Abstract
We have previously found that in failing human hearts, Rho-associated coiled-coil protein kinase 1 (ROCK1) is processed by caspase-3 into an active isoform, ROCKΔ1. The purpose of the current investigation was to elucidate the pathological consequences of truncated ROCK1 accumulation in the heart, the associated molecular mechanism of ROCKΔ1-mediated cardiac phenotype, and the molecular signaling between Rho kinase activation in cardiomyocytes and extracellular matrix response. We generated transgenic mice expressing ROCKΔ1 in cardiomyocytes to mimic the situation observed in human heart disease, whereas an additional kinase-deficient mouse was generated as a control. The ROCKΔ1 transgenic mice developed fibrotic cardiomyopathy with diastolic dysfunction. Transgenic hearts displayed activated TGFβ1 and NF-κB signaling and a release of a subset of cytokines and were susceptible to angiotensin II stress. Treatment with a Rho kinase inhibitor attenuated the fibrotic phenotype. Cardiac fibroblasts differentiated into myofibroblasts when cocultured with transgenic cardiomyocytes but not with wild-type cardiomyocytes. Inhibitors of Rho kinase as well as TGFβR1 and NF-κB decreased these effects. The serum response factor-dependent TGFβ1 regulation was shown to be responsible for the Rho kinase-mediated activation of TGFβ1 signaling. We conclude that ROCKΔ1 is a novel fibrotic factor. Activation of TGFβ1 and NF-κB signaling contributes to the Rho kinase-mediated pathological fibrosis.—Yang, X., Li, Q., Lin, X., Ma, Y., Yue, X., Tao, Z., Wang, F., Mckeehan, W. L., Wei, L., Schwartz, R. J., Chang, J. Mechanism of fibrotic cardiomyopathy in mice expressing truncated Rho-associated coiled-coil protein kinase 1.
Keywords: myocardial fibrosis, transgenic mice, serum response factor, TGFβ1
Cardiac fibrosis is a major maladaptive response to hemodynamic stress and various fibrotic stimuli. It leads to ventricular wall stiffness through excessive deposition of extracellular matrix proteins associated with many pathological conditions. Rho-associated coiled-coil protein kinase 1 (ROCK1) has been suggested to be involved in this process (1–3).
The Rho kinase family has 2 members: ROCK1 (p160ROCK) and ROCK2 (ROKα). Downstream mediators of Rho kinase best characterized in vivo are myosin light chain phosphatase, myosin light chain (MLC), and LIM kinase. ROCK1 protein consists of an N-terminal kinase domain, followed by an extended coiled-coil domain that includes a RhoA binding site and an inhibitory C-terminal pleckstrin homology (PH)/cysteine-rich domain. Under physiological conditions, RhoA binding leads to a change in kinase conformation and dissociation of the inhibitory C-terminal domain from the N-terminal kinase domain, which results in the Rho kinase activation.
Under pathological conditions, such as ischemia/reperfusion, hypertrophy, and myocardial infarction, a constitutively active Rho kinase is generated by proteolytic cleavage of the inhibitory C terminus by caspase-3 (4–6). We were the first to demonstrate proteolytic ROCK1 cleavage and accumulation of constitutively active cleaved ROCK1 isoform, ROCKΔ1, in failing human hearts (7). Although this clinical observation provided the evidence of Rho kinase activation in patients with heart failure, the pathological significance of ROCKΔ1 and the associated molecular mechanism of cardiac remodeling remained unknown.
The role of Rho kinase in cardiac fibrosis was primarily determined in the loss-of-function studies, including our own ROCK1-knockout study. We demonstrated that genetic deletion of ROCK1 attenuated aortic banding-induced fibrotic cardiomyopathy (1). The same conclusion was independently reported for the ROCK1 haploinsufficient mice by Rikitake et al. (2). Using the same ROCK1-null mouse model, Haudek et al. (3) found that ROCK1 facilitated differentiation of fibroblast precursor cells, thus contributing to nonadaptive cardiac fibrosis. On the other hand, application of Rho kinase inhibitors, Fasudil, and statins rescued the fibrotic phenotype in various animal models (8, 9). Although all of these studies implicated the profibrotic effect of ROCK1, there was no direct evidence that an increase in the Rho kinase activity in cardiomyocytes (CMs) per se was sufficient to initiate the fibrotic response and lead to fibrotic cardiomyopathy.
To further elucidate the underlying molecular signaling from cardiomyocytes that activate cardiac fibroblasts (CFs) and initiate the fibrotic process, in this study we generated transgenic mice to express ROCKΔ1 in the heart to recapitulate the situation observed in human heart disease. Mutant mice displayed extensive cardiac fibrosis. We identified TGFβ1 as a new serum response factor (SRF)-regulated gene. ROCKΔ1 promoted cardiac fibrosis by modulating SRF activity, which resulted in up-regulation of TGFβ1. At the same time, ROCKΔ1 activated NF-κB signaling, which led to a release of a subset of cytokines. The activation of both TGFβ1 and NF-κB signaling in cardiomyocytes promoted myofibroblast differentiation and contributed to the Rho kinase-mediated fibrotic cardiomyopathy. These results provide the in vivo and in vitro evidence that truncated ROCK1 is a unique and potent fibrotic factor.
MATERIALS AND METHODS
Cell isolation, culture, plasmid constructs, and gene transient transfection
Neonatal rat and mouse CMs and CFs were isolated as described previously (10). Ventricles from 2-d-old rats and mice were extracted, followed by digestion with collagenase (75 U/ml; Worthington, Lakewood, NJ, USA), pancreatin (0.6 mg/ml; Sigma-Aldrich, St. Louis, MO, USA), and Liberase blendzyme 4 (05401135001; Roche Diagnostics, Indianapolis, IN, USA). Cells were then plated and incubated for 1 h, and nonadherent CMs were collected using a gentle wash, while rapidly attaching CFs were left in the culture dish. The CMs in the collected wash medium were spun down and resuspended in DF-10 culture medium (DMEM/F12, adjusted to 17 mM sodium bicarbonate, 2 mM l-glutamine, and 10% FBS). Mouse myoblast C2C12 cells were cultured in DMEM with 10% FBS. In the experiments involving cell treatment with chemical inhibitors Y27632, SB431542, and caffeic acid phenylethyl ester (CAPE), the inhibitors were directly added to the culture medium at concentrations of 25, 50, and 20 μM, respectively.
For expression of human ROCKΔ1 with the C-terminal GFP tag, its cDNA was subcloned into pcDNA3.1(−)/Myc-His B (Life Technologies, Carlsbad, CA, USA). To generate the mutant kinase-deficient ROCKΔ1 (ROCKΔ1-KD), lysine-105 in the kinase domain was mutated to alanine using the QuickChange site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA, USA). SRF and N-terminal SRF (SRF-N) expression vectors were generated in our previous study (11). All of the gene transient transfection experiments were conducted by the Neon Transfection System (MPK5000; Life Technologies).
Generation of transgenic ROCKΔ1 and ROCKΔ1-KD mouse lines and conditional SRF-null mice
The ROCKΔ1 and ROCKΔ1-KD cDNA were subcloned into the α-myosin heavy chain (α-MHC) promoter expression vector. The DNA fragment including α-MHC-V5-His-ROCKΔ1-SV40p(A) or α-MHC-V5-His-ROCKΔ1-KD-SV40p(A) was cut out by PacI and EcoRV for pronuclear microinjection with FVB background. DNAs isolated from mouse tails were used for genotyping purposes.
Constitutive SRF knockout resulted in early embryonic lethality due to several defects in mesoderm formation (12). To avoid the lethality and evaluate TGFβ1 expression level under SRF-null conditions, SRFloxP/loxP mice (a generous gift from Alfred Nordheim, University of Tuebingin, Tuebingen, Germany) were bred with αMHC-MerCreMer mice (Jackson Laboratory, Bar Harbor, ME, USA) to generate SRFloxP/loxP/Mer−CreMer mice. SRF deletion in CMs was achieved by application of tamoxifen (20 μg/g body weight; intraperitoneal injection for 4 d; ref. 13). All animal experiments were conducted in 8- to 12-wk-old male mice with age-matched littermates as controls. All experiments with animals were approved by the Institutional Animal Care and Use Committee of the Texas A&M Health Science Center–Houston.
Cardiac function assessment by echocardiography and Doppler
For cardiovascular measurements, a Vevo770 High-Resolution Micro-Imaging System (VisualSonics, Toronto, ON, Canada) with 30-MHz probe (RMV-707B) was used. Male mice, 8–12 wk of age, were anesthetized with 3–4% isoflurane, then switched to 1–1.5% isoflurane mixed with 100% oxygen. At the level of the papillary muscles, 2-dimensional guided M-mode echocardiography was obtained from anterior (+septum) and posterior walls. Peak E or peak of the fused transmitral diastolic flow was evaluated as a reference to calculate isovolume relation time (IVRT) for diastolic function.
Angiotensin II (ANG II) pump implantation and Fasudil treatment
ALZET osmotic pumps (Model 1003D; Alzet, Cupertino, CA, USA) filled with ANG II (3 mg/kg/d) were implanted into tunneled layer under the skin of 10- to 12-wk-old male mice for 2 wk. For the rescue experiment, mice were allowed access to drinking water containing Fasudil (∼100 mg/kg/d; LC Laboratories, Woburn, MA, USA) for 4 wk starting at the time of ANG II pump implantation.
Sirius Red staining, ELISA, immunostaining, and immunoblotting
To detect collagen deposition, paraffin sections of the hearts were stained by picrosirius red (26357-02; Electron Microscopy Sciences, Hatfield, PA, USA). Pictures were taken under ×20 microscope objective from 5 fields, 1 from the top of an interventricular septum and 4 from a left ventricle wall. A total of 20 staining pictures from 4 mouse hearts in each group were quantified for collagen deposition by SigmaScan Pro Image Analysis 5.0.0 software (SPSS, Chicago, IL, USA).
TGFβ1 and IL-1β concentrations in mouse serum were assessed by the mouse TGF-β 1 DuoSet (DY1679) and mouse IL-1β/IL-1F2 Quantikine (MLB00B) ELISA kits (R&D Systems, Minneapolis, MN, USA). The optical density was analyzed by a microplate reader at the wavelength of 450 nm.
For immunostaining, cells on glass coverslips were briefly fixed by 4% paraformaldehyde and then permeabilized by PBS containing 0.1% Triton X-100. The primary anti-smooth muscle actin (SMA; A2547; Sigma-Aldrich) and anti-F4/80 (ab6640; Abcam, Cambridge, MA, USA) antibodies and the secondary goat anti-mouse IgG antibody coupled with Alexa Fluor 488 and 594 (Life Technologies) were used. Nuclei were visualized by DAPI staining, and the images were acquired by fluorescence microscopy.
Protein samples for Western blot analysis were extracted and separated as described previously (10). Commercially available antibodies were from the following sources: collagen I (sc-8784), connective tissue growth factor (sc-14940), collagen III (sc-8781), ROCK1 (C terminus: sc-5560), MLC (sc-12896), and SRF (sc-335) from Santa Cruz Biotechnology (Santa Cruz, CA, USA); ROCK2 (610623) from BD Biosciences (Sparks, MD, USA); ROCK1 (N terminus, A300-457A) from Bethyl Laboratory (Montgomery, TX, USA); and Smad2/3 (9523), myosin phosphatase target subunit 1 (MYPT1; 5143), and p65 (436700) from Cell Signaling Technology (Beverly, MA USA). Equivalent protein loading was verified by intensity of the GAPDH blot.
Quantitative PCR (qPCR) analysis
Transcripts were quantified by qPCR analysis (StepOnePlus; Life Technologies) using the SYBR green method with a MasterMix buffer system containing Taq polymerase, as described previously (10). Total RNAs was prepared by Trizol extraction. The forward and reverse PCR primers were as follows (5′ to 3′): for ROCK1, ATGCCATGTTAAGTGCCACA and TCTTGTTGACAGCGTTCGAG; for CTGF, ACCCGAGTTACCAATGACAATACC and CCGCAGAACTTAGCCCTGTATG; for collagen III, TGAATGGTGGTTTTCAGTTCAG and TATTCAAAGACTGTCTTGCTCC; for collagen I, TGTTCAGCTTTGTGGACCTC and GCCATTGTGGCAGATACAGA; for atrial natriuretic peptide (ANP), CCCCGACCCACGCCAGCAT and TGCGGCCCCTGCTTCCTCA; for brain natriuretic peptide, GCGGCATGGATCTCCTGAAGGTG and AGCCCAAACGACTGACGGATCC; for TGFβ1, GACTCTCCACCTGCAAGACC and ACTGCTTCCCGAATGTCTGA; for SRF, ATCATGAAGAAGGCCTATGA and CTTCAGTGTGTCCTTGGTTT; for IL-1β, TGTGAAATGCCACCTTTTGA and TGTCCTCATCCTGGAAGGTC; for TNF-α, CCACCACGCTCTTCTGTCTA and GGTTGTCTTTGAGATCCATGC; for NF-κB(p65), AGGTGTATTTCACGGGACCA and CATAGGTCCTTTTGCGCTTC; for α-SMA, GCCCCCCTGAACCCCAAAGCTA and CGGCCAGGTCCAGACGCATGAT; and for GAPDH, GGTGAAGGTCGGTGTGAACGGATTT and GCAGAAGGGGCGGAGATGATGA. GAPDH expression levels were used for qPCR normalization. Expression levels were determined by the 2−ΔΔCt threshold cycle method.
Electrophoretic mobility shift assay (EMSA)
The 32P-labeled 19- to 20-bp DNA duplexes containing the individual serum response elements (SREs) from the 5-kb region upstream of the TGFβ1 gene were identified (Genomatix, Munich, Germany) and used as probes. The positive SRE probe was derived from the mouse c-fos promoter containing one SRE, which was also used to assess the endogenous SRF binding affinity in the animal hearts and cell culture studies. The mutant SRE probe was used to validate the specificity of binding. The probes were as follows (5′ to 3′): for a positive SRE probe, CAGGATGTCCATATTAGGACATCTGC and GCAGATGTCCTAATATGGACATCCTG; for SRE1, ACACCCCAGATAGGGGACAT and ATGTCCCCTATCTGGGGTGT; for SRE2, TGTTTCCAAATGTGGCCAGC and GCTGGCCACATTTGGAAACA; for SRE3, CATGGCCTTATAGGGCAGAG and CTCTGCCCTATAAGGCCATG; for SRE4, CATCTCCAGGTGTGGTCCC and GGGACCACACCTGGAGATG; for SRE5, ACGCCCCTATTCCGGACCA and TGGTCCGGAATAGGGGCGT; and for the mutant SRE3, CATGGAATTATAGAACAGAG and CTCTGTTCTATAATTCCATG. Experiments examining the supershift by the anti-SRF antibody and competition with unlabeled probe were conducted to verify the SRF-DNA interaction. Nuclear extracts were used for each experiment.
Luciferase assay
The 5-kb region upstream of the TGFβ1 gene containing the SRE3 site was subcloned into a pGL3-basic vector expressing the luciferase reporter gene (Promega, Madison, WI, USA). A similar vector with the SRE3 mutant was constructed as a mutant reporter. Generation of the SRF expression vector and transient transfection were described previously (11). The luciferase assay was conducted 36 h post-transfection, and 20 out of 60 μl of the lysis supernatant was used with a Monolight 3010 luminometer (BD Biosciences) for the measurement of luciferase activity. Each sample was measured 3 times. All results were normalized to β-galactosidase activity (MRX Revelation; Dynex Technologies, Chantilly, VA, USA).
Chromatin immunoprecipitation (ChIP) assay
The SRF expression vector was transfected into C2C12 cells. After 36 h, cells were gently fixed with formaldehyde and lysed by sonication. The specific SRF-DNA complex was immunoprecipitated using an anti-SRF antibody. The identity of the DNA fragment isolated in the complex with SRF was determined by PCR, and the final PCR product was then sequenced to confirm the presence of the SRE motif (13).
Assessment of G-actin/F-actin ratio
Neonatal rat CMs were lysed in the lysis buffer (BK037; Cytoskeleton, Denver, CO, USA), and the cellular debris was removed by a brief spin. The supernatant was then centrifuged at 100,000 g for 1 h to separate F-actin from soluble G-actin. The insoluble F-actin (in a pellet) and the soluble G-actin (in a supernatant) were quantified by Western blot with an actin antibody.
Statistical analysis
Data are expressed as means ± se. In multiple group comparisons, 1-way ANOVA followed by Student-Newman-Keuls method was used. In 2- group comparisons, nonpaired t test was used. All of the analyses were conducted by SigmaPlot 11.0 (Systat, San Jose, CA, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
Mice expressing truncated Rho kinase ROCKΔ1 develop fibrotic cardiomyopathy with impaired diastolic function
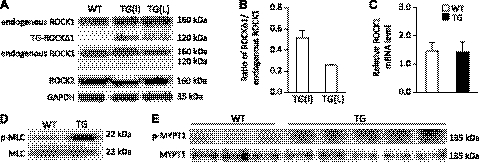
To investigate the role of truncated ROCKΔ1 in failing human hearts in vivo, we generated 2 transgenic mouse lines expressing low and intermediate levels of ROCKΔ1 protein specifically in the hearts. This was achieved by placing the ROCKΔ1 gene under the control of the α-MHC promoter. Verification by antibodies to the N and C termini of ROCK1 confirmed the expression of transgene ROCKΔ1 and the absence of changes in the endogenous ROCK1 and ROCK2 protein expression levels in the transgenic heart (Fig. 1A). The relative expression levels of ROCKΔ1 protein over the endogenous ROCK1 are presented in Fig. 1B. The unchanged level of endogenous ROCK1 transcript was also confirmed by qPCR analysis (Fig. 1C). Noticeable increases in phosphorylation levels of 2 direct targets of ROCK1, MLC, and MYPT1 indicated an increase in the Rho kinase activity in the transgenic heart (Fig. 1D, E).
Figure 1.
Generation of transgenic ROCKΔ1 mice along with elevated Rho kinase activity. A) Two transgenic mouse lines expressing intermediate (I) and low (L) levels of ROCKΔ1 protein in the heart were generated and assessed by Western blot with the antibody specific against the N (top panel) and C termini of ROCK1 (middle panel), respectively. Transgene ROCKΔ1 was detected only by anti-ROCK1 N-terminal and not by anti-ROCK1 C-terminal antibody, confirming the truncated ROCKΔ1 expression (without C-terminal domain). Endogenous full-length ROCK1 protein can be detected by both antibodies. B) Relative expression levels of ROCKΔ1 protein over the endogenous ROCK1. No effect on endogenous ROCK1 and ROCK2 protein expression was observed in the transgenic (TG) heart. C) Endogenous ROCK1 mRNA level (low-expression mouse line) was also assessed by qPCR, and the data were pooled from 10 mice/group with analyses for each mouse in triplicate. D, E) Hyperphosphorylation of 2 Rho kinase direct substrates, MLC (D) and MYPT1 (E), was detected in the transgenic heart of the low-expression mouse line. WT, wild type.
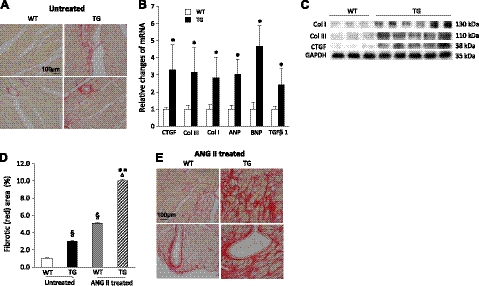
Mice were viable, and the hearts from both lines developed obvious fibrotic cardiomyopathy by the age of 12 wk, when they were dissected. Since we did not observe significant differences in phosphorylation levels of MLC and MYPT1 or in the severity of cardiac fibrosis between the mouse lines, the low-ROCKΔ1-expression mouse line was used for the remainder of this study. The transgenic mice showed no significant differences in heart weight indexes (Supplemental Table S1) compared with the control wild-type mice. However, marked increases in collagen deposition were observed in the transgenic heart in the interstitial and perivascular regions compared with the control (Fig. 2A). Along with the fibrotic histology phenotype, expression of fibrotic and stress factors were assessed in the animal hearts. A >2-fold increase in CTGF, collagen I, collagen III, ANP, BNP, and TGFβ1 transcripts were detected by qPCR in the transgenic heart compared with the control (Fig. 2B). The increases in collagen I, collagen III, and CTGF protein expression levels were also observed in the transgenic myocardium (Fig. 2C). The fibrotic area (collagen deposition) as a fraction of the total analyzed area was quantified in Fig. 2D (untreated group), which shows a near 2-fold increase in the transgenic heart compared with the control wild-type heart.
Figure 2.
ROCKΔ1 transgenic mice developed interstitial and perivascular fibrotic cardiomyopathy. A) Heart sections were stained with Sirius red. Transgenic (TG) heart showed noticeable collagen deposition (red) in interstitial and perivascular regions compared with the wild-type (WT) heart under physiological (untreated) conditions. B, C) Significant increases in fibrotic and stress factors were detected in the transgenic hearts by qPCR (B) and immunoblot analyses (C). qPCR data were pooled from 10 mice/group with analyses for each mouse in triplicate. D) Quantification of fibrotic areas. E) Even more severe fibrotic ardiomyopathy was observed with Sirius red staining in the mutant heart after ANG II treatment compared with the WT heart. col, collagen. *P < 0.01 vs. WT; **P < 0.01 vs. ANG II-treated WT; §P < 0.01 vs. untreated WT; ΔP < 0.01 vs. untreated TG.
Cardiac function was assessed by echocardiography and Doppler analyses (Table 1; untreated group). The transgenic heart showed a significant increase in IVRT (23.66 ms) compared with the control (19.41 ms). No differences in parameters for systolic function and cardiac dimensions such as ejection fraction, fractional shortening, stroke volume, left ventricle inner dimension, and left ventricle posterior wall thickness were observed. The prolonged IVRT suggested a compromised heart compliance and diastolic dysfunction.
Table 1.
Impaired diastolic function in the transgenic heart elevated by echocardiography and attenuated by Fasudil treatment
| Parameter | Untreated |
Ang II treated |
Fasudil treated, TG | ||
|---|---|---|---|---|---|
| WT | TG | WT | TG | ||
| n | 9 | 9 | 6 | 6 | 5 |
| Body weight (g) | 29.39 ± 0.39 | 30.96 ± 0.47 | 27.13 ± 0.81 | 26.32 ± 1.99 | 29.68 ± 0.66 |
| HR (beats/min) | 390 ± 5 | 388 ± 6 | 401 ± 2 | 402 ± 2 | 398 ± 3 |
| IVS;d (mm) | 0.99 ± 0.05 | 0.94 ± 0.04 | 1.01 ± 0.03 | 1.13 ± 0.05 | 0.96 ± 0.05 |
| LVID;d (mm) | 3.26 ± 0.12 | 3.35 ± 0.09 | 3.18 ± 0.10 | 3.03 ± 0.14 | 3.16 ± 0.13 |
| LVPW;d (mm) | 1.06 ± 0.07 | 1.12 ± 0.05 | 1.11 ± 0.04 | 1.29 ± 0.12 | 1.00 ± 0.04 |
| IVS;s (mm) | 1.40 ± 0.07 | 1.35 ± 0.04 | 1.32 ± 0.03 | 1.46 ± 0.08 | 1.44 ± 0.04 |
| LVID;s (mm) | 2.01 ± 0.16 | 2.03 ± 0.12 | 2.16 ± 0.08 | 2.13 ± 0.14 | 1.79 ± 0.12 |
| LVPW;s (mm) | 1.48 ± 0.06 | 1.56 ± 0.05 | 1.43 ± 0.05 | 1.56 ± 0.07 | 1.47 ± 0.08 |
| Stroke volume (μl) | 35 ± 3 | 34 ± 2 | 29 ± 3 | 24 ± 2** | 35 ± 3 |
| Ejection fraction (%) | 78 ± 4 | 76 ± 3 | 66 ± 4* | 65 ± 3** | 83 ± 2 |
| Fractional shortening (%) | 47 ± 4 | 44 ± 2 | 36 ± 3* | 35 ± 2 | 51 ± 2 |
| Cardiac output (ml/min) | 14.52 ± 1.19 | 13.58 ± 0.73 | 11.81 ± 1.40 | 9.59 ± 0.97** | 13.87 ± 1.14 |
| IVRT (ms) | 19.41 ± 0.60 | 23.66 ± 0.53* | 24.06 ± 0.50* | 26.90 ± 0.60**,# | 20.15 ± 0.52** |
A significant increase in isovolume relaxation time (IVRT) was displayed in the mutant transgenic (TG) heart compared to the wild-type (WT) heart under physiological (untreated) conditions. Cardiac functions deteriorated after Ang II treatment. Diastolic dysfunction was improved by Fasudil treatment. HR, heart rate; d: diastolic; s, systolic; IVS, interventricular septum thickness; LVID, left ventricle inner dimension; LVPW, left ventricle posterior wall thickness. n = numbers of 8- to 12-wk-old male mice used for analysis.
P < 0.05 vs. untreated WT;
P < 0.05 vs. untreated TG;
P < 0. vs. Ang II-treated WT.
Mice expressing truncated Rho kinase ROCKΔ1 are predisposed to stress, and treatment with the Rho kinase inhibitor attenuates ROCKΔ1-mediated fibrotic cardiomyopathy
To demonstrate that the transgenic ROCKΔ1 mice were hypersensitive to fibrotic stimuli, animals were treated with ANG II. As expected, the wild-type mice developed noticeable cardiac fibrosis. The ROCKΔ1 transgenic mice, however, developed much more severe cardiac fibrosis compared with the controls (Fig. 2D, E). Consistent with the histology change, a further prolonged IVRT from 23.66 to 26.90 ms was detected in the mutant mice, indicating a deteriorated diastolic dysfunction (Table 1, ANG II treated). A compromised systolic function was also detected in both groups after ANG II treatment. This result suggested that the mutant hearts were predisposed to fibrotic stress.
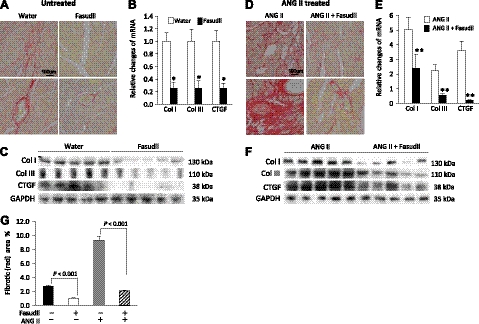
Fasudil is a known clinically used Rho kinase inhibitor. To understand whether the cardiac fibrosis in the transgenic animal was directly associated with activated Rho kinase, animals with and without ANG II challenge were treated with Fasudil. As shown in Fig. 3, Fasudil treatment reduced the development of cardiac fibrosis in the transgenic mice under both the baseline (Fig. 3A) and ANG II treatment conditions (Fig. 3D). Fasudil treatment also significantly curtailed increases in collagen I, collagen III, and CTGF transcripts (Fig. 3B, E) and their protein expression levels (Fig. 3C, F). Histology quantification (Fig. 3G) showed that without ANG II challenge, collagen deposition in the mutant heart, after Fasudil treatment, returned to 1 from 2.8% (relative to the wild-type mouse baseline level in Fig. 2D). Diastolic function also improved (Table 1, Fasudil treated). In ANG II-treated mutant mice, the fibrosis area also declined from 9.3 to 2.1% (Fig. 3G), suggesting that the Rho kinase activation was responsible for the fibrotic cardiomyopathy and that inhibition of the Rho kinase activity attenuated the fibrotic phenotype and rescued cardiac diastolic function.
Figure 3.
A–F) Cardiac fibrosis in the transgenic heart, both untreated (A–C) and treated with ANG II (D–F), was attenuated by the Rho kinase inhibitor Fasudil. Significant decreases in collagen depositions (A, D; Sirius red staining) and fibrotic factors were observed after Fasudil treatment in the transgenic hearts analyzed by qPCR (B, E) and immunoblot analysis (C, F). qPCR data were pooled from 10 mice/group with triplicate analyses for each mouse. G) Quantification of fibrotic area over total analyzed heart area. *P < 0.001 vs. water-treated group; **P < 0.001 vs. ANG II-treated group.
To rule out the possibility that the development of fibrotic cardiomyopathy in the ROCKΔ1 transgenic heart was due to a nonspecific disruption of cardiac function during transgene introduction, an additional transgenic mouse was generated, ROCKΔ1-KD, expressing truncated ROCK1 with the kinase deficiency. No increase in MYPT1 phosphorylation was detected in this control transgenic mouse (Supplemental Fig. S1A), indicating that the Rho kinase activity remained at the same level as in the wild-type animal. No obvious cardiac fibrosis was observed in the control transgenic heart (Supplemental Fig. S1B), and no hypersensitivity to ANG II treatment, compared with the wild-type control, was observed in the kinase-deficient transgenic animals (Supplemental Fig. S1C). The mRNA levels of collagen I, collagen III, CTGF, and TGFβ1 in the heart (Supplemental Fig. S1D) and the serum concentrations of TGF β1 (Supplemental Fig. S1E) and IL-1β (Supplemental Fig. S1F) in ROCKΔ1-KD mice remained at the same levels as in the wild-type controls. Echocardiography analysis showed no cardiac dysfunction in the ROCKΔ1-KD heart (Supplemental Table S2).
TGFβ1 is a new target gene of SRF, and ROCK1 positively regulates TGFβ1 expression through SRF activation
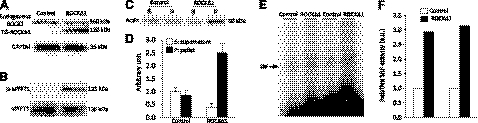
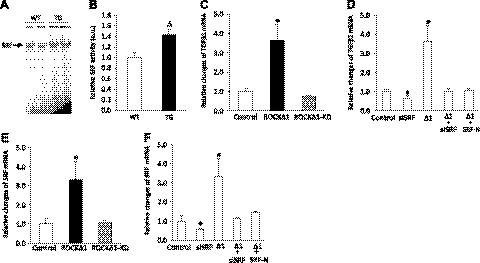
TGFβ1 is a key mediator of fibrogenic response in many tissues. To explore the molecular mechanism underlying the Rho kinase-mediated cardiac fibrosis, we investigated transcriptional regulation of the TGFβ1 gene. It is well known that Rho kinase-controlled actin dynamics modulates SRF activity in non-CMs (14–16). To test whether this were the case in CMs, we transduced a ROCKΔ1 expression plasmid into neonatal rat CMs. The ROCKΔ1 protein expression (Fig. 4A) was accompanied by an increase in the Rho kinase activity detected by hyperphosphorylation of MYPT1 (Fig. 4B). The ratio between polymerized F-actin and unpolymerized G-actin increased with the introduction of ROCKΔ1 (Fig. 4C, D). In parallel, we detected an enhanced SRF binding activity in the same cohort of CMs (Fig. 4E, F). Thus, these data from CMs are consistent with the earlier results from non-CMs, that the Rho kinase activation facilitates actin assembly, which eventually leads to SRF activation.
Figure 4.
ROCKΔ1 facilitated actin assembly and promoted SRF activation in cardiomyocytes. A, B) Immunoblot displayed ROCKΔ1 (TG-ROCKΔ1) protein expression by transient transfection in cardiomyocytes with the ROCKΔ1 expression plasmid (A), which led to an obvious increase in the Rho kinase activity shown by MYPT1 hyperphosphorylation (B). C) A significant increase in the ratio between polymerized F-actin (in a pellet) and unpolymerized G-actin (in a supernatant) was detected by ROCKΔ1 transfection. D) Immunoblot densitometry was summarized from 2 repeated experiments. E) Same cohort of cardiomyocytes showed enhanced SRF activity in the EMSA assay. F) Densitometry was summarized from E. S, supernatant; P, pellet.
Next, we assessed the DNA binding activity of SRF by the EMSA assay, in which the c-fos SRE served as an SRF-binding site. Figure 5A, B shows that compared with the wild-type control heart, SRF activity in the nuclear extract from the transgenic heart was ∼40% higher. Since the TGFβ1 expression more than doubled in the transgenic hearts compared with the wild-type hearts (Fig. 2B), we hypothesized that SRF might be involved in ROCKΔ1-mediated TGFβ1 regulation. To test this hypothesis, we transfected C2C12 myoblasts with the ROCKΔ1 expression plasmid and found a manifest increase in the TGFβ1 mRNA (Fig. 5C), consistent with the observation made in ROCKΔ1 transgenic heart presented in Fig. 2B. We then knocked down SRF expression to about half of the control level using the short interfering (si)RNA and found that ROCKΔ1-mediated TGFβ1 up-regulation was diminished (Fig. 5D), suggesting that TGFβ1 might be an SRF target gene and thus positively regulated by SRF. In addition, the TGFβ1 regulation by SRF was demonstrated by introducing SRF-N, a dominant negative SRF isoform (11). The ROCKΔ1-mediated up-regulation of TGFβ1 expression was impeded when cells were cotransfected with the dominant negative SRF-N expression plasmid (Fig. 5D). For an additional control, the kinase-deficient ROCKΔ1 mutant isoform ROCKΔ1-KD was used, which failed to increase the TGFβ1 transcript level (Fig. 5C). Consistent with our mouse study, transient transfection of ROCKΔ1 but not ROCKΔ1-KD also resulted in up-regulation of the SRF transcript (Fig. 5E), and this up-regulation was attenuated by SRF knockdown or by SRF-N (Fig. 5F) since SRF is a self-regulated transcription factor. These data provide evidence that SRF participates in ROCKΔ1-mediated TGFβ1 up-regulation.
Figure 5.
Up-regulation of TGFβ and SRF by ROCKΔ1. A, B) Enhanced SRF binding to the c-fos SRE was assessed by EMSA assay in the transgenic (TG) heart (A), and the assay intensity was quantified from 6 mice/group (B). C–F) qPCR analysis of TGFβ1 and SRF transcripts. Significant increases in TGFβ1 (C, D) and SRF (E, F) mRNA levels were observed in C2C12 myoblasts transfected with the ROCKΔ1 expression plasmid (C, E), and both increases were diminished by the SRF deficiency induced by the siRNA specific for SRF or by a dominant negative SRF-N (D, F). qPCR data were pooled from 3 repeated experiments in each group with triplicate analyses for each time. Δ1, ROCKΔ1; ROCKΔ1KD, mutant ROCKΔ1 with kinase deficiency; SRF-N, N-terminal SRF (a dominant negative SRF isoform); a.u., arbitrary unit. *P < 0.01 vs. control; ΔP < 0.01 vs. wild type (WT).
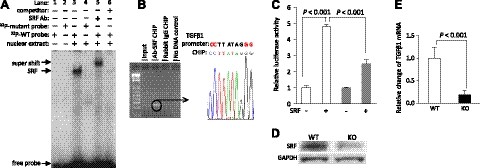
To verify the regulatory role of SRF in TGFβ1 expression, we analyzed the 5-kb sequence upstream of the TGFβ1 gene using computational biology approaches and identified 5 putative SRF-binding sites, SRE1 to SRE5. The SRE consensus sequence is CC(A/T)6GG with an AT-rich middle core. To determine whether SRF binds to the predicted SREs, EMSA assay was performed. 32P-labeled DNA duplexes containing the SREs were used as probes for the EMSA screen (11). A strong bandshift was observed only for SRE3 (data not shown). This suggested that SRF might bind to the TGFβ1 promoter via SRE3. To verify the SRF-SRE3 interaction, a comprehensive EMSA was conducted (Fig. 6A). As expected, a bandshift was observed on incubation of SRF-containing nuclear extract the wild-type SRE3 (lane 3), while no SRF binding was observed to the mutant SRE3 probe (lane 4), thus validating the binding specificity. A supershift band was obtained by adding an anti-SRF antibody (lane 5), confirming the SRF-SRE3 interaction. Finally, an excess of unlabeled wild-type SRE3 probe was included in the SRF-SRE3 binding reaction, which resulted in the abolishment of the SRF-SRE3 bandshift (lane 6). This experiment confirmed that SRF was able to bind SRE motif in the TGFβ1 promoter region in vitro.
Figure 6.
Analysis of the TGFβ1 promoter region revealed TGFβ1 as a direct target gene of SRF. A, B) SRF binding to the SRE site in the TGFβ1 promoter region was demonstrated by the EMSA assay in vitro (A) and verified by the ChIP assay with sequencing assessment in vivo (B). C) Addition of SRF activated luciferase activity driven by TGFβ1 promoter, and mutation of the SRE (solid gray bar) diminished the SRF-mediated reporter activity. Data represent the average of 3 experiments with triplicate measurements in every experiment. D) A significant decrease in the SRF protein expression was achieved in the cardiac-specific SRF-knockout heart. E) In parallel with the decline in the SRF expression level, repressed TGFβ1 transcript was detected in the SRF-null heart. qPCR data represent the average of 10 mice/group.
To verify that SRF interacts with the TGFβ1 promoter region in vivo, ChIP assay was performed (13). The specific SRF-DNA complex was immunoprecipitated using an anti-SRF antibody. Sequencing analysis revealed that the DNA fragment isolated in the complex with SRF contained part of the TGFβ1 5′-upstream sequence with the SRE3 site (Fig. 6B). Thus, the in vivo interaction of SRF with the SRE3 site in the TGFβ1 promoter region was confirmed.
To evaluate functional significance of SRF interaction with TGFβ1 promoter, the 5-kb fragment of the TGFβ1 5′-upstream sequence was subcloned into a reporter vector to control luciferase expression. The magnitude of the SRF effect on transcriptional regulation can be deduced from the changes in luciferase activity. Cotransfection of the reporter and SRF-expressing vectors led to a >4-fold increase in luciferase activity compared with the baseline control without SRF expression (Fig. 6C). In a parallel experiment, the proactive effect of SRF on TGFβ1 promoter was significantly attenuated by the mutation of the SRE3 in the TGFβ1 promoter. These results suggest that the TGFβ1 is regulated by SRF.
To further demonstrate TGFβ1 as an SRF direct target gene, we assessed the TGFβ1 expression in the myocardium of cardiac-specific SRF-knockout mice by using a conditional SRF allele harboring loxP sites (SRFloxP/loxP) flanking SRF exon 1 (17) and an inducible αMHC-MerCre transgene that activates in CMs to delete the SRFloxP/loxP allele (13). The mouse hearts were analyzed 10 wk after tamoxifen induction. Based on the Western blot analysis, the SRF level in the heart was reduced by >90% (Fig. 6D). Along with the decrease in SRF expression, the TGFβ1 mRNA level in the SRF knockout heart declined to ∼20% of the control heart level (Fig. 6E), in support of the above conclusion. Taken together, TGFβ1 is one of the genes regulated by SRF, and ROCKΔ1-mediated SRF activation contributes to TGFβ1 up-regulation.
Profibrotic TGFβ1 and NF-κB signaling are activated in the transgenic heart and contribute to the ROCKΔ1-mediated fibrotic cardiomyopathy
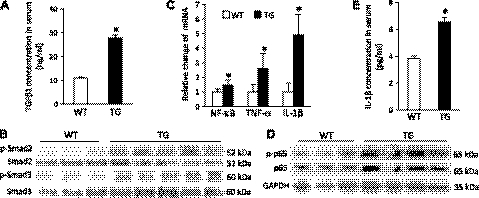
A >2-fold increase in TGFβ1 concentration in serum was detected in the ROCKΔ1 transgenic animals compared with the wild-type controls (Fig. 7A). The downstream proteins Smad2 and Smad3 were hyperphosphorylated in the transgenic heart (Fig. 7B). Meanwhile, qPCR showed up-regulation of the NF-κB transcript level (Fig. 7C), whereas an immunoblot analysis showed a general increase in the protein p65 level and its hyperphosphorylation in the transgenic heart (Fig. 7D). In parallel with the enhanced TGFβ and NF-κB signaling, analyses of the proinflammatory cytokines TNF-α, IL-1β in the heart (Fig. 7C), and IL-1β in serum (Fig. 7E) showed significant increases in concentrations of these cytokines in the transgenic animals.
Figure 7.
Activation of TGFβ1 and proinflammatory NF-κB signaling in the transgenic animals. Marked increases in TGFβ1 (A) and IL-1β (E) serum concentrations in transgenic mice were observed compared with the wild-type (WT) mice. Western blots revealed hyperphosphorylated Smad2 (B) and p65 and up-regulated p65 protein expression (D) in transgenic (TG) hearts, and proinflammatory factors were analyzed by qPCR (C). Data were pooled from 10 mice/group with triplicate analyses for each mouse. *P < 0.01 vs. WT.
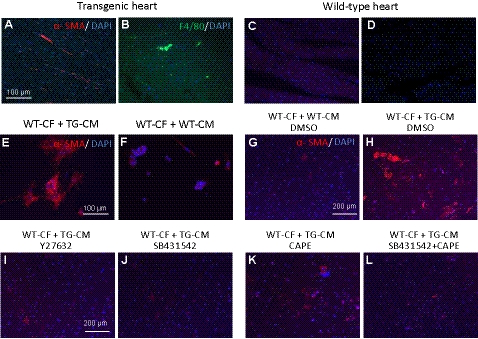
The fibrotic and inflammatory responses in the mutant heart were further characterized by the expression of α-SMA and macrophage marker F4/80. Both markers were overtly displayed by immunostaining in the transgenic heart (Fig. 8A, B) compared with the wild-type heart (Fig. 8C, D). The up-regulation of α-SMA mRNA was quantified by qPCR analysis, which showed a >50% increase in the level of α-SMA transcript in the transgenic heart compared with the wild-type heart (Supplemental Fig. S2A).
Figure 8.
Activation of cardiac fibroblasts and inflammatory response in the transgenic heart, and attenuation of the fibrotic response by Rho kinase, TGFβ, and NF-κB signaling inhibitors. A–D) Visualization of α-SMA and macrophage marker F4/80 expression in left ventricles by immunostaining showed that both factors were up-regulated in the transgenic (TG) heart (A, B) compared with the wild type (WT; C, D). E–H) α-SMA expression was also induced in the WT CFs when cocultured with transgenic CMs (E, H) but not with the WT cardiomyocytes (F, G). I–L) Induction of α-SMA was attenuated by treatment with inhibitors Y27632 (I), SB431542 (J), CAPE (K), or both SB431542 and CAPE (L), respectively.
To elucidate the molecular signaling by which the activation of Rho kinase in CMs of the ROCKΔ1 transgenic heart initiates the fibrotic response, the wild-type CFs were cocultured with transgenic CMs. As a control, wild-type CFs were also cocultured with wild-type CMs. The α-SMA expression was assessed by immunostaining. Large amounts of CFs were activated and induced to express α-SMA by the transgenic CMs (Fig. 8E) but not by the wild-type CMs (Fig. 8F). Meanwhile, other fibrotic factors, including α-SMA, were assessed by qPCR, and all of them were significantly up-regulated in the same cohort of cocultured cells (Supplemental Fig. S2B). Under the same conditions, when we added the Rho kinase inhibitor Y27632 (Fig. 8I), the TGFβ receptor 1 inhibitor SB431542 (Fig. 8J), the NF-κB inhibitor CAPE (Fig. 8K), or a combination of SB431542 and CAPE (Fig. 8L) to the culture medium, the α-SMA expression induced in the wild-type fibroblasts by transgenic CMs was significantly diminished. These results support the hypothesis that the profibrotic TGFβ1 and NF-κB signaling contribute to the ROCKΔ1-mediated fibrotic cardiomyopathy.
DISCUSSION
Role of Rho kinase in heart diseases
Rho kinase activity has been shown to closely associate with various cardiovascular diseases. Up-regulation of the Rho kinase expression and activity was observed in the failing hearts of Dahl salt-sensitive hypertensive rats (18) and in hypertrophic rat hearts induced by angiotensin II (19). Administration of Rho kinase inhibitors led to the regression of pathological remodeling and improvement of endothelial function in both rat models (18, 19). In our previous study, constitutive activation of Rho kinase, due to its cleavage by caspase-3 to produce a protein (ROCKΔ1) that lacks its inhibitory C-terminal domain, was observed in the failing myocardium from heart failure patients and was reversed by ventricular unloading (7). In this study, we established a unique mouse model expressing ROCKΔ1 to mimic the conditions in human heart failure patients. Mutant mice developed overt fibrotic cardiomyopathy with diastolic dysfunction recapitulating cardiac remodeling progress in patients with heart failure. The transgenic heart was predisposed to the stress challenge. Treatment with the clinically available Rho kinase inhibitor Fasudil reduced the fibrotic development. These results provided direct evidence that activation of Rho kinase in CMs per se is sufficient to initiate pathological cardiac remodeling.
Communication between CMs and CFs in pathological cardiac remodeling
In our transgenic ROCKΔ1 animal, a >2-fold increase in TGFβ1 expression was observed in both myocardium and circulating serum along with Smad2 and Smad3 phosphorylation. In the coculture study, the transgenic CMs facilitated cardiac fibroblast differentiation. An addition of TGFβ receptor 1 inhibitor blocked the transgenic cardiomyocyte-mediated fibroblast maturation, suggesting that TGFβ1 is a critical mediator between CMs and CFs.
TGFβ1 is an important cytokine in mediating fibrogenic response, including cardiac fibrosis (20, 21). TGFβ1 protein is secreted through the autocrine/paracrine pattern by most mammalian cells including CMs. Many stress stimuli, such as ANG II, myocardial infarction, and pressure overload can activate TGFβ1 signaling, initiate fibrogenic process, and eventually result in excessive collagen deposition (22). In cardiac fibrosis, TGFβ1 is involved in facilitating differentiation of CFs into myofibroblasts, increasing collagen synthesis, recruiting bone marrow-derived endothelial cells, promoting endothelial-to-mesenchymal transition, and facilitating inflammatory cells infiltration and cytokine synthesis, etc. (23, 24).
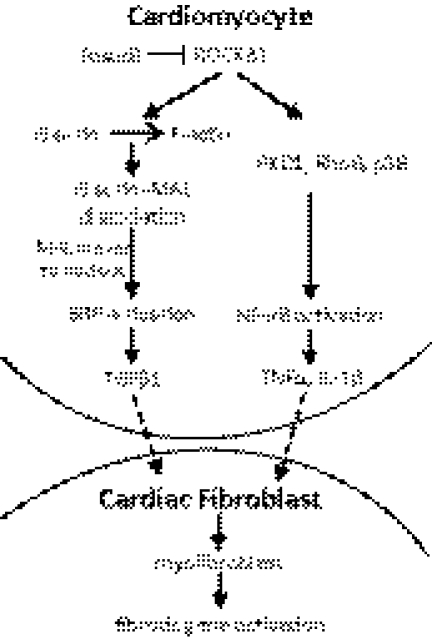
Up-regulation of a subset of profibrotic cytokines including TNF-α and IL-1β in ROCKΔ1 transgenic heart is another cardiac fibrosis mechanism revealed by this study. Increases in the NF-κB activity and expression level promoted these profibrotic cytokines. The NF-κB signaling inhibitor blocked ROCKΔ1-mediated fibrotic gene expression. Recent studies demonstrated that inhibition of RhoA/ROCK signaling blocked the activation of NF-κB by preventing the phosphorylation of NF-κB (Ser-536) and IkBα in endothelial cells (25). The protein kinase D1 and RhoB were identified as mediators that relayed ROCK-induced NF-κB activation (26, 27). Rho kinase was also shown to activate p38 MAP kinase in macrophages (28), which could potentiate NF-κB activity. Individual activation of p38 MAP and NF-κB has been demonstrated to closely associate with cardiac fibrosis. Their profibrotic effects were shown in animal experiments on overexpression of p38 MAP or inhibition of NF-κB (29–31). Note that the inhibitory effect of CAPE in the coculture experiment was not as potent as that of SB431542, suggesting a possible additional indirect role of NF-κB in this Rho kinase-induced fibrotic response, which should be investigated in the future study. Taken together, we propose the mechanism that activation of TGFβ1 and NF-κB signaling along with a release of a subset of potent cytokines from CMs induces cardiac fibroblast differentiation and facilitates collagen deposition and macrophage infiltration. Both pathways synergistically contribute to the Rho-kinase-mediated pathological fibrosis (Fig. 9).
Figure 9.
Proposed mechanism of ROCKΔ1-mediated fibrotic program. In cardiomyocytes, active ROCK1 facilitates actin assembly and potentiates SRF activity by promoting an entry of the SRF accessory factor MAL into the nucleus to form the SRF-MAL complex. SRF binds to the TGFβ1 promoter/enhancer, thus directing the TGFβ1 expression. Meanwhile, Rho kinase activates the NF-κB signaling and induces a subset of cytokines indicative of the profibrotic and inflammatory program. Activation of both signaling pathways initiates a fibrotic response in cardiac fibroblasts and promotes fibrotic remodeling. PKD1, protein kinase D1.
Stress-responsive regulation of TGFβ1 by ROCK1 via SRF
Despite accumulating evidence of the linkage between TGFβ1 and Rho kinase activation, the precise mechanism of TGFβ1 activation by activated ROCK1 is unclear. There have been limited studies focused on the cis-regulation of TGFβ1. Several early studies revealed the TGFβ1 self-activation mechanism through transcription factor AP-1 in cancer or fibroblast cell lines (32, 33). The self-activation mechanism was also suggested to contribute to the RhoA/Rho kinase-mediated TGFβ1 signaling activity in glomerular mesangial cells, specialized kidney pericytes (34). However, the question still remains as to whether Rho kinase activates AP-1.
In this study, we have demonstrated a new cis-regulatory element of TGFβ1, the SRF-dependent TGFβ1 regulation. SRF is an ancient transcription factor that regulates a broad range of genes. At least 3 signaling pathways are known to modulate its activity. One involves Ets transcription factors, the second involves SRF phosphorylation, and the third is through the Rho kinase-controlled actin dynamics (14–16, 35). The detailed mechanism of Rho kinase-mediated SRF activation has been well documented in non-CMs (14–16, 36). The activation of ROCK1 facilitates actin assembly from unpolymerized G-actin to polymerized F-actin. The dissociation of G-actin-MAL (myocardin-related protein) complex results in MAL localization in the nucleus. MAL is an SRF cofactor; the formation of SRF-MAL enhances SRF-dependent expression of genes (Fig. 9 and ref. 37).
In this study, we also observed such Rho kinase-mediated actin dynamics and its consequential SRF activation in neonatal rat CMs. Based on these observations, we hypothesized that SRF might be a candidate transcription factor involved in transcriptional regulation of TGFβ1. Using bioinformatics and EMSA, luciferase, and ChIP analyses, we demonstrated an evolutionarily conserved, cis-regulatory element containing the SRF-binding site in the promoter/enhancer region of the TGFβ1 gene. The regulatory role of SRF in TGFβ1 expression was further demonstrated in vivo by genetic deletion of SRF in the mouse heart, which resulted in a marked decrease in the TGFβ1 transcript level. Consistent with this SRF-null animal study, cells lacking SRF expression failed to respond to the ROCKΔ1-mediated TGFβ1 up-regulation. These results reveal a novel regulatory mechanism of TGFβ1 and explain how Rho kinase initiates TGFβ1 signaling pathway (Fig. 9).
To summarize, here we demonstrated that ROCKΔ1 is a novel profibrotic factor. Mouse hearts expressing ROCKΔ1 developed spontaneous fibrotic cardiomyopathy. Coculture studies of CMs with CFs revealed that activation of TGFβ1 and NF-κB signaling contributed to the ROCKΔ1-mediated fibrotic program. The transcriptional regulation and knockout animal studies revealed that TGFβ1 is a new target gene of SRF. ROCKΔ1 initiated the TGFβ1 signaling through modulation of SRF activity (Fig. 9). These results provide a mechanistic basis for therapeutic applications of Rho kinase inhibitors in human heart failure treatments. Our finding that ROCKΔ1 is a potent profibrotic factor has a clinical significance. Certainly, long-term caspase inhibition has severe side effects. However, selective inhibition of ROCK1 combined with a short-term treatment with caspase inhibitors might have significant beneficial effects and therapeutic potential.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Vladimir N. Potaman and Alexis M. Boggs for editorial assistance on the manuscript.
This work was supported by U.S. National Heart, Lung, and Blood Institute grants R01-HL-102314, R21-HL-094844, and K02-HL-098956 and American Heart Association grant-in-aid 0855030F (to J.C.); and National Science Foundation of China grant NSFC 30860103 (to Q.L.). The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- α-MHC
- α-myosin heavy chain
- ANG II
- angiotensin II
- ANP
- atrial natriuretic peptide
- CAPE
- caffeic acid phenylethyl ester
- CF
- cardiac fibroblast
- ChIP
- chromatin immunoprecipitation
- CM
- cardiomyocyte
- EMSA
- electrophoretic mobility shift assay
- IVRT
- isovolume relation time
- MAL
- myocardin-related protein
- MLC
- myosin light chain
- MYPT1
- myosin phosphatase target subunit 1
- ROCK1
- Rho-associated coiled-coil protein kinase 1
- ROCKΔ1
- cleaved Rho-associated coiled-coil protein kinase 1 isoform (N-terminal part)
- ROCKΔ1-KD
- kinase-deficient cleaved Rho-associated coiled-coil protein kinase 1 isoform
- SRE
- serum response element
- SRF
- serum response factor
- SRF-N
- N-terminal serum response factor
- SMA
- smooth muscle actin.
REFERENCES
- 1. Zhang Y. M., Bo J., Taffet G. E., Chang J., Shi J., Reddy A. K., Michael L. H., Schneider M. D., Entman M. L., Schwartz R. J., Wei L. (2006) Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J. 20, 916–925 [DOI] [PubMed] [Google Scholar]
- 2. Rikitake Y., Oyama N., Wang C. Y., Noma K., Satoh M., Kim H. H., Liao J. K. (2005) Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/- haploinsufficient mice. Circulation 112, 2959–2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haudek S. B., Gupta D., Dewald O., Schwartz R. J., Wei L., Trial J., Entman M. L. (2009) Rho kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovasc. Res. 83, 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sebbagh M., Renvoize C., Hamelin J., Riche N., Bertoglio J., Breard J. (2001) Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat. Cell Biol. 3, 346–352 [DOI] [PubMed] [Google Scholar]
- 5. Coleman M. L., Sahai E. A., Yeo M., Bosch M., Dewar A., Olson M. F. (2001) Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol. 3, 339–345 [DOI] [PubMed] [Google Scholar]
- 6. Dorn G. W., 2nd (2009) Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc. Res. 81, 465–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang J., Xie M., Shah V. R., Schneider M. D., Entman M. L., Wei L., Schwartz R. J. (2006) Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc. Natl. Acad. Sci. U. S. A. 103, 14495–14500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hattori T., Shimokawa H., Higashi M., Hiroki J., Mukai Y., Tsutsui H., Kaibuchi K., Takeshita A. (2004) Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation 109, 2234–2239 [DOI] [PubMed] [Google Scholar]
- 9. Zhai Y., Gao X., Wu Q., Peng L., Lin J., Zuo Z. (2008) Fluvastatin decreases cardiac fibrosis possibly through regulation of TGF-beta(1)/Smad 7 expression in the spontaneously hypertensive rats. Eur. J. Pharmacol. 587, 196–203 [DOI] [PubMed] [Google Scholar]
- 10. Li Q., Lin X., Yang X., Chang J. (2010) NFATc4 is negatively regulated in miR-133a-mediated cardiomyocyte hypertrophic repression. Am. J. Physiol. Heart Circ. Physiol. 298, H1340–H1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang J., Wei L., Otani T., Youker K. A., Entman M. L., Schwartz R. J. (2003) Inhibitory cardiac transcription factor, SRF-N, is generated by caspase 3 cleavage in human heart failure and attenuated by ventricular unloading. Circulation 108, 407–413 [DOI] [PubMed] [Google Scholar]
- 12. Arsenian S., Weinhold B., Oelgeschlager M., Ruther U., Nordheim A. (1998) Serum response factor is essential for mesoderm formation during mouse embryogenesis. EMBO J. 17, 6289–6299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin X., Yang X., Li Q., Ma Y., Cui S., He D., Schwartz R. J., Chang J. (2012) Protein tyrosine phosphatase-like a regulates myoblast proliferation and differentiation through myog and cell cycling signaling pathway. Mol. Cell. Biol. 32, 297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sotiropoulos A., Gineitis D., Copeland J., Treisman R. (1999) Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell 98, 159–169 [DOI] [PubMed] [Google Scholar]
- 15. Miralles F., Posern G., Zaromytidou A. I., Treisman R. (2003) Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113, 329–342 [DOI] [PubMed] [Google Scholar]
- 16. Vartiainen M. K., Guettler S., Larijani B., Treisman R. (2007) Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316, 1749–1752 [DOI] [PubMed] [Google Scholar]
- 17. Wiebel F. F., Rennekampff V., Vintersten K., Nordheim A. (2002) Generation of mice carrying conditional knockout alleles for the transcription factor SRF. Genesis 32, 124–126 [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi N., Horinaka S., Mita S., Nakano S., Honda T., Yoshida K., Kobayashi T., Matsuoka H. (2002) Critical role of Rho-kinase pathway for cardiac performance and remodeling in failing rat hearts. Cardiovasc. Res. 55, 757–767 [DOI] [PubMed] [Google Scholar]
- 19. Higashi M., Shimokawa H., Hattori T., Hiroki J., Mukai Y., Morikawa K., Ichiki T., Takahashi S., Takeshita A. (2003) Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ. Res. 93, 767–775 [DOI] [PubMed] [Google Scholar]
- 20. Li G., Li R. K., Mickle D. A., Weisel R. D., Merante F., Ball W. T., Christakis G. T., Cusimano R. J., Williams W. G. (1998) Elevated insulin-like growth factor-I and transforming growth factor-beta 1 and their receptors in patients with idiopathic hypertrophic obstructive cardiomyopathy. A possible mechanism. Circulation 98, II144–149; discussion II149–150 [PubMed] [Google Scholar]
- 21. Waltenberger J., Lundin L., Oberg K., Wilander E., Miyazono K., Heldin C. H., Funa K. (1993) Involvement of transforming growth factor-beta in the formation of fibrotic lesions in carcinoid heart disease. Am. J. Pathol. 142, 71–78 [PMC free article] [PubMed] [Google Scholar]
- 22. Leask A. (2007) TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc. Res. 74, 207–212 [DOI] [PubMed] [Google Scholar]
- 23. Deten A., Volz H. C., Briest W., Zimmer H. G. (2002) Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc. Res. 55, 329–340 [DOI] [PubMed] [Google Scholar]
- 24. Nian M., Lee P., Khaper N., Liu P. (2004) Inflammatory cytokines and postmyocardial infarction remodeling. Circ. Res. 94, 1543–1553 [DOI] [PubMed] [Google Scholar]
- 25. Anwar K. N., Fazal F., Malik A. B., Rahman A. (2004) RhoA/Rho-associated kinase pathway selectively regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via activation of I kappa B kinase beta and phosphorylation of RelA/p65. J. Immunol. 173, 6965–6972 [DOI] [PubMed] [Google Scholar]
- 26. Cowell C. F., Yan I. K., Eiseler T., Leightner A. C., Doppler H., Storz P. (2009) Loss of cell-cell contacts induces NF-kappaB via RhoA-mediated activation of protein kinase D1. J. Cell. Biochem. 106, 714–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez P. L., Sahay S., Olabisi O. O., Whitehead I. P. (2007) ROCK I-mediated activation of NF-kappaB by RhoB. Cell. Signal. 19, 2361–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lemarie A., Bourdonnay E., Morzadec C., Fardel O., Vernhet L. (2008) Inorganic arsenic activates reduced NADPH oxidase in human primary macrophages through a Rho kinase/p38 kinase pathway. J. Immunol. 180, 6010–6017 [DOI] [PubMed] [Google Scholar]
- 29. Liao P., Georgakopoulos D., Kovacs A., Zheng M., Lerner D., Pu H., Saffitz J., Chien K., Xiao R. P., Kass D. A., Wang Y. (2001) The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc. Natl. Acad. Sci. U. S. A. 98, 12283–12288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li M., Georgakopoulos D., Lu G., Hester L., Kass D. A., Hasday J., Wang Y. (2005) p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation 111, 2494–2502 [DOI] [PubMed] [Google Scholar]
- 31. Onai Y., Suzuki J., Maejima Y., Haraguchi G., Muto S., Itai A., Isobe M. (2007) Inhibition of NF-{kappa}B improves left ventricular remodeling and cardiac dysfunction after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 292, H530–H538 [DOI] [PubMed] [Google Scholar]
- 32. Kim S. J., Angel P., Lafyatis R., Hattori K., Kim K. Y., Sporn M. B., Karin M., Roberts A. B. (1990) Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol. Cell. Biol. 10, 1492–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim S. J., Jeang K. T., Glick A. B., Sporn M. B., Roberts A. B. (1989) Promoter sequences of the human transforming growth factor-beta 1 gene responsive to transforming growth factor-beta 1 autoinduction. J. Biol. Chem. 264, 7041–7045 [PubMed] [Google Scholar]
- 34. Peng F., Wu D., Gao B., Ingram A. J., Zhang B., Chorneyko K., McKenzie R., Krepinsky J. C. (2008) RhoA/Rho-kinase contribute to the pathogenesis of diabetic renal disease. Diabetes 57, 1683–1692 [DOI] [PubMed] [Google Scholar]
- 35. Iyer D., Chang D., Marx J., Wei L., Olson E. N., Parmacek M. S., Balasubramanyam A., Schwartz R. J. (2006) Serum response factor MADS box serine-162 phosphorylation switches proliferation and myogenic gene programs. Proc. Natl. Acad. Sci. U. S. A. 103, 4516–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuwahara K., Barrientos T., Pipes G. C., Li S., Olson E. N. (2005) Muscle-specific signaling mechanism that links actin dynamics to serum response factor. Mol. Cell. Biol. 25, 3173–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Small E. M., Thatcher J. E., Sutherland L. B., Kinoshita H., Gerard R. D., Richardson J. A., Dimaio J. M., Sadek H., Kuwahara K., Olson E. N. (2010) Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ. Res. 107, 294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.