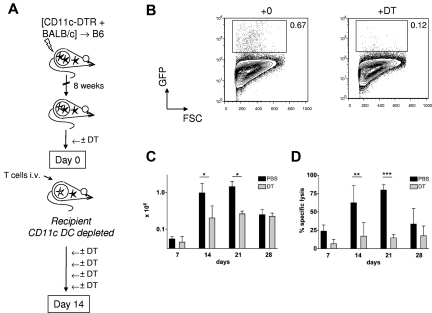
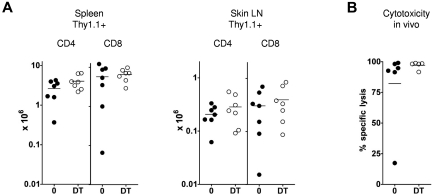
In selection of an appropriate model to examine the role of LCs in mediating cutaneous injury, we exploited a model of graft-versus-host reactivity where both LCs and other, non-LC, DC populations would be capable of presenting alloantigen. We have shown previously that delayed transfer of donor T cells to MHC-mismatched mixed chimeras (MCs, where donor and recipient hematopoietic cells coexist) readily elicits antihost cytotoxic T lymphocyte (CTL) reactivity.15,20 Infused donor T cells are primed directly by host-strain BM-derived antigen-presenting cells21; and, in line with their known efficiency in priming adaptive immunity to foreign antigens, host conventional CD11chigh DCs are capable of initiating the response.22 To determine whether host conventional DC populations were also required for priming, we adapted this model to permit inducible and specific depletion of host-derived CD11chigh cells at the time of donor splenocyte transfer (Figure 1A). In these experiments, B6 mice were reconstituted with a mixture of T cell-depleted BALB/c and CD11c.DTR/green fluorescent protein (GFP) BM. CD11c.DTR/GFP mice are on a host-type B6 background and express a high affinity diphtheria toxin receptor (DTR) sequence downstream of the CD11c promoter.18 After 8 weeks, intraperitoneal injections of DT were given every 72 hours from day −2 to day 10 around the time of delayed transfer of 3 × 107 BALB/c Thy1.1+ splenocytes. As shown in Figure 1B and C, efficient depletion of host conventional DCs led to reduced accumulation of donor Thy1.1+ CD8 cells in the spleen. To examine how host CD11c+ cell depletion influenced CTL reactivity against hematopoietic targets, we injected a 1:1 mixture of host and donor B cells, differentially labeled with CFSE, at timed intervals after T-cell transfer to MCs that received DT or PBS. After 16 hours, recipient spleens were recovered and the relative survival of host and donor B cells determined. As shown in Figure 1D, host CD11c+ cells were required to prime significant antihost CTL reactivity against host hematopoietic cells in vivo. Consistent with this, no mice converted to full donor chimerism in the DT-treated chimeras, whereas all control chimeras fully converted (data not shown). We can also infer from these data that radio-resistant host LCs were not sufficient to prime allogeneic T cells because they would not have been depleted using this experimental approach.
Figure 1.
Host CD11chighcells are required for induction of graft-versus-host reactivity after delayed T-cell transfer to allogeneic chimeras. (A) Lethally irradiated B6 mice were reconstituted with a mix of CD11c.DTR/GFP and BALB/c BM, 8 weeks before infusion of 3 × 107 BALB/c Thy1.1+ splenocytes. DT or PBS was given intraperitoneally to recipient mice on day −2 and days 1, 4, 7, and 10 after splenocyte transfer. (B) Contour plots showing GFP reporter expression (specific for host CD11chigh DCs) in dermal suspensions of CD11c.DTR/GFP mice treated with DT or control 24 hours previously (mean percentages ± SD of GFP-expressing cells among live cells were 0.72 ± 0.35 in PBS-treated mice vs 0.08 ± 0.05 in DT-treated mice (n = 4-6 mice per group); data pooled from 4 independent experiments. P < .01. In the spleen of MCs at day 7, mean percentages ± SD of host CD11chighGFP+ among live splenocytes were 0.09 ± 0.04 versus 0.01 ± 0.01 (n = 3 or 4 mice per group); data pooled from 2 independent experiments. P < .05. (C) Absolute numbers (mean ± SD) of Thy1.1+ CD8 cells in recipient spleen of [CD11c.DTR/GFP +BALB/c] → B6 MCs treated with (gray bars) or without (black bars) DT. (D) Specific cytotoxicity in vivo (mean ± SD) directed against B6 strain B cells in the same experiment shown in panel C. (C-D) Data are pooled from 2 or 3 independent experiments (day 7, n = 2 or 3 mice per group; day 14, n = 6 or 7 mice per group; day 21, n = 3 mice per group; day 28, n = 3 mice per group). *P < .05. **P < .01. ***P < .001.
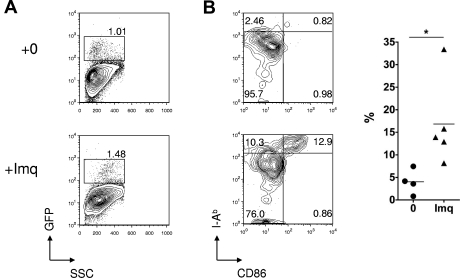
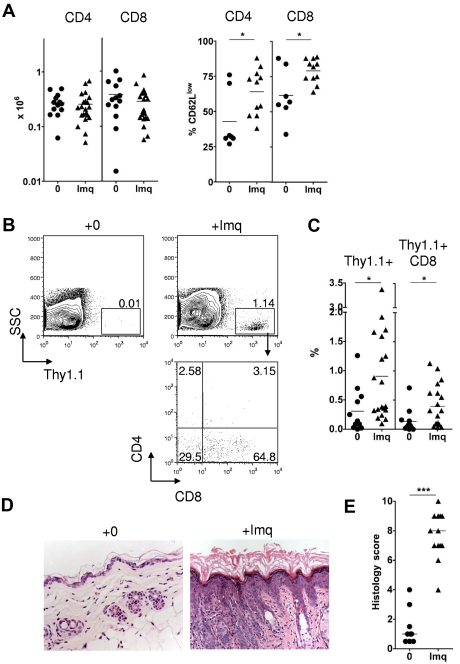
Although CTL activity against host hematopoietic elements is readily elicited in this model, GVHD does not occur in the absence of inflammation.15 In contrast, inflammation induced by imiquimod treatment of the skin of MC at the time of splenocyte transfer leads to local T-cell infiltration and induction of localized GVHD.15 Imiquimod treatment induces LC activation as a result of interactions in trans with other accessory cells, such as mast cells that express TLR-7/TLR-8.23 Figure 2 demonstrates that LC activation also occurred when imiquimod was applied at the low doses (3 mg) used in our experimental protocol. To characterize the donor T-cell response in more detail, we transferred BALB/c Thy1.1+ splenocytes to either control or imiquimod-treated MCs and then tracked T-cell numbers and phenotype in the draining LN and skin on day 14. As shown in Figure 3A, imiquimod treatment had no effect on the absolute numbers of Thy1.1+ T cells in the draining LN but sharply increased the frequency of donor CD4 and CD8 cells with a CD62Llow effector phenotype. This was associated with enhanced recruitment, primarily of Thy1.1+ CD8 cells, to the epidermis (Figure 3B-C) and in accordance with our previously published findings,15 recruitment of effector CTLs induced cutaneous injury as evidenced by epidermal thickening, dyskeratosis, and an inflammatory cell infiltrate (Figure 3D-E).
Figure 2.
Effect of topical imiquimod on LC frequency and phenotype in the skin epithelium. (A) Contour plots showing GFP reporter expression (specific for host LCs) in skin epithelia of Langerin.DTR/GFP mice treated 24 hours previously with topical imiquimod (Imq) or control. (B) Left: Contour plots showing dual staining for CD86 and MHC class II in gated LCs from mice treated 24 hours previously with topical imiquimod or control. Right: Summary data showing the percentage of CD11b+ LCs that were CD86high MHCIIhigh, in control or imiquimod-treated mice (n = 4 or 5 mice per group); data pooled from 3 independent experiments. *P < .05. The mean is indicated by the horizontal bar.
Figure 3.
Topical imiquimod induces recruitment of donor CD8 cells and GVHD in the skin of allogeneic chimeras. MCs were treated with 3 mg topical imiquimod (Imq) or nil in an area of approximately 2.25 cm2 above the base of the tail at day 0, day 5, and day 10 after donor splenocyte transfer. (A) Left: Absolute numbers of Thy1.1+ CD4 or CD8 cells in the skin draining LN at day 14 (n = 13-21 mice per group); data pooled from 8 independent experiments. The mean is indicated by the horizontal bar. Right: Percentage of gated Thy1.1+ CD4 or CD8 cells on day 14 that were CD62Llow (n = 7-11 mice per group); data pooled from 3 independent experiments. *P < .05. The mean is indicated by the horizontal bar. (B) Representative contour plots (top row) showing accumulation of Thy1.1+ cells in the epidermis at day 14. Plot in the bottom row represents CD4 and CD8 expression in gated Thy1.1+ cells of imiquimod-treated MCs. (C) Summary data showing accumulation of total Thy1.1+ cells (left) or Thy1.1+ CD8 cells (right) in the epidermis of control or imiquimod-treated MCs (n = 12-19 mice per group); data pooled from 8 independent experiments. *P < .05. The mean is indicated by the horizontal bar. (D) Representative histologic sections showing skin histology on day 14 in imiquimod-treated and control mice (original magnification ×200). (E) Summary data of histologic GVHD score as evaluated single blind (n = 9-13 per group); data pooled from 4 inde-pendent experiments. ***P < .001. The median is indicated by the horizontal bar.
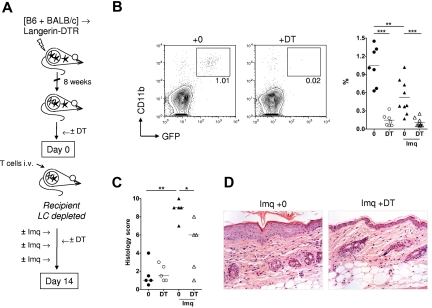
To determine the role of LCs in the development of cutaneous injury, we transferred 3 × 107 BALB/c Thy1.1+ splenocytes to established [B6 plus BALB/c] → Langerin.DTR/GFP MCs that were treated on the back skin with 3 mg imiquimod on days 0, 5, and 10 or left untreated, and received DT or PBS on day −2 and day 4 (Figure 4A). Langerin.DTR/GFP mice are on a host-type B6 background and express the DTR sequence downstream of the Langerin promoter.24 DT treatment of MCs was highly effective at depleting radio-resistant GFP+ LCs within the epidermis for the duration of the experiment (Figure 4B). Of note, after irradiation and reconstitution of MCs, radiosensitive DC populations from the Langerin.DTR host (including dermal Langerin+ DCs) would be replaced with cell populations derived from wild-type B6 and BALB/c BM cells and were therefore not depleted using this approach. As shown in Figure 4C and D, DT-induced host LC depletion in imiquimod-treated chimeras led to a significant, partial reduction in the degree of cutaneous GVHD (as evaluated by single blind scoring of histologic sections). Similar results were observed in an independent experiment involving ear pinna skin, suggesting that this LC function was similar across the murine epidermis (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Thus, host LCs were required for development of TLR-induced cutaneous GVHD under conditions where other DC populations could prime the response.
Figure 4.
Host LCs are required for the development of TLR-induced cutaneous GVHD. (A) Lethally irradiated Langerin.DTR/GFP mice were reconstituted with a mix of B6 and BALB/c BM, 8 weeks before infusion of 3 × 107 BALB/c Thy1.1+ splenocytes. DT or PBS was given intraperitoneally to recipient mice on day −2 and day 4 after splenocyte transfer. MCs were treated topically with imiquimod (Imq) or nil on day 0, day 5, and day 10. (B) Left: Representative contour plots showing depletion of CD11b+ GFP+ recipient LCs from the epidermis of imiquimod-treated MCs at day 14. Right: Summary data showing the percentage of CD11b+ GFP+ cells in the epidermis of MCs at day 14, with or without DT and with or without imiquimod (n = 7-9 mice per group); data pooled from 4 independent experiments. **P < .01. ***P < .001. The mean is indicated by the horizontal bar. (C) Summary data of histologic GVHD score as evaluated single blind (n = 5 mice per group); data pooled from 2 independent experiments. *P < .05. **P < .01. The median is indicated by the horizontal bar. (D) Photomicrographs of representative histologic sections (original magnification ×200) of skin histology on day 14 in imiquimod-treated [B6 + BALB/c] → Langerin.DTR MCs given DT or control.
LCs are not required for activation or skin homing of donor CD8 cells
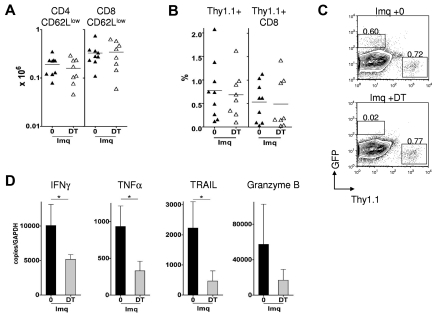
Next, we performed a series of experiments to determine the mechanism underlying LC involvement in the development of cutaneous injury. Because LCs traffic to the draining LN only in low numbers in the steady state,24,25 we reasoned that they would be dispensable for priming the systemic graft-versus-host response in the absence of inflammation. Indeed, when donor splenocytes were transferred to MCs without topical application of imiquimod, LC depletion had no effect on the accumulation of donor T cells in the spleen and skin-draining LNs (Figure 5A). Furthermore, the CTL response against host hematopoietic cells in vivo was equivalent in the presence or absence of LCs (Figure 5B). In contrast to the steady state, repetitive local application of imiquimod led to a reduction in the frequency of LCs in the epidermis (Figure 4B) and is reported to enhance migration of activated LCs to the draining LN.23 Unexpectedly, however, we found that LC depletion had no effect on the absolute numbers (or frequency) of CD62Llow donor T cells in the skin-draining LN (Figure 6A; and not shown). Furthermore, the degree of epithelial infiltration by donor CTLs was similar in LC-depleted and nondepleted MCs (Figure 6B-C). Taken together, these data indicated that LCs were required for the induction of cutaneous injury but were dispensable for the priming and recruitment phase of the response, where other antigen-presenting cell populations were sufficient to fulfill the role.
Figure 5.
Host LCs are not required for priming graft-versus-host reactivity in the steady state. DT or PBS was given intraperitoneally to [B6 + BALB/c] → Langerin.DTR MCs on day −2 and day 4 after splenocyte transfer. (A) Absolute numbers of Thy1.1+ CD4 and CD8 cells in recipient spleen and LN on day 14 in MCs treated with or without DT (n = 7 mice per group); data pooled from 4 independent experiments. The mean is indicated by the horizontal bar. (B) Specific cytotoxicity in vivo directed against B6 strain B cells on day 14 (n = 5 or 6 mice per group); data pooled from 3 independent experiments. The mean is indicated by the horizontal bar.
Figure 6.
Host LCs are required for licensing CTLs recruited to the skin epidermis. [B6 + BALB/c] →Langerin.DTR/GFP MCs were treated topically with imiquimod (Imq) and with or without DT by intraperitoneal injection on day −2 and day 4 after donor splenocyte transfer. (A) Absolute numbers of Thy1.1+ CD62Llow CD4 or CD8 cells in the skin draining LN (n = 8 or 9 mice per group); data pooled from 4 independent experiments. The mean is indicated by the horizontal bar. (B) Summary data showing accumulation of total Thy1.1+ cells (left) or Thy1.1+ CD8 cells (right) in the epidermis of imiquimod-treated MCs, with DT or PBS (n = 8 or 9 mice per group); data pooled from 4 independent experiments. The mean is indicated by the horizontal bar. (C) Representative contour plots showing frequency of GFP-expressing cells (representing host LCs) and of Thy1.1+ cells in epidermal suspensions derived from imiquimod-treated MCs after splenocyte transfer, and after DT or PBS. (D) Thy1.1+ CD8 cells were flow-sorted on day 14 from pooled skin epithelia of imiquimod-treated MCs given DT or PBS. Graphs represent mean plus or minus SD mRNA quantification for each transcript in the DT and control groups compared with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL; n = 3 or 4 mice per group per experiment); data pooled from 3 independent experiments. *P < .05.
LCs license effector CTLs recruited to the epidermis
In other models involving adoptive transfer of CD8 cells specific for self-antigens, recruitment of activated CD8 cells to antigen-expressing tissues occurs without injury, unless inflammation is provoked.26,27 In the current model, CD8 cells accessing inflamed skin were unable to induce GVHD in the absence of LCs, even though they had already acquired some cytotoxic functions. We reasoned that LCs, activated via TLR signaling, might be involved in regulating the acquisition of effector functions by CD8 cells infiltrating the epidermis. In initial experiments to test this concept, we attempted to visualize cytokine expression by donor T cells within intact epithelial sheets. However, we found that the inflammatory process significantly disrupted the epithelial layer, and this was not technically feasible. Therefore, we flow-sorted Thy1.1+ CD8+ fractions at the peak of the response from epithelia of imiquimod-treated chimeras where LCs were either present or absent. The number of recovered Thy1.1+ CD8+ cells from each epithelium was very low (median, 1.4 × 103; range, 0.5-4.6 × 103) and not suitable for evaluation of protein expression or direct CTL function. We therefore extracted mRNA from pooled cellular fractions (3 or 4 mice per group per experiment) and performed quantitative polymerase chain reaction for effector molecules associated with CTL function. As shown in Figure 6D, donor CD8 cells from LC-depleted chimeras displayed reductions in the expression of transcripts for several key effector molecules involved in graft-versus-host injury, including IFN-γ, TNF-α, and TNF-related apoptosis-inducing ligand. A similar trend was also observed for granzyme B mRNA expression. This same pattern was observed in 3 independent experiments. Taken together with our findings in Figure 4, these data demonstrate a critical requirement for LCs in licensing epidermis-infiltrating CD8 CTLs to become fully competent effectors and induce injury.
Discussion
In this study, we have demonstrated a nonredundant role for LCs during the effector phase of a CTL response that led to cutaneous GVHD in the context of TLR-induced inflammation. In the absence of host LCs, alloreactive CD8 cells were primed within the draining LN and readily accessed inflamed skin yet failed to induce GVHD, even though they had already acquired some cytotoxic functions. Instead, LCs acted to confer full functional competence to activated CD8 cells and drive the development of tissue injury. Thus, the checkpoint regulating the induction of cutaneous injury by donor CTLs required the presence of both inflammation and host LCs.
Our observations are consistent with other recent studies where the functions of T cells were enhanced on further interaction with DC populations during an effector or memory response in tissues, such as the dorsal ganglia, lung, or skin.28–31 In all of the studies published to date, the “second hit” was provided by CD11c+ DCs; and to our knowledge, our data are the first demonstration that LCs can also fulfill this role. For example, after subcutaneous vaccination with peptide and incomplete Freund adjuvant, T-cell receptor-transgenic CD4 cells required cognate interaction with CD11c+CD11bhigh dermal DCs at the injected site to undergo differentiation into effector cells.29 Furthermore, in the MRL.Faslpr model of lupus, autoreactive CD4 cells entered affected tissues but then failed to expand or develop effector functions in the constitutive absence of CD11chigh DCs.30 Of note, and in contrast to our findings, LCs were demonstrated to be unnecessary in driving either CD4 effector differentiation in the incomplete Freund adjuvant /peptide model29 or dermatitis in the MRL.Faslpr mice.30 Factors, including the nature of the initial stimuli, the cellular effectors involved, or the relative abundance or location of the different DCs, could potentially account for these differences. However, taken together, these findings suggest that either LCs or conventional DCs can augment the functions of skin-infiltrating T cells and that this requirement depends on the precise experimental context.
To date, few other studies have addressed the role of LCs during the effector phase of the response. Our findings in the TLR-induced GVHD model differ from those we have reported previously in experiments involving contact hypersensitivity, where we showed that Langerin+ DCs were not required during the elicitation phase of the response.3 Although both the contact hypersensitivity and GVHD models we used are “classic” delayed-type hypersensitivity responses, they are distinct in several key respects that may be relevant to the differences observed in LC function. For example, antigen presentation by LCs in contact hypersensitivity is limited by the efficiency of haptenized self-peptide loading to MHC, and other skin DC populations may be more proficient at this task.32 In contrast, the graft-versus-host response is directed at MHC alloantigens expressed by all host LCs. A second issue is our use of a powerful TLR agonist to provoke GVHD. Imiquimod, through interactions with other accessory cells, induces profound changes in LC function,23 whereas haptens are probably less effective immunostimulants in the absence of TLR signaling.33,34 Both these factors have the potential to influence the nature of the interaction between LCs and CTLs during the effector phase of the response. Their location in the epidermis, where they extend dendritic processes to the external stratum corneum,35 potentially exposes LC populations more than other skin DCs to commensal or pathogenic organisms that activate distinct pathogen-associated molecular pattern recognition pathways. It will therefore be of interest for future studies to determine how innate recognition of viruses or bacteria influences the functions of LCs in mediating tissue injury.
In the model we describe here, LCs are abundant at the site of injury (Figure 4B), but we do not know whether the final step in CTL differentiation requires direct interaction with LCs in the skin and/or is mediated by soluble factors. Our findings suggest that examination of postpriming or in situ functions of LCs may be just as important as exploring their role at the initiation of a response. Indeed, atopic dermatitis is associated with marked LC proliferation in the epidermis,11 and persistence and expansion of LCs at the site of cutaneous inflammation might also be important in other T cell-mediated skin inflammatory states. Further investigations of the mechanisms by which LCs control the functions of incoming inflammatory cells inside the epidermis may thus prove to be a fruitful area of research, relevant to a number of human diseases.
Supplementary Material
Acknowledgment
This work was supported by Leukemia and Lymphoma Research, United Kingdom.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.L.B. conceived the study, designed and performed experiments, analyzed data, and wrote the paper; F.F.-A. designed and performed experiments, analyzed data, and cowrote the paper; T.C., C.T., H.G., L.C., and B.F. performed experiments; T.K.M. designed and performed experiments; F.G. designed experiments and cowrote the paper; and R.C. conceived the study, performed and oversaw the data analysis, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Clare L. Bennett, Department of Haematology, UCL Royal Free campus, Rowland Hill St, London, NW3 2PF United Kingdom; e-mail: c.bennett@medsch.ucl.ac.uk.
References
- 1.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8(12):935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Witmer MD, Nussenzweig MC, Chen LL, Schlesinger S, Cohn ZA. Dendritic cells of the mouse: identification and characterization. J Invest Dermatol. 1980;75(1):14–16. doi: 10.1111/1523-1747.ep12521052. [DOI] [PubMed] [Google Scholar]
- 3.Bennett CL, Noordegraaf M, Martina CA, Clausen BE. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179(10):6830–6835. doi: 10.4049/jimmunol.179.10.6830. [DOI] [PubMed] [Google Scholar]
- 4.Nagao K, Ginhoux F, Leitner WW, et al. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci U S A. 2009;106(9):3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bursch LS, Wang L, Igyarto B, et al. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204(13):3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginhoux F, Collin MP, Bogunovic M, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204(13):3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204(13):3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Bursch LS, Kissenpfennig A, Malissen B, Jameson SC, Hogquist KA. Langerin expressing cells promote skin immune responses under defined conditions. J Immunol. 2008;180(7):4722–4727. doi: 10.4049/jimmunol.180.7.4722. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23(6):611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 10.de Witte L, Nabatov A, Pion M, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13(3):367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 11.Chorro L, Sarde A, Li M, et al. Langerhans cell (LCs) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LCs network. J Exp Med. 2009;206(13):3089–3100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayerova D, Parke EA, Bursch LS, Odumade OA, Hogquist KA. Langerhans cells activate naive self-antigen-specific CD8 T cells in the steady state. Immunity. 2004;21(3):391–400. doi: 10.1016/j.immuni.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Merad M, Hoffmann P, Ranheim E, et al. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10(5):510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Kaplan DH, Matte-Martone C, et al. Langerhans cells are not required for graft-versus-host disease. Blood. 2011;117(2):697–707. doi: 10.1182/blood-2010-07-299073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakraverty R, Cote D, Buchli J, et al. An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues. J Exp Med. 2006;203(8):2021–2031. doi: 10.1084/jem.20060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flutter B, Edwards N, Fallah-Arani F, et al. Nonhematopoietic antigen blocks memory programming of alloreactive CD8+ T cells and drives their eventual exhaustion in mouse models of bone marrow transplantation. J Clin Invest. 2010;120(11):3855–3868. doi: 10.1172/JCI41446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett CL, van Rijn E, Jung S, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169(4):569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung S, Unutmaz D, Wong P, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdi R, Means TK, Luster AD. Chemokines in islet allograft rejection. Diabetes Metab Res Rev. 2003;19(3):186–190. doi: 10.1002/dmrr.362. [DOI] [PubMed] [Google Scholar]
- 20.Chakraverty R, Flutter B, Fallah-Arani F, et al. The host environment regulates the function of CD8+ graft-versus-host-reactive effector cells. J Immunol. 2008;181(10):6820–6828. doi: 10.4049/jimmunol.181.10.6820. [DOI] [PubMed] [Google Scholar]
- 21.Chakraverty R, Eom HS, Sachs J, et al. Host MHC class II+ antigen-presenting cells and CD4 cells are required for CD8-mediated graft-versus-leukemia responses following delayed donor leukocyte infusions. Blood. 2006;108(1):2106–2113. doi: 10.1182/blood-2006-03-007427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia G, Truitt RL, Johnson BD. Graft-versus-leukemia and graft-versus-host reactions after donor lymphocyte infusion are initiated by host-type antigen-presenting cells and regulated by regulatory T cells in early and long-term chimeras. Biol Blood Marrow Transplant. 2006;12(4):397–407. doi: 10.1016/j.bbmt.2005.11.519. [DOI] [PubMed] [Google Scholar]
- 23.Heib V, Becker M, Warger T, et al. Mast cells are crucial for early inflammation, migration of Langerhans cells, and CTL responses following topical application of TLR7 ligand in mice. Blood. 2007;110(3):946–953. doi: 10.1182/blood-2006-07-036889. [DOI] [PubMed] [Google Scholar]
- 24.Kissenpfennig A, Henri S, Dubois B, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22(5):643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Henri S, Poulin LF, Tamoutounour S, et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med. 2009;207(1):189–206. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Lefrancois L. Intestinal epithelial antigen induces mucosal CD8 T cell tolerance, activation, and inflammatory response. J Immunol. 2004;173(7):4324–4330. doi: 10.4049/jimmunol.173.7.4324. [DOI] [PubMed] [Google Scholar]
- 27.Vezys V, Lefrancois L. Cutting edge: inflammatory signals drive organ-specific autoimmunity to normally cross-tolerizing endogenous antigen. J Immunol. 2002;169(12):6677–6680. doi: 10.4049/jimmunol.169.12.6677. [DOI] [PubMed] [Google Scholar]
- 28.McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med. 2008;205(7):1635–1646. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLachlan JB, Catron DM, Moon JJ, Jenkins MK. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity. 2009;30(2):277–288. doi: 10.1016/j.immuni.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 2010;33(6):967–978. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010;31(12):446–451. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin SF, Dudda JC, Bachtanian E, et al. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J Exp Med. 2008;205(9):2151–2162. doi: 10.1084/jem.20070509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt M, Raghavan B, Muller V, et al. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol. 2010;11(9):814–819. doi: 10.1038/ni.1919. [DOI] [PubMed] [Google Scholar]
- 35.Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med. 2009;206(13):2937–2946. doi: 10.1084/jem.20091527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.