Abstract
We previously reported that RanBP9 binds low-density lipoprotein receptor-related protein (LRP), amyloid precursor protein (APP), and BACE1 and robustly increased Aβ generation in a variety of cell lines and primary neuronal cultures. To confirm the physiological/ pathological significance of this phenotype in vivo, we successfully generated transgenic mice overexpressing RanBP9 as well as RanBP9-null mice. Here we show that RanBP9 overexpression resulted in >2-fold increase in Aβ40 levels as early as 4 mo of age. A sustained increase in Aβ40 levels was seen at 12 mo of age in both CHAPS-soluble and formic acid (FA)-soluble brain fractions. In addition, Aβ42 levels were also significantly increased in FA-soluble fractions at 12 mo of age. More important, increased Aβ levels were translated to increased deposition of amyloid plaques. In addition, RanBP9 overexpression significantly decreased the levels of synaptophysin and PSD-95 proteins. Conversely, RanBP9-null mice showed increased levels of synaptophysin, PSD-95, and drebrin A protein levels. Given that loss of synapses is the best pathological correlate of cognitive deficits in Alzheimer's disease (AD), increased Aβ levels by RanBP9 observed in the present study provides compelling evidence that RanBP9 may indeed play a key role in the etiology of AD. If so, RanBP9 provides a great opportunity to develop novel therapy for AD.—Lakshmana, M. K., Hayes, C. D., Bennett, S. P., Bianchi, E., Reddy, K. M., Koo, E. H., Kang, D. E. Role of RanBP9 on amyloidogenic processing of APP and synaptic protein levels in the mouse brain.
Keywords: Alzheimer's disease, transgenic mice, null mice, synapses
Alzheimer's disease (AD) is an age-associated neurodegenerative disease characterized pathologically by the presence of intracellular neurofibrillary tangles and extracellular amyloid plaques made up of amyloid β (Aβ) protein, a 38- to 43-aa peptide derived from amyloid precursor protein (APP). Recent statistics suggest that ∼5.3 million patients are affected by AD in the United States alone, and by 2050, 11–16 million individuals are predicted to develop AD (1). While death rates due to stroke, heart disease, and cancer have a decreasing trend, deaths related to AD have actually increased by 47% between 2000 and 2006 (1). In the past 2 decades, intense research has unraveled how Aβ is produced in terms of its precursor protein and the enzymes involved in its generation. It is now clear that Aβ is produced by the consecutive actions of β- and γ-secretases, which releases Aβ from its precursor protein, APP (2, 3). An alternative cleavage by α-secretase at the 17th amino acid of the Aβ sequence prevents the generation of intact Aβ. As of today, no effective therapy exists for AD, and the available treatments can neither slow nor reverse disease progression, as they are not designed to treat the underlying cause of AD. Though hundreds of clinical trials have been attempted so far, including those specifically targeting β-secretase and γ-secretases, more recent results from such trials are increasingly becoming dismal. What is even more disturbing is that instead of improving the cognitive measures, many patients experienced worsening of the symptoms and some patients even developed skin cancer after treatment with semagacestat, a γ-secretase inhibitor (4, 5). The most likely explanation for the failure of γ-secretase inhibitors in the clinical trials is because γ-secretase has dozens of target proteins, particularly notch signaling, which is crucial for cell-cell communication, immune system formation, and cell proliferation and survival (6). Therefore, alternative targets that may modulate Aβ generation without directly inhibiting γ-secretase or β-secretases are very important at this juncture.
The low-density lipoprotein receptor-related protein (LRP) and two of its key ligands, apoE and α2-macroglobulin, are genetically associated with late onset AD (7–9). Increasing evidence suggests that LRP facilitates amyloidogenic processing of APP, although the precise mechanisms are still unclear. We recently demonstrated that the last 37 aa of LRP (LRP-C37), without the NPXY motifs, is necessary and sufficient to increase Aβ production (10). Since LRP-C37 alone was a potent inducer of Aβ production, we used this domain as bait in a yeast 2-hybrid screen, which resulted in the identification of Ran-binding protein 9 (RanBP9, RanBPM; ref. 10). RanBP9 is a multidomain scaffolding protein implicated in a variety of functions, including integration of cell surface receptors with intracellular signaling targets (11). At present, the biology of RanBP9 is completely unknown. RanBP9 is expressed ubiquitously in all tissues, including brain (12). RanBP9 is localized throughout the cell, with a significant fraction found near the inner surface of the plasma membrane and cytoplasm (12, 13), which is the potential site for interactions with the membrane receptors. In addition, RanBP9 has been shown to regulate cell morphology (14) and adhesion (15). More important, when overexpressed in NGF-dependent dorsal root ganglion (DRG) neurons, RanBP9 almost completely blocked the formation of neurites, and by contrast, reducing RanBP9 function either through dominant negative mutant or siRNAs increased the basal outgrowth of neurites (16). Similarly, RanBP9 has been shown to link neural cell adhesion molecule L1 with the extracellular signal-regulated kinase/MAPK pathway and inhibit L1-mediated neurite outgrowth in the cerebellar primary neurons (13). RanBP9 is also a ligand for Rho-GEF (17), and many studies have shown an essential role for Rho-like GTPases in regulating spine and dendritic morphology (18).
Among several clones identified in the yeast 2-hybrid assay binding to LRP-C37 domain, only RanBP9 increased Aβ generation in a variety of cells, including primary neuronal cultures. We subsequently found that RanBP9 not only interacted with LRP but also with APP and BACE-1 and that overexpression of RanBP9 robustly increased Aβ secretion from both wild-type APP (APPwt) and APP with Swedish mutation (APPswe). Although the exact mechanism for increasing Aβ generation is not yet clear, RanBP9 reduced cell surface levels of APP, accelerated APP internalization, and strongly enhanced BACE-1 cleavage of APP and Aβ generation (19), suggesting that RanBP9 appears to increase Aβ generation through β-secretase processing of APP in the endocytic pathway. Surprisingly, RanBP9-N60, a 60-kDa proteolytically derived species of RanBP9, which binds APP, LRP, and BACE-1 more strongly than the full-length, was increased 6-fold in the brains of patients with AD (20). To gain a better insight on the species physiological/pathological significance of this phenotype in vivo, we successfully generated transgenic mice overexpressing RanBP9, as well as RanBP9-null mice, by gene-trap strategy.
Here we show that RanBP9 overexpression caused significantly increased Aβ levels at 4 and 12 mo of age, which were also accompanied by increased number of amyloid plaques. RanBP9 overexpression in APdE9 mice increased both Aβ40 and Aβ42 levels. In addition, we found a trend toward decreased levels of some presynaptic and postsynaptic proteins in RanBP9-overexpressing transgenic mice. In contrast, RanBP9-null mice showed an increased trend in the amount of some presynaptic and postsynaptic proteins, which implies that RanBP9 might play an essential role not only in increasing amyloid plaques but also in decreasing the number of functional synapses. The increased Aβ levels by RanBP9 in APdE9 mice might be responsible for reductions in some of the synaptic proteins.
MATERIALS AND METHODS
Chemicals and antibodies
Thioflavin S (cat. no. T1892), 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-gal; B4252), EGTA (E4378), and glutaraldehyde (G-7776) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The polyclonal antibody CT15 (against C-terminal 15 residues of APP), the monoclonal antibodies 4G8 (against residues 17–24 of Aβ sequence) and 26D6 (against residues 1–12 of Aβ), and the polyclonal antibody 63G (against the middle region of APP) have been described previously (21, 22). The monoclonal antibody 6E10 (SIG-39300, recognizing residues 1–17 of Aβ sequence) was obtained from Covance Research (Denver, CO, USA). Polyclonal anti-secreted derivative of APP (sAPP)β-WT antibody (18957) was purchased from IBL Co. Ltd (Gunma, Japan). Monoclonal anti-drebrin antibody (D029-3) was purchased from MBL international corporation (Woburn, MA, USA). PSD-95 polyclonal antibody (EP2652Y) was obtained from Epitomics (Burlingame, CA, USA). Polyclonal Rab3A antibody (15029-1-AP) was purchased from Protein Tech Group Inc. (Chicago, IL, USA). Anti-synaptophysin mouse monoclonal antibody (573822) was purchased from EMD Chemicals Inc. (Gibbstown, NJ, USA). Mouse monoclonal antibody against α-tubulin (A01410) was purchased from Genscript USA Inc. (Piscataway, NJ, USA). Rabbit polyclonal antibody against Gap-43 (AB5220) was obtained from Millipore (Temecula, CA, USA). Anti-spinophilin/neurabin rabbit polyclonal antibody (N5162) and anti-flag-tag antibody (M2; F3165) were purchased from Sigma-Aldrich. Monoclonal antibody against RanBP9 was produced by immunizing mice with a peptide corresponding to aa 146–729 of RanBP9, as described previously (23). Secondary antibodies, such as anti-mouse and anti-rabbit IgGs, were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA).
Primary neuronal cultures and RanBP9 immunocytochemistry
Primary hippocampal neurons were derived from postnatal day 1 (P1) pups as described in detail previously (19). For immunocytochemical staining, the hippocampal cultures were grown until 18–20 d, when a complex network of processes developed. The cell cultures were placed on ice, washed 3× in cold phosphate buffered saline (PBS), and fixed in 4% paraformaldehyde (PFA). After washing with 3× PBS, the cells were permeabilized with 0.02% Triton X-100, and blocked in 4% normal goat serum (NGS) for 30 min. The monoclonal anti-RanBP9 primary antibody that is being used in our laboratory for immunoblot detection of RanBP9 (23) was ineffective for immunostaining, and therefore we purchased a polyclonal anti-RanBP9 antibody from a commercial source (71-001; BioAcademia, Osaka, Japan). Initially, in a pilot study, the primary antibody was used at various concentrations to find the right dilution, and 1:250 was found appropriate to obtain specific signal without background. The primary antibody was incubated overnight, and immunoreactivity was visualized using a cy3-conjugated anti-rabbit secondary antibody. Coverslips were mounted on Permafluor (Thermo Scientific, Waltham, MA, USA), and images were obtained using an Olympus confocal microscope (Olympus, Tokyo, Japan).
Generation and characterization of transgenic mice with overexpression of flag-tagged RanBP9
We generated RanBP9-transgenic mice by cloning 3x-flag-RanBP9 cDNA in the mouse thy-1 gene cassette in the pTSC21K plasmid generously provided by Prof. J. W. Gordon (Mount Sinai School of Medicine, New York, NY, USA). See Supplemental Data for the detailed description.
Generation and characterization of RanBP9-null mice by gene-trap strategy
Gene trapping is a method to introduce mutations into embryonic stem cells by inserting a gene-trap vector construct through electroporation. RanBP9-null mice were generated by injecting a gene-trap clone into blastocysts, as described in detail in Supplemental Data.
Tissue extraction and immunoblotting
Because the RanBP9−/− mice die on the P2, we extracted cortical brain tissue from P1 pups to be used in immunoblots for presynaptic and postsynaptic proteins. However, for RanBP9-transgenic mice, we used cortical brain tissue from 4- and 12-mo-old adult mice to prepare the lysates. In brief, we anesthetized the mice with isoflurane, decapitated immediately, and rapidly removed the brain tissue into 1% Nonidet P-40 buffer (50 mM Tris-HCl, pH 8.0; 150 mM NaCl; 0.02% sodium azide; 400 nM microcystine-LR; 0.5 mM sodium vanadate; and 1% sodium Nonidet P-40) containing complete protease inhibitor cocktail for use with mammalian cell and tissue extracts (Sigma-Aldrich). Tissue was homogenized using Power Gen 125 (Fisher Scientific, Pittsburgh, PA, USA) and centrifuged at 100,000 g for 1 h. Protein concentrations from each sample were measured by BCA method (Pierce Biotechnology Inc., Rockford, IL, USA). Equal amounts of proteins were loaded into each well and subjected to SDS-PAGE electrophoresis. The proteins were then transferred onto polyvinylidene fluoride membranes, blocked with 5% milk, and incubated overnight with primary antibodies, followed by 1 h incubation with HRP-conjugated secondary antibodies. The protein signals were detected using Super Signal West Pico Chemiluminescent substrate (Pierce).
Quantification of Aβ by ELISA
For measurements of Aβ, mice were sacrificed under isoflurane anesthesia, and hemibrains from APdE9 and APdE9/RanBP9 mice were weighed and homogenized in 1% 3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonate (CHAPSO)/PBS (plus protease inhibitors), then centrifuged at 100,000 g for 60 min at 4°C, and the supernatants were used immediately or stored frozen at −80°C. The pellet was used for formic acid (FA) extraction. To measure Aβ1–40, Ab9 (N-terminal mAb, epitope Aβ1–16) was used as capture antibody, and HRP-conjugated 13-1-1 mAb (epitope Aβ35-40) was used as detection antibody. Synthetic Aβ1-40 at 0, 1.56, 3.125, 6.25, 12.5, 25, 50, 100, and 200 pmol concentrations was used as standard. To develop color, 1-step Ultra 3,3′5,5′-tetramethyl benzidine (TMB)-ELISA solution (Thermo Scientific) was used, and plates were read at λ 450 nm. Aβ42 levels were measured using an ELISA kit from Invitrogen (KHB3441; Invitrogen, Carlsbad, CA, USA) following the manufacturer's protocol. Briefly, 1 ml of 70% FA was added to the pellet and homogenized again at 100,000 g for 10 s (×3) on ice. Clear samples were transferred to another tube using a syringe. The samples were then diluted 1:10 with standard diluent buffer. The Aβ1-42 standards were diluted in 90% standard diluent buffer and 10% of brain extraction buffer, and concentrations of 0, 5, 10, 20, 40, 80 and 160 pg/ml were used to prepare the standard curve for each assay. To the 50 μl of standards or samples in the wells, 50 μl of Aβ42 detection antibody was added and incubated for 3 h at 4°C with shaking. After washing, 100 μl of anti-rabbit IgG HRP working solution was added, followed by 100 μl of stabilized chromogen solution. The plates were then incubated for 30 min, 100 μl of stop solution was added, and the plates were read at λ 450 nm. Aβ levels were calculated per milligram of protein and expressed as percentage change from controls.
Staining of amyloid plaques
Mice were anesthetized by isoflurane and perfused using a mixture of 4% PFA and glutaraldehyde. Brain sections were cut, and once the sections were adhered to slides, they were placed in citrate buffer (10 mM, pH 6.0) at 95–100°C for 5 min and rinsed with distilled water 2× for 5 min. The slides were then immersed in 1% Thioflavin S solution (Sigma-Aldrich) prepared in water for 5 min, and then differentiated in 70% ethanol for 5 min, rinsed again in water 2× for 5 min, and coverslipped with Sure Mount mounting medium (EMS, Hatfield, PA, USA) and placed at 4°C overnight before imaging. For staining with 4G8 antibody, standard immunohistochemical methods were used. To quantify plaques, the brain level of sections cut was fixed for all mice at the region of motor cortex and hippocampus. A fixed thickness of 15-μm coronal sections at regular intervals was maintained in all animals. The amyloid plaques were quantified from throughout the sections from 5 sections/mouse (n=6/genotype), and mean values were generated for each mouse. Pictures were then montaged and, for quantification by ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA), the color images were converted in to HSV format and 8-bit channels. Plaques were quantified in an unbiased manner by an investigator blinded to the genotype of the samples. Plaque burden was calculated based on the percentage of the area covered by the plaques over the total area of the brain sections.
Statistical analysis
Immunoblot signal intensity as well as average number of plaques per section were quantified using ImageJ software. Data were analyzed by Student's t test for presynaptic and postsynaptic proteins in wild-type (WT), RanBP9-transgenic, and RanBP9−/− mice and ANOVA followed by post hoc Tukey's test for synaptophysin and PSD-95 levels in WT, APdE9, and APdE9/RanBP9 mice using Instat3 software (GraphPad Software, San Diego, CA, USA). We used 2-tailed P values assuming populations may have different standard errors. The data presented are means ± se. The data were considered significant at values of P < 0.05.
RESULTS
Generation of transgenic mice overexpressing flag-tagged RanBP9
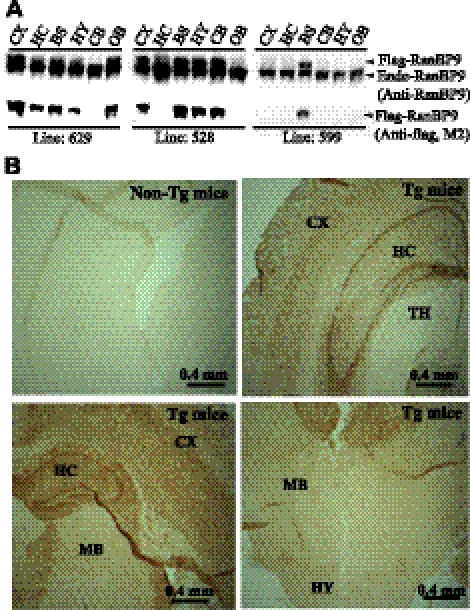
We generated RanBP9-transgenic mice by cloning 3x-flag-RanBP9 cDNA in the mouse thy-1 gene cassette in the pTSC21K plasmid. To avoid possible prenatal lethality, we used thy-1 promoter to restrict RanBP9 expression to the postnatal/adult brain, since RanBP9 is implicated in many vital functions. We established 3 lines of RanBP9-transgenic mice (lines 629, 528, and 599) based on differential protein expression levels in the brain regions. Line 629 expresses exogenous flag-tagged RanBP9 in all brain regions studied except cerebellum. Expression level was more in cortex (108% of endogenous levels) than brainstem (37%) followed by olfactory bulb (36%), hippocampus (32%), and hypothalamus (21%) (Fig. 1A). Line 528 expressed exogenous flag-RanBP9 only in cortex (70% of endogenous levels), brainstem (65%), hypothalamus (60%), cerebellum (58%), and hippocampus (31%), and was undetectable in other brain regions, such as the olfactory bulb (Fig. 1A). Line 599 expresses flag-tagged RanBP9 protein only in brainstem (58% of endogenous levels). We also confirmed transgene expression in cortex, hippocampus, thalamus, and hypothalamus by staining for RanBP9 by immunohistochemistry (Fig. 1B). Overall, transgenic mice expressing flag-tagged RanBP9 looked similar to nontransgenic control mice without any apparent phenotype, and their body and brain weights were also did not differ from the control mice.
Figure 1.
Characterization of RanBP9-transgenic lines for the expression of flag-tagged RanBP9 protein. A) Immunoblot detection of RanBP9 protein expression by both RanBP9-specific monoclonal antibody (anti-RanBP9) as well as anti-flag antibody (M2) in line 629 revealed protein expression in the cortex (CX), hippocampus (HC), brainstem (BS), hypothalamus (HY), and olfactory bulb (OB) but not in cerebellum (CB). Line 528 showed RanBP9 expression in CX, BS, HY, and CB but not in HC and OB. Line 599 showed RanBP9 protein expression only in the BS. B) Immunohistochemical staining of brain sections by flag antibody (M2) from the nontransgenic (non-Tg mice) and transgenic mouse (Tg mice) line 629 (12 mo of age) also showed RanBP9-specific immunoreactivity in the thalamus (TH), midbrain (MB), CX, HC, and HY.
RanBP9 overexpression increases Aβ generation in vivo in the APdE9 double-transgenic mice
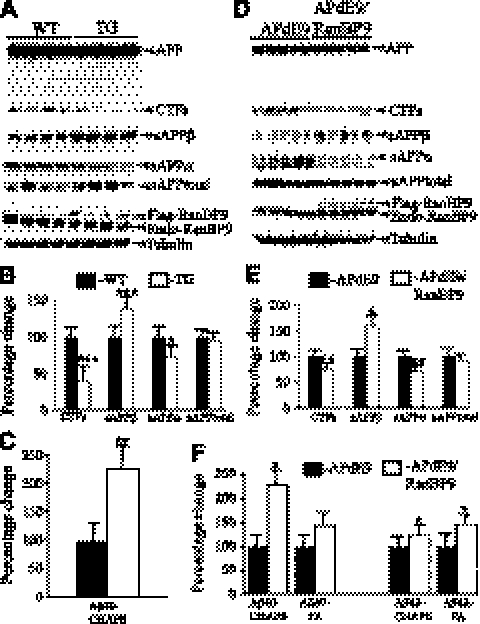
To confirm our previous observation in cell cultures that RanBP9 strongly increases Aβ generation, we crossed B6.Cg-Tg, APPswe, PSEN1dE9 (APdE9) double-transgenic mice (strain C57BL/6×C3H, F2) as a robust model of AD (24) with RanBP9-transgenic mice (strain C57BL/6) and thus generated APdE9/RanBP9 triple-transgenic mice overexpressing chimeric mouse and human APP (Mo/HuAPP695swe) and a mutant human presenilin 1 (PS1-dE9) and flag-tagged RanBP9. As an initial analysis, we compared levels of APP and C-terminal fragments (CTFs) at 4 mo of age in RanBP9-transgenic mice, line 629, and the age-matched WT mice (Fig. 2A–C). We found that the total CTF levels were significantly decreased in the RanBP9-transgenic mice compared to WT mice by 60% and that of sAPPα levels by 28%. On the contrary, sAPPβ levels were increased by 40%. More important, RanBP9 overexpression increased Aβ40 by >2-fold by 122% over the APdE9 control mice (Fig. 2C, P<0.01). We could not detect Aβ42 at this age by our methods. We also compared APP metabolites between APdE9 double-transgenic and APdE9/RanBP9 triple-transgenic mice (Fig. 2D–F). RanBP9 overexpression in the triple-transgenic mice decreased CTF levels by 24% and sAPPα levels by 29%, but sAPPβ levels were increased by 59%. At 12 mo, CHAPSO-soluble Aβ40 was increased by 127% (Fig. 2F, P<0.05) and that of FA-extracted Aβ40 was increased by 42% (Fig. 2F, P<0.05) in APdE9/RanBP9 triple-transgenic mice when compared to APdE9 double-transgenic mice. Similarly, at 12 mo, CHAPSO-soluble Aβ42 was increased by 22% and that of FA-soluble Aβ42 was increased by 45% (Fig. 2F, P<0.05). Since we used the whole hemi brain for quantification of Aβ40 and Aβ42, the net effect of RanBP9 on Aβ levels may have been diluted, especially for the CHAPSO-soluble fraction and its region-specific differential accumulation of amyloid plaques.
Figure 2.
RanBP9 overexpression increases amyloidogenic processing of APP in vivo. A–C) APP metabolites measured in 4-mo-old WT and RanBP9-transgenic (TG) mice. A) Lysates prepared from cortical tissue using Nonidet P-40 buffer were subjected to immunoblotting and probed with specific antibodies. B) RanBP9 overexpression decreased levels of CTF by 60%, sAPPα by 28%, but sAPPβ levels were increased by 40%, while sAPPtotal levels were not altered (n=4/group). C) CHAPSO-soluble Aβ40 was extracted from hemibrains and quantified by ELISA. RanBP9 overexpression in APdE9/RanBP9 triple-transgenic mice (n=4, age 4 mo) increased Aβ40 levels by 122% compared to APdE9 double-transgenic mice (n=4, age 4 mo). D–F) APP metabolites in 12-mo-old APdE9 and APdE9/RanBP9 mice. D) Cortical lysates were subjected to immunoblotting for quantification of APP metabolites. E) RanBP9 overexpression in APdE9 mice decreased levels of CTF by 24%, sAPPα by 29%, but sAPPβ levels were increased by 59%, while sAPPtotal levels were not altered (n=5/group, age 12 mo). F) CHAPSO-soluble and FA-soluble Aβ was extracted from hemibrains and quantified by ELISA (n=6/group, age 12 mo). Aβ40 was increased in both CHAPSO-soluble and FA-soluble fractions, while Aβ42 was increased only in FA-soluble fractions. Aβ was quantified per milligram protein. Values are expressed as percentage change from APdE9 control. Data are means ± se. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; Student's t test.
Increased Aβ levels are associated with increased amyloid plaques
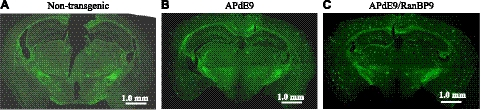
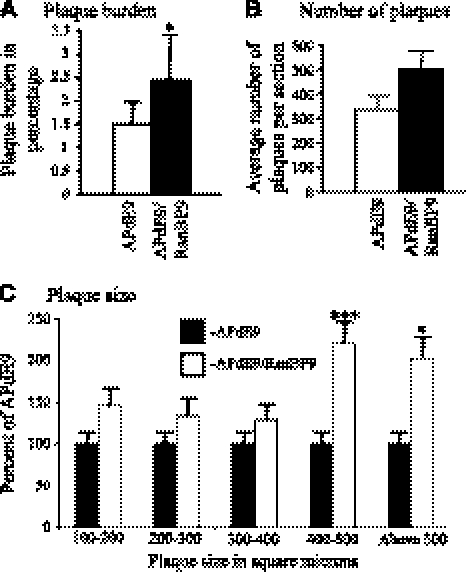
To validate the increased amyloidogenic processing of APP and Aβ generation by RanBP9 as evidenced by the CHAPSO-soluble and FA-soluble Aβ levels in APdE9/RanBP9 mice, we quantified the number of amyloid plaques in APdE9 mice with or without overexpression of RanBP9. As in the other models of AD, APdE9-transgenic mice also displayed more plaques in the cortex and hippocampus compared to subcortical and other brain regions (Fig. 3B). Quantitative analysis of thioflavin S-stained sections revealed significant increases in the number of plaques by 66% in the triple-transgenic mice compared to the double-transgenic mice (Figs. 3C and 4B). We analyzed 5 sections from each of 6 mice from each group of double- and triple-transgenic mice. As the plaque load, i.e., the brain area occupied by Aβ plaques, and plaque size are indicative of severity of Aβ pathology (25), we calculated the plaque burden by estimating the area occupied by plaques over the total brain region used for plaque quantification. The triple-transgenic mice showed an increase of plaque burden to 2.45% from 1.5% in the APdE9 double-transgenic mice (Fig. 4B; P<0.05). A representative picture for each of the nontransgenic (Fig. 3A), APdE9 double-transgenic (Fig. 3B), and APdE9/RanBP9 triple-transgenic mice (Fig. 3C) is shown. A more detailed analysis of the sizes of plaques affected by RanBP9 overexpression revealed that the larger plaques were increased more than the smaller plaques. To calculate this, we categorized the sizes of plaques based on square micrometers from ImageJ analysis and divided them into those that measured 100–200, 200–300, 300–400, 400–500, and >500 μm2. Interestingly, those plaques measuring 400–500 and >500 μm2were increased by 121 and 103% in APdE9/RanBP9 mice compared to APdE9 mice, respectively, compared to an increase of 29 to 46% for smaller plaques (Fig. 4C and Supplemental Table S1). Thus, the overall increase in the number of plaques by RanBP9 is accounted for by larger plaques. Increased plaques are also correlated with increased amounts of both Aβ40 and Aβ42, especially in the FA-soluble fractions. This finding suggests that RanBP9 indeed increases amyloidogenic processing of APP and Aβ generation in vivo in the mouse brains.
Figure 3.
RanBP9 overexpression increases total Aβ plaque burden in APdE9/RanBP9 mice. Coronal sections stained with thioflavin S demonstrates the absence of plaques in nontransgenic mice (A), numerous plaques in APdE9 mice (B), and even more plaques in APdE9/RanBP9 triple-transgenic mice (C). Plaques are particularly increased in the hippocampus and cortical brain areas (the data are mean ± sem, n=6, for each group). The section shown is at the level of interaural, 1.86 mm; Bregma, −1.94 mm (Fig. 47 in Franklin and Paxinos, ref. 40).
Figure 4.
Quantification of plaque burden and average number of plaques per section revealed increased plaque burden when RanBP9 is overexpressed. A) ImageJ quantification of plaque burden revealed 1.5% of the total brain area in APdE9 mice, which increased to 2.45% in APdE9/RanBP9 triple-transgenic mice. B) ImageJ quantification of average number of plaques per section showed 66% increase in plaque numbers in APdE9/RanBP9 triple-transgenic mice as compared to APdE9 double-transgenic mice. C) ImageJ analysis of plaque size (μm2) showed that RanBP9 increased plaques measuring 400–500 and >500 μm2 by121 and 103%, respectively, whereas plaques measuring 100–200 μm2 increased only by 46%, those of 200–300 μm2 by 34%, and those measuring 300–400 μm2 increased only by 29%. Five sections from each of 6 mice/group were used for plaque quantification. Data are presented as means ± se (n=6/group). *P < 0.05, ***P < 0.001; Student's t test.
RanBP9 is localized to neurite and dendritic processes
Endogenous RanBP9 has been shown to be localized in the cytoplasm, cell membrane, and nucleus of a variety of cells. Biochemical fractionation experiments have revealed that the concentration of RanBP9 in the nucleus is ∼50% of that present in the cytoplasm in non-neuronal cell lines (14). This subcellular distribution pattern of RanBP9 in multiple compartments is consistent with its implication in a large number of functions. However, the subcellular localization of endogenous RanBP9 in primary neuronal cultures is completely unknown. Therefore, we performed immunocytochemistry to determine the exact intracellular localization of RanBP9 in hippocampal neurons. RanBP9 immunoreactivity was distributed throughout the hippocampal neuron (Fig. 5B), similar to previous findings for many other non-neuronal cell types. However, one interesting observation in this study is the immunoreactivity of RanBP9 throughout neurites with a punctate appearance (Fig. 5C). The negative controls, either without the secondary or primary antibodies, resulted in no signal (Fig. 5A). A closer examination of the cell body revealed that the signal intensity in the center of the cell (probably the nucleus) is much lower than in the rest of the cell, including the membrane. We also stained adult mouse brain sections for RanBP9, which also revealed immunoreactivity throughout the neuron as well as dendritic processes (Fig. 5D, E). The fact that RanBP9 immunoreactivity is found in neurites in addition to cell bodies implies that RanBP9 may have an important function either in the formation or maintenance of neurites in the primary neurons or dendrites and axons in general in the adult brain.
Figure 5.
RanBP9 protein is localized throughout the neuron, including neurites. Primary hippocampal neurons from P1 pups were cultured for 18–20 d in vitro to allow for the formation of complex network of neurites and then stained with RanBP9 antibody. A) Negative control without the primary antibody revealed no immunoreactivity, indicating that the immunofluorescence signal observed in B is specific to RanBP9. B) Two neurons showing robust expression of RanBP9 protein throughout the neuron, though to a lesser extent at the center of the neuron. C) Complex network of neurites showing punctate staining for RanBP9. D, E) staining of neurons in an adult mouse brain cortex (D) and hippocampus (E), showing the presence of RanBP9 protein throughout the neuron, including processes.
Synaptic protein levels are decreased in RanBP9-transgenic mice
In DRG neurons, overexpression of RanBP9 blocked the formation of neurites, whereas reducing RanBP9 function increased the basal outgrowth of neurites (16). This, together with the evidence that sAPPα plays a critical role in the neurite outgrowth (26, 27) and that RanBP9 significantly decreases sAPPα levels, prompted us to ask whether RanBP9 is also critical in regulating synaptic proteins and synapses. As an indicator of alterations in presynaptic terminals, we measured the levels of synaptophysin, Gap43, and Rab3a both in the transgenic mice overexpressing flag-tagged RanBP9 and the RanBP9-null mice. Synaptophysin levels were decreased by ∼26%, which was statistically highly significant (Fig. 6A, B; P<0.001). These results are in agreement with the previous observations that overexpression of RanBP9 significantly decreased the growth and branching of neurites in both DRG neurons and cerebellar primary neurons (16). The reduced growth and branching of neurites are expected to decrease spine density and number of synapses and, therefore, reduce the amount of synaptic proteins. The most affected of the three presynaptic proteins by RanBP9 overexpression is synaptophysin, an essential molecule implicated in neurotransmitter vesicle docking, fusion, and exocytosis.
Figure 6.
RanBP9 overexpression decreases presynaptic protein at 12 mo of age. A) Brain homogenates from RanBP9-transgenic (TG) and age-matched WT controls prepared using Nonidet P-40 lysis buffer were subjected to SDS-PAGE electrophoresis and probed with antibodies against presynaptic proteins, synaptophysin, gap-43, and Rab-3A. Flag antibody detected expression of flag-tagged RanBP9 in TG mice but not in WT mice. Immunoblotting using RanBP9 specific monoclonal antibody showed expression of both flag-tagged RanBP9 and endogenous RanBP9 in TG mice, while WT mice showed expression of endogenous RanBP9 only. B) ImageJ quantification showed decreased levels of synaptophysin by 26%, gap-43 by 16.6%, and Rab-3A by 4.15%. Data are presented as means ± se (n=4/group). ***P < 0.001 vs. WT; Student's t test.
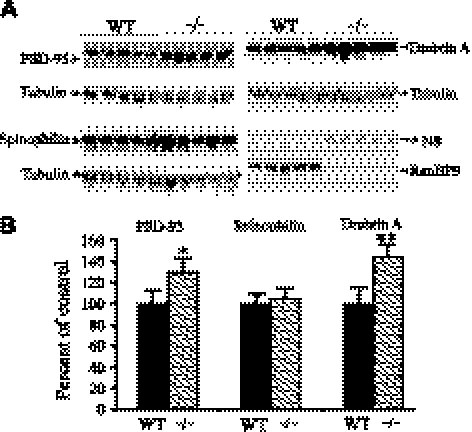
To obtain a global picture on the effect of RanBP9 on the synaptic machinery, in addition to presynaptic proteins, we also quantified the levels of postsynaptic protein markers, such as PSD-95, spinophilin, and drebrin A. Similar to presynaptic protein synaptophysin, the postsynaptic protein level was also reduced under RanBP9-overexpression conditions (Fig. 7A, B). PSD-95 is the most affected of all three proteins studied and was reduced by 19%, which was also statistically significant (P<0.01; n=4/genotype), but drebrin A and spinophilin levels were not altered (Fig. 7A, B). Flag antibody was used to detect exogenous flag-tagged RanBP9, while RanBP9-specific monoclonal antibody detected both the endogenous and exogenous RanBP9 (Figs. 6A and 7A). Overall, RanBP9 overexpression reduced the levels of both presynaptic and postsynaptic protein.
Figure 7.
RanBP9 overexpression decreases postsynaptic protein at 12 mo of age. A) Brain homogenates from RanBP9-transgenic (TG) and age-matched WT controls prepared using Nonidet P-40 lysis buffer were subjected to SDS-PAGE electrophoresis and probed with antibodies against postsynaptic proteins PSD-95, spinophilin, and drebrin A. Same blot used in Fig. 9 for presynaptic proteins is also shown here for the detection of flag-tagged RanBP9 and endogenous RanBP9. B) ImageJ quantification showed decreased levels of PSD-95 by 19%, spinophilin by 7%, and drebrin A by 10%. Data are presented as means ± se (n=4/group). **P < 0.01 vs. WT; Student's t test.
Generation of RanBP9-null mice by gene-trap strategy
Gene trapping is a method to introduce mutations into embryonic stem cells by inserting a gene-trap vector construct through electroporation. An ES clone, RHA056, having the insertion of the cassette within the second intron at the farthest 5′ end of the gene, was obtained from the Mutant Mouse Regional Resource Center (MMRRC; University of California, Davis, CA, USA). On blastocyst injection and subsequent characterization, we could successfully derive RanBP9 heterozygous mice followed by RanBP9 homozygous mice by mating male and female RanBP9 heterozygous mouse lines.
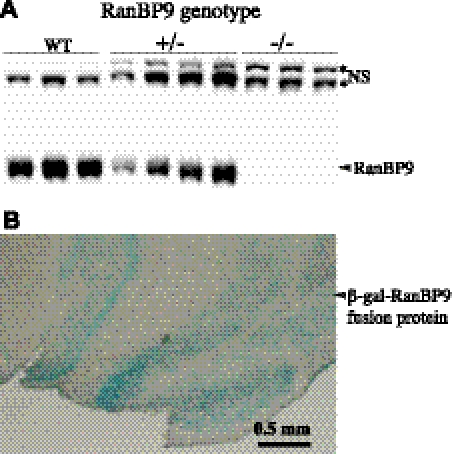
The germline transmission of the mutated RanBP9 allele was confirmed by PCR amplification of β-gal gene. β-Galactosidase activity was also confirmed on coronal brain sections of heterozygous mice by staining with X-gal. A moderate β-gal staining in the heterozygous mouse brains and robust staining in the homozygous mouse brains in a pattern identical to RanBP9 mRNA distribution (http://mouse.brain-map.org/experiment/show/69817940) was observed (Fig. 8B). Finally, a 50% reduction in the levels of RanBP9 protein in the heterozygous mouse brains and complete absence of RanBP9 protein in the homozygous mouse brains were confirmed by immunoblots using RanBP9 specific monoclonal antibody (Fig. 8A). Unfortunately, since RanBP9 homozygous mice survive only until P2, we used 1-d-old pups for the quantification of synaptic proteins. Very rarely, RanBP9 homozygous mice survive up to P10. In-depth analysis of homozygous pups is currently being carried out to identify the exact reason for the neonatal lethality.
Figure 8.
RanBP9-null mice show complete absence of RanBP9 protein. A) Nonidet P-40 lysates from WT, RanBP9 heterozygous (+/−), and RanBP9 homozygous (−/−) mice subjected to immunoblotting revealed presence of endogenous RanBP9 protein in WT, reduced levels in +/−, and complete absence of RanBP9 protein in −/− mice. *NS, nonspecific band. B) Immunohistochemical staining of cortical brain sections from RanBP9−/− mice with X-gal revealed expression of RanBP9-β-gal fusion protein.
Synaptic protein levels are increased in RanBP9-null mice
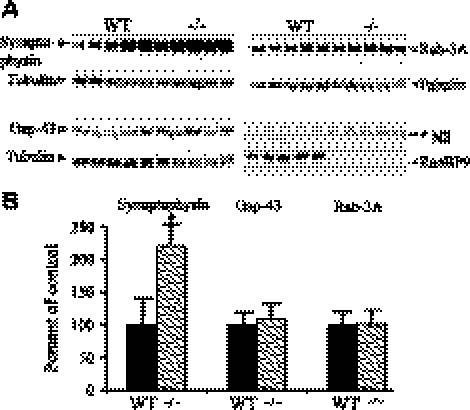
Contrary to the effect of RanBP9 in the 12-mo-old transgenic mice, which showed decreased trend in the synaptic protein levels, RanBP9-null mice showed increased levels of synaptic proteins. Because RanBP9-deficient homozygous mice do not survive beyond P2, as RanBP9 appears to be critical for postnatal growth, we used P1 mouse brains for the immunoblot quantitation of synaptic proteins. Complete absence of RanBP9 protein was evident in RanBP9−/− mice (Figs. 9A and 10A). RanBP9−/−mice showed a 122% increase in synaptophysin levels, which was statistically significant (Fig. 9A, B; P<0.05), but gap43 and Rab3a levels were not significantly altered.
Figure 9.
Neonatal RanBP9-null mice showed increased presynaptic proteins. A) Brain homogenates from RanBP9-null mice (−/−) and age-matched WT controls prepared using Nonidet P-40 lysis buffer were subjected to SDS-PAGE electrophoresis and probed with antibodies against presynaptic proteins, synaptophysin, gap-43, and Rab-3A. Monoclonal RanBP9 antibody detected endogenous RanBP9 protein only in WT mice, while RanBP9-null mice showed complete absence of RanBP9 protein. *NS, nonspecific band. B) ImageJ quantification showed that synaptophysin was increased by 122%, but gap-43 and Rab-3A levels were not altered. Data are presented as means ± se (n=5/group). *P < 0.05 vs. WT; Student's t test.
Figure 10.
Neonatal RanBP9-null mice showed increased postsynaptic proteins. A) Brain homogenates from RanBP9-null mice (−/−) and age-matched WT controls prepared using Nonidet P-40 lysis buffer were subjected to SDS-PAGE electrophoresis and probed with antibodies against postsynaptic proteins PSD-95, spinophilin, and drebrin A. Same blot used in Fig. 11 for presynaptic proteins is also shown here for the absence of endogenous RanBP9. Monoclonal RanBP9 antibody detected endogenous RanBP9 protein only in WT mice, while RanBP9-null mice showed complete absence of RanBP9 protein. *NS, nonspecific band. B) ImageJ quantification showed that PSD-95 level was increased by 30% and drebrin A by 45%, with no change in spinophilin levels. Data are presented as means ± se (n=5/group). *P < 0.05, **P < 0.01 vs. WT; Student's t test.
Interestingly, like the presynaptic protein synaptophysin, the levels of postsynaptic protein PSD-95 were increased significantly by 30% (Fig. 10A, B; P<0.05), and drebrin A levels by 45%, which was also statistically significant (Fig. 10A, B; P<0.01). However, spinophilin levels were not changed from the control levels.
Synaptic protein levels are reduced in APdE9 mice
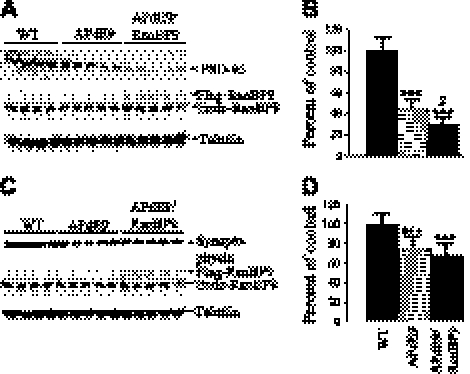
To test whether RanBP9 has any additive effect on the levels of synaptic proteins under APP/PS1-expression conditions, we quantified the levels of synaptophysin and PSD-95 in APdE9 and APdE9/RanBP9 mice compared with those of age-matched WT controls. PSD-95 levels were decreased by 55% in APdE9 mice vs. WT mice, and the levels were further reduced by 71% in APdE9/RanBP9 mice (Fig. 11A, B; P<0.001). Thus, RanBP9 overexpression reduced PSD-95 levels further, by 17% (P<0.05; APdE9 vs. APdE9/RanBP9 mice). Synaptophysin levels were also reduced in APdE9 mice by 25%, and in APdE9/RanBP9 mice by 32%, both of which were significant when compared to WT mice (Fig. 11C, D; P<0.001) but were not significant among APdE9 and APdE9/RanBP9 groups.
Figure 11.
Synaptic protein levels are altered in APP-transgenic mice. Brain homogenates from APdE9 double-transgenic mice, APdE9/RanBP9 triple-transgenic mice, and age-matched WT controls prepared using Nonidet P-40 lysis buffer were subjected to SDS-PAGE electrophoresis and probed with antibodies against postsynaptic protein, PSD-95 (A, B) and presynaptic protein, synaptophysin (C, D). Same blot was reprobed to detect flag-tagged RanBP9 and endogenous RanBP9. ImageJ quantification showed decreased levels of PSD-95 (B) by 55% in APdE9 mice compared to WT mice, and the levels were further decreased in APdE9/RanBP9 mice to ∼71%; synaptophysin levels (D) were decreased by 25% in APdE9 mice and by 32% in APdE9/RanBP9 triple-transgenic mice. Data are presented as means ± se (n=4/group for WT; n=5/group for APdE9 and APdE9/RanBP9). ***P < 0.001 vs. WT, $P < 0.05 vs. APdE9/RanBP9; ANOVA followed by post hoc Tukey's test.
DISCUSSION
In this study, data from both immunohistochemistry and biochemical experiments have provided compelling evidence that RanBP9 increases amyloidogenic processing of APP and amyloid plaques in vivo, confirming our previous results in cell cultures (19, 20). We also analyzed several markers of synaptic structure and found that overexpression of RanBP9 reduces and genetic ablation of RanBP9 increases key synaptic proteins, which suggests that RanBP9 has an essential role in the regulation of synapses. As AD is characterized not only by the presence of amyloid plaques but most importantly by massive loss of synapses, the current results on the effect of RanBP9 on both amyloid plaques and synaptic proteins strongly implicate that RanBP9 plays a crucial role in the pathogenesis of AD. Increased PSD-95 levels in APdE9/RanBP9 mice further strengthens this conclusion.
The present study revealed that RanBP9 increases Aβ40 levels by >2-fold as early as 4 mo of age, and that increase is stably maintained until 12 mo of age. Aβ42 levels, however, showed an increase of ∼50% in FA-soluble fractions only, which is much lower than the 4-fold increase previously observed in cell cultures (19). The smaller increase of Aβ levels observed in the RanBP9-Tg mice probably depends on the amount of transgene expression. Although the thy1 promoter is not as strong as other promoters, we used it mainly to restrict transgene expression in the CNS. The transgenic line 629, which was used for Aβ measurements, showed transgene expression levels lower than the endogenous levels in some brain regions. Nevertheless, these data clearly demonstrate that RanBP9 increases Aβ generation in vivo. How could RanBP9 increase amyloidogenic processing of APP and amyloid plaque burden? Consistent with our previous data in cell cultures, the present in vivo study in mice suggests that RanBP9 promotes β-secretase processing of APP at the expense of α-secretase because RanBP9 overexpression increased the levels of sAPPβ, whereas sAPPα levels were significantly reduced. We could also reproduce decreased CTF levels as early as 4 mo of age, which is also consistent with previous cell culture studies. Moreover, increased Aβ40 and Aβ42 levels were translated into increased plaque burden. It appears that RanBP9 increases the number of larger plaques much more than smaller ones. However, Aβ levels in the absence of RanBP9 could not be measured since RanBP9 homozygous mice do not survive and therefore we could not breed them with APdE9 mice.
RanBP9, through its SPRY domain, physically interacts with APP, BACE1, and LRP, and they promote interaction with each other (19). For example, RanBP9 promotes the interaction between APP and LRP, and LRP promotes the interaction between APP and RanBP9. It is important to note that all 3 proteins with which RanBP9 interacts are crucial in the pathogenesis of AD. Therefore, RanBP9 might directly influence APP trafficking and thus mediate amyloidogenic processing of APP. Since β-site cleavage preferentially occurs in the endocytic vesicles (28, 29), we believe that increased Aβ production induced by RanBP9 overexpression is because of its influence on APP endocytic trafficking. Reduced levels of sAPPα in the RanBP9-transgenic mice also reflect reduced APP levels at the plasma membrane since APP is primarily cleaved by α-secretase at the plasma membrane. In support of this notion we found increased endocytosis of APP and reduced APP levels at the plasma membrane when RanBP9 is overexpressed in CHO cells (19). Since RanBP9 binds BACE1, it is also possible that RanBP9 might directly influence BACE1 and facilitate APP cleavage. In addition, though APP is not associated with lipid rafts under normal conditions, overexpression of RanBP9 facilitates APP association into lipid rafts, where BACE1 and γ-secretases are enriched. Taken together, these data suggest that RanBP9 either directly or indirectly enhances β-site cleavage of APP and Aβ generation, which, in turn, leads to increased deposition of amyloid plaques, as observed in the present study.
Another interesting finding in this study is the influence of RanBP9 on synaptic protein levels. Loss of specific synaptic connections is now considered the likely basis for the initiation and progression of AD, because it reflects the cognitive impairment more closely than any other pathological findings (30, 31). Multiple reasons exist for suspecting that RanBP9 is a critical regulator of synapses. First, and most important, RanBP9 protein is present throughout the network of neurites in addition to cell bodies, appearing as bright punctate spots in the primary hippocampal neurons. Therefore, unless RanBP9 has an important function in the dendrites, substantial amounts of RanBP9 in the neurites are not expected. Second, RanBP9 has been shown to drastically reduce the growth and branching of neurites from DRG neurons (16) and cerebellar primary neurons (13). Third, since Rho-like GTPases are known to regulate dendritic and spine morphology (18), RanBP9, being a ligand of Rho-GEF (17), is also expected to play a critical role in the maintenance of spine and dendritic morphology. Finally, RanBP9 also binds the product of the X-linked mental retardation gene, FMRK (32) and, since dendritic and spine defects are the most prominent alterations in mental retardation besides cognitive deficits, RanBP9, as a common protein binding to both APP and FMRK, is more likely to have an essential role in these defects. In line with these predictions, we found a trend toward decreased amounts of multiple presynaptic and postsynaptic proteins, reaching statistical significance for synaptophysin and PSD-95, when RanBP9 is overexpressed. Alteration in both presynaptic and postsynaptic proteins suggests that RanBP9 might be critical in the regulation of whole synapse formation. Overall, the results showed that the absence of RanBP9 protein in the knockout mice increased the presynaptic as well as postsynaptic proteins, while overexpression of RanBP9 in the transgenic mice decreased both the presynaptic and postsynaptic proteins. In patients with AD, reduced synaptophysin levels were the major correlate of cognitive deficits (30). Conversely, we observed increased levels of 1 presynaptic and 2 postsynaptic proteins, reaching statistical significance for synaptophysin, PSD95, and drebrin A in RanBP9−/− mice. Drebrin A is an actin-binding protein substantially reduced in AD brains (33, 34). Drebrin A reduction also correlates well with the severity of cognitive impairment (35). The effects of RanBP9 on multiple presynaptic and postsynaptic proteins clearly suggest that RanBP9 is a critical regulator of the whole synaptic structure. The change in the levels of synaptic proteins directly reflects the changes in the synaptic structure. Therefore, it may be presumed that activation or overexpression of RanBP9 decreases the number of synapses and the absence of RanBP9 protein increases the number of synapses. Since synaptic protein levels are an estimate of synaptic density, we suggest that RanBp9 plays an essential role in the loss of synapses in AD. It is also important to note that since whole brain was used for immunoblot quantification of synaptic proteins, it might have diluted the net effect, and the changes in synaptic proteins in specific brain regions, such as the hippocampus, might be even more pronounced. These results further strengthen our assertion that RanBP9 plays a critical role in AD pathogenesis.
RanBP9 appears to have multiple roles in AD pathogenesis and progression. First, RanBP9-N60, a proteolytically cleaved product of RanBP9, is increased 6-fold in AD brains (20), which, in turn, can be expected to increase Aβ and plaque burden, because RanBP9-N60 increases Aβ levels more than RanBP9-FL in cell cultures (20). Increased RanBP9 also reduces sAPPα levels in vivo, as demonstrated in the present study and also previously in CHO cells (19). Reduced levels of sAPPα per se can have a profound effect on synapses. In line with these findings, note that patients with AD display significantly lower levels of sAPPα in their cerebrospinal fluid compared to age-matched normal individuals (36, 37). Thus, not only too much Aβ but also too little functional sAPPα might be of potential pathogenic significance. This finding is especially true in light of existing knowledge that exogenous administration of sAPPα into both rat and mouse brain increases synaptophysin immunoreactivity and memory retention (38). Also, in vivo evidence indicates that increasing α-secretase-mediated APP cleavage in a mouse model generated by crossing transgenic mice overexpressing ADAM-10 (α-secretase) with transgenic mice expressing the APPV717I mutation led to alleviation of LTP and cognitive deficits because of reductions in the secretion of Aβ and also most likely because of increased sAPPα (39). This abundant evidence suggests a critical role for sAPPα in synaptogenesis, and the fact that RanBP9 significantly decreases sAPPα levels is yet another compelling bit of evidence to suggest that RanBP9 has an important role in the synaptic damage in AD. Second, RanBP9 can directly reduce number of functional synapses through its effect on multiple proteins, including L1 receptor, integrin receptor, and Rho-GTPases. Reduced synaptic protein levels observed in the present study support such a conclusion. Finally, genetic association studies revealed that like LRP and two of its ligands, apoE and α2-macroglobulin, RanBP9 is also genetically associated with late-onset AD in Caucasian as well as Chinese populations (unpublished results). These multiple pieces of evidences point to a critical role for RanBP9 in the pathogenesis of AD.
In summary, our data have revealed for the first time that RanBP9 plays a critical role in vivo in the amyloidogenic processing of APP, plaque deposition as well as loss of synaptic proteins and synapses. Though the precise mechanism by which RanBP9 increases Aβ is not clear, RanBP9 appears to promote β-secretase processing of APP by targeting APP to secretase-rich lipid raft microdomains. So far, only RanBP9, LRP, and ApoER2 have been shown to target APP to lipid rafts. This gives us an enormous opportunity for a novel mechanism-based therapeutic approach for AD.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institute of Aging/National Institutes of Health grants to M.L.K. (1R03AG032064-01 and 1R01AG036859-01) and D.E.K. (1R01AG033055-01A1 and 1K02AG031920-10A1) and by a National Research Foundation -World Class University -Neurocytomics (Korea) grant to D.E.K.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- Aβ
- amyloid β
- AD
- Alzheimer's disease
- APP
- amyloid precursor protein
- APPswe
- amyloid precursor protein with Swedish mutation
- APPwt
- wild-type amyloid precursor protein
- CHAPSO
- 3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonate
- CTF
- C-terminal fragment
- DRG
- dorsal root ganglion
- FA
- formic acid
- LRP
- low-density lipoprotein receptor-related protein
- LRP-C37
- last 37 amino acids of low-density lipoprotein receptor-related protein
- NGS
- normal goat serum
- PFA
- paraformaldehyde
- RanBP9
- Ran-binding protein 9
- sAPP
- secreted derivative of amyloid precursor protein
- X-gal
- 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside.
REFERENCES
- 1. Alzheimer's Association (2010) 2010 Alzheimer's disease facts and figures. Alzheimers Dement. 6, 158–194 [DOI] [PubMed] [Google Scholar]
- 2. Cole S. L., Vassar R. (2008) The role of amyloid precursor protein processing by BACE1, the beta secretase in Alzheimer's disease pathogenesis. J. Biol. Chem. 283, 29621–29625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Strooper B., Annaert W. (2000) Proteolytic processing and cell biological functions of the amyloid precursor protein. J. Cell Sci. 113, 1857–1870 [DOI] [PubMed] [Google Scholar]
- 4. Cummings J. (2010) What can be inferred from the interruption of the semagacestat trial for treatment of Alzheimer's disease. Biol. Psych. 68, 876–878 [DOI] [PubMed] [Google Scholar]
- 5. Extance A. (2010) Alzheimer's failure raises questions about disease modifying strategies. Nat. Rev. Drug Discov. 9, 749–751 [DOI] [PubMed] [Google Scholar]
- 6. Haapasalo A., Kovacs D. M. (2011) The many substrates of presenilin/γ-secretase. J. Alzheimer's Dis. 25, 3–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kang D. E., Pietrzik C. U., Baum I., Chevallier N., Merriem D. E., Kounnas M. Z., Wagner S. L., Troncoso J.C., Kawas C. H., Katzman R., Koo E. H. (2000) Modulation of amyloid beta protein clearance and Alzheimer's disease susceptibility by the LDL receptor-related protein pathway. J. Clin. Invest. 106, 1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blacker D., Wilcox M. A., Laird N. M., Rodes L., Horwath S. M., Go R. C., Perry R., Watson B., Jr., Bassette S. S., McInnis M. G. (1998) Alpha-2 macroglobulin is genetically associated with Alzheimer's disease. Nat. Genet. 19, 1904–1911 [DOI] [PubMed] [Google Scholar]
- 9. Schmechel D. E., Saunders A. M., Strittmatter W. J., Crain B. J., Hulette C. M., Joo S. H., Pericak- Vance M. A., Goldgaber D., Roses A. D. (1993) Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 90, 9649–9653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lakshmana M. K., Chen E., Yoon I. S., Kang D. E. (2008) C-terminal 37 residues of LRP promotes the amyloidogenic processing of APP independent of Fe65. J. Cell. Mol. Med. 12, 2665–2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murrin L. C., Talbot J. N. (2007) RanBPM, a scaffolding protein in the immune and nervous systems. J. Neuroimmune Pharmacol. 2, 290–295 [DOI] [PubMed] [Google Scholar]
- 12. Denti S., Sirri A., Cheli A., Rogge L., Innamorati G., Putignano S., Fabbri M., Pardi R., Bianchi E. (2004) RanBPM is a phosphoprotein that associates with the plasma membrane and interacts with the integrin LFA-1. J. Biol. Chem. 279, 13027–13034 [DOI] [PubMed] [Google Scholar]
- 13. Cheng L., Lemmon S., Lemmon V. (2005) RanBPM is an L1-interacting protein that regulates L1- mediated mitogen-activated protein kinase. J. Neurochem. 94, 1102–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valiyaveettil M., Bentley A. A., Gursahaney P., Hussien R., Chakravarti R., Kureishy N., Prag S., Adams J. C. (2008) Novel role of the muskelin-RanBP9 complex as a nucleocytoplasmic mediator of cell morphology regulation. J. Cell Biol. 182, 727–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dansereau D. A., Lasko P. (2008) RanBPM regulates cell shape, arrangement, and capacity of the female germline stem cell niche in Drosophila melanogaster. J. Cell Biol. 182, 963–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Togashi H., Schmidt E. F., Strittmatter S. M. (2006) RanBPM contributes to Semaphorin3A signaling through plexin-A receptor. J. Neurosci. 26, 4961–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bowman A. L., Catino D. H., Strong J. C., Randall W. R., Kontrogianni-Konstantopoulos A., Bloch R. J. (2008) The rhoguanine nucleotide exchange factor domain of obscurin regulates assembly of titin at the Z-disk through interactions with Ran binding protein 9. Mol. Biol. Cell 19, 3782–3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakayama A. Y., Harms M. B., Luo L. (2000) Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J. Neurosci. 20, 5329–5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lakshmana M. K., Yoon I. S., Chen E., Park S. A., Bianchi E., Koo E. H., Kang D. E. (2009) Novel role of RanBP9 in BACE1 processing of APP and amyloid beta peptide generation. J. Biol. Chem. 284, 1863–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lakshmana M. K., Chung J. Y., Wickramarachchi S., Tak E., Bianchi E., Koo E. H., Kang D. E. (2009) A fragment of the scaffolding protein RanBP9 is increased in Alzheimer's disease brains and strongly potentiates amyloid beta peptide generation. FASEB J. 24, 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoon I. S., Pietrzik C. U., Kang D. E., Koo E. H. (2005) Sequences from the low density lipoprotein receptor-related protein (LRP) cytoplasmic domain enhance amyloid beta protein production via the beta-secretase pathway without altering amyloid precursor protein/LRP nuclear signaling. J. Biol. Chem. 280, 20140–20147 [DOI] [PubMed] [Google Scholar]
- 22. Yoon I. S., Chen E., Busse T., Repetto E., Lakshmana M. K., Koo E. H., Kang D. E. (2007) Low-density lipoprotein receptor-related protein promotes amyloid precursor protein trafficking to lipid rafts in the endocytic pathway. FASEB J. 21, 2742–2752 [DOI] [PubMed] [Google Scholar]
- 23. Denti S., Sirri A., Cheli A., Rogge L., Innamorati G., Putignano S., Fabbri M., Pardi R., Bianchi E. (2004) J. Biol. Chem. 279, 13027–13034 [DOI] [PubMed] [Google Scholar]
- 24. Jankowsky J. L., Fadale D. J., Anderson J., Xu G. M., Gonzales V., Jenkins N. A., Copeland N. G., Lee M. K., Younkin L. H., Wagner S. L., Younkin S. G., Borchelt D. R. (2004) Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ secretase. Hum. Mol. Genet. 13, 159–170 [DOI] [PubMed] [Google Scholar]
- 25. Christensen M. A., Zhou W., Qing H., Lehman A., Philipsen S., Song W. (2004) Transcriptional regulation of BACE1, the beta-amyloid precursor protein beta secretase by sp1. Mol. Cell. Biol. 24, 865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gakhar-Koppole N., Hundeshagen P., Mandl C., Weyer S. W., Allinquant B., Müller U., Ciccolini F. (2008) Activity requires soluble amyloid precursor protein alpha to promote neurite outgrowth in neural stem cell derived neurons via activation of the MAPK pathway. Eur. J. Neurosci. 28, 871–882 [DOI] [PubMed] [Google Scholar]
- 27. Quast T., Wehner S., Kirfel G., Jaeger K., De Luca M., Herzog V. (2003) sAPP as a regulator of dendrite motility and melanin release in epidermal melanocytes and melanoma cells. FASEB J. 17, 1739–1741 [DOI] [PubMed] [Google Scholar]
- 28. Koo E. H., Squazzo S. L. (1994) Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J. Biol. Chem. 269, 17386–17389 [PubMed] [Google Scholar]
- 29. Refolo L. M., Sambamurti K., Efthimiopoulos S., Pappolla M. A., Robakis N. K. (1995) Evidence that secretase cleavage of cell surface Alzheimer amyloid precursor occurs after normal endocytic internalization. J. Neurosci. Res. 40, 694–706 [DOI] [PubMed] [Google Scholar]
- 30. Terry R. D., Masliah E., Salmon D. P., Butters N., De Teresa R., Hill R., Hansen L. A., Katzman R. (1991) Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 [DOI] [PubMed] [Google Scholar]
- 31. Selkoe D. J. (2002) Alzheimer's disease is a synaptic failure. Science 298, 789–791 [DOI] [PubMed] [Google Scholar]
- 32. Menon R. P., Gibson T. J., Pastore A. (2004) The C terminus of fragile X mental retardation protein interacts with the multi-domain Ran-binding protein in the microtubule-organising centre. J. Mol. Biol. 343, 43–53 [DOI] [PubMed] [Google Scholar]
- 33. Harigaya Y., Shoji M., Shirao T., Hirai S. (1996) Disappearance of actin-binding protein, drebrin, from hippocampal synapses in Alzheimer's disease. J. Neurosc. Res. 43, 87–92 [DOI] [PubMed] [Google Scholar]
- 34. Hatanpaa K., Isaacs K. R., Shirao T., Brady D. R., Rapoport S. L. (1999) Loss of proteins regulating synaptic plasticity in normal aging of the human brain and in Alzheimer's disease. J. Neuropathol. Exp. Neurol. 58, 637–643 [DOI] [PubMed] [Google Scholar]
- 35. Counts S. E., Nadeem M., Lad S. P., Wuu J., Mufson E. J. (2006) Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J. Neuropathol. Exp. Neurol. 65, 592–601 [DOI] [PubMed] [Google Scholar]
- 36. Sennvik K., Fastbom J., Blomberg M., Wahlund L. O., Winblad B., Benedikz E. (1995) Levels of alpha- and beta-secretase cleaved amyloid precursor protein in the cerebrospinal fluid of Alzheimer's disease patients. Neurosci. Lett. 278, 169–172 [DOI] [PubMed] [Google Scholar]
- 37. Lannfelt L., Basun H., Wagner S. L. (1995) Decreased alpha-secretase-cleaved amyloid precursor protein as a diagnostic marker for Alzheimer's disease. Nat. Med. 1, 829–832 [DOI] [PubMed] [Google Scholar]
- 38. Roch J. M., Masliah E., Roch-Levecq A. C., Sundsmo M. P., Otero D. A., Veinbergs I., Saitoh T. (1994) Increase of synaptic density and memory retention by a peptide representing the trophic domain of the amyloid beta/A4 protein precursor. Proc. Natl. Acad. Sci. U. S. A. 91, 7450–7454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Postina R., Schroeder A., Dewachter I., Bohl J., Schmitt U., Kojro E., Prinzen C., Endres K., Hiemke C., Blessing M., Flamez P., Dequenne A., Godaux E., van Leuven F., Fahrenholz F. A. (2004) Disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J. Clin. Invest. 113, 1456–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Franklin K. B. J., Paxinos G. (1997) The Mouse Brain in Stereotaxic Coordinates, Academic Press, San Diego, CA, USA [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.