Abstract
Renal inflammation modulates angiotensinogen (AGT) production in renal proximal tubular cells (RPTCs) via inflammatory cytokines, including interleukin-6, tumor necrosis factor α, and interferon-γ (IFN-γ). Among these, the effects of IFN-γ on AGT regulation in RPTCs are incompletely delineated. This study aimed to elucidate mechanisms by which IFN-γ regulates AGT expression in RPTCs. RPTCs were incubated with or without IFN-γ up to 48 h. AGT expression, STAT1 and STAT3 activities, and SOCS1 expression were evaluated. RNA interference studies against STAT1, SOCS1, and STAT3 were performed to elucidate a signaling cascade. IFN-γ decreased AGT expression at 6 h (0.61±0.05, ratio to control) and 12 h (0.47±0.03). In contrast, longer exposure for 24 and 48 h increased AGT expression (1.76±0.18, EC50=3.4 ng/ml, and 1.45±0.08, respectively). IFN-γ treatment for 6 h strongly induced STAT1 phosphorylation and SOCS1 augmentation, and decreased STAT3 activity. However, STAT1 phosphorylation and SOCS1 augmentation waned at 24 h, while STAT3 activity increased. RNA interference studies revealed that activation of STAT1-SOCS1 axis decreased STAT3 activity. Thus, IFN-γ biphasically regulates AGT expression in RPTCs via STAT3 activity modulated by STAT1-SOCS1 axis, suggesting the STAT1-SOCS1 axis is important in IFN-γ-induced activation of the intrarenal renin-angiotensin system.—Satou, R., Miyata, K., Gonzalez-Villalobos, R. A., Ingelfinger, J. R., Navar, L. G., Kobori, H. Interferon-γ biphasically regulates angiotensinogen expression via a JAK-STAT pathway and suppressor of cytokine signaling 1 (SOCS1) in renal proximal tubular cells.
Keywords: kidney, inflammation, hypertension
The renin-angiotensin system (RAS) is critical for blood pressure regulation, as well as electrolyte and body fluid homeostasis (1). Chronic angiotensin II (Ang II) infusions induce hypertension, and when combined with a high-salt diet, create an inflammatory response leading to intrarenal T-cell infiltration and increased formation of interferon-γ (IFN-γ) from activated T cells, natural killer cells, and macrophages (2, 3). Thus, Ang II contributes not only to the development of hypertension but also to the development of renal inflammation (4). Previous studies have demonstrated that renin and angiotensinogen (AGT), the precursor of Ang II, are expressed in multiple tissues (5, 6) and that the local RAS functions in individual organs in a tissue-specific manner (5, 7, 8). In the kidney, intrarenal Ang II is derived from intrarenal AGT, which is produced mainly in renal proximal tubular cells (RPTCs; refs. 9, 10). Overexpression of rat AGT using a kidney androgen-regulated protein promoter in mouse RPTCs increases blood pressure in the transgenic mice (11). Intrarenal AGT is increased in several forms of clinical and experimental hypertension (12–15). Therefore, AGT expression in the RPTCs plays an important role in regulating intrarenal Ang II levels (16). However, the molecular basis of such AGT regulation is not well understood.
IFN-γ induces gene transcription via activation of a Janus activated kinase (JAK) and the signal transducers and activators of transcription (STAT) pathway (3, 17, 18). The IFN-γ receptor contains two subunits, IFN-γ receptor 1 (IFNGR1) and IFNGR2 (3). IFNGR1 contributes to activation of JAK1, and IFNGR2 is important in the activation of JAK2 (3). Once activated, JAKs induce STAT activation, leading to gene transcription (3). Although it is known that IFN-γ increases AGT expression in hepatocytes via activation of the STAT1 pathway (19), the contribution and mechanism of IFN-γ to AGT regulation in the kidney have not been established.
Activation of the JAK-STAT pathway also induces suppressor of cytokine signaling (SOCS) expression, and SOCSs act as internal suppressors of the JAK-STAT pathway (20). Thus, the JAK-STAT pathway and SOCSs may be considered a negative feedback system (21). In the SOCS family, SOCS1 and SOCS3 have been well characterized (21). SOCS1 can interact directly with JAK1 and JAK2, and inhibit their phosphorylation, leading to suppression of JAK-dependent phosphorylation of receptors and STATs (21). SOCS1 binds directly to JAKs and the IFN-γ receptor (22, 23). In contrast, SOCS3 requires gp130 to inhibit JAK activities (22, 24, 25), and IFN-γ receptor does not possess the gp130 protein (22). Therefore, SOCS1 appears to be the predominant inhibitor of IFN-γ signaling (22). Of interest, overexpression of SOCS1 and SOCS3 in diabetic rats suppressed activation of intrarenal JAK-STAT pathway and abrogated the development of diabetic nephropathy (26). Overexpression of SOCS1 alone suppresses a proinflammatory factor and the JAK-STAT pathway and ameliorates renal injury in diabetic nephropathy (27). Such findings indicate that SOCS1 plays an important protective role counteracting renal injury.
Given the role of the RAS in the kidney, including intrarenal inflammation, we hypothesized that IFN-γ regulates AGT expression via the JAK-STAT pathway and SOCS1 in RPTCs. Therefore, this study was performed to demonstrate the contribution of IFN-γ to AGT expression and to elucidate the mechanisms by which IFN-γ regulates AGT expression in rat RPTCs.
MATERIALS AND METHODS
Cell culture
Immortalized rat RPTCs were used in this study (28). The cells were cultured in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated FCS (Invitrogen) and were plated at a density of 2 × 105 cells/well in 6-well plates. Prior to stimulation, the cells were serum-starved for 24 h. RPTCs were subsequently treated with 0–20 ng/ml rat recombinant IFN-γ (PeproTech, Rocky Hill, NJ, USA) for up to 48 h in a medium containing 1% serum. In the experiments, cells that were not treated with IFN-γ (0 ng/ml) served as control groups.
Antibodies
A rabbit anti-phospho-STAT1 (Tyr-701) antibody, a rabbit anti-phospho-STAT1 (Ser-727) antibody, a mouse anti-phospho-STAT3 (Tyr-705) antibody, a rabbit anti-phospho-STAT3 (Ser-727) antibody, a rabbit anti-STAT1 antibody, a rabbit anti-STAT3 antibody, a rabbit anti-phospho-JAK2 antibody, and a rabbit anti-JAK2 antibody were obtained from Cell Signaling Technology (Danvers, MA, USA). A rabbit anti-rat AGT antibody and a rabbit anti-SOCS1 antibody were purchased from IBL (Takasaki, Japan). A mouse anti-β-actin antibody was purchased from Abcam (Danvers, MA, USA). IRDye-labeled anti-mouse IgG and anti-rabbit IgG antibodies were obtained from Li-Cor (Lincoln, NE, USA), as secondary antibodies in Western blot analysis.
Real-time quantitative RT-PCR (qRT-PCR)
Real-time qRT-PCR was performed to evaluate rat AGT mRNA and rat SOCS1 mRNA expression using the TaqMan PCR system. For total RNA isolation, treated cells were washed with 3 ml of PBS. PBS was aspirated, and total RNA was isolated from the cells using the Bio-Robot EZ 1 (Qiagen, Valencia, CA, USA). Subsequently, qRT-PCR was performed as described previously (29, 30). All samples were analyzed in triplicate, and the data were normalized based on expression levels of rat β-actin mRNA.
AGT enzyme-linked immunosorbent assay (ELISA)
To quantify rat AGT protein in the culture medium, rat AGT ELISA (31) was performed as described previously (32, 33). RPTCs were cultured in 6-well plates with 1.5 ml/well of medium. After treatment of cells with 20 ng/ml IFN-γ, the medium was collected at 6 and 12 h. The total volume of medium was measured; total AGT protein levels were calculated based on the medium volume. The total AGT protein levels were normalized on the basis of cell number.
Western blot analysis
AGT protein levels were determined using Western blot analysis. In addition, phosphorylation levels of STAT1 and STAT3, and expression levels of STAT1, STAT3, and SOCS1, were detected using Western blot analysis in order to elucidate participation of the transcriptional factors in AGT regulation by IFN-γ in RPTCs. The Western blots were performed as described previously (34). After treatment, the cells were harvested with 80 μl lysis buffer containing 1% Triton X-100, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 1 mM Na3VO4, and 0.25% protease inhibitor cocktail (Sigma, St. Louis, MO, USA). The lysates were sonicated 3 times for 10 s each and centrifuged at 13,000 rpm at 4°C for 30 min. Total protein concentration of the supernatant was quantified using the Micro BCA protein assay kit (Pierce, Rockford, IL, USA). Then, 20 μg of total protein was applied to a precast NuPAGE 4–12% gel (Invitrogen). The separated proteins were transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). After incubation of the membrane with primary and secondly antibodies, detection and analysis were performed using the Odyssey system (Li-Cor). Data were normalized on the basis of rat β-actin protein expression levels.
Electrophoresis mobility shift assay (EMSA)
Binding activity of STAT3 to DNA was tested using EMSA. After treatments with IFN-γ, the cells were washed with 3-ml cold PBS, and the cells were harvested. Nuclear protein extraction was performed using the NE-PER kit (Pierce). The protein concentration was determined using the Micro BCA protein assay kit (Pierce). Nuclear protein (3 μg) was incubated with STAT3 IRDye-700 (Li-Cor) for 30 min. Reagents included in the EMSA buffer kit (Li-Cor) were used for the reaction. The reaction was applied to 6% Novex DNA Retardation Gel (Invitrogen). The detection was performed using the Odyssey system. The shift band was previously identified as STAT3-DNA complex (32).
RNA interference
The contributions of STAT1, SOCS1, and STAT3 to changes in AGT expression by IFN-γ treatment were examined using small interference RNA (siRNA) technology, as described previously (34). RPTCs were plated on 6-well plates with siPORT NeoFX (Ambion, Austin, TX, USA) containing rat negative control siRNA (Ambion), STAT1-siRNA (Ambion; sense sequence 5′-CCUUUGUGGUGGAACGACATT-3′), SOCS1-siRNA (Ambion; sense sequence 5′-CGAGCAUUCGUGUGCACUUTT-3′) or STAT3-siRNA (Ambion; sense sequence 5′-GCAGAGUUCAAGCACCUGATT-3′). The final concentrations of the siRNAs were 40 nM each because the concentration was almost the maximum effective dose. Opti-MEM I medium (Invitrogen) was used for these transfections. After 48 h transfection of siRNA, cells were harvested to determine suppression of STAT1, SOCS1, and STAT3 protein expression using Western blot analysis. A separate group of cells was also harvested after 6 h IFN-γ treatment to evaluate the contributions of STAT1, SOCS1, and STAT3 to the regulation of AGT expression by IFN-γ treatment using qRT-PCR and Western blot analysis.
Statistical analysis
Data are expressed as means ± se. The data were analyzed using Student's t test or 1-way ANOVA followed by post hoc Bonferroni/Dunn multiple-comparison test. A value of P < 0.05 was considered statistically significant.
RESULTS
Effects of IFN-γ on AGT mRNA expression
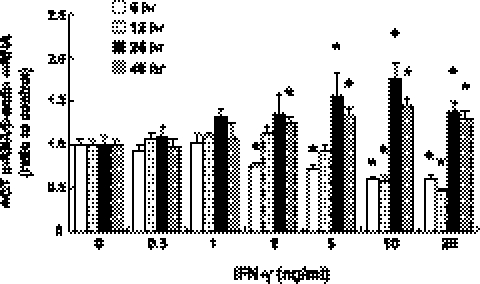
Treatment with IFN-γ in concentrations of ≥2 ng/ml suppressed AGT mRNA expression at 6 h (Fig. 1, open bar, with maximum suppression; 0.61±0.05, ratio to control, at 20 ng/ml IFN-γ, n=4). Treatment for 12 h also caused AGT mRNA suppression but only with 10 and 20 ng/ml IFN-γ (Fig. 1, light shaded bar, 0.47±0.03, ratio to control, n=4). In contrast, prolonged treatment with IFN-γ increased AGT mRNA expression at 24 h (Fig. 1, solid bar; 1.76±0.18, ratio to control, with 10 ng/ml IFN-γ, n=4) and 48 h (Fig. 1, dark shaded bar; 1.45±0.08, ratio to control, with 10 ng/ml IFN-γ). The EC50 value for the IFN-γ-induced AGT augmentation at 24 h was 3.4 ng/ml. The maximum augmentation of AGT mRNA expression was observed in cells treated with 10 ng/ml IFN-γ, while 20 ng/ml IFN-γ at 24 and 48 h elicited smaller responses.
Figure 1.
Effects of IFN-γ treatment on AGT mRNA expression. RPTCs were treated with 0–20 ng/ml IFN-γ for 6 h (open bars, n=4), 12 h (light shaded bars, n=4), 24 h (solid bars, n=4), and 48 h (dark shaded bars, n=4). After these treatments, AGT mRNA was measured by qRT-PCR. Expression levels of AGT mRNA were normalized on the basis of rat β-actin mRNA levels. Data are expressed as relative values compared with the control group at each time point and represent means ± se. *P < 0.05 vs. control group.
Effects of IFN-γ on AGT protein expression
Figure 2 shows the effects of IFN-γ treatment on AGT protein expression at 6 and 12 h. In both the control and IFN-γ-treated group, accumulated AGT protein levels in the culture medium at 12 h were higher than those at 6 h, showing the continued production and secretion of AGT protein (Fig. 2). However, AGT protein levels were suppressed by 20 ng/ml IFN-γ treatment at 6 h (0.27±0.01 vs. 0.20±0.01 ng AGT/105 cells, n=4) and 12 h (0.59±0.03 vs. 0.35±0.01 ng AGT/105 cells, n=4). AGT protein levels were suppressed by IFN-γ treatment initially, but prolonged treatment augmented AGT expression (Figs. 1 and 2A). Therefore, to evaluate whether the sustained IFN-γ treatment increases accumulated AGT protein levels in the culture medium is difficult. AGT protein levels in the cell lysates were determined using Western blot analysis. At 24 h, IFN-γ treatment (20 ng/ml) significantly increased AGT protein levels (Fig. 2B; 1.70±0.14, ratio to control, n=4).
Figure 2.
Effects of IFN-γ treatment on AGT protein expression. A) AGT protein levels in medium were measured by rat AGT ELISA. Medium was collected at 6 and 12 h (n=4). Total volume of medium was measured, and total AGT protein levels were calculated based on the medium volume. Total AGT protein levels were normalized on the basis of cell number. B) AGT protein levels in cell lysates were determined by Western blot analysis. After 24 h-treatment with IFN-γ, the cells were harvested (n=4). Expression levels were normalized on the basis of β-actin levels. Open bars indicate control groups; solid bars indicate IFN-γ-treated groups. Data are expressed as means ± se. *P < 0.05 vs. control group.
STAT1 activation by IFN-γ
To elucidate the molecular mechanism underlying the regulation of AGT expression by IFN-γ treatment, 10 ng/ml IFN-γ treatment was used, because the maximum augmentation of AGT expression at 24 and 48 h was observed by 10 ng/ml IFN-γ treatment (Fig. 1). Phosphorylation levels of STAT1 (Tyr-701, Fig. 3A) and STAT1 (Ser-727, Fig. 3B) by 10 ng/ml IFN-γ treatment were determined at 6, 12, and 24 h. Phosphorylation of STAT1 (Tyr-701) in controls was not detected at any time. Treatment with IFN-γ induced the phosphorylation of STAT1 (Tyr-701) at 6, 12, and 24 h (Fig. 3A). However, the phosphorylation levels of STAT1 at 24 h were decreased after the initial peak at 6 h (Fig. 3A). Phosphorylation of STAT1 (Ser-727) was also induced by the IFN-γ treatment (Fig. 3B). The phosphorylation levels at Ser-727 were enhanced until 24 h (Fig. 3B). In addition, total STAT1 expression levels were augmented by the IFN-γ treatment, as previously reported (35), and the augmentation of STAT1 expression levels was enhanced until 24 h (Fig. 3C). When the phosphorylation levels of STAT1 (Tyr-701) were normalized on the basis of total STAT1 expression levels, the decrease in the phosphorylation levels at 24 h compared with that at 6 h were also observed (Fig. 3D). In contrast, when the phosphorylation levels at Ser-727 were normalized on the basis of total STAT1 expression levels, induction of the phosphorylation at 12 and 24 h was not observed (Fig. 3E).
Figure 3.
STAT1 activation by IFN-γ treatment. A–C) RPTCs were treated with 10 ng/ml IFN-γ for 6, 12, and 24 h. After treatment, STAT1 phosphorylation at Tyr-701 (A, n=4) and Ser-727 (B, n=4) and expression levels (C, n=4) were evaluated by Western blot analysis and normalized based on β-actin levels. D, E). Phosphorylation levels at Tyr-701 (D) and Ser-727 (E) were normalized on the basis of total STAT1 levels. ND, nondetectable; C, control group. I, IFN-γ-treated group. Data are expressed as means ± se. *P < 0.05 vs. control group.
STAT3 activation by IFN-γ
In contrast to the induction of phosphorylation of STAT1 by IFN-γ treatment, STAT3 (Tyr-705) phosphorylation was decreased by the IFN-γ treatment at 6 h (Fig. 4A; 0.98±0.07 vs. 0.71±0.03 density units, n=4) and at 12 h (Fig. 4A; 0.98±0.04 vs. 0.75±0.04 density units, n=4), but increased at 24 h (Fig. 4A; 1.07±0.07 vs. 1.55±0.05 density units, n=4). Phosphorylation of STAT3 at Ser-727 showed the same pattern with the phosphorylation at Tyr 705 (Fig. 4B). The IFN-γ treatment did not change total STAT3 expression levels (Fig. 4C). Thus, the same result was obtained when the phosphorylation levels of STAT3 were normalized on the basis of total STAT3 expression levels (Fig. 4D, E). Since the change in STAT3 activity was correlated with change in AGT expression (Fig. 1), we hypothesized that IFN-γ regulates AGT expression in a STAT3-dependent mechanism. Therefore, DNA-binding activity of STAT3 was evaluated by EMSA. The IFN-γ treatment suppressed the DNA-binding activity of STAT3 at 6 and 12 h (Fig. 4F). However, the treatment augmented the STAT3 activity at 24 h (Fig. 4F).
Figure 4.
STAT3 activation by IFN-γ treatment. A–C) RPTCs were treated with 10 ng/ml IFN-γ for 6, 12, and 24 h. After treatment, STAT3 phosphorylation at Tyr-705 (A, n=4) and Ser-727 (B, n=4) and expression levels (C, n=4) were evaluated by Western blot analysis and normalized on the basis of β-actin levels. D, E). Phosphorylation levels at Tyr-701 (D) and Ser-727 (E) were normalized on the basis of total STAT3 levels. F) DNA-binding activity of STAT3 was also evaluated using EMSA. C, control group; I, IFN-γ-treated group; N, no protein control. Data are expressed as means ± se. *P < 0.05 vs. control group.
Effects of IFN-γ on SOCS1 expression
As shown in Fig. 5, IFN-γ treatment increased SOCS1 mRNA expression in a dose-dependent manner at 6 h (Fig. 5A; 24.30±0.61, ratio to control, with 20 ng/ml IFN-γ, n=4) and at 12 h (Fig. 5B; 18.49±0.84, ratio to control, with 20 ng/ml IFN-γ, n=4). Only 20 ng/ml IFN-γ treatment augmented SOCS1 mRNA expression at 24 h (Fig. 5C; 1.98±0.14, ratio to control, with 20 ng/ml IFN-γ, n=4), and the augmentation level was smaller than those at 6 h and 12 h. SOCS1 protein levels in the cell lysates were determined using Western blot analysis. Six hours of IFN-γ treatment (20 ng/ml) significantly increased AGT protein levels (Fig. 5D; 9.36±0.85, ratio to control, n=4) although the magnitude of SOCS1 protein augmentation by IFN-γ treatment was smaller than SOCS1 mRNA augmentation.
Figure 5.
Effects of IFN-γ treatment on SOCS1 expression. A–C) RPTCs were treated with 0–20 ng/ml IFN-γ for 6 h (A, n=4), 12 h (B, n=4), and 24 h (C, n=4). After these treatments, SOCS1 mRNA was measured by qRT-PCR. Expression levels of SOCS1 mRNA were normalized on the basis of rat β-actin mRNA levels. Inset: different range of y axis for results at 24 h (C). D) Cells were treated with 20 ng/ml IFN-γ for 24 h; thereafter, SOCS1 protein levels were determined using Western blot analysis (n=4). Data are expressed as relative values compared with the control group at each time point and represent means ± se. *P < 0.05 vs. control group.
Contribution of JAK2 to the regulation of AGT expression by IFN-γ
As our time control experiments demonstrated that IFN-γ treatment (10 ng/ml) markedly increased STAT1 activity (Fig. 3) and SOCS1 expression (Fig. 5) at 6 h but not 24 h, we selected 6 h-IFN-γ treatment for studies to delineate the roles of STAT1, STAT3, and SOCS1 in the regulation of AGT expression. To test whether JAK2 is involved in the AGT regulation by IFN-γ treatment, the cells were pretreated with 1 μM AG490, a JAK2 inhibitor for 90 min prior to treatment with IFN-γ for 6 h. Treatment with 10 ng/ml IFN-γ induced JAK2 phosphorylation (Fig. 6A; n=4). The phosphorylation was attenuated by the AG490 treatment (Fig. 6A). The treatment with IFN-γ augmented SOCS1 expression at 6 h (Fig. 6B) as shown in Fig. 5. The SOCS1 augmentation was significantly attenuated by the AG490 treatment (Fig. 6B; n=4). AGT expression levels were suppressed by IFN-γ treatment at 6 h, and this suppression was also attenuated by the AG490 treatment (Fig. 6C).
Figure 6.
Contribution of JAK2 to the regulation of AGT expression by IFN-γ treatment. RPTCs were pretreated with 1 μM AG490 for 90 min prior to 6 h IFN-γ treatment (10 ng/ml). After these treatments, JAK2 phosphorylation (A, n=4), SOCS1 mRNA (B, n=4) and AGT mRNA (C, n=4) levels were determined. Phosphorylation levels of JAK2 were normalized on the basis of total JAK2 levels. Expression levels of SOCS1 mRNA and AGT mRNA were normalized on the basis of rat β-actin mRNA levels. Data are expressed as relative values compared with the control group and represent means ± se. *P < 0.05 vs. control group.
STAT1 is pivotal in the regulation of AGT expression by IFN-γ
To elucidate the role of STAT1 in the regulation of AGT by IFN-γ treatment, STAT1 expression was suppressed using STAT1-siRNA. As described in Fig. 7A; treatment with STAT1-siRNA suppressed the basal STAT1 protein expression [0.42±0.03, ratio to rat negative control siRNA (NC)-treated group, n=4]. At 6 h, there was no effect of treatment with NC on the expected increases in SOCS1 expression (Fig. 7B, left panel; 7.63±0.45, ratio to control, n=4) or the expected reductions on STAT3 phosphorylation (Fig. 7C, left panel; 0.68±0.03, ratio to control, n=4). In contrast, targeting STAT1 by siRNA had a reverse effect. Treatment with STAT1-siRNA not only reduced STAT1 expression but also reduced the expected activation of SOCS1 (Fig. 7B, right panel; 3.39±0.28, ratio to control, n=4) and caused a paradoxical increase in STAT3 phosphorylation (Fig. 7C, right panel; 1.75±0.08, ratio to control, n=4). These results demonstrate that STAT1 phosphorylation is upstream of the activation of SOCS1 and the inactivation of STAT3. AGT expression was suppressed by 10 ng/ml IFN-γ treatment at 6 h in the NC group (Fig. 7D, left panel; 0.36±0.03, ratio to control, n=4), and this suppression was attenuated by STAT1-siRNA (Fig. 7D, right panel; 0.70±0.05, ratio to control, n=4).
Figure 7.
STAT1 is pivotal in the regulation of AGT expression by IFN-γ treatment. A) To elucidate the roles of STAT1 in the regulation of AGT expression by IFN-γ treatment, basal STAT1 expression was suppressed using STAT1-siRNA (n=4). B–D) Cells were then treated with 10 ng/ml IFN-γ for 6 h, and SOCS1 mRNA (B, n=4) and AGT mRNA (D, n=4) levels were measured by qRT-PCR; STAT3 phosphorylation levels (C, n=4) were also evaluated by Western blot analysis. Expression levels of STAT1 and phosphorylation levels of STAT3 were normalized on the basis of β-actin protein levels. SOCS1 and AGT mRNA levels were normalized on the basis of β-actin mRNA levels. NC, negative control siRNA-treated group; ST1-si, STAT1-siRNA-treated group. Data are expressed as relative values compared with NC group or each control group and represent means ± se. *P < 0.05 vs. control group.
SOCS1 regulates AGT expression and STAT3 phosphorylation by IFN-γ
Figure 8A shows that treatment with SOCS1-siRNA suppressed the basal SOCS1 protein expression (0.42±0.05, ratio to NC group, n=4). The suppression of SOCS1 expression by siRNA did not affect STAT1 phosphorylation (Fig. 8B; n=4). In a similar way to STAT1-siRNA, suppression of AGT expression by the IFN-γ treatment (Fig. 8D, left panel) was attenuated in the SOCS1-siRNA group (Fig. 8D, right panel; 1.13±0.14, ratio to control, n=4). Notably, IFN-γ treatment suppressed STAT3 phosphorylation (Fig. 8C, left panel), and a paradoxical increase in STAT3 phosphorylation was observed by the SOCS1-siRNA treatment (Fig. 8C, right panel; 2.04±0.01, ratio to control, n=4).
Figure 8.
SOCS1 regulates AGT expression and STAT3 phosphorylation by IFN-γ treatment. A) RPTCs were treated with SOCS1-siRNA (n=4). B–D) Cells were then treated with 10 ng/ml IFN-γ for 6 h, and STAT1 phosphorylation levels (B, n=4) and STAT3 phosphorylation levels (C, n=4) were evaluated by Western blot analysis; AGT mRNA (D, n=4) levels were measured by qRT-PCR. Expression levels of SOCS1 and phosphorylation levels of STATs were normalized on the basis of β-actin protein levels. AGT mRNA levels were normalized on the basis of β-actin mRNA levels. ND, nondetectable; NC, negative control siRNA-treated group; SO1-si, SOCS1-siRNA-treated group. Data are expressed as relative values compared with NC group or each control group and represent means ± se. *P < 0.05 vs. control group.
Roles of STAT3 in the regulation of AGT expression by IFN-γ
Contribution of STAT3 to the regulation of AGT was also tested using STAT3-siRNA. Treatment with STAT3-siRNA suppressed the basal STAT3 protein expression (Fig. 9A; 0.46±0.05, ratio to NC group, n=4). The suppression of STAT3 expression by siRNA did not affect STAT1 phosphorylation (Fig. 9B; n=4). At 24 h, STAT3 suppression by STAT3-siRNA reduced basal levels of AGT expression (Fig. 9D; 0.63±0.05, ratio to NC group, n=4). Moreover, as opposed to STAT1-siRNA treatment, down-regulation of STAT3 by STAT3-siRNA did not affect IFN-γ-induced SOCS1 up-regulation (Fig. 9C). These results demonstrate that STAT3 is involved in the long-term regulation of AGT expression and that SOCS1 is upstream of STAT3 in this pathway.
Figure 9.
Roles of STAT3 in the regulation of AGT expression by IFN-γ treatment. A) Contribution of STAT3 to the regulation of AGT was tested using STAT3-siRNA (n=4). B–D) After treatment of RPTCs with STAT3-siRNA, cells were treated with 10 ng/ml IFN-γ for 6 h. STAT1 phosphorylation levels (B, n=4) were determined by Western blot analysis; SOCS1 (C, n=4) and AGT (D, n=4) mRNA levels were measured by qRT-PCR. Phosphorylation levels of STAT1 and expression levels of SOCS1 and AGT mRNA were normalized based on β-actin levels. ND, nondetectable; NC, negative control siRNA-treated group; ST3-si, STAT3-siRNA-treated group. Data are expressed as relative values compared with NC group or each control group and represent means ± se. *P < 0.05 vs. control group.
DISCUSSION
IFN-γ is one of the most important known proinflammatory factors, and there is a clear relationship between inflammation and the RAS (1, 2); however, little is known with regard to the effects of this cytokine on AGT expression in kidney cells. An effect of IFN-γ on AGT regulation has been reported in a study using hepatocytes (19). That study demonstrated that AGT expression is augmented by IFN-γ via activation of STAT1 in hepatocytes, and reported that the molecular mechanism is independent from STAT3 pathway (19). However, there is no information about the regulation of AGT expression by IFN-γ in other cell types, including renal cells. AGT regulation in the kidneys is key in the development of a number of conditions, including hypertension (11). In the present study, IFN-γ appears to regulate AGT expression in a biphasic manner, ultimately augmenting AGT expression in RPTCs. These responses appear to be mediated by the interplay between STAT1-SOCS1 and STAT3, a novel observation that is consonant with previous publications concerning IFN-γ effects on hepatic AGT expression (19). Biphasic effects of IFN-γ are also reported on other targets such as p47-phox in polymorphonuclear leukocytes (36).
To elucidate the mechanisms underlying the biphasic effects of IFN-γ on AGT expression in RPTCs, we focused on the role of a STAT1-SOCS1 pathway in the regulation of AGT expression. In the present study, IFN-γ-induced STAT1 phosphorylation at Tyr-701 weakened until 24 h, and phosphorylation at Ser-727 was enhanced until 24 h. It has been demonstrated that the phosphorylation at Ser-727 plays key roles in the transcription activity of STAT1 (37). In addition, phosphorylation of STAT1 at Tyr-701 is also required for dimerization, nuclear translocation, and DNA binding (38). Therefore, as discussed, it would appear that the decrease in phosphorylation levels of STAT1 at Tyr-701 resulted in the reduction of SOCS1 augmentation at 24 h in the present study. Furthermore, the phosphorylation levels of serine residue in STAT1 at 12 and 24 h were not higher than control when they are normalized based on total STAT1 levels. Opposite behaviors were observed in STAT3 activity and AGT expression, which showed an early down-regulation and a late increase, suggesting that STAT1 and SOCS1 were involved in mediating such effects (Fig. 10). This was corroborated by our RNA interference experiments. The results using STAT1-siRNA and SOCS1-siRNA indicate that STAT1 activity regulates the expression of SOCS1 and STAT3 activity and AGT expression and that SOCS1 is downstream to STAT1, but upstream to STAT3 and AGT regulation in the mechanism. The results of STAT3 suppression by siRNA suggest that STAT3 is downstream of SOCS1, which was reported in breast cancer cells (39) and that STAT3 directly regulates AGT expression in RPTCs. The results are consistent with previous findings demonstrating that STAT3 contributes to AGT regulation (30, 40–42).
Figure 10.
Schematic summary of the proposed AGT regulation by IFN-γ in RPTCs based on the results of the present study. IFN-γ activates both STAT1 and STAT3; however, the activation of STAT1 induces SOCS1 augmentation, which suppresses STAT3 activation and AGT augmentation induced by IFN-γ in RPTCs.
STAT1 binds to the rat AGT gene promoter at a low level and STAT3 binds to rat AGT gene promoter at high level in Ang II-treated myocytes (43). This may explain why the activated STAT1 by IFN-γ treatment did not induce augmentation of AGT expression in RPTCs in the present study.
Because RPTCs are the predominant sources of intrarenal AGT (9, 10), AGT regulation in these cells contributes to intrarenal Ang II production and development of hypertension (11, 44). Furthermore, an inappropriate increase in intrarenal RAS activity plays an important role in the development of renal inflammation (2, 13). The present study demonstrates that IFN-γ ultimately augments AGT expression in RPTCs, which may lead to increases in intrarenal Ang II levels and exacerbation of renal inflammation. It also suggests that the STAT1-SOCS1 pathway serves as an internal suppressor limiting the augmentation of intrarenal AGT levels via activation of the JAK-STAT pathway, which may lead to new strategies to treat hypertension and kidney diseases associated with an activated intrarenal RAS.
Acknowledgments
The authors acknowledge the valuable comments and excellent technical assistance of Dr. Maki Urushihara, Dr. Masumi Kamiyama, Dr. Daisuke Inui, Dr. Omar W. Acres, Ms. Akemi Katsurada, and Ms. Ayumi Kitano (Tulane University).
This study was supported by grants from the U.S. National Institute of Diabetes and Digestive and Kidney Diseases (R01DK072408 and K99DK083455), the National Center for Research Resources (P20RR017659), and the National Heart, Lung, and Blood Institute (R01HL026371). The authors declare no conflicts of interest.
Footnotes
- AGT
- angiotensinogen
- Ang II
- angiotensin II
- ELISA
- enzyme-linked immunosorbent assay
- EMSA
- electrophoresis mobility shift assay
- IFN-γ
- interferon-γ
- IFNGR
- IFN-γ receptor
- JAK
- Janus activated kinase, NC, negative control siRNA, qRT-PCR, quantitative RT-PCR
- RAS
- renin-angiotensin system
- RPTC
- renal proximal tubular cell
- siRNA
- small interference RNA
- SOCS
- suppressor of cytokine signaling
- STAT
- signal transducers and activators of transcription.
REFERENCES
- 1. Navar L. G., Prieto M. C., Satou R., Kobori H. (2011) Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr. Opin. Pharmacol. 11, 180–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crowley S. D., Frey C. W., Gould S. K., Griffiths R., Ruiz P., Burchette J. L., Howell D. N., Makhanova N., Yan M., Kim H. S., Tharaux P. L., Coffman T. M. (2008) Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am. J. Physiol. Renal Physiol. 295, F515–F524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saha B., Jyothi Prasanna S., Chandrasekar B., Nandi D. (2009) Gene modulation and immunoregulatory roles of interferon gamma. Cytokine 50, 1–14 [DOI] [PubMed] [Google Scholar]
- 4. Kobori H., Nangaku M., Navar L. G., Nishiyama A. (2007) The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev. 59, 251–287 [DOI] [PubMed] [Google Scholar]
- 5. Dzau V. J., Re R. (1994) Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circulation 89, 493–498 [DOI] [PubMed] [Google Scholar]
- 6. Tamura K., Tanimoto K., Takahashi S., Sagara M., Fukamizu A., Murakami K. (1992) Structure and expression of the mouse angiotensinogen gene. Jpn. Heart J. 33, 113–124 [DOI] [PubMed] [Google Scholar]
- 7. Paul M., Poyan Mehr A., Kreutz R. (2006) Physiology of local renin-angiotensin systems. Physiol. Rev. 86, 747–803 [DOI] [PubMed] [Google Scholar]
- 8. Re R. N. (2004) Tissue renin angiotensin systems. Med. Clin. North Am. 88, 19–38 [DOI] [PubMed] [Google Scholar]
- 9. Ingelfinger J. R., Zuo W. M., Fon E. A., Ellison K. E., Dzau V. J. (1990) In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J. Clin. Invest. 85, 417–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Terada Y., Tomita K., Nonoguchi H., Marumo F. (1993) PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int. 43, 1251–1259 [DOI] [PubMed] [Google Scholar]
- 11. Sachetelli S., Liu Q., Zhang S. L., Liu F., Hsieh T. J., Brezniceanu M. L., Guo D. F., Filep J. G., Ingelfinger J. R., Sigmund C. D., Hamet P., Chan J. S. (2006) RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 69, 1016–1023 [DOI] [PubMed] [Google Scholar]
- 12. Kobori H., Harrison-Bernard L. M., Navar L. G. (2001) Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J. Am. Soc. Nephrol. 12, 431–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kobori H., Ozawa Y., Satou R., Katsurada A., Miyata K., Ohashi N., Hase N., Suzaki Y., Sigmund C. D., Navar L. G. (2007) Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am. J. Physiol. Renal Physiol. 293, F938–F945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gonzalez-Villalobos R. A., Seth D. M., Satou R., Horton H., Ohashi N., Miyata K., Katsurada A., Tran D. V., Kobori H., Navar L. G. (2008) Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am. J. Physiol. Renal Physiol. 295, F772–F779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kobori H., Alper A. B., Jr., Shenava R., Katsurada A., Saito T., Ohashi N., Urushihara M., Miyata K., Satou R., Hamm L. L., Navar L. G. (2009) Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension 53, 344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Navar L. G., Kobori H., Prieto M. C., Gonzalez-Villalobos R. A. (2011) Intratubular renin-angiotensin system in hypertension. Hypertension 57, 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takeda K., Akira S. (2000) STAT family of transcription factors in cytokine-mediated biological responses. Cytokine Growth Factor Rev. 11, 199–207 [DOI] [PubMed] [Google Scholar]
- 18. Krejci P., Prochazkova J., Bryja V., Jelinkova P., Pejchalova K., Kozubik A., Thompson L. M., Wilcox W. R. (2009) Fibroblast growth factor inhibits interferon gamma-STAT1 and interleukin 6-STAT3 signaling in chondrocytes. Cell. Signal. 21, 151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jain S., Shah M., Li Y., Vinukonda G., Sehgal P. B., Kumar A. (2006) Upregulation of human angiotensinogen (AGT) gene transcription by interferon-gamma: involvement of the STAT1-binding motif in the AGT promoter. Biochim. Biophys. Acta 1759, 340–347 [DOI] [PubMed] [Google Scholar]
- 20. Larsen L., Ropke C. (2002) Suppressors of cytokine signalling: SOCS. APMIS 110, 833–844 [DOI] [PubMed] [Google Scholar]
- 21. Kile B. T., Alexander W. S. (2001) The suppressors of cytokine signalling (SOCS). Cell. Mol. Life Sci. 58, 1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DiGiandomenico A., Wylezinski L. S., Hawiger J. (2009) Intracellular delivery of a cell-penetrating SOCS1 that targets IFN-gamma signaling. Sci. Signal. 2, ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qing Y., Costa-Pereira A. P., Watling D., Stark G. R. (2005) Role of tyrosine 441 of interferon-gamma receptor subunit 1 in SOCS-1-mediated attenuation of STAT1 activation. J. Biol. Chem. 280, 1849–1853 [DOI] [PubMed] [Google Scholar]
- 24. Kubo M., Hanada T., Yoshimura A. (2003) Suppressors of cytokine signaling and immunity. Nat. Immunol. 4, 1169–1176 [DOI] [PubMed] [Google Scholar]
- 25. Fujimoto M., Naka T. (2003) Regulation of cytokine signaling by SOCS family molecules. Trends Immunol. 24, 659–666 [DOI] [PubMed] [Google Scholar]
- 26. Ortiz-Munoz G., Lopez-Parra V., Lopez-Franco O., Fernandez-Vizarra P., Mallavia B., Flores C., Sanz A., Blanco J., Mezzano S., Ortiz A., Egido J., Gomez-Guerrero C. (2010) Suppressors of cytokine signaling abrogate diabetic nephropathy. J. Am. Soc. Nephrol. 21, 763–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi Y., Du C., Zhang Y., Ren Y., Hao J., Zhao S., Yao F., Duan H. (2010) Suppressor of cytokine signaling-1 ameliorates expression of MCP-1 in diabetic nephropathy. Am. J. Nephrol. 31, 380–388 [DOI] [PubMed] [Google Scholar]
- 28. Ingelfinger J. R., Jung F., Diamant D., Haveran L., Lee E., Brem A., Tang S. S. (1999) Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am. J. Physiol. 276, F218–F227 [DOI] [PubMed] [Google Scholar]
- 29. Kobori H., Ozawa Y., Suzaki Y., Nishiyama A. (2005) Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J. Am. Soc. Nephrol. 16, 2073–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Satou R., Gonzalez-Villalobos R. A., Miyata K., Ohashi N., Urushihara M., Acres O. W., Navar L. G., Kobori H. (2009) IL-6 augments angiotensinogen in primary cultured renal proximal tubular cells. Mol. Cell. Endocrinol. 311, 24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kobori H., Katsurada A., Miyata K., Ohashi N., Satou R., Saito T., Hagiwara Y., Miyashita K., Navar L. G. (2008) Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am. J. Physiol. Renal Physiol. 294, F1257–F1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Satou R., Gonzalez-Villalobos R. A., Miyata K., Ohashi N., Katsurada A., Navar L. G., Kobori H. (2008) Costimulation with angiotensin II and interleukin 6 augments angiotensinogen expression in cultured human renal proximal tubular cells. Am. J. Physiol. Renal Physiol. 295, F283–F289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Katsurada A., Hagiwara Y., Miyashita K., Satou R., Miyata K., Ohashi N., Navar L. G., Kobori H. (2007) Novel sandwich ELISA for human angiotensinogen. Am. J. Physiol. Renal Physiol. 293, F956–F960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Satou R., Miyata K., Katsurada A., Navar L. G., Kobori H. (2010) Tumor necrosis factor-α suppresses angiotensinogen expression through formation of a p50/p50 homodimer in human renal proximal tubular cells. Am. J. Physiol. Cell Physiol. 299, C750–C759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheon H., Stark G. R. (2009) Unphosphorylated STAT1 prolongs the expression of interferon-induced immune regulatory genes. Proc. Natl. Acad. Sci. U. S. A. 106, 9373–9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Amezaga M. A., Bazzoni F., Sorio C., Rossi F., Cassatella M. A. (1992) Evidence for the involvement of distinct signal transduction pathways in the regulation of constitutive and interferon gamma-dependent gene expression of NADPH oxidase components (gp91-phox, p47-phox, and p22-phox) and high-affinity receptor for IgG (Fc gamma R-I) in human polymorphonuclear leukocytes. Blood 79, 735–744 [PubMed] [Google Scholar]
- 37. Tsuboi H., Wakamatsu E., Iizuka M., Nakamura Y., Sugihara M., Suzuki T., Ogishima H., Hayashi T., Goto D., Ito S., Matsumoto I., Sumida T. (2011) Importance of serine727 phosphorylated STAT1 in IFN gamma-induced signaling and apoptosis of human salivary gland cells. Int. J. Rheum. Dis. 14, 86–91 [DOI] [PubMed] [Google Scholar]
- 38. Decker T., Kovarik P. (2000) Serine phosphorylation of STATs. Oncogene 19, 2628–2637 [DOI] [PubMed] [Google Scholar]
- 39. Park Y., Shon S. K., Kim A., Kim K. I., Yang Y., Cho D. H., Lee M. S., Lim J. S. (2007) SOCS1 induced by NDRG2 expression negatively regulates STAT3 activation in breast cancer cells. Biochem. Biophys. Res. Commun. 363, 361–367 [DOI] [PubMed] [Google Scholar]
- 40. Ray S., Boldogh I., Brasier A. R. (2005) STAT3 NH2-terminal acetylation is activated by the hepatic acute-phase response and required for IL-6 induction of angiotensinogen. Gastroenterology 129, 1616–1632 [DOI] [PubMed] [Google Scholar]
- 41. Jain S., Li Y., Patil S., Kumar A. (2007) HNF-1alpha plays an important role in IL-6-induced expression of the human angiotensinogen gene. Am. J. Physiol. Cell Physiol. 293, C401–C410 [DOI] [PubMed] [Google Scholar]
- 42. Fukuzawa J., Booz G. W., Hunt R. A., Shimizu N., Karoor V., Baker K. M., Dostal D. E. (2000) Cardiotrophin-1 increases angiotensinogen mRNA in rat cardiac myocytes through STAT3: an autocrine loop for hypertrophy. Hypertension 35, 1191–1196 [DOI] [PubMed] [Google Scholar]
- 43. Mascareno E., Dhar M., Siddiqui M. A. (1998) Signal transduction and activator of transcription (STAT) protein-dependent activation of angiotensinogen promoter: a cellular signal for hypertrophy in cardiac muscle. Proc. Natl. Acad. Sci. U. S. A. 95, 5590–5594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gonzalez-Villalobos R. A., Billet S., Kim C., Satou R., Fuchs S., Bernstein K. E., Navar L. G. (2011) Intrarenal angiotensin-converting enzyme induces hypertension in response to angiotensin I infusion. J. Am. Soc. Nephrol. 22, 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]